Influence of Physico-Chemical Factors on the Efficiency and Metabolite Profile of Adult Pinus radiata D. Don Bud Organogenesis
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
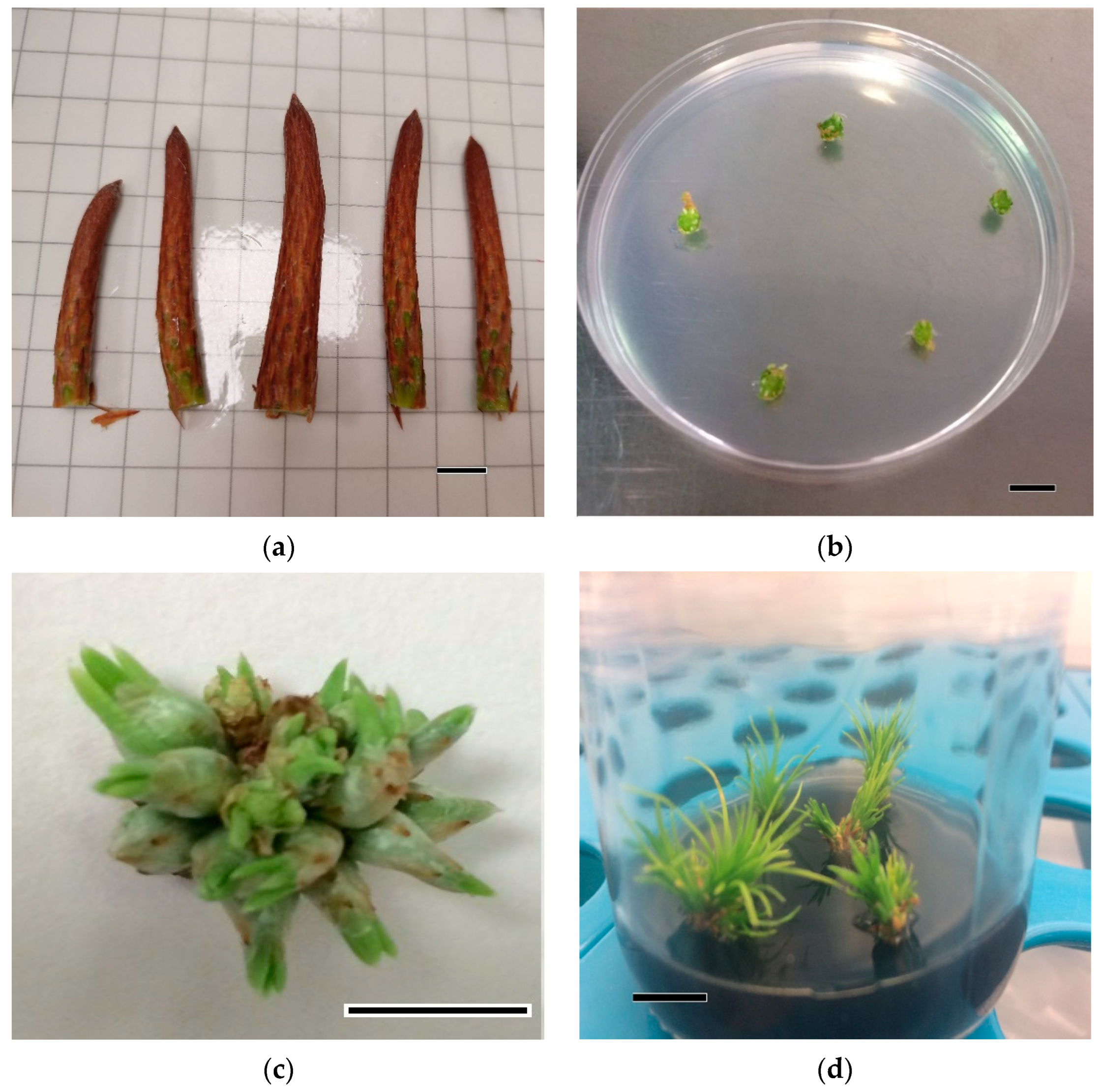
2.2. Organogenic Process
2.2.1. Shoot Growth and Elongation
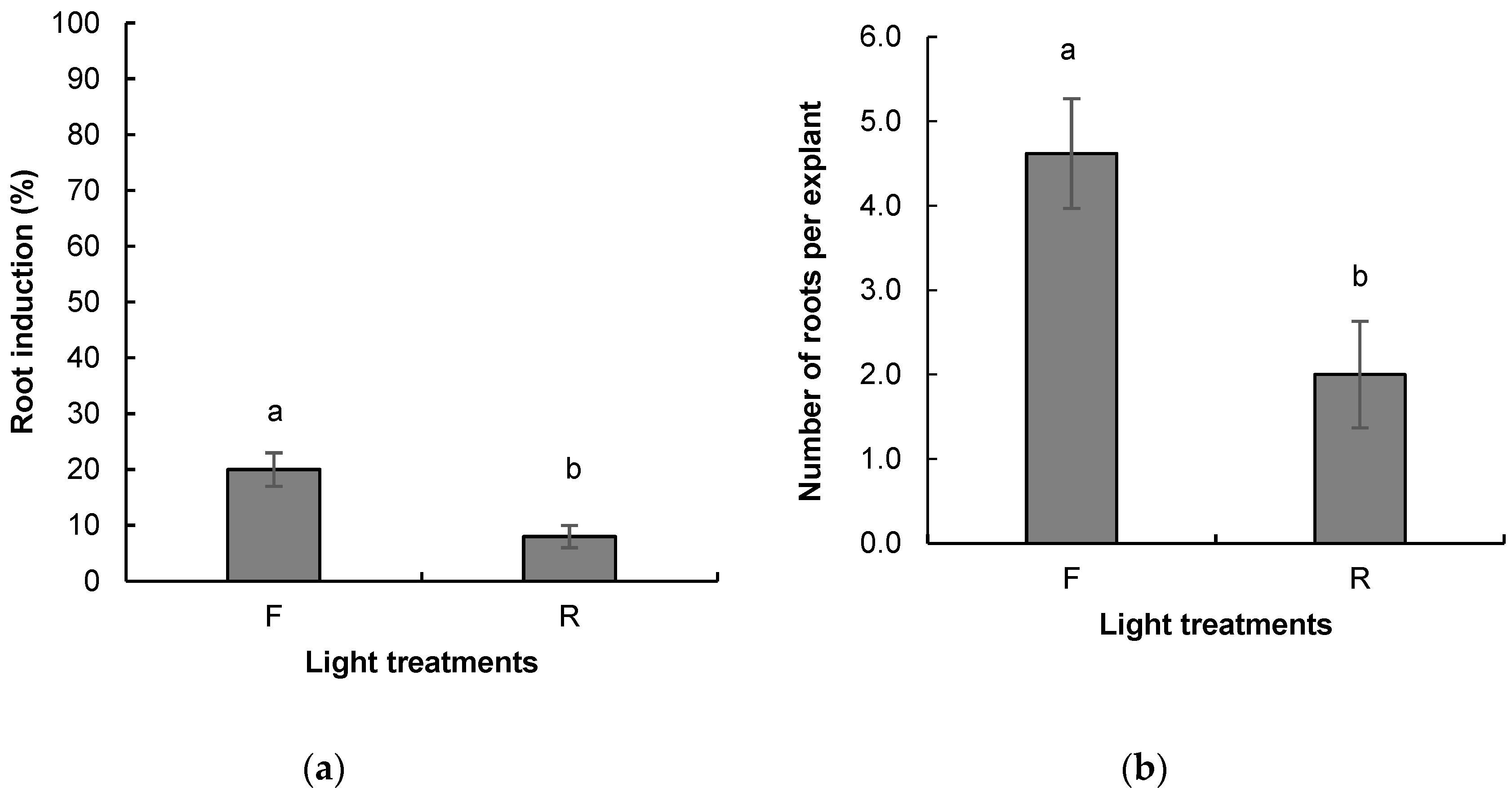
2.2.2. Root Induction and Acclimatization of Rooted Plants
2.3. Metabolite Extraction and Soluble Sugar and Amino Acid Quantification
2.4. Data Collection and Statistical Analysis
3. Results
3.1. Organogenic Process
Rooting Induction and Acclimatization of Rooted Plants
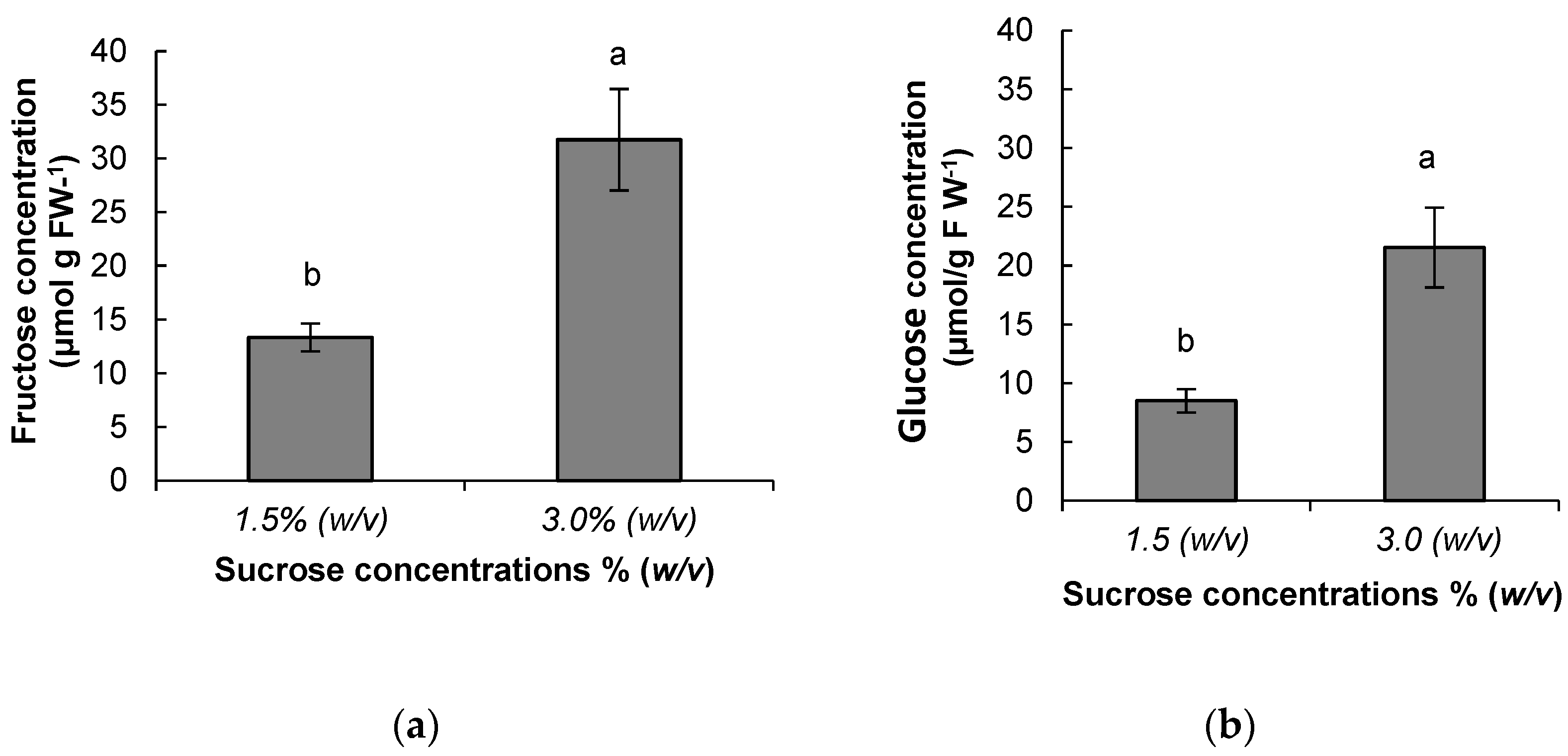
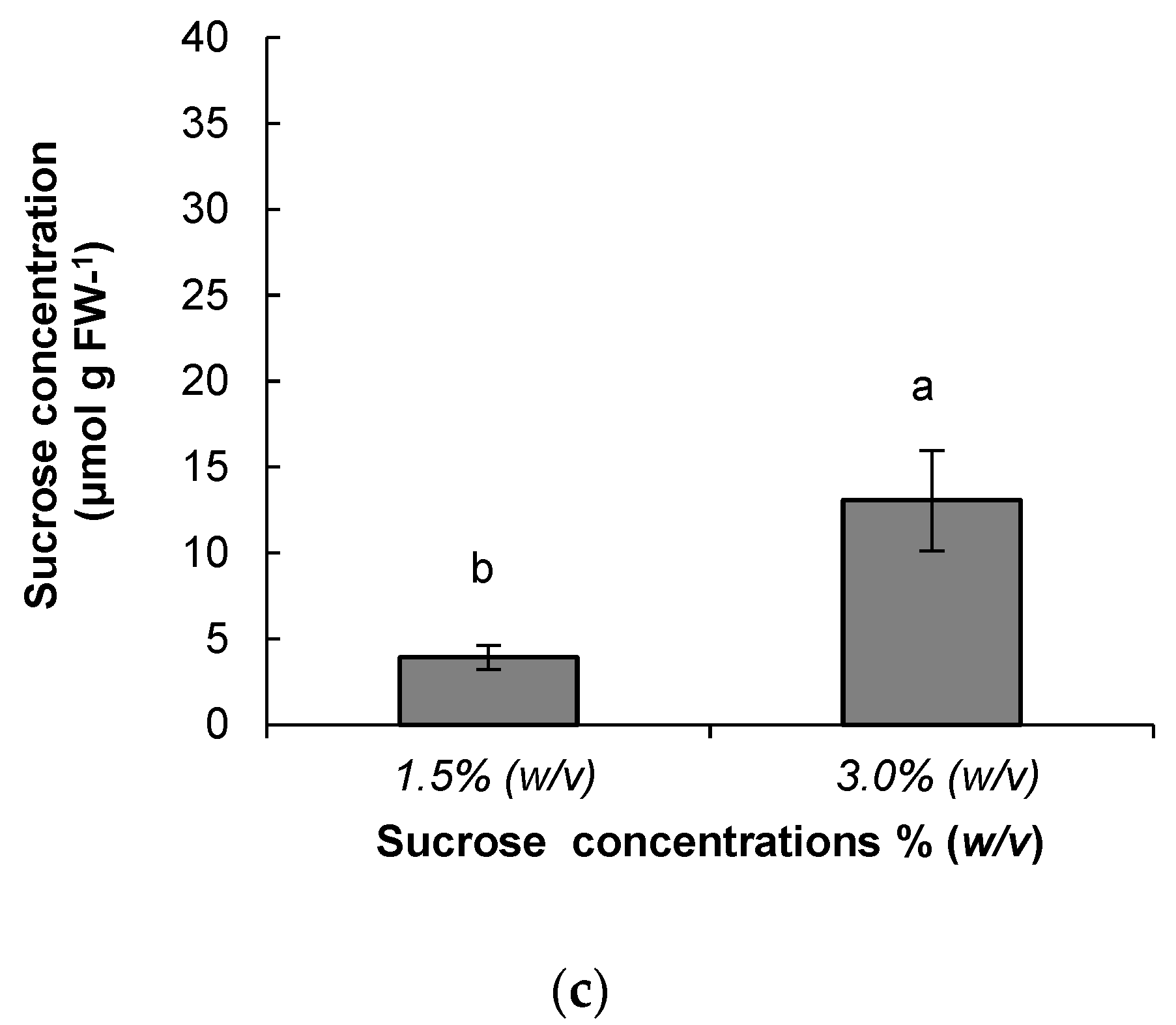
3.2. Metabolite Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Neale, D.B.; Wheeler, N.C. The Conifers. In The Conifers: Genomes, Variation and Evolution; Springer Nature: Cham, Switzerland, 2019; pp. 1–21. [Google Scholar] [CrossRef]
- Farjon, A. The Kew review: Conifers of the world. Kew Bull. 2018, 73, 8. [Google Scholar] [CrossRef]
- Escandón, M.; Valledor, L.; Pascual, J.; Pinto, G.; Cañal, M.J.; Meijón, M. System-wide analysis of short-term response to high temperature in Pinus radiata. J. Exp. Bot. 2017, 68, 3629–3641. [Google Scholar] [CrossRef]
- Castander-Olarieta, A.; Montalbán, I.A.; De Medeiros Oliveira, E.; Dell’Aversana, E.; D’Amelia, L.; Carillo, P.; Steiner, N.; Fraga, H.P.F.; Guerra, M.P.; Goicoa, T.; et al. Effect of thermal stress on tissue ultrastructure and metabolite profiles during initiation of radiata pine somatic embryogenesis. Front. Plant Sci. 2019, 9, 2004. [Google Scholar] [CrossRef]
- Allen, C.D.; Macalady, A.K.; Chenchouni, H.; Bachelet, D.; McDowell, N.; Vennetier, M.; Kitzberger, T.; Rigling, A.; Breshears, D.D.; Hogg (Ted), E.H.; et al. A global overview of drought and heat-induced tree mortality reveals emerging climate change risks for forests. For. Ecol. Manag. 2010, 259, 660–684. [Google Scholar] [CrossRef]
- Jankovský, L.; Palovčíková, D.; Dvořák, M.; Tomšovský, M. Records of Brown spot needle blight related to Lecanosticta acicola in the Czech Republic. Plant Prot. Sci. 2009, 45, 16–18. [Google Scholar] [CrossRef]
- Woods, A.J.; Martín-García, J.; Bulman, L.; Vasconcelos, M.W.; Boberg, J.; La Porta, N.; Peredo, H.; Vergara, G.; Ahumada, R.; Brown, A.; et al. Dothistroma needle blight, weather and possible climatic triggers for the disease’s recent emergence. For. Pathol. 2016, 46, 443–452. [Google Scholar] [CrossRef]
- Reed, B.M.; Sarasan, V.; Kane, M.; Bunn, E.; Pence, V.C. Biodiversity conservation and conservation biotechnology tools. In Vitro Cell. Dev. Biol. Plant. 2011, 47, 1–4. [Google Scholar] [CrossRef]
- De Diego, N.; Montalbán, I.A.; Fernandez de Larrinoa, E.; Moncaleán, P. In vitro regeneration of Pinus pinaster adult trees. Can. J. For. Res. 2008, 38, 2607–2615. [Google Scholar] [CrossRef]
- De Diego, N.; Montalbán, I.A.; Moncaleán, P. In vitro regeneration of adult Pinus sylvestris L. Trees. S. Afr. J. Bot. 2010, 76, 158–162. [Google Scholar] [CrossRef]
- Cortizo, M.; de Diego, N.; Moncaleán, P.; Ordás, R.J. Micropropagation of adult stone pine (Pinus pinea L.). Trees Struct. Funct. 2009, 23, 835–842. [Google Scholar] [CrossRef]
- Phillips, G.C. In vitro morphogenesis in plants—Recent advances. In Vitro Cell. Dev. Biol. Plant. 2004, 40, 342–345. [Google Scholar] [CrossRef]
- Kulus, D.; Woźny, A. Influence of light conditions on the morphogenetic and biochemical response of selected ornamental plant species under in vitro conditions: A mini-review. BioTechnologia 2020, 101, 75–83. [Google Scholar] [CrossRef]
- De Almeida, M.; de Almeida, C.V.; Graner, E.M.; Brondani, G.E.; de Abreu-Tarazi, M.F. Pre-procambial cells are niches for pluripotent and totipotent stem-like cells for organogenesis and somatic embryogenesis in the peach palm: A histological study. Plant Cell Rep. 2012, 31, 1495–1515. [Google Scholar] [CrossRef] [PubMed]
- Thorpe, T.; Stasolla, C.; Yeung, E.C.; de Klerk, G.J.; Roberts, A.; George, E.F. The components of plant tissue culture media II: Organic additions, osmotic and pH effects, and support systems. In Plant Propagation by Tissue Culture, 3rd ed.; George, E.F., Hall, M.A., de Klerk, G.J., Eds.; Springer: Dordrecht, The Netherlands, 2008; pp. 115–173. [Google Scholar]
- Larraburu, E.E.; Correa, G.S.; Llorente, B.E. In vitro development of yellow lapacho (Bignoniaceae) using high-power light emitting diode. Rev. Arvore 2018, 42, e420508. [Google Scholar] [CrossRef]
- Jao, R.C.; Fang, W. An adjustable light source for photo-phyto related research and young plant production. Appl. Eng. Agric. 2003, 19, 601–608. [Google Scholar] [CrossRef]
- Riikonen, J.; Kettunen, N.; Gritsevich, M.; Hakala, T.; Särkkä, L.; Tahvonen, R. Growth and development of Norway spruce and Scots pine seedlings under different light spectra. Environ. Exp. Bot. 2016, 121, 112–120. [Google Scholar] [CrossRef]
- Astolfi, S.; Marianello, C.; Grego, S.; Bellarosa, R. Preliminary investigation of LED lighting as growth light for seedlings from different tree species in growth chambers. Not. Bot. Horti. Agrobot. Cluj-Napoca 2012, 40, 31–38. [Google Scholar] [CrossRef]
- Kumar, N.; Reddy, M.P. In vitro plant propagation: A review. J. For. Environ. Sci. 2011, 27, 61–72. [Google Scholar] [CrossRef]
- Narváez, I.; Martín, C.; Jiménez-Díaz, R.M.; Mercado, J.A.; Pliego-Alfaro, F. Plant regeneration via somatic embryogenesis in mature wild olive genotypes resistant to the defoliating pathotype of Verticillium dahliae. Front. Plant Sci. 2019, 10, 1471. [Google Scholar] [CrossRef]
- Montalbán, I.A.; De Diego, N.; Igartua, E.A.; Setién, A.; Moncaleán, P. A combined pathway of somatic embryogenesis and organogenesis to regenerate radiata pine plants. Plant Biotechnol. Rep. 2011, 5, 177–186. [Google Scholar] [CrossRef]
- Ragonezi, C.; Klimaszewska, K.; Castro, M.R.; Lima, M.; de Oliveira, P.; Zavattieri, M.A. Adventitious rooting of conifers: Influence of physical and chemical factors. Trees Struct. Funct. 2010, 24, 975–992. [Google Scholar] [CrossRef]
- Montalbán, I.A.; De Diego, N.; Moncaleán, P. Testing novel cytokinins for improved in vitro adventitious shoots formation and subsequent ex vitro performance in Pinus radiata. Forestry 2011, 84, 363–373. [Google Scholar] [CrossRef]
- Pereira, C.; Montalbán, I.A.; Pedrosa, A.; Tavares, J.; Pestryakov, A.; Bogdanchikova, N.; Canhoto, J.; Moncaleán, P. Regeneration of Pinus halepensis (Mill.) through organogenesis from apical shoot buds. Forests 2021, 12, 363. [Google Scholar] [CrossRef]
- Stephano-Hornedo, J.L.; Torres-Gutiérrez, O.; Toledano-Magaña, Y.; Gradilla-Martínez, I.; Pestryakov, A.; Sánchez-González, A.; García-Ramos, J.C.; Bogdanchikova, N. ArgovitTM silver nanoparticles to fight Huanglongbing disease in Mexican limes (Citrus aurantifolia Swingle). RSC Adv. 2020, 10, 6146–6155. [Google Scholar] [CrossRef] [PubMed]
- Quoirin, M.; Lepoivre, P. Études des milieux adaptés aux cultures in vitro de Prunus. Acta Hortic. 1977, 78, 437–442. [Google Scholar] [CrossRef]
- Aitken-Christie, J.; Singh, A.P.; Davis, H. Multiplication of meristematic tissue: A new tissue culture system for radiata pine. In Genetic Manipulation of Woody Plants; Hanover, J.W., Keathley, D.E., Eds.; Plenum Publishing Corp: New York, NY, USA, 1988; pp. 413–432. [Google Scholar] [CrossRef]
- Valledor, L.; Escandón, M.; Meijón, M.; Nukarinen, E.; Cañal, M.J.; Weckwerth, W. A universal protocol for the combined isolation of metabolites, DNA, long RNAs, small RNAs, and proteins from plants and microorganisms. Plant J. 2014, 79, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Castander-Olarieta, A.; Pereira, C.; Montalbán, I.A.; Mendes, V.M.; Correia, S.; Suárez-Álvarez, S.; Manadas, B.; Canhoto, J.; Moncaleán, P. Proteome-wide analysis of heat-stress in Pinus radiata somatic embryos reveals a combined response of sugar metabolism and translational regulation mechanisms. Front. Plant Sci. 2021, 12, 631239. [Google Scholar] [CrossRef]
- Coleman, G.D.; Ernst, S.G. In vitro shoot regeneration of Populus deltoides: Effect of cytokinin and genotype. Plant Cell Rep. 1989, 8, 459–462. [Google Scholar] [CrossRef]
- Jiménez, V.M. Involvement of plant hormones and plant growth regulators on in vitro somatic embryogenesis. Plant Growth Regul. 2005, 47, 91–110. [Google Scholar] [CrossRef]
- Fehér, A. Why somatic plant cells start to form embryos? In Somatic Embryogenesis; Mujib, A., Šamaj, J., Eds.; Plant Cell Monographs; Springer: Berlin/Heidelberg, Germany, 2005; Volume 2, pp. 85–101. [Google Scholar] [CrossRef]
- Montalbán, I.A.; Novák, O.; Rolčik, J.; Strnad, M.; Moncaleán, P. Endogenous cytokinin and auxin profiles during in vitro organogenesis from vegetative buds of Pinus radiata Adult Trees. Physiol. Plant. 2013, 148, 214–231. [Google Scholar] [CrossRef]
- Cézar, T.M.; Higa, A.R.; Koehler, H.S.; Ribas, L.L.F. Influence of culture medium, explant length and genotype on micropropagation of Pinus taeda L. Ciênc. Florest. 2015, 25, 13–22. [Google Scholar] [CrossRef]
- Parzymies, M. Nano-silver particles reduce contaminations in tissue culture but decrease regeneration rate and slows down growth and development of Aldrovanda vesiculosa explants. Appl. Sci. 2021, 11, 3653. [Google Scholar] [CrossRef]
- Tung, H.T.; Thuong, T.T.; Cuong, D.M.; Luan, V.Q.; Hien, V.T.; Hieu, T.; Nam, N.B.; Phuong, H.T.N.; Van The Vinh, B.; Khai, H.D.; et al. Silver nanoparticles improved explant disinfection, in vitro growth, runner formation and limited ethylene accumulation during micropropagation of strawberry (Fragaria × Ananassa). Plant Cell. Tissue Organ Cult. 2021, 145, 393–403. [Google Scholar] [CrossRef]
- Rojas-Vargas, A.; Castander-Olarieta, A.; Moncaleán, P.; Montalbán, I.A. Optimization of the micropropagation of elite adult trees of Sequoia sempervirens: Forest species of interest in the Basque Country, Spain. Rev. Bionatura. 2021, 6, 1511–1519. [Google Scholar] [CrossRef]
- Traore, A.; Xing, Z.; Bonser, A.; Carlson, J. Optimizing a protocol for sterilization and in vitro establishment of vegetative buds from mature douglas fir trees. J. Am. Soc. Hortic. Sci. 2005, 40, 1464–1468. [Google Scholar] [CrossRef]
- Pastelín-Solano, M.C.; Ramírez-Mosqueda, M.A.; Bogdanchikova, N.; Castro-González, C.G.; Bello-Bello, J.J. Silver nanoparticles affect the micropropagation of vanilla (Vanilla planifolia Jacks. Ex Andrews). Agrociencia 2020, 54, 1–13. [Google Scholar]
- Andújar, I.; González, N.; García-Ramos, J.C.; Bogdanchikova, N.; Pestryakov, A.; Escalona, M.; Concepción, O. ArgovitTM Silver nanoparticles reduce contamination levels and improve morphological growth in the in vitro culture of Psidium friedrichsthalianum (O. Berg) Nied. SN Appl. Sci. 2020, 2, 2110. [Google Scholar] [CrossRef]
- Andersone, U.; Ievinsh, G. Changes of morphogenic competence in mature Pinus sylvestris L. buds in vitro. Ann. Bot. 2002, 90, 293–298. [Google Scholar] [CrossRef]
- Corredoira, E.; San-José, M.C.; Vieitez, A.M. Induction of somatic embryogenesis from different explants of shoot cultures derived from young Quercus alba Trees. Trees Struct. Funct. 2012, 26, 881–891. [Google Scholar] [CrossRef]
- Khanam, M.N.; Anis, M. Organogenesis and efficient in vitro plantlet regeneration from nodal segments of Allamanda cathartica L. using TDZ and ultrasound assisted extraction of quercetin. Plant Cell. Tissue Organ Cult. 2018, 134, 241–250. [Google Scholar] [CrossRef]
- Bonga, J.M. The effect of various culture media on the formation of embryo-like structures in cultures derived from explants taken from mature Larix decidua. Plant Cell. Tissue Organ Cult. 2004, 77, 43–48. [Google Scholar] [CrossRef]
- Bonga, J.M. Can explant choice help resolve recalcitrance problems in in vitro propagation, a problem still acute especially for adult conifers? Trees Struct. Funct. 2017, 31, 781–789. [Google Scholar] [CrossRef]
- Phillips, G.C.; Garda, M. Plant tissue culture media and practices: An overview. In Vitro Cell. Dev. Biol. Plant. 2019, 55, 242–257. [Google Scholar] [CrossRef]
- Valverde-Cerdas, L.; Rojas-Vargas, A.; Hine-Gómez, A. In vitro propagation of Albizia guachapele, Cedrela odorata, Platymiscium pinnatum and Guaiacum sanctum. Plant Tissue Cult. Biotechnol. 2008, 18, 151–156. [Google Scholar] [CrossRef]
- Moncaleán, P.; Alonso, P.; Centeno, M.L.; Cortizo, M.; Rodríguez, A.; Fernández, B.; Ordás, R.J. Organogenic responses of Pinus pinea cotyledons to hormonal treatments: BA metabolism and cytokinin content. Tree Physiol. 2005, 25, 1–9. [Google Scholar] [CrossRef][Green Version]
- Juan-Vicedo, J.; Serrano-Martinez, F.; Cano-Castillo, M.; Casas, J.L. In vitro propagation, genetic assessment, and, medium-term conservation of coastal endangered species Tetraclinis articulata (Vahl) Masters (Cupressaceae) from adult trees. Plants 2022, 11, 187. [Google Scholar] [CrossRef]
- Wendling, I.; Trueman, S.J.; Xavier, A. Maturation and related aspects in clonal forestry-part II: Reinvigoration, rejuvenation and juvenility maintenance. New For. 2014, 45, 473–486. [Google Scholar] [CrossRef]
- Álvarez, J.M.; Majada, J.; Ordás, R.J. An improved micropropagation protocol for maritime pine (Pinus pinaster Ait.) isolated cotyledons. Forestry 2009, 82, 175–184. [Google Scholar] [CrossRef]
- Nunes, S.; Sousa, D.; Pereira, V.T.; Correia, S.; Marum, L.; Santos, C.; Dias, M.C. Efficient protocol for in vitro mass micropropagation of slash pine. In Vitro Cell. Dev. Biol. Plant. 2018, 54, 175–183. [Google Scholar] [CrossRef]
- Bairu, M.W.; Kane, M.E. Physiological and developmental problems encountered by in vitro cultured plants. Plant Growth Regul. 2011, 63, 101–103. [Google Scholar] [CrossRef]
- Kalia, R.K.; Arya, S.; Kalia, S.; Arya, I.D. Plantlet regeneration from fascicular buds of seedling shoot apices of Pinus roxburghii Sarg. Biol. Plant. 2007, 51, 653–659. [Google Scholar] [CrossRef]
- De Oliveira, L.F.; Ribas, L.L.F.; Quoirin, M.; Koehler, H.S.; Amano, E.; Higa, A.R. Micropropagation of Pinus taeda L. from juvenile material. Tree For. Sci. Biotechnol. 2012, 6, 96–101. [Google Scholar]
- Patel, A.K.; Lodha, D.; Shekhawat, N.S. An improved micropropagation protocol for the ex situ conservation of Mitragyna parvifolia (Roxb.) Korth (Rubiaceae): An endangered tree of pharmaceutical importance. In Vitro Cell. Dev. Biol. Plant. 2020, 56, 817–826. [Google Scholar] [CrossRef]
- Soumare, A.; Boubekri, K.; Lyamlouli, K.; Hafidi, M.; Ouhdouch, Y.; Kouisni, L. Efficacy of phosphate solubilizing actinobacteria to improve rock phosphate agronomic effectiveness and plant growth promotion. Rhizosphere 2021, 17, 100284. [Google Scholar] [CrossRef]
- Gibson, S.I. Plant sugar-response pathways. part of a complex regulatory web. Plant Physiol. 2000, 124, 1532–1539. [Google Scholar] [CrossRef]
- Calamar, A.; De Klerk, G.J. Effect of sucrose on adventitious root regeneration in apple. Plant Cell. Tissue Organ Cult. 2002, 70, 207–212. [Google Scholar] [CrossRef]
- Ahmed, S.; Sharma, A.; Bhushan, B.; Singh, A.K.; Wali, V.K. Effect of carbohydrate source, pH and supporting media on in vitro rooting of banana (Musa Spp.) cv. Grand naine plantlets. Afr. J. Agric. Res. 2014, 9, 1135–1140. [Google Scholar] [CrossRef][Green Version]
- Sumaryono, W.M.; Ratnadewi, D. Effect of carbohydrate source on growth and performance of in vitro sago palm (Metroxylon sagu Rottb.) plantlets. HAYATI J. Biosci. 2012, 19, 88–92. [Google Scholar] [CrossRef]
- Corrêa, L.D.R.; Paim, D.C.; Schwambach, J.; Fett-Neto, A.G. Carbohydrates as regulatory factors on the rooting of Eucalyptus saligna Smith and Eucalyptus globulus Labill. Plant Growth Regul. 2005, 45, 63–73. [Google Scholar] [CrossRef]
- Zavattieri, A.; Lima, M.; Sobral, V.; Oliveira, P.; Costa, A. Effects of carbon source, carbon concentration and culture conditions on in vitro rooting of Pinus pinea L. microshoots. Acta Hortic. 2009, 812, 173–180. [Google Scholar] [CrossRef]
- Gupta, S.; Jatothu, B. Fundamentals and applications of light-emitting diodes (LEDs) in in vitro plant growth and morphogenesis. Plant Biotechnol. Rep. 2013, 7, 211–220. [Google Scholar] [CrossRef]
- Moon, H.K.; Park, S.Y.; Kim, Y.W.; Kim, C.S. Growth of Tsuru-Rindo (Tripterospermum japonicum) cultured in vitro under various sources of light-emitting diode (LED) irradiation. J. Plant Biol. 2006, 49, 174–179. [Google Scholar] [CrossRef]
- Li, H.; Xu, Z.; Tang, C. Effect of light-emitting diodes on growth and morphogenesis of upland cotton (Gossypium hirsutum L.) plantlets in vitro. Plant Cell. Tissue Organ Cult. 2010, 103, 155–163. [Google Scholar] [CrossRef]
- Shin, K.S.; Murthy, H.N.; Heo, J.W.; Hahn, E.J.; Paek, K.Y. The effect of light quality on the growth and development of in vitro cultured Doritaenopsis plants. Acta Physiol. Plant. 2008, 30, 339–343. [Google Scholar] [CrossRef]
- Budiarto, K. Spectral quality affects morphogenesis on Anthurium plantlet during in vitro culture. Agrivita 2010, 32, 234–240. [Google Scholar] [CrossRef]
- Alvarenga, I.C.A.; Pacheco, F.V.; Silva, S.T.; Bertolucci, S.K.V.; Pinto, J.E.B.P. In vitro culture of Achillea millefolium L.: Quality and intensity of light on growth and production of volatiles. Plant Cell. Tissue Organ Cult. 2015, 122, 299–308. [Google Scholar] [CrossRef]
- Jo, E.A.; Tewari, R.K.; Hahn, E.J.; Paek, K.Y. Effect of photoperiod and light intensity on in vitro propagation of Alocasia amazonica. Plant Biotechnol. Rep. 2008, 2, 207–212. [Google Scholar] [CrossRef]
- Poudel, P.R.; Kataoka, I.; Mochioka, R. Effect of red- and blue-light-emitting diodes on growth and morphogenesis of grapes. Plant Cell. Tissue Organ Cult. 2008, 92, 147–153. [Google Scholar] [CrossRef]
- Yaseen, M.; Ahmad, T.; Sablok, G.; Standardi, A.; Hafiz, I.A. Review: Role of carbon sources for in vitro plant growth and development. Mol. Biol. Rep. 2013, 40, 2837–2849. [Google Scholar] [CrossRef]
- Moradi, P.; Ford-Lloyd, B.; Pritchard, J. Metabolomic approach reveals the biochemical mechanisms underlying drought stress tolerance in thyme. Anal. Biochem. 2017, 527, 49–62. [Google Scholar] [CrossRef]
- Jo, E.A.; Tewari, R.K.; Hahn, E.J.; Paek, K.Y. In vitro sucrose concentration affects growth and acclimatization of Alocasia amazonica plantlets. Plant Cell. Tissue Organ Cult. 2009, 96, 307–315. [Google Scholar] [CrossRef]
- Mingozzi, M.; Morini, S.; Lucchesini, M.; Mensuali-Sodi, A. Effects of leaf soluble sugars content and net photosynthetic rate of quince donor shoots on subsequent morphogenesis in leaf explants. Biol. Plant. 2011, 55, 237–242. [Google Scholar] [CrossRef]
- Hildebrandt, T.M.; Nunes Nesi, A.; Araújo, W.L.; Braun, H.P. Amino acid catabolism in plants. Mol. Plant. 2015, 8, 1563–1579. [Google Scholar] [CrossRef]
- Campalans, A.; Messeguer, R.; Goday, A.; Pagès, M. Plant responses to drought, from ABA signal transduction events to the action of the induced proteins. Plant Physiol. Biochem. 1999, 37, 327–340. [Google Scholar] [CrossRef]
- Nuccio, M.L.; Rhodes, D.; McNeil, S.D.; Hanson, A.D. Metabolic engineering of plants for osmotic stress resistance. Curr. Opin. Plant Biol. 1999, 2, 128–134. [Google Scholar] [CrossRef]
- Silva, N.C.B.; Macedo, A.F.; Lage, C.L.S.; Esquibel, M.A.; Sato, A. Developmental effects of additional ultraviolet a radiation, growth regulators and tyrosine in Alternanthera brasiliana (L.) Kuntze cultured in vitro. Braz. Arch. Biol. Technol. 2005, 48, 779–786. [Google Scholar] [CrossRef]
- Zhang, J.X.; Ma, L.Q.; Yu, H.S.; Zhang, H.; Wang, H.T.; Qin, Y.F.; Shi, G.L.; Wang, Y.N. A tyrosine decarboxylase catalyzes the initial reaction of the salidroside biosynthesis pathway in Rhodiola sachalinensis. Plant Cell Rep. 2011, 30, 1443–1453. [Google Scholar] [CrossRef]
- Xu, J.J.; Fang, X.; Li, C.Y.; Yang, L.; Chen, X.Y. General and Specialized Tyrosine Metabolism Pathways in Plants. Abiotech 2020, 1, 97–105. [Google Scholar] [CrossRef]
- Joshi, V.; Joung, J.G.; Fei, Z.; Jander, G. Interdependence of threonine, methionine and isoleucine metabolism in plants: Accumulation and transcriptional regulation under abiotic stress. Amino Acids 2010, 39, 933–947. [Google Scholar] [CrossRef]
- Muthuramalingam, P.; Krishnan, S.R.; Pandian, S.; Mareeswaran, N.; Aruni, W.; Pandian, S.K.; Ramesh, M. Global analysis of threonine metabolism genes unravel key players in rice to improve the abiotic stress tolerance. Sci. Rep. 2018, 8, 9270. [Google Scholar] [CrossRef]
- Krasensky, J.; Jonak, C. Drought, salt, and temperature stress-induced metabolic rearrangements and regulatory networks. J. Exp. Bot. 2012, 63, 1593–1608. [Google Scholar] [CrossRef]
- Lugan, R.; Niogret, M.F.; Leport, L.; Guégan, J.P.; Larher, F.R.; Savouré, A.; Kopka, J.; Bouchereau, A. Metabolome and water homeostasis analysis of Thellungiella salsuginea suggests that dehydration tolerance is a key response to osmotic stress in this halophyte. Plant J. 2010, 64, 215–229. [Google Scholar] [CrossRef]
- Oliva, M.; Guy, A.; Galili, G.; Dor, E.; Schweitzer, R.; Amir, R.; Hacham, Y. Enhanced production of aromatic amino acids in tobacco plants leads to increased phenylpropanoid metabolites and tolerance to stresses. Front. Plant Sci. 2021, 11, 604349. [Google Scholar] [CrossRef]
- KC, S.; Liu, M.; Zhang, Q.; Fan, K.; Shi, Y.; Ruan, J. Metabolic Changes of Amino Acids and Flavonoids in Tea Plants in Response to Inorganic Phosphate Limitation. Int. J. Mol. Sci. 2018, 19, 3683. [Google Scholar] [CrossRef]





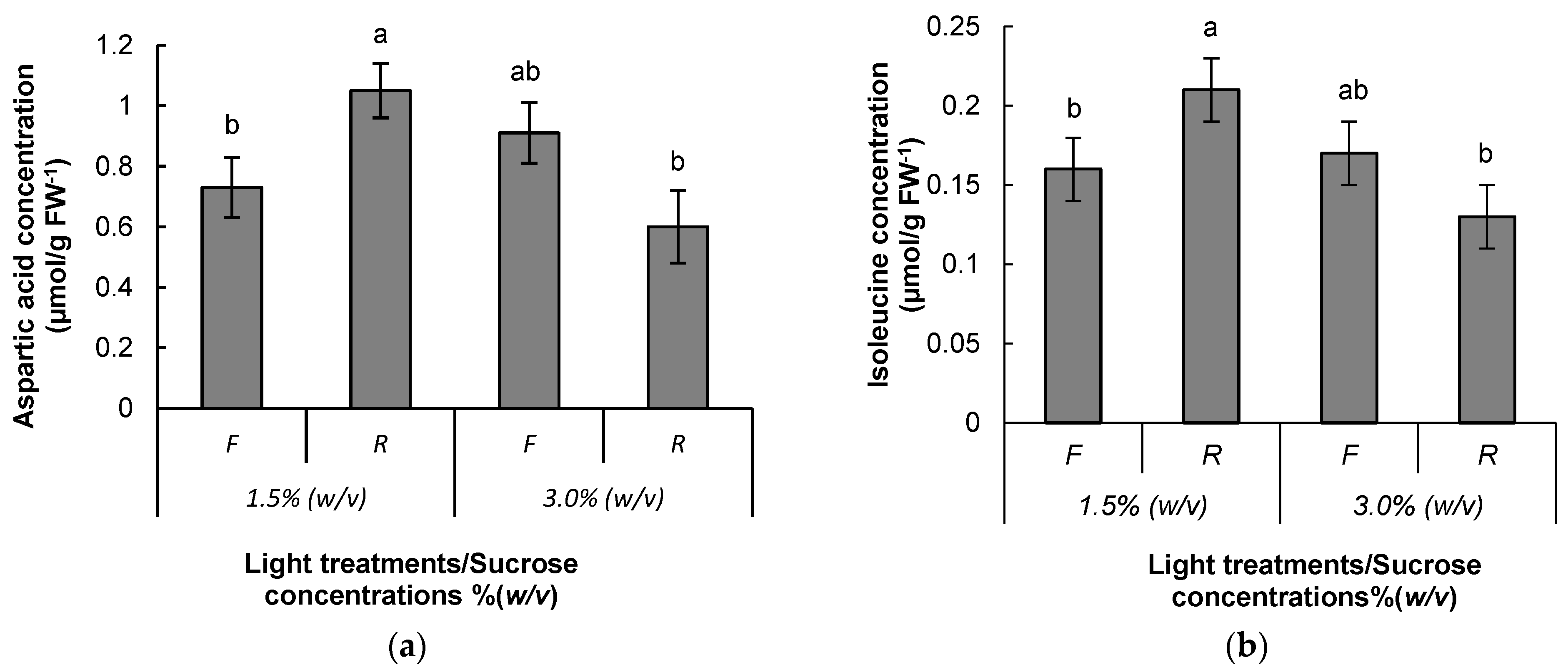
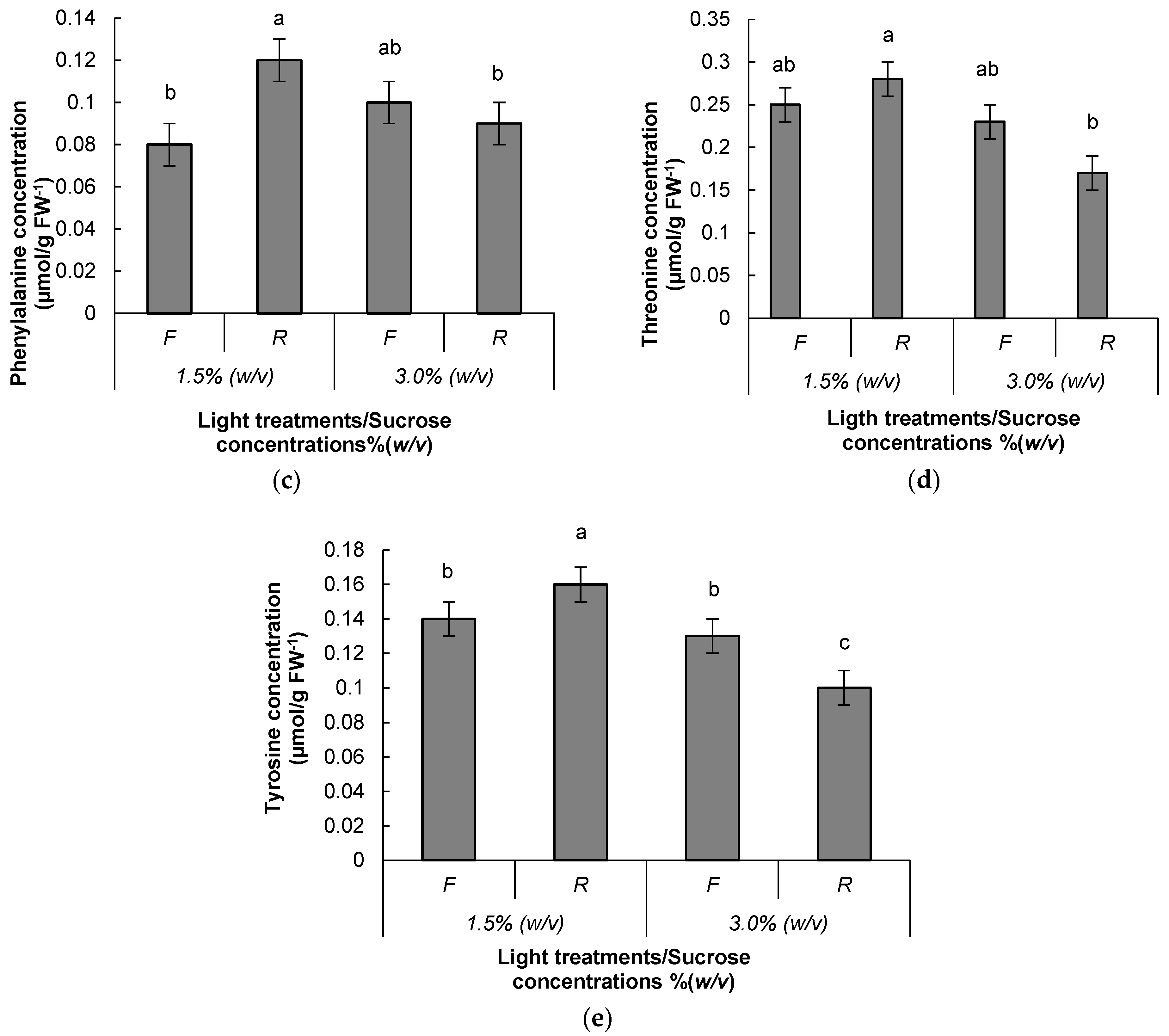
| Carbohydrates (μmol g FW−1) | Retention Time (min) | BA Concentration (μM) | Light Treatment | ||
|---|---|---|---|---|---|
| 22 | 44 | White Fluorescent | Red LEDs | ||
| Fructose | 18.076 | 21.36 ± 6.28 | 22.71 ± 2.90 | 24.91 ± 5.21 | 19.20 ± 2.59 |
| Galactose | 16.096 | 4.82 ± 2.18 | 2.75 ± 0.28 | 2.96 ± 0.37 | 4.25 ± 1.82 |
| Glucose | 14.653 | 13.66 ± 4.37 | 15.50 ± 2.17 | 16.70 ± 3.57 | 12.66 ± 2.19 |
| Mannitol | 22.911 | 7.59 ± 0.45 | 7.04 ± 0.44 | 7.36 ± 0.36 | 7.15 ± 0.55 |
| Sucrose | 12.640 | 12.29 ± 3.82 | 5.67 ± 0.83 | 10.73 ± 3.30 | 5.70 ± 0.99 |
| Carbohydrates (μmol g FW−1) | Retention Time (min) | BA Concentration (μM) | Light Treatment | Sucrose Concentration (w/v) | |||
|---|---|---|---|---|---|---|---|
| 22 | 44 | White Fluorescent | Red LEDs | 1.5% | 3.0% | ||
| Fructose | 18.076 | 25.74 ± 7.18 | 22.87 ± 1.97 | 25.98 ± 4.79 | 21.57 ± 2.36 | 27.17 ± 4.44 | 19.58 ± 1.95 |
| Galactose | 16.096 | 3.12 ± 0.59 | 3.87 ± 0.59 | 4.00 ± 0.47 | 3.19 ± 0.75 | 3.42 ± 0.66 | 3.86 ± 0.53 |
| Glucose | 14.653 | 26.44 ±15.01 | 14.41 ± 1.55 | 24.22 ± 9.88 | 12.46 ± 1.37 | 22.50 9.11 | 13.51±2.28 |
| Mannitol | 22.911 | 5.38 ± 0.75 | 5.85 ± 0.36 | 5.66 ± 0.56 | 5.71 ± 0.41 | 5.83 ± 0.56 | 5.49 ± 0.33 |
| Sucrose | 12.640 | 10.10 ± 4.24 | 5.29 ± 0.79 | 8.63 ± 2.88 | 5.15 ± 0.94 | 8.38 ± 2.71 | 5.12 ± 0.79 |
| Free Amino Acids (μmol g FW−1) | Retention Time (min) | BA Concentration (μM) | Light Treatment | Sucrose Concentration (w/v) | |||
|---|---|---|---|---|---|---|---|
| 22 | 44 | White Fluorescent | Red LEDs | 1.5% | 3.0% | ||
| Alanine | 5.323 | 19.14 ± 9.72 | 16.08 ±4.82 | 15.40 ± 6.71 | 19.43 ± 6.64 | 11.98 ± 4.54 | 22.54 ± 8.08 |
| Arginine | 5.035 | 29.98 ± 10.39 | 28.22 ±7.34 | 34.10 ± 8.77 | 22.8 ± 7.72 | 22.45 ± 6.74 | 35.34 ± 9.64 |
| Asparagine | 3.115 | 13.72 ± 8.27 | 24.78 ±8.18 | 16.09 ± 7.67 | 25.70 ± 9.39 | 30.53 ± 9.68 | 10.52 ± 6.05 |
| Aspartic acid | 1.204 | 0.57 ± 0.12 | 0.61 ± 0.07 | 0.68 ± 0.10 | 0.50 ± 0.05 | 0.60 ± 0.08 | 0.59 ± 0.10 |
| Cysteine | 7.140 | 3.47 ± 0.82 | 4.36 ± 0.55 | 4.61 ± 0.67 | 3.32 ± 0.60 | 3.95 ± 0.79 | 4.07 ± 0.53 |
| Glutamic acid | 1.849 | 9.31 ± 8.07 | 5.55 ± 2.64 | 11.78 ± 6.17 | 1.42 ± 0.10 | 3.60 ± 2.04 | 10.39 ± 6.54 |
| Glutamine | 3.827 | 52.78 ± 16.66 | 70.16 ±8.58 | 65.37 ± 12.38 | 61.26 ± 11.19 | 63.54 ± 12.57 | 63.41 ± 11.29 |
| Glycine | 4.231 | 0.11 ± 0.02 | 0.12 ± 0.01 | 0.13 ± 0.02 | 0.10 ± 0.01 | 0.10 ± 0.02 | 0.13 ± 0.01 |
| Hydroxyproline | 10.330 | 0.02 ± 0.01 | 0.03 ± 0.01 | 0.02 ± 0.01 | 0.03 ± 0.01 | 0.03 ± 0.01 | 0.03 ± 0.01 |
| Histidine | 4.016 | 0.15 ± 0.04 | 0.18 ± 0.02 | 0.18 ± 0.03 | 0.16 ± 0.02 | 0.18 ± 0.03 | 0.17 ± 0.02 |
| Isoleucine | 9.079 | 0.08 ± 0.01 | 0.10 ± 0.01 | 0.09 ± 0.01 | 0.09 ± 0.01 | 0.10 ± 0.01 | 0.09 ± 0.01 |
| Leucine | 9.592 | 0.09 ± 0.02 | 0.11 ± 0.01 | 0.11 ± 0.01 | 0.10 ± 0.01 | 0.10 ± 0.01 | 0.11 ± 0.01 |
| Lysine | 10.018 | 0.14 ± 0.03 | 0.17 ± 0.02 | 0.16 ± 0.03 | 0.16 ± 0.01 | 0.17 ± 0.02 | 0.14 ± 0.02 |
| Methionine | 7.905 | 0.02 ± 0.01 | 0.03 ± 0.01 | 0.02 ± 0.01 | 0.03 ± 0.01 | 0.02 ± 0.01 | 0.03 ± 0.01 |
| Phenylalanine | 8.927 | 0.05 ± 0.01 | 0.06 ± 4.9−3 | 0.06 ± 0.01 | 0.06 ± 0.01 | 0.05 ± 0.01 | 0.06 ± 4.3−3 |
| Proline | 12.543 | 0.27 ± 0.07 | 0.30 ± 0.08 | 0.33 ± 0.09 | 0.23 ± 0.06 | 0.25 ± 0.07 | 0.31 ± 0.08 |
| Serine | 3.335 | 3.82 ± 3.16 | 1.01 ± 0.11 | 3.18 ± 2.24 | 0.83 ± 0.12 | 0.92 ± 0.14 | 3.27 ± 2.42 |
| Threonine | 4.386 | 0.12 ± 0.02 | 0.15 ± 0.01 | 0.14 ± 0.02 | 0.12 ± 0.01 | 0.13 ± 0.02 | 0.14 ± 0.02 |
| Tryptophan | 8.653 | 0.05 ± 0.01 | 0.06 ± 0.01 | 0.05 ± 0.01 | 0.06 ± 5.0−3 | 0.05 ± 0.01 | 0.06 ± 4.6−3 |
| Tyrosine | 6.334 | 0.06 ± 0.01 | 0.09 ± 0.01 | 0.08 ± 0.01 | 0.08 ± 0.01 | 0.08 ± 0.01 | 0.08 ± 0.01 |
| Valine | 7.760 | 0.29 ± 0.07 | 0.40 ± 0.04 | 0.38 ± 0.05 | 0.33 ± 0.04 | 0.32 ± 0.05 | 0.39 ± 0.05 |
| Free Amino Acids (μmol g FW−1) | Retention Time (min) | BA Concentration (μM) | Light Treatment | Sucrose Concentration (w/v) | |||
|---|---|---|---|---|---|---|---|
| 22 | 44 | White Fluorescent | Red LEDs | 1.5% | 3.0% | ||
| Alanine | 5.323 | 27.12 ± 9.12 | 15.29 ± 5.17 | 18.89 ± 7.55 | 19.96 ± 5.62 | 24.53 ± 6.87 | 12.74 ± 5.63 |
| Arginine | 5.035 | 67.60 ± 24.44 | 63.97 ± 15.2 | 79.68 ± 19.55 | 49.47 ± 16.80 | 68.70 ± 15.72 | 60.71 ± 23.06 |
| Asparagine | 3.115 | 0.76 ± 0.09 | 3.98 ± 3.30 | 0.73 ± 0.07 | 5.17 ± 4.50 | 4.56 ± 3.80 | 0.64 ± 0.06 |
| Aspartic acid | 1.204 | 0.81 ± 0.08 | 0.87 ± 0.08 | 0.82 ± 0.04 | 0.89 ± 0.12 | 0.90 ± 0.09 | 0.78 ± 0.06 |
| Cystine | 7.140 | 7.08 ± 0.74 | 7.39 ± 0.53 | 7.17 ± 0.60 | 7.41 ± 0.62 | 7.74 ± 0.66 | 6.69 ± 0.42 |
| Glutamic acid | 1.849 | 19.35 ± 10.99 | 27.51 ± 6.85 | 20.38 ± 8.18 | 29.34 ± 8.35 | 31.59 ± 9.19 | 15.67 ± 5.12 |
| Glutamine | 3.827 | 52.86 ± 12.07 | 39.56 ± 6.66 | 49.22 ± 10.51 | 38.70 ± 5.38 | 46.58 ± 5.06 | 41.08 ± 12.57 |
| Glycine | 4.231 | 0.20 ± 0.02 | 0.18 ± 0.02 | 0.18 ± 0.02 | 0.19 ± 0.03 | 0.20 ± 0.03 | 0.17 ± 0.01 |
| Hydroxyproline | 10.330 | 0.05 ± 0.01 | 0.09 ± 0.01 | 0.07 ± 0.01 | 0.08 ± 0.01 | 0.09 ± 0.02 | 0.06 ± 0.0047 |
| Histidine | 4.016 | 0.24 ± 0.03 | 0.23 ± 0.02 | 0.23 ± 0.03 | 0.23 ± 0.03 | 0.26 ± 0.03 | 0.20 ± 0.02 |
| Isoleucine | 9.079 | 0.17 ± 0.01 | 0.17 ± 0.02 | 0.16 ± 0.01 | 0.18 ± 0.02 | 0.19 ± 0.02 | 0.15 ± 0.01 |
| Leucine | 9.592 | 0.22 ± 0.02 | 0.21 ± 0.02 | 0.20 ± 0.01 | 0.22 ± 0.03 | 0.22 ± 0.02 | 0.19 ± 0.01 |
| Lysine | 10.018 | 0.41 ± 0.16 | 0.25 ± 0.04 | 0.36 ± 0.11 | 0.25 ± 0.04 | 0.30 ± 0.04 | 0.32 ± 0.13 |
| Methionine | 7.905 | 0.02 ± 0.01 | 0.04 ± 0.01 | 0.03 ± 0.01 | 0.04 ± 0.01 | 0.03 ± 0.01 | 0.03 ± 0.01 |
| Phenylalanine | 8.927 | 0.11 ± 0.01 | 0.10 ± 0.01 | 0.09 ± 0.01 | 0.11 ± 0.01 | 0.10 ± 0.01 | 0.10 ± 0.01 |
| Proline | 12.543 | 1.00 ± 0.18 | 1.02 ± 0.26 | 0.91 ± 0.17 | 1.12 ± 0.32 | 1.28 ± 0.28 | 0.67 ± 0.13 |
| Serine | 3.335 | 1.44 ± 0.14 | 1.41 ± 0.15 | 1.31 ± 0.11 | 1.53 ± 0.19 | 1.60 ± 0.16 | 1.19 ± 0.09 |
| Threonine | 4.386 | 0.23 ± 0.02 | 0.24 ± 0.02 | 0.24 ± 0.01 | 0.24 ± 0.03 | 0.27 ± 0.02 a | 0.21 ± 0.01 b |
| Tryptophan | 8.653 | 0.15 ± 0.02 | 0.12 ± 0.01 | 0.14 ± 0.01 | 0.12 ± 0.02 | 0.13 ± 0.01 | 0.13 ± 0.01 |
| Tyrosine | 6.334 | 0.14 ± 0.01 | 0.13 ± 0.01 | 0.13 ± 4.0−3 | 0.14 ± 0.01 | 0.15 ± 0.01 a | 0.12 ± 0.01 b |
| Valine | 7.760 | 0.63 ± 0.09 | 0.56 ± 0.06 | 0.56 ± 0.05 | 0.61 ± 0.09 | 0.63 ± 0.07 | 0.52 ± 0.06 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rojas-Vargas, A.; Castander-Olarieta, A.; Montalbán, I.A.; Moncaleán, P. Influence of Physico-Chemical Factors on the Efficiency and Metabolite Profile of Adult Pinus radiata D. Don Bud Organogenesis. Forests 2022, 13, 1455. https://doi.org/10.3390/f13091455
Rojas-Vargas A, Castander-Olarieta A, Montalbán IA, Moncaleán P. Influence of Physico-Chemical Factors on the Efficiency and Metabolite Profile of Adult Pinus radiata D. Don Bud Organogenesis. Forests. 2022; 13(9):1455. https://doi.org/10.3390/f13091455
Chicago/Turabian StyleRojas-Vargas, Alejandra, Ander Castander-Olarieta, Itziar A. Montalbán, and Paloma Moncaleán. 2022. "Influence of Physico-Chemical Factors on the Efficiency and Metabolite Profile of Adult Pinus radiata D. Don Bud Organogenesis" Forests 13, no. 9: 1455. https://doi.org/10.3390/f13091455
APA StyleRojas-Vargas, A., Castander-Olarieta, A., Montalbán, I. A., & Moncaleán, P. (2022). Influence of Physico-Chemical Factors on the Efficiency and Metabolite Profile of Adult Pinus radiata D. Don Bud Organogenesis. Forests, 13(9), 1455. https://doi.org/10.3390/f13091455








