Ecological Response of Urban Forest Carbon Density to Site Conditions: A Case Study of a Typical Karst Mountainous Regions in Southwest China
Abstract
:1. Introduction
2. Materials and Methods
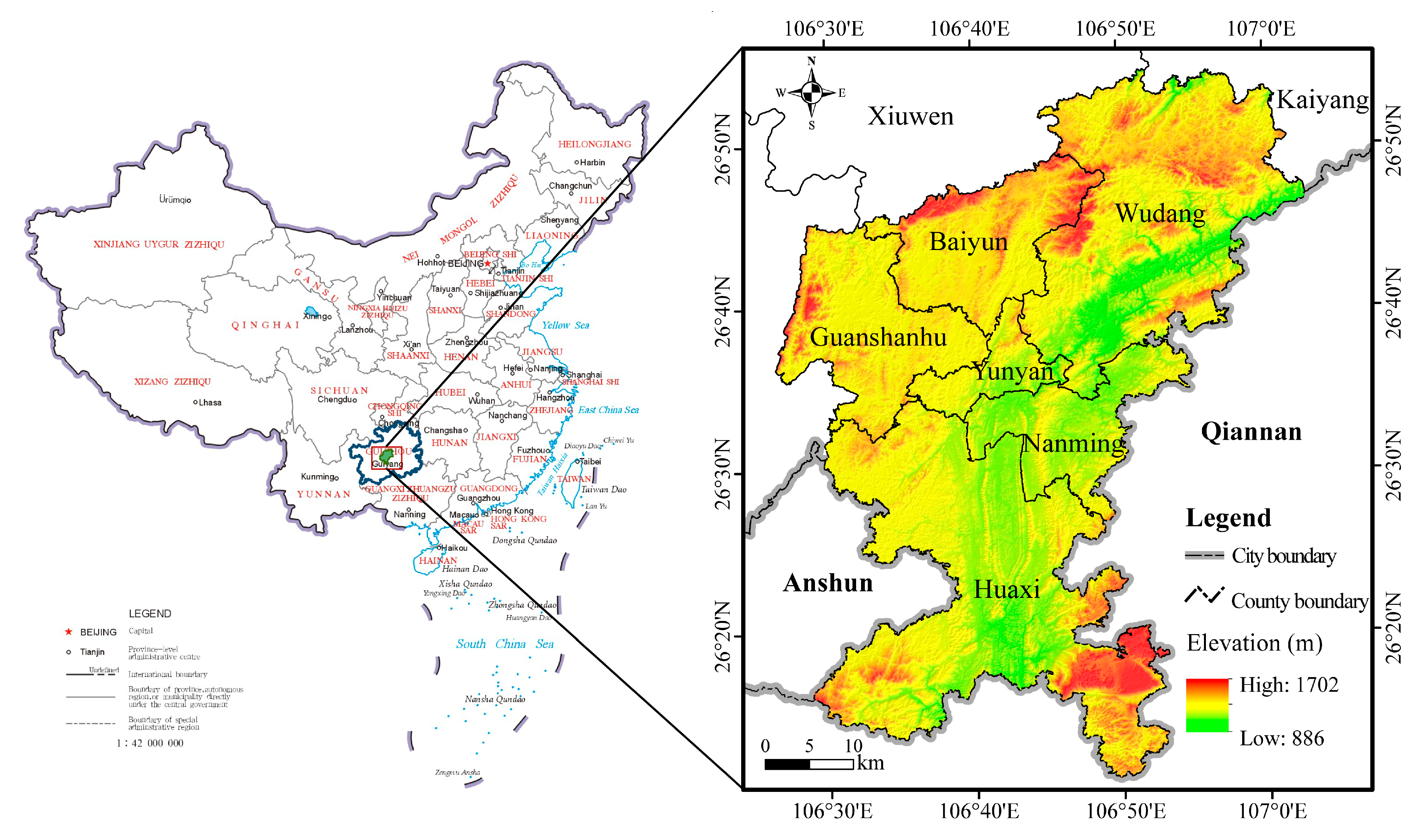
2.1. Study Area
2.2. Data Collection and Processing
2.2.1. Carbon Storage and Carbon Density
2.2.2. Site Conditions
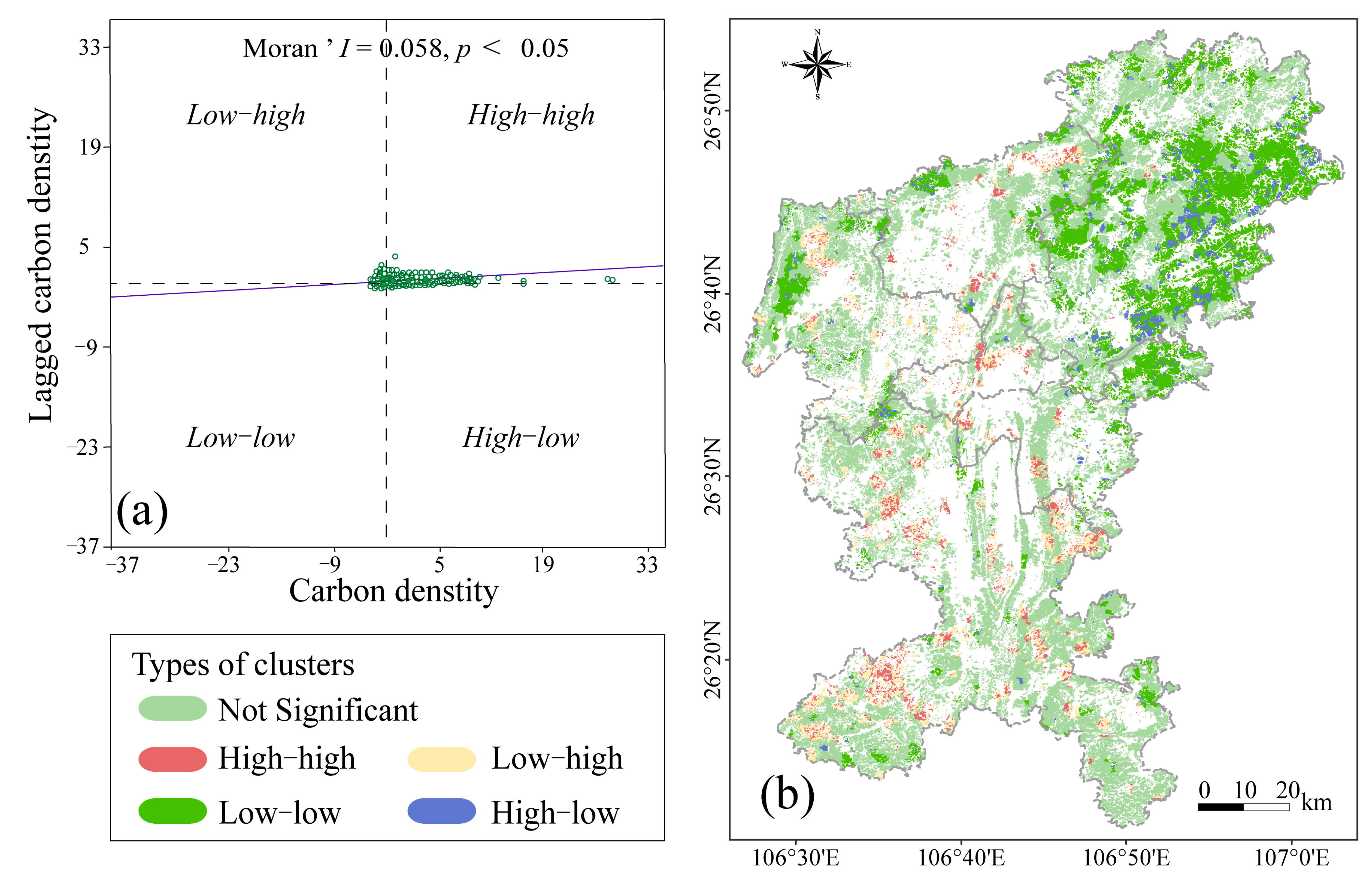
2.3. Spatial Autocorrelation Analysis
2.4. Random Forest Model
2.5. Spatial Autoregression Model
3. Results
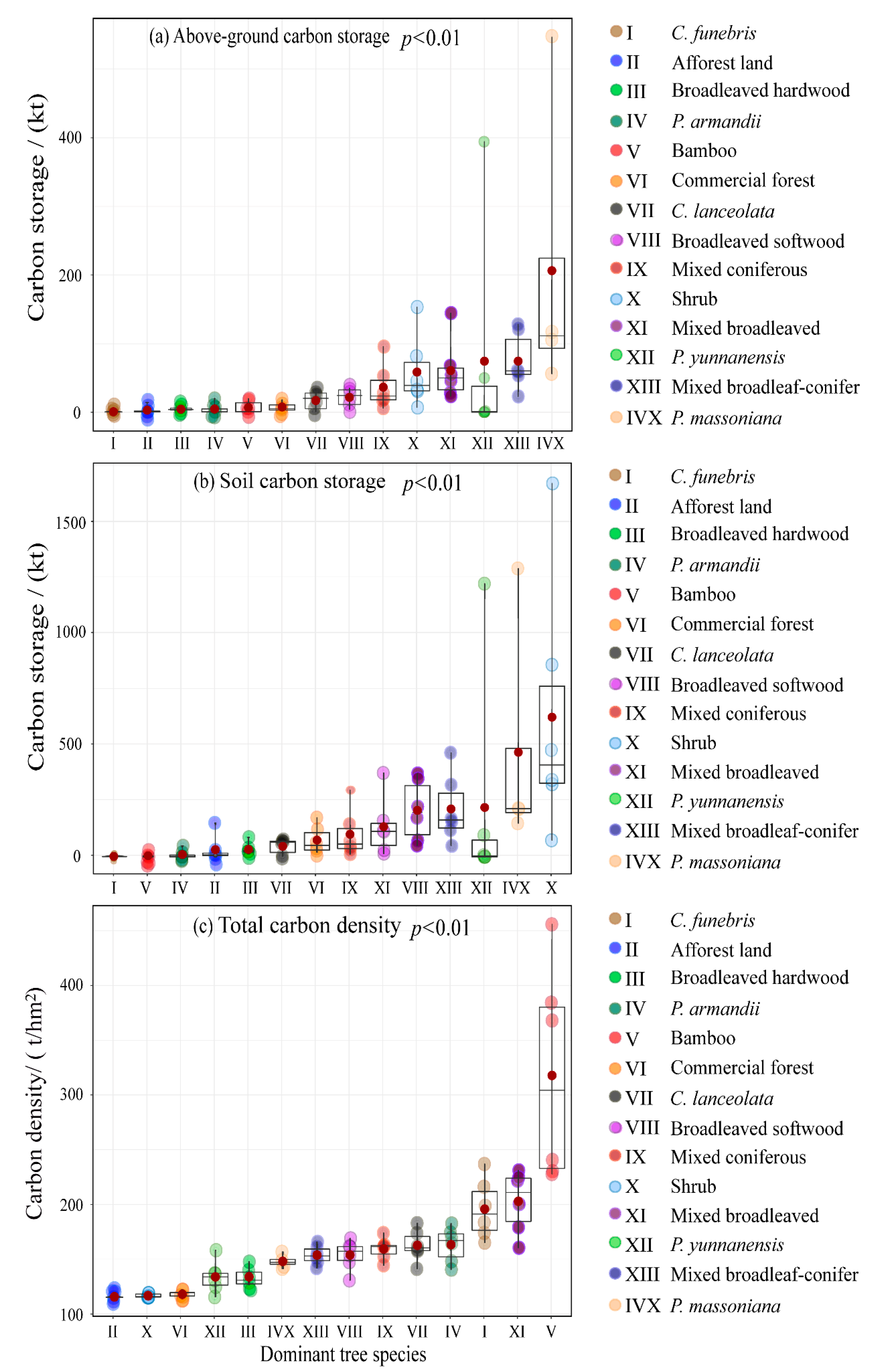
3.1. Carbon Density Assessment in Different Forest Types
3.2. Spatial Heterogeneity of Carbon Density
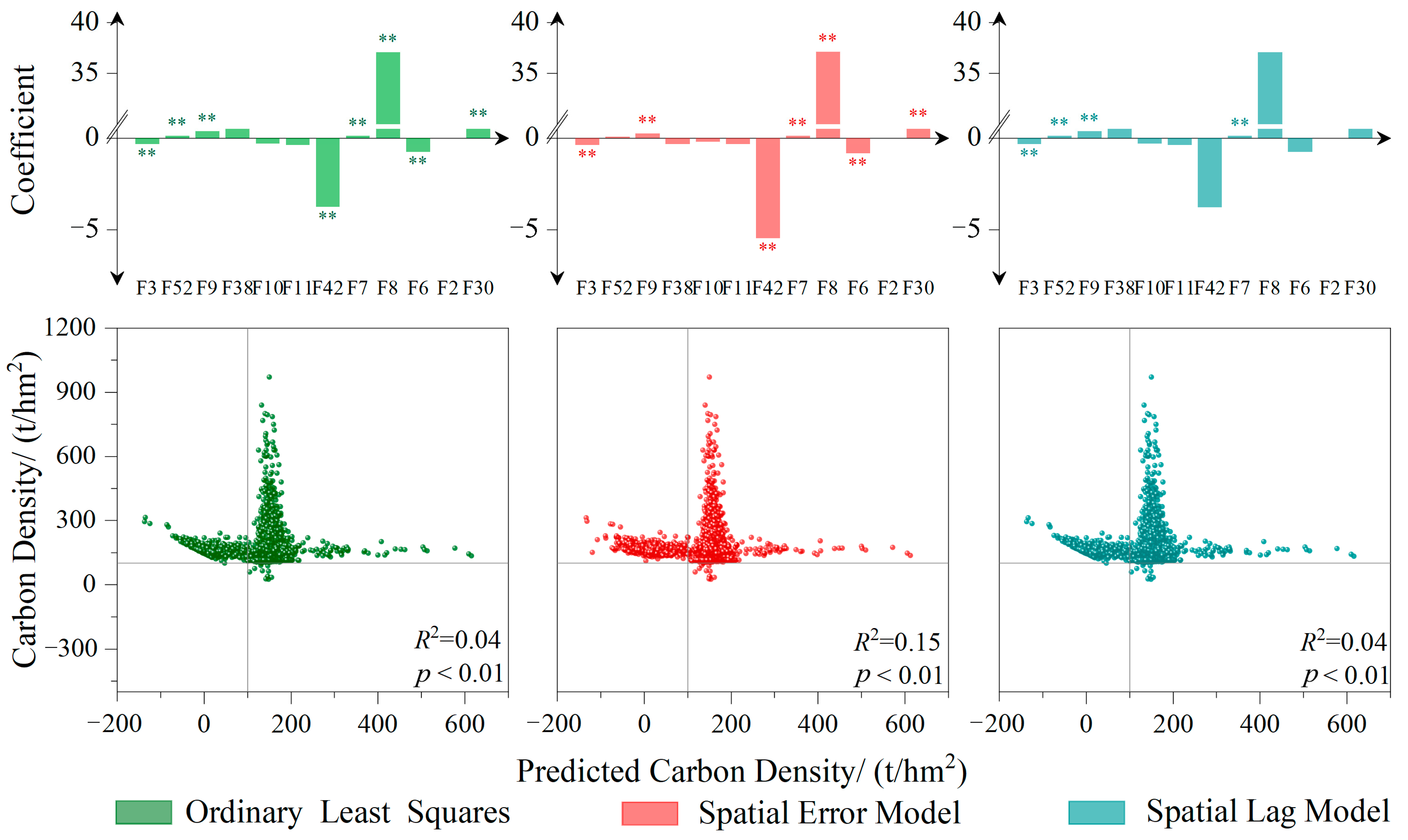
3.3. Relative Importance of Site Conditions on the Spatial Distribution of Carbon Density
3.4. Driving Factors of the Spatial Differentiation on Carbon Density
4. Discussion
4.1. Urban Forest Carbon Sequestration Capacity
4.2. Response of Carbon Sequestration Ability to Site Conditions
4.3. Limitations and Future Work
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gogoi, A.; Ahirwal, J.; Sahoo, U.K. Evaluation of ecosystem carbon storage in major forest types of Eastern Himalaya: Implications for carbon sink management. J. Environ. Manag. 2022, 302, 113972. [Google Scholar] [CrossRef] [PubMed]
- He, N.; Wen, D.; Zhu, J.; Tang, X.; Xu, L.; Zhang, L.; Hu, H.; Huang, M.; Yu, G. Vegetation carbon sequestration in Chinese forests from 2010 to 2050. Glob. Chang. Biol. 2017, 23, 1575–1584. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Wang, Q.; Fan, X. An Integrative Approach to Study How Driving Factors Control Biomass Carbon Density for Natural Mountain Forests, in China’s Loess Plateau. Forests 2022, 13, 1114. [Google Scholar] [CrossRef]
- Chen, L.C.; Guan, X.; Li, H.M.; Wang, Q.K.; Zhang, W.D.; Yang, Q.P.; Wang, S.L. Spatiotemporal patterns of carbon storage in forest ecosystems in Hunan Province, China. For. Ecol. Manag. 2019, 432, 656–666. [Google Scholar] [CrossRef]
- Fang, J.; Chen, A.; Peng, C.; Zhao, S.; Ci, L. Changes in forest biomass carbon storage in China between 1949 and 1998. Science 2001, 292, 2320–2322. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Ji, X.; Deane, D.C.; Wu, L.; Chen, S. Spatiotemporal distribution and driving factors of forest biomass carbon storage in China: 1977–2013. Forests 2017, 8, 263. [Google Scholar] [CrossRef]
- Li, X.; OuYang, X.; Liu, Q. Carbon storage of forest v.vegetation and its geographical pattern in China’s Jiangxi Province during 2001–2005. J. Nat. Resour. 2011, 26, 655–665. [Google Scholar] [CrossRef]
- Zhang, L.J.; Lai, G.H.; Zeng, W.S.; Zou, W.T.; Yi, S.J. Effect of Climate on Carbon Storage Growth Models for Three Major Coniferous Plantations in China Based on National Forest Inventory Data. Forests 2022, 13, 882. [Google Scholar] [CrossRef]
- Skovsgaard, J.P.; Vanclay, J.K. Forest site productivity: A review of spatial and temporal variability in natural site conditions. Forestry 2013, 86, 305–315. [Google Scholar] [CrossRef]
- Nave, L.E.; DeLyser, K.; Domke, G.M.; Holub, S.M.; Janowiak, M.K.; Kittler, B.; Ontl, T.A.; Sprague, E.; Sucre, E.B.; Walters, B.F.; et al. Disturbance and management effects on forest soil organic carbon stocks in the Pacific Northwest. Ecol. Appl. 2022, 32, e2611. [Google Scholar] [CrossRef]
- Huang, J.; Ge, Z.; Huang, Y.; Tang, X.; Shi, Z.; Lai, P.; Song, Z.; Hao, B.; Yang, H.; Ma, M. Climate change and ecological engineering jointly induced vegetation greening in global karst regions from 2001 to 2020. Plant Soil 2022, 475, 193–212. [Google Scholar] [CrossRef]
- Zhang, C.S.; Deng, Q.H.; Liu, A.B.; Liu, C.L.; Xie, G.D. Effects of Stand Structure and Topography on Forest Vegetation Carbon Density in Jiangxi Province. Forests 2021, 12, 1483. [Google Scholar] [CrossRef]
- Shi, W.Y.; Chen, Y.Z.; Feng, X.M. Identifying the terrestrial carbon benefits from ecosystem restoration in ecologically fragile regions. Agric. Ecosyst. Environ. 2020, 296, 106889. [Google Scholar] [CrossRef]
- Peng, T.; Deng, H. Research on the sustainable development process of low-carbon pilot cities: The case study of Guiyang, a low-carbon pilot city in south-west China. Environ. Dev. Sustain. 2021, 23, 2382–2403. [Google Scholar] [CrossRef]
- Liu, L.; Zeng, F.P.; Song, T.Q.; Wang, K.L.; Du, H. Stand Structure and Abiotic Factors Modulate Karst Forest Biomass in Southwest China. Forests 2020, 11, 443. [Google Scholar] [CrossRef]
- Zhou, B.; Liao, Z.; Chen, S.; Jia, H.; Zhu, J.; Fei, X. Net Primary Productivity of Forest Ecosystems in the Southwest Karst Region from the Perspective of Carbon Neutralization. Forests 2022, 13, 1367. [Google Scholar] [CrossRef]
- Zhang, C.; Zeng, F.; Zeng, Z.; Du, H.; Su, L.; Zhang, L.; Lu, M.; Zhang, H. Impact of Selected Environmental Factors on Variation in Leaf and Branch Traits on Endangered Karst Woody Plants of Southwest China. Forests 2022, 13, 1080. [Google Scholar] [CrossRef]
- Jiang, Y.; Meng, T.; Xu, W.; Tan, W.; Zhang, Q.; Liu, Y. Community characteristics of endangered Gymnosperms and their topographic relationships in a karst mountaintop region. Acta Ecol. Sin. 2021, 41, 1868–1877. [Google Scholar] [CrossRef]
- Song, X.W.; Gao, Y.; Wen, X.F.; Guo, D.L.; Yu, G.R.; He, N.P.; Zhang, J.Z. Carbon sequestration potential and its eco-service function in the karst area, China. J. Geogr. Sci. 2017, 27, 967–980. [Google Scholar] [CrossRef]
- Zhou, B.; Wang, G.; Tang, Y.; Zhang, W.; Zang, R. Zoning of degraded natural forests in mountain areas of Southwest China. For. Res. Beijing 2010, 23, 612–616. [Google Scholar]
- Liu, L.; Xiong, K.; Luo, Y. Advances in evaluation of sustainable development capability in karst region. Guizhou Agric. Sci. 2012, 41, 67–72. [Google Scholar] [CrossRef]
- Liu, Y.; Huang, X.; Yang, H.; Zhong, T. Environmental effects of land-use/cover change caused by urbanization and policies in Southwest China Karst area–A case study of Guiyang. Habitat Int. 2014, 44, 339–348. [Google Scholar] [CrossRef]
- Mingjun, L.; Lifei, Y.; Huanbai, X.; Chaojun, N.; Zongsheng, H.; Du Mingfeng, Z.; Jianhua, S. The Prediction of Forest Carbon Sequestration Dynamics in Guizhou Province and Relevant Influencing Factors. Pak. J. Bot. 2018, 50, 1157–1170. [Google Scholar] [CrossRef]
- Yang, J.; Luo, X.; Lu, S.; Yang, Y.; Yang, J. Effects of compositional and configurational heterogeneity of the urban matrix on the species richness of woody plants in urban remnant forest patches. Landsc. Ecol. 2022, 37, 619–632. [Google Scholar] [CrossRef]
- Lu, J.; Feng, Z.K.; Zhu, Y. Estimation of Forest Biomass and Carbon Storage in China Based on Forest Resources Inventory Data. Forests 2019, 10, 650. [Google Scholar] [CrossRef]
- Li, M.; Du, M.; Yu, L. Carbon storage and density of forest vegetation and its spatial distribution pattern in Guizhou Province. J. Northwest. For. Univ. 2016, 31, 48–54. [Google Scholar] [CrossRef]
- Tian, X.L.; Xia, J.; Xia, H.B.; Ni, J. Forest biomass an.nd its spatial pattern in Guizhou Province. Chin. J. Appl. Ecol. 2011, 22, 287–294. [Google Scholar]
- Zhou, J.; Xu, S. Analysis of the current status and dynamics of forest carbon storage in Yunnan Province. For. Invent. Plan. 2016, 41, 17–23. [Google Scholar]
- Yu, D.; Shi, X.; Sun, W.; Wang, H.; Liu, Q.; Zhao, Y. Estimation of China soil organic carbon storage and density based on 1: 1,000,000 soil database. Chin. J. Appl. Ecol. 2005, 16, 2279–2283. [Google Scholar]
- Bedane, G.A.; Feyisa, G.L.; Senbeta, F. Spatial distribution of above ground carbon density in Harana Forest, Ethiopia. Ecol. Process. 2022, 11, 1–13. [Google Scholar] [CrossRef]
- Shangguan, W.; Dai, Y.; Liu, B.; Zhu, A.; Duan, Q.; Wu, L.; Ji, D.; Ye, A.; Yuan, H.; Zhang, Q. A China data set of soil properties for land surface modeling. J. Adv. Model. Earth Syst. 2013, 5, 212–224. [Google Scholar] [CrossRef]
- Cui, Y.; Li, L.; Chen, L.; Zhang, Y.; Cheng, L.; Zhou, X.; Yang, X. Land-use carbon emissions estimation for the Yangtze River Delta Urban Agglomeration using 1994–2016 Landsat image data. Remote Sens. 2018, 10, 1334. [Google Scholar] [CrossRef]
- Hu, X.; Ma, C.; Huang, P.; Guo, X. Ecological vulnerability assessment based on AHP-PSR method and analysis of its single parameter sensitivity and spatial autocorrelation for ecological protection–A case of Weifang City, China. Ecol. Indic. 2021, 125, 107464. [Google Scholar] [CrossRef]
- Li, W.; Dong, F.; Ji, Z. Research on coordination level and influencing factors spatial heterogeneity of China’s urban CO2 emissions. Sustain. Cities Soc. 2021, 75, 103323. [Google Scholar] [CrossRef]
- Breiman, L. Bagging predictors. Mach. Learn. 1996, 24, 123–140. [Google Scholar] [CrossRef]
- Fang, X.; Li, X.; Zhang, Y.; Zhao, Y.; Qian, J.; Hao, C.; Zhou, J.; Wu, Y. Random forest-based understanding and predicting of the impacts of anthropogenic nutrient inputs on the water quality of a tropical lagoon. Environ. Res. Lett. 2021, 16, 055003. [Google Scholar] [CrossRef]
- Han, H.; Guo, X.; Yu, H. Variable selection using mean decrease accuracy and mean decrease gini based on random forest. In Proceedings of the 2016 7th IEEE International Conference on Software Engineering and Service Science (Icsess), Beijing, China, 26–28 August 2016; IEEE: New York, NY, USA, 2016; pp. 219–224. [Google Scholar]
- Zhang, L.; Huettmann, F.; Zhang, X.; Liu, S.; Sun, P.; Yu, Z.; Mi, C. The use of classification and regression algorithms using the random forests method with presence-only data to model species’ distribution. MethodsX 2019, 6, 2281–2292. [Google Scholar] [CrossRef]
- Guo, J.; Han, G.; Xie, Y.; Cai, Z.; Zhao, Y. Exploring the relationships between urban spatial form factors and land surface temperature in mountainous area: A case study in Chongqing city, China. Sustain. Cities Soc. 2020, 61, 102286. [Google Scholar] [CrossRef]
- Conway, D.; Li, C.Q.; Wolch, J.; Kahle, C.; Jerrett, M. A spatial autocorrelation approach for examining the effects of urban greenspace on residential property values. J. Real Estate Financ. Econ. 2010, 41, 150–169. [Google Scholar] [CrossRef]
- Gu, Y.L.; You, X.Y. A spatial quantile regression model for driving mechanism of urban heat island by considering the spatial dependence and heterogeneity: An example of Beijing, China. Sustain. Cities Soc. 2022, 79, 103692. [Google Scholar] [CrossRef]
- Lorenz, K.; Lal, R. Carbon Sequestration in Forest Ecosystems; Springer: Berlin/Heidelberg, Germany, 2009; pp. 103–146. [Google Scholar]
- Guan, J.; Du, S.; Cheng, J.; Wu, C.; Li, G.; Deng, L.; Zhang, J.; He, Q.; Shi, W. Current stocks and rate of sequestration of forest carbon in Gansu Province, China. Chin. J. Plant Ecol. 2016, 40, 304. [Google Scholar] [CrossRef]
- Sun, W.; Liu, X. Review on carbon storage estimation of forest ecosystem and applications in China. For. Ecosyst. 2020, 7, 1–14. [Google Scholar] [CrossRef]
- Guo, C.; Wu, Y.; Ni, J.; Guo, Y. Forest carbon storage in Guizhou Province based on field measurement dataset. Acta Geochim. 2019, 38, 8–21. [Google Scholar] [CrossRef]
- Wei, Y.; Li, M.; Chen, H.; Lewis, B.J.; Yu, D.; Zhou, L.; Zhou, W.; Fang, X.; Zhao, W.; Dai, L. Variation in carbon storage and its distribution by stand age and forest type in boreal and temperate forests in northeastern China. PLoS ONE 2013, 8, e72201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, Y.; Shao, Q.; Shi, T.; Lu, Z.; Wu, G. Spatiotemporal dynamics of forest ecosystem carbon budget in Guizhou: Customisation and application of the CBM-CFS3 model for China. Carbon Balance Manag. 2022, 17, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Xiang, M.; Wang, C.; Tan, Y.; Yang, J.; Duan, L.; Fang, Y.; Li, W.; Shu, Y.; Liu, M. Spatio-temporal evolution and driving factors of carbon storage in the Western Sichuan Plateau. Sci. Rep. 2022, 12, 8114. [Google Scholar] [CrossRef]
- Yen, T.-M.; Lee, J.-S. Comparing aboveground carbon sequestration between moso bamboo (Phyllostachys heterocycla) and China fir (Cunninghamia lanceolata) forests based on the allometric model. For. Ecol. Manag. 2011, 261, 995–1002. [Google Scholar] [CrossRef]
- Sun, L.N.; Wang, M.B.; Fan, X.H. Spatial pattern and driving factors of biomass carbon density for natural and planted coniferous forests in mountainous terrain, eastern Loess Plateau of China. For. Ecosyst. 2020, 7, 1–13. [Google Scholar] [CrossRef]
- Liu, F.; Wu, H.Y.; Zhao, Y.G.; Li, D.C.; Yang, J.L.; Song, X.D.; Shi, Z.; Zhu, A.X.; Zhang, G.L. Mapping high resolution National Soil Information Grids of China. Sci. Bull. 2022, 67, 328–340. [Google Scholar] [CrossRef]
- Zald, H.S.; Spies, T.A.; Seidl, R.; Pabst, R.J.; Olsen, K.A.; Steel, E.A. Complex mountain terrain and disturbance history drive variation in forest aboveground live carbon density in the western Oregon Cascades, USA. For. Ecol. Manag. 2016, 366, 193–207. [Google Scholar] [CrossRef]
- Olorunfemi, I.E.; Fasinmirin, J.T.; Akinola, F.F. Soil physico-chemical properties and fertility status of long-term land use and cover changes: A case study in forest vegetative zone of Nigeria. Eurasian J. Soil Sci. 2018, 7, 133–150. [Google Scholar] [CrossRef]
- Fasinmirin, J.; Olorunfemi, I. Comparison of hydraulic conductivity of soils of the forest vegetative zone of Nigeria. Appl. Trop. Agric. 2012, 17, 64–77. [Google Scholar]
- Wiesmeier, M.; Urbanski, L.; Hobley, E.; Lang, B.; von Lützow, M.; Marin-Spiotta, E.; van Wesemael, B.; Rabot, E.; Ließ, M.; Garcia-Franco, N. Soil organic carbon storage as a key function of soils-A review of drivers and indicators at various scales. Geoderma 2019, 333, 149–162. [Google Scholar] [CrossRef]
- Liu, R.; Wang, M.; Chen, W. The influence of urbanization on organic carbon sequestration and cycling in soils of Beijing. Landsc. Urban Plan. 2018, 169, 241–249. [Google Scholar] [CrossRef]
- Liu, X.; Li, T.; Zhang, S.; Jia, Y.; Li, Y.; Xu, X. The role of land use, construction and road on terrestrial carbon stocks in a newly urbanized area of western Chengdu, China. Landsc. Urban Plan. 2016, 147, 88–95. [Google Scholar] [CrossRef]
- Ding, Z.; Zheng, H.; Li, H.; Yu, P.J.; Man, W.D.; Liu, M.Y.; Tang, X.G.; Liu, Y. Afforestation-driven increases in terrestrial gross primary productivity are partly offset by urban expansion in Southwest China. Ecol. Indic. 2021, 127, 107641. [Google Scholar] [CrossRef]
- Lorenz, K.; Lal, R. Carbon storage in some urban forest soils of Columbus, Ohio, USA. In Carbon Sequestration in Urban Ecosystems; Springer: Dordrecht, Netherlands, 2012; pp. 139–158. [Google Scholar]
- Zhu, Z.P.; Li, J.Y.; Chen, Z.R. Green space equity: Spatial distribution of urban green spaces and correlation with urbanization in Xiamen, China. Environ. Dev. Sustain. 2022, 24, 1–21. [Google Scholar] [CrossRef]
- Berhe, A.A.; Harte, J.; Harden, J.W.; Torn, M.S. The significance of the erosion-induced terrestrial carbon sink. BioScience 2007, 57, 337–346. [Google Scholar] [CrossRef]
- Pan, Y.; Luo, T.; Birdsey, R.; Hom, J.; Melillo, J. New estimates of carbon storage and sequestration in China’s forests: Effects of age–class and method on inventory-based carbon estimation. Clim. Change 2004, 67, 211–236. [Google Scholar] [CrossRef]

| Dominant Tree Species (Group) | Volume–Biomass Equations | Carbon Content/% | Reference |
|---|---|---|---|
| Afforest land | 50.00 | [27] | |
| Bamboo | 46.69 | [5] | |
| Broadleaved hardwood | 49.34 | [27] | |
| Broadleaved softwood | 49.56 | [28] | |
| Commercial forest | 45.51 | [5] | |
| Cunninghamia lanceolata (Lamb.) Hook. | 55.28 | [5] | |
| Cupressus funebris Endl. | 51.00 | [5] | |
| Mixed broadleaf-conifer | 49.78 | [5] | |
| Mixed broadleaved | 49.00 | [5] | |
| Mixed conifer | 51.00 | [5] | |
| Pinus armandii Franch. | 52.25 | [5] | |
| Pinus massoniana Lamb. | 59.84 | [5] | |
| Pinus yunnanensis Franch. | 51.13 | [5] | |
| Shrub | 50.47 | [5] |
| Stand Indicators | Short Name | Resolution | Data Sources |
|---|---|---|---|
| Aspect | F1 | 30 m | http://www.gscloud.cn/ (accessed on 10 April 2022) |
| Elevation (m) | F2 | 30 m | http://www.gscloud.cn/ (accessed on 10 April 2022) |
| Slope (°) | F3 | 30 m | http://www.gscloud.cn/ (accessed on 10 April 2022) |
| Slope position | F4 | 30 m | http://www.gscloud.cn/ (accessed on 10 April 2022) |
| Terrain relief (°) | F5 | 30 m | http://www.gscloud.cn/ (accessed on 10 April 2022) |
| Construction density (m2/hectare) | F6 | 30 m | Forest inventory data |
| Population density (people/hectare) | F7 | 100 m | https://www.worldpop.org/ (accessed on 28 April 2022) |
| Road network density (m/hectare) | F8 | 30 m | https://www.openstreetmap.org/ (accessed on 28 April 2022) |
| Vegetation coverage (%) | F9 | 30 m | Forest inventory data |
| Cation exchange capacity at 0 cm (me/100 g) | F10 | 30 arc-second | National Tibetan Plateau Data Center |
| Cation exchange capacity at 4.5 cm (me/100 g) | F11 | 30 arc-second | National Tibetan Plateau Data Center |
| Cation exchange capacity at 9.1 cm (me/100 g) | F12 | 30 arc-second | National Tibetan Plateau Data Center |
| Cation exchange capacity at 16.6 cm (me/100 g) | F13 | 30 arc-second | National Tibetan Plateau Data Center |
| Cation exchange capacity at 28.9 cm (me/100 g) | F14 | 30 arc-second | National Tibetan Plateau Data Center |
| Cation exchange capacity at 49.3 cm (me/100 g) | F15 | 30 arc-second | National Tibetan Plateau Data Center |
| Clay fraction at 0 cm (%) | F16 | 30 arc-second | National Tibetan Plateau Data Center |
| Clay fraction at 4.5 cm (%) | F17 | 30 arc-second | National Tibetan Plateau Data Center |
| Clay fraction at 9.1 cm (%) | F18 | 30 arc-second | National Tibetan Plateau Data Center |
| Clay fraction at 16.6 cm (%) | F19 | 30 arc-second | National Tibetan Plateau Data Center |
| Clay fraction at 28.9 cm (%) | F20 | 30 arc-second | National Tibetan Plateau Data Center |
| Clay fraction at 49.3 cm (%) | F21 | 30 arc-second | National Tibetan Plateau Data Center |
| Sand fraction at 0 cm (%) | F22 | 30 arc-second | National Tibetan Plateau Data Center |
| Sand fraction at 4.5 cm (%) | F23 | 30 arc-second | National Tibetan Plateau Data Center |
| Sand fraction at 9.1 cm (%) | F24 | 30 arc-second | National Tibetan Plateau Data Center |
| Sand fraction at 16.6 cm (%) | F25 | 30 arc-second | National Tibetan Plateau Data Center |
| Sand fraction at 28.9 cm (%) | F26 | 30 arc-second | National Tibetan Plateau Data Center |
| Sand fraction at 49.3 cm (%) | F27 | 30 arc-second | National Tibetan Plateau Data Center |
| Silt fraction at 0 cm (%) | F28 | 30 arc-second | National Tibetan Plateau Data Center |
| Silt fraction at 4.5 cm (%) | F29 | 30 arc-second | National Tibetan Plateau Data Center |
| Silt fraction at 9.1 cm (%) | F30 | 30 arc-second | National Tibetan Plateau Data Center |
| Silt fraction at 16.6 cm (%) | F31 | 30 arc-second | National Tibetan Plateau Data Center |
| Silt fraction at 28.9 cm (%) | F32 | 30 arc-second | National Tibetan Plateau Data Center |
| Silt fraction at 49.3 cm (%) | F33 | 30 arc-second | National Tibetan Plateau Data Center |
| Soil organic matter at 0 cm (%) | F34 | 30 arc-second | National Tibetan Plateau Data Center |
| Soil organic matter at 4.5 cm (%) | F35 | 30 arc-second | National Tibetan Plateau Data Center |
| Soil organic matter at 9.1 cm (%) | F36 | 30 arc-second | National Tibetan Plateau Data Center |
| Soil organic matter at 16.6 cm (%) | F37 | 30 arc-second | National Tibetan Plateau Data Center |
| Soil organic matter at 28.9 cm (%) | F38 | 30 arc-second | National Tibetan Plateau Data Center |
| Soil organic matter at 49.3 cm (%) | F39 | 30 arc-second | National Tibetan Plateau Data Center |
| Soil pH at 0 cm | F40 | 30 arc-second | National Tibetan Plateau Data Center |
| Soil pH at 4.5 cm | F41 | 30 arc-second | National Tibetan Plateau Data Center |
| Soil pH at 9.1 cm | F42 | 30 arc-second | National Tibetan Plateau Data Center |
| Soil pH at 16.6 cm | F43 | 30 arc-second | National Tibetan Plateau Data Center |
| Soil pH at 28.9 cm | F44 | 30 arc-second | National Tibetan Plateau Data Center |
| Soil pH at 49.3 cm | F45 | 30 arc-second | National Tibetan Plateau Data Center |
| Soil gravel content at 0 cm (%) | F46 | 30 arc-second | National Tibetan Plateau Data Center |
| Soil gravel content at 4.5 cm (%) | F47 | 30 arc-second | National Tibetan Plateau Data Center |
| Soil gravel content at 9.1 cm (%) | F48 | 30 arc-second | National Tibetan Plateau Data Center |
| Soil gravel content at 16.6 cm (%) | F49 | 30 arc-second | National Tibetan Plateau Data Center |
| Soil gravel content at 28.9 cm (%) | F50 | 30 arc-second | National Tibetan Plateau Data Center |
| Soil gravel content at 49.3 cm (%) | F51 | 30 arc-second | National Tibetan Plateau Data Center |
| Soil parent materials | F52 | 30 m | Forest inventory data |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, X.; Hu, C.; Wang, Z. Ecological Response of Urban Forest Carbon Density to Site Conditions: A Case Study of a Typical Karst Mountainous Regions in Southwest China. Forests 2022, 13, 1484. https://doi.org/10.3390/f13091484
Zhou X, Hu C, Wang Z. Ecological Response of Urban Forest Carbon Density to Site Conditions: A Case Study of a Typical Karst Mountainous Regions in Southwest China. Forests. 2022; 13(9):1484. https://doi.org/10.3390/f13091484
Chicago/Turabian StyleZhou, Xuexia, Changyue Hu, and Zhijie Wang. 2022. "Ecological Response of Urban Forest Carbon Density to Site Conditions: A Case Study of a Typical Karst Mountainous Regions in Southwest China" Forests 13, no. 9: 1484. https://doi.org/10.3390/f13091484
APA StyleZhou, X., Hu, C., & Wang, Z. (2022). Ecological Response of Urban Forest Carbon Density to Site Conditions: A Case Study of a Typical Karst Mountainous Regions in Southwest China. Forests, 13(9), 1484. https://doi.org/10.3390/f13091484





