Foliar Application of Selenium Reduces Cadmium Accumulation in Walnut Seedlings
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Growth Conditions
2.2. Determination of Cd and Se
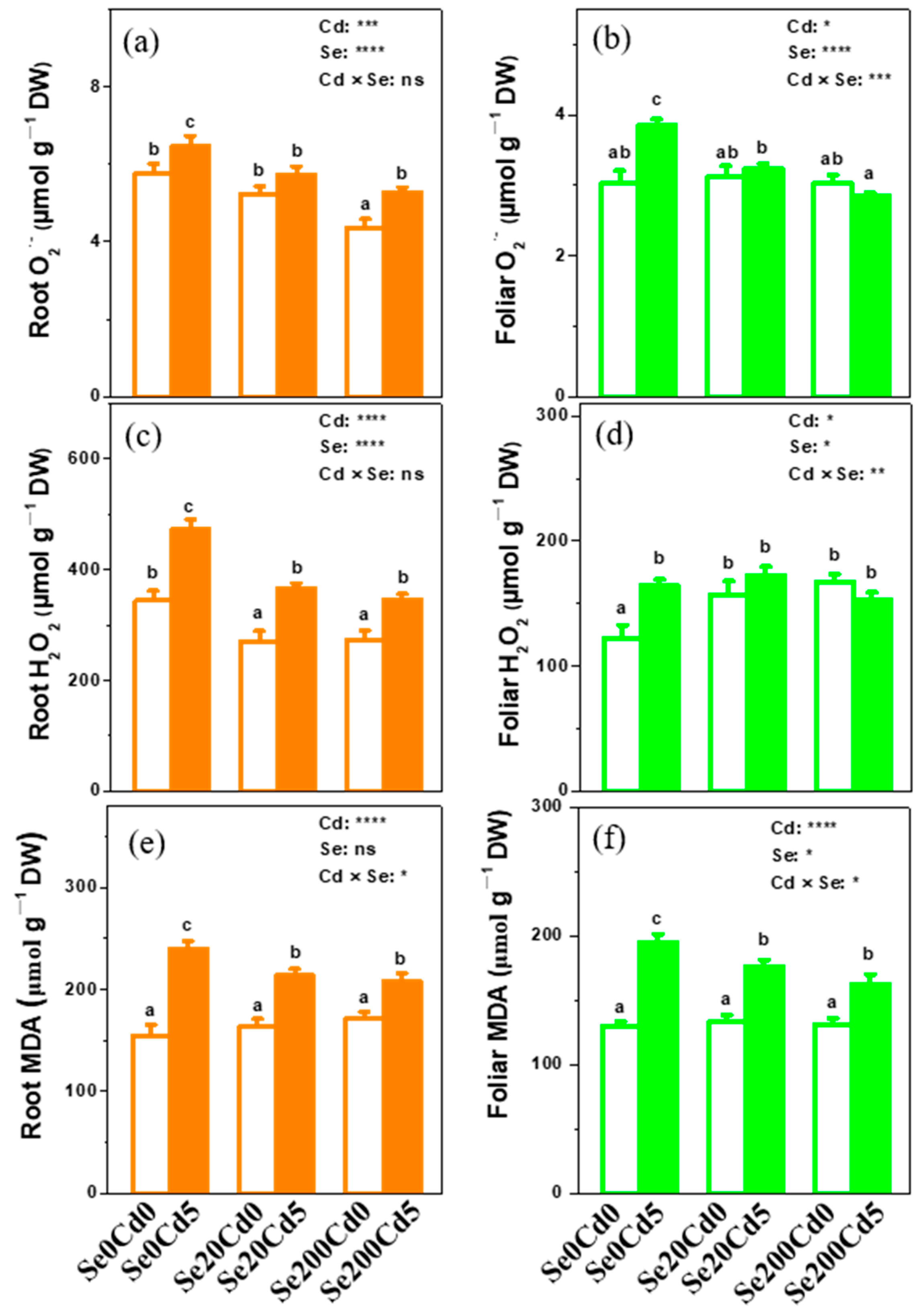
2.3. Analysis of O2•−, H2O2, and MDA
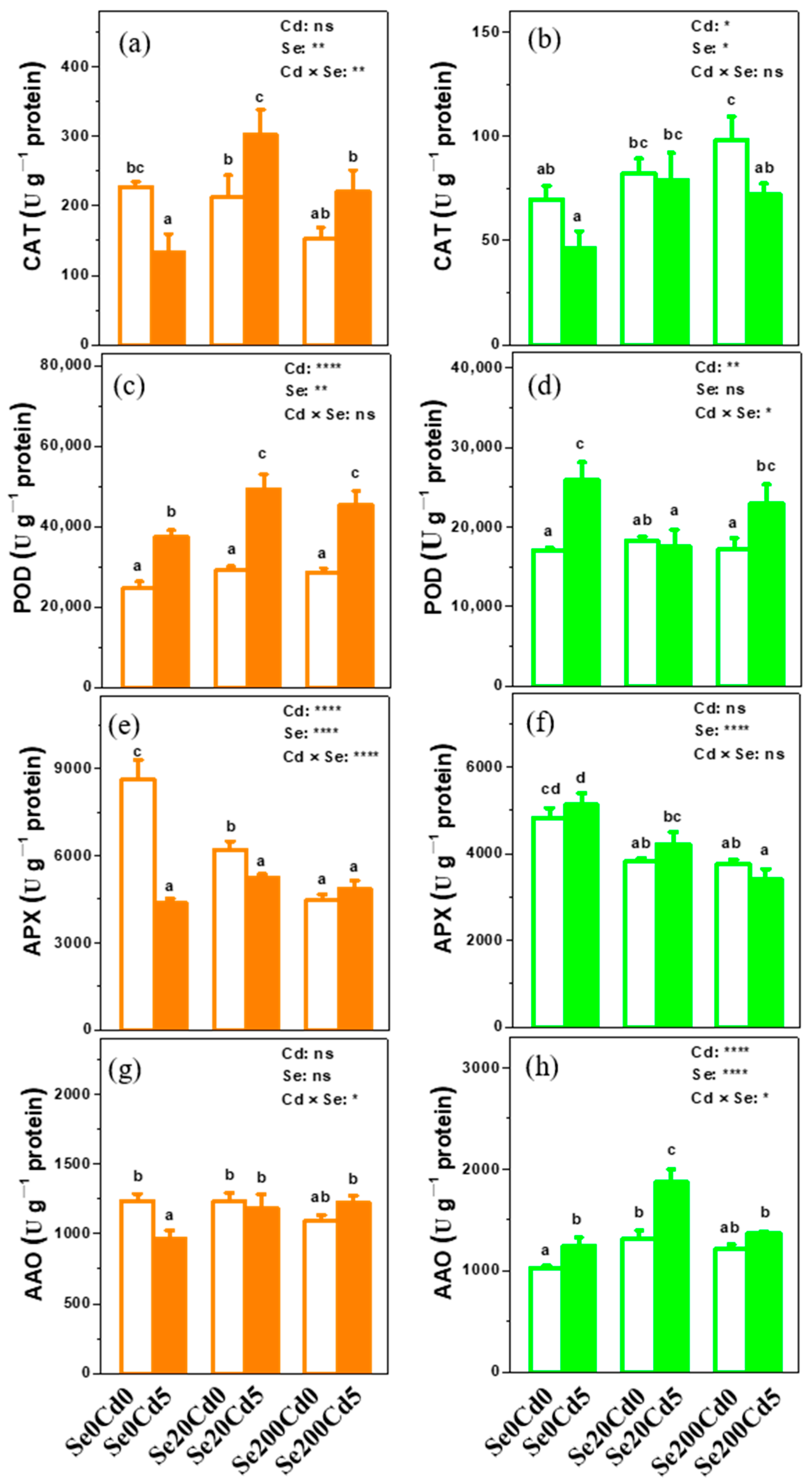
2.4. Assays of Antioxidant Enzyme Activities
2.5. Statistical Analysis
3. Results
3.1. Effect of Se and Cd on the Growth of Xinfeng Walnut
3.2. The Se and Cd Accumulations in Walnuts
3.3. Effects of Se Application on O2•−, H2O2, and MDA Contents in Xinfeng Walnut
3.4. Effects of Se Application on the Antioxidant Enzyme Activities in Xinfeng Walnut
4. Discussion
4.1. Se Application Promoted Xinfeng Walnut Growth and Reduced Cd Accumulation
4.2. Se Application Enhanced Antioxidant Enzyme Activities and Alleviated Cd-Induced Oxidative Stress
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Luo, Z.B.; He, J.; Polle, A.; Rennenberg, H. Heavy metal accumulation and signal transduction in herbaceous and woody plants: Paving the way for enhancing phytoremediation efficiency. Biotechnol. Adv. 2016, 34, 1131–1148. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Wan, H.; Qin, S.; He, J.; Lyu, D.; Li, H. Net cadmium flux and gene expression in relation to differences in cadmium accumulation and translocation in four apple rootstocks. Environ. Exp. Bot. 2016, 130, 95–105. [Google Scholar] [CrossRef]
- Fang, B.; Zhu, X.Q. High content of five heavy metals in four fruits: Evidence from a case study of Pujiang County, Zhejiang Province, China. Food Control 2014, 39, 62–67. [Google Scholar] [CrossRef]
- Liang, Y.; Zeng, T.; Tian, J.; Yan, J.; Lan, Z.; Chen, J.; Xin, X.; Lei, B.; Cai, Z. Long-term environmental cadmium exposure induced serum metabolic changes related to renal and liver dysfunctions in a female cohort from Southwest China. Sci. Total Environ. 2021, 798, 149379. [Google Scholar] [CrossRef] [PubMed]
- Rizwan, M.; Ali, S.; Adrees, M.; Rizvi, H.; Zia-Ur-Rehman, M.; Hannan, F.; Qayyum, M.F.; Hafeez, F.; Ok, Y.S. Cadmium stress in rice: Toxic effects, tolerance mechanisms, and management: A critical review. Environ. Sci. Pollut. Res. 2016, 23, 17859–17879. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Liu, J.; Cheng, S. Heavy metals in apple orchard soils and fruits and their health risks in Liaodong Peninsula, Northeast China. Environ. Monit. Assess. 2015, 187, 4178. [Google Scholar] [CrossRef] [PubMed]
- Shahid, M.A.; Balal, R.M.; Khan, N.; Zotarelli, L.; Garcia-Sanchez, F. Selenium impedes cadmium and arsenic toxicity in potato by modulating carbohydrate and nitrogen metabolism. Ecotoxicol. Environ. Saf. 2019, 180, 588–599. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.; Yu, Y.; Wang, Q.; Qiao, Y.; Li, H. Cadmium uptake dynamics and translocation in rice seedling: Influence of different forms of selenium. Ecotoxicol. Environ. Saf. 2016, 133, 127–134. [Google Scholar] [CrossRef]
- Tian, S.; Liang, S.; Qiao, K.; Wang, F.; Zhang, Y.; Chai, T. Co-expression of multiple heavy metal transporters changes the translocation, accumulation, and potential oxidative stress of Cd and Zn in rice (Oryza sativa). J. Hazard. Mater. 2019, 380, 120853. [Google Scholar] [CrossRef]
- Xu, L.L.; Fan, Z.Y.; Dong, Y.J.; Kong, J.; Bai, X.Y. Effects of exogenous salicylic acid and nitric oxide on physiological characteristics of two peanut cultivars under cadmium stress. Biol. Plant. 2015, 59, 171–182. [Google Scholar] [CrossRef]
- Aradhya, M.K.; Potter, D.; Simon, C.J. Cladistic Biogeography of Juglans (Juglandaceae) Based on Chloroplast DNA Intergenic Spacer Sequences. In Darwins Harvest. New Approaches Origins; Columbia University Press: New York, NY, USA, 2004; pp. 143–170. [Google Scholar]
- Martínez-García, P.; Crepeau, M.W.; Puiu, D.; Gonzalez-Ibeas, D.; Whalen, J.; Stevens, K.A.; Paul, R.; Butterfield, T.S.; Britton, M.T.; Reagan, R.L. The walnut (Juglans regia) genome sequence reveals diversity in genes coding for the biosynthesis of non-structural polyphenols. Plant J. 2016, 87, 507–532. [Google Scholar] [CrossRef]
- Peng, Y.; Yao, J. Investigation and evaluation on heavy metal content and pesticide residues of three edible forest products in central Sichuan province. Agric. Biotechnol. 2019, 8, 108–112. [Google Scholar]
- Arpadjan, S.A.; Momchilova, S.A.; Elenkova, D.B.; Blagoeva, E.C. Essential and toxic microelement profile of walnut (Juglans regia L.) cultivars grown in industrially contaminated area—Evaluation for human nutrition and health. J. Food Nutr. Res. 2013, 52, 121–127. [Google Scholar]
- Muller, A.; Muller, C.C.; Lyra, F.; Mello, P.A.; Mesko, M.F.; Muller, E.I.; Flores, E. Determination of Toxic Elements in Nuts by Inductively Coupled Plasma Mass Spectrometry after Microwave-Induced Combustion. Food Anal. Methods 2013, 6, 258–264. [Google Scholar] [CrossRef]
- Kafaoglu, B.; Fisher, A.; Hill, S.; Kara, D. Determination and evaluation of element bioaccessibility in some nuts and seeds by in-vitro gastro-intestinal method. J. Food Compos. Anal. 2016, 45, 58–65. [Google Scholar] [CrossRef]
- Moreda-Pineiro, J.; Herbello-Hermelo, P.; Dominguez-Gonzalez, R.; Bermejo-Barrera, P.; Moreda-Pineiro, A. Bioavailability assessment of essential and toxic metals in edible nuts and seeds. Food Chem. 2016, 205, 146–154. [Google Scholar] [CrossRef]
- White, P.J. Selenium accumulation by plants. Ann. Bot. 2016, 117, 217–235. [Google Scholar] [CrossRef]
- Dinh, Q.T.; Cui, Z.; Huang, J.; Tran, T.A.T.; Wang, D.; Yang, W.; Zhou, F.; Wang, M.; Yu, D.; Liang, D. Selenium distribution in the Chinese environment and its relationship with human health: A review. Environ. Int. 2018, 112, 294–309. [Google Scholar] [CrossRef]
- Filek, M.; Gzyl-Malcher, B.; Zembala, M.; Bednarska, E.; Laggner, P.; Kriechbaum, M. Effect of selenium on characteristics of rape chloroplasts modified by cadmium. J. Plant Physiol. 2010, 167, 28–33. [Google Scholar] [CrossRef]
- Handa, N.; Kohli, S.K.; Sharma, A.; Thukral, A.K.; Bhardwaj, R.; Alyemeni, M.N.; Wijaya, L.; Ahmad, P.A. Selenium ameliorates chromium toxicity through modifications in pigment system, antioxidative capacity, osmotic system, and metal chelators in Brassica juncea seedlings. S. Afr. J. Bot. 2018, 119, 1–10. [Google Scholar] [CrossRef]
- Zhao, Y.; Hu, C.; Wang, X.; Qing, X.; Wang, P.; Zhang, Y.; Zhang, X.; Zhao, X. Selenium alleviated chromium stress in Chinese cabbage (Brassica campestris L. ssp. Pekinensis) by regulating root morphology and metal element uptake. Ecotoxicol. Environ. Saf. 2019, 173, 314–321. [Google Scholar] [CrossRef]
- Nawaz, F.; Ashraf, M.Y.; Ahmad, R.; Waraich, E.A.; Shabbir, R.N.; Bukhari, M.A. Supplemental selenium improves wheat grain yield and quality through alterations in biochemical processes under normal and water deficit conditions. Food Chem. 2015, 175, 350–357. [Google Scholar] [CrossRef]
- Gao, M.; Zhou, J.; Liu, H.; Zhang, W.; Hu, Y.; Liang, J.; Zhou, J. Foliar spraying with silicon and selenium reduces cadmium uptake and mitigates cadmium toxicity in rice. Sci. Total Environ. 2018, 631–632, 1100–1108. [Google Scholar] [CrossRef]
- Sun, H.; Wang, X.; Wang, Y.; Wei, Y.; Wang, G. Alleviation of cadmium toxicity in cucumber (Cucumis sativus) seedlings by the application of selenium. Span. J. Agric. Res. 2016, 133, 114–126. [Google Scholar] [CrossRef]
- Yu, Y.; Wan, Y.; Wang, Q.; Li, H. Effect of humic acid-based amendments with foliar application of Zn and Se on Cd accumulation in tobacco. Ecotoxicol. Environ. Saf. 2017, 138, 286–291. [Google Scholar] [CrossRef]
- Yu, Y.; Yuan, S.; Zhuang, J.; Wan, Y.; Wang, Q.; Zhang, J.; Li, H. Effect of selenium on the uptake kinetics and accumulation of and oxidative stress induced by cadmium in Brassica chinensis. Ecotoxicol. Environ. Saf. 2018, 162, 571–580. [Google Scholar] [CrossRef]
- Harvey, M.A.; Erskine, P.D.; Harris, H.H.; Brown, G.K.; Pilon-Smits, E.; Casey, L.W.; Echevarria, G.; Ent, A. Distribution and chemical form of selenium in Neptunia amplexicaulis from Central Queensland, Australia. Metallomics 2020, 12, 514–527. [Google Scholar] [CrossRef]
- Shi, Z.; Pan, P.; Feng, Y.; Kan, Z.; Li, Z.; Wei, F. Environmental water chemistry and possible correlation with Kaschin-Beck Disease (KBD) in northwestern Sichuan, China. Environ. Int. 2017, 99, 282–292. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, H.R.; Yang, L.S.; Li, Y.H.; Wei, B.G.; Yu, J.P.; Feng, F.J. Distribution and translocation of selenium from soil to highland barley in the Tibetan Plateau Kashin-Beck disease area. Environ. Geochem. Health 2017, 39, 221–229. [Google Scholar] [CrossRef]
- Hussain, B.; Lin, Q.; Hamid, Y.; Sanaullah, M.; Di, L.; Hashmi, M.; Khan, M.B.; He, Z.; Yang, X. Foliage application of selenium and silicon nanoparticles alleviates Cd and Pb toxicity in rice (Oryza sativa L.). Sci. Total Environ. 2020, 712, 136497. [Google Scholar] [CrossRef]
- Esringu, A.; Turan, M.; Cangnul, A. Remediation of Pb and Cd polluted soils with fulvic acid. Forests 2021, 12, 1608. [Google Scholar] [CrossRef]
- Ding, S.; Ma, C.; Shi, W.; Liu, W.; Lu, Y.; Liu, Q.; Luo, Z.B. Exogenous glutathione enhances cadmium accumulation and alleviates its toxicity in Populus canescens. Tree Physiol. 2017, 37, 1697–1712. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lei, Y.; Korpelainen, H.; Li, C. Physiological and biochemical responses to high Mn concentrations in two contrasting Populus cathayana populations. Chemosphere 2007, 68, 686–694. [Google Scholar] [CrossRef]
- He, J.; Ma, C.; Ma, Y.; Li, H.; Kang, J.; Liu, T.; Polle, A.; Peng, C.; Luo, Z.B. Cadmium tolerance in six poplar species. Environ. Sci. Pollut. Res. 2013, 20, 163–174. [Google Scholar] [CrossRef]
- Luo, J.; Zhou, J.-J.; Masclaux-Daubresse, C.; Wang, N.; Wang, H.; Zheng, B. Morphological and physiological responses to contrasting nitrogen regimes in Populus cathayana is linked to resources allocation and carbon/nitrogen partition. Environ. Exp. Bot. 2019, 162, 247–255. [Google Scholar] [CrossRef]
- He, J.; Qin, J.; Long, L.; Ma, Y.; Li, H.; Li, K.; Jiang, X.; Liu, T.; Polle, A.; Liang, Z.; et al. Net cadmium flux and accumulation reveal tissue-specific oxidative stress and detoxification in Populus canescens. Physiol. Plant 2011, 143, 50–63. [Google Scholar] [CrossRef]
- Chen, L.; Han, Y.; Jiang, H.; Korpelainen, H.; Li, C. Nitrogen nutrient status induces sexual differences in responses to cadmium in Populus yunnanensis. J. Exp. Bot. 2011, 62, 5037–5050. [Google Scholar] [CrossRef]
- Tamás, L.; Bočová, B.; Huttová, J.; Mistrík, I.; Ollé, M. Cadmium-induced inhibition of apoplastic ascorbate oxidase in barley roots. Plant. Growth Regul. 2006, 48, 41–49. [Google Scholar] [CrossRef]
- Gallego, S.M.; Pena, L.B.; Barcia, R.A.; Azpilicueta, C.E.; Iannone, M.F.; Rosales, E.P.; Zawoznik, M.S.; Groppa, M.D.; Benavides, M.P. Unravelling cadmium toxicity and tolerance in plants: Insight into regulatory mechanisms. Environ. Exp. Bot. 2012, 83, 33–46. [Google Scholar] [CrossRef]
- Reeves, R.D.; Baker, A.J.M.; Jaffre, T.; Erskine, P.D.; Echevarria, G.; van der Ent, A. A global database for plants that hyperaccumulate metal and metalloid trace elements. New Phytol. 2017, 218, 407–411. [Google Scholar] [CrossRef]
- Zhou, W.; Qiu, B. Effects of cadmium hyperaccumulation on physiological characteristics of Sedum alfredii Hance (Crassulaceae). Plant Sci. 2005, 169, 737–745. [Google Scholar] [CrossRef]
- Konlechner, C.; Tuerktas, M.; Langer, I.; Vaculík, M.; Wenzel, W.W.; Puschenreiter, M.; Hauser, M.T. Expression of zinc and cadmium responsive genes in leaves of willow (Salix caprea L.) genotypes with different accumulation characteristics. Environ. Pollut. 2013, 178, 121–127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robinson, B.H.; Mills, T.M.; Petit, D.; Fung, L.E.; Green, S.R.; Clothier, B.E. Natural and induced cadmium-accumulation in poplar and willow: Implications for phytoremediation. Plant Soil 2000, 227, 301–306. [Google Scholar] [CrossRef]
- Unterbrunner, R.; Puschenreiter, M.; Sommer, P.; Wieshammer, G.; Tlustos, P.; Zupan, M.; Wenzel, W.W. Heavy metal accumulation in trees growing on contaminated sites in Central Europe. Environ. Pollut. 2007, 148, 107–114. [Google Scholar] [CrossRef]
- Zhang, Z.C.; Chen, B.X.; Qiu, B.S. Phytochelatin synthesis plays a similar role in shoots of the cadmium hyperaccumulator Sedum alfredii as in non-resistant plants. Plant Cell Environ. 2010, 33, 1248–1255. [Google Scholar] [CrossRef]
- He, X.L.; Fan, S.K.; Zhu, J.; Guan, M.Y.; Liu, X.X.; Zhang, Y.S.; Jin, C.W. Iron supply prevents Cd uptake in Arabidopsis by inhibiting IRT1 expression and favoring competition between Fe and Cd uptake. Plant Soil 2017, 41, 446–453. [Google Scholar] [CrossRef]
- Saidi, I.; Chtourou, Y.; Djebali, W. Selenium alleviates cadmium toxicity by preventing oxidative stress in sunflower (Helianthus annuus) seedlings. J. Plant Physiol. 2014, 171, 85–91. [Google Scholar] [CrossRef]
- Fodor, F.; Gáspár, L.; Morales, F.; Gogorcena, Y.; Lucena, J.J.; Cseh, E.; Kröpfl, K.; Abadía, J.; Sárvári, E. Effects of two iron sources on iron and cadmium allocation in poplar (Populus alba) plants exposed to cadmium. Tree Physiol. 2005, 25, 1173–1180. [Google Scholar] [CrossRef]
- Alfthan, G.; Eurola, M.; Ekholm, P.; Venalainen, E.; Root, T.; Korkalainen, K.; Hartikainen, H.; Salminen, P.; Hietaniemi, V.; Aspila, P.; et al. Effects of nationwide addition of selenium to fertilizers on foods, and animal and human health in Finland: From deficiency to optimal selenium status of the population. J. Trace. Elem. Med. Biol. 2015, 31, 142–147. [Google Scholar] [CrossRef]
- Li, S.; Chen, J.; Islam, E.; Wang, Y.; Wu, J.; Ye, Z.; Yan, W.; Peng, D.; Liu, D. Cadmium-induced oxidative stress, response of antioxidants and detection of intracellular cadmium in organs of moso bamboo (Phyllostachys pubescens) seedlings. Chemosphere 2016, 153, 107–114. [Google Scholar] [CrossRef]
- Del, R.D.; Stewart, A.J.; Pellegrini, N. A review of recent studies on malondialdehyde as toxic molecule and biological marker of oxidative stress. Nutr. Metab. Cardiovas. 2005, 15, 316–328. [Google Scholar] [CrossRef]
- Kanazawa, S.; Sano, S.; Koshiba, T.; Ushimaru, T. Changes in antioxidative enzymes in cucumber cotyledons during natural senescence: Comparison with those during dark-induced senescence. Physiol. Plant. 2000, 109, 211–216. [Google Scholar] [CrossRef]
- Sun, Y.; Zhou, Q.; Lin, W.; Liu, W. Cadmium tolerance and accumulation characteristics of Bidens pilosa L. as a potential Cd-hyperaccumulator. J. Hazard. Mater. 2009, 161, 808–814. [Google Scholar] [CrossRef]
- Pignocchi, C.; Fletcher, J.M.; Wilkinson, J.E.; Barnes, J.D.; Foyer, C.H. The function of ascorbate oxidase in tobacco. Plant Physiol. 2003, 132, 1631–1641. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Treatment | Se (μM) | Cd (mM) |
|---|---|---|
| Se0Cd0 | 0 | 0 |
| Se0Cd5 | 0 | 5 |
| Se20Cd0 | 20 | 0 |
| Se20Cd5 | 20 | 5 |
| Se200Cd0 | 200 | 0 |
| Se200Cd5 | 200 | 5 |
| Se (μM) | Cd (mM) | Root (g) | Wood (g) | Bark (g) | Leaves (g) |
|---|---|---|---|---|---|
| 0 | 0 | 10.99 ± 0.24 d | 3.34 ± 0.12 b | 2.35 ± 0.13 b | 10.79 ± 0.62 c |
| 5 | 6.37 ± 0.30 a | 2.99 ± 0.24 ab | 1.96 ± 0.07 a | 7.16 ± 0.12 a | |
| 20 | 0 | 11.25 ± 0.80 d | 4.02 ± 0.15 c | 2.76 ± 0.14 c | 12.65 ± 0.42 d |
| 5 | 8.26 ± 0.30 b | 2.79 ± 0.24 a | 2.01 ± 0.07 a | 7.86 ± 0.94 ab | |
| 200 | 0 | 9.58 ± 0.39 c | 3.18 ± 0.13 ab | 2.17 ± 0.09 ab | 8.96 ± 0.27 b |
| 5 | 9.63 ± 0.42 c | 3.11 ± 0.13 ab | 2.14 ± 0.10 ab | 8.86 ± 0.36 b | |
| p-values | Cd | **** | *** | **** | **** |
| Se | * | ns | * | * | |
| Cd × Se | **** | ** | ** | *** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, B.; Zhang, D.; Wang, W.; Song, Y.; Lu, M.; Ding, S. Foliar Application of Selenium Reduces Cadmium Accumulation in Walnut Seedlings. Forests 2022, 13, 1493. https://doi.org/10.3390/f13091493
Wang B, Zhang D, Wang W, Song Y, Lu M, Ding S. Foliar Application of Selenium Reduces Cadmium Accumulation in Walnut Seedlings. Forests. 2022; 13(9):1493. https://doi.org/10.3390/f13091493
Chicago/Turabian StyleWang, Bingwen, Dangquan Zhang, Wenfeng Wang, Yukun Song, Mengfei Lu, and Shen Ding. 2022. "Foliar Application of Selenium Reduces Cadmium Accumulation in Walnut Seedlings" Forests 13, no. 9: 1493. https://doi.org/10.3390/f13091493
APA StyleWang, B., Zhang, D., Wang, W., Song, Y., Lu, M., & Ding, S. (2022). Foliar Application of Selenium Reduces Cadmium Accumulation in Walnut Seedlings. Forests, 13(9), 1493. https://doi.org/10.3390/f13091493





