Ecological Niche Overlap and Prediction of the Potential Distribution of Two Sympatric Ficus (Moraceae) Species in the Indo-Burma Region
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area and Collection of Species Occurrence Data
2.2. Environmental Variables
2.3. Ecological Niche Model
3. Results
3.1. Model Performance
3.2. Environmental Variable Importance
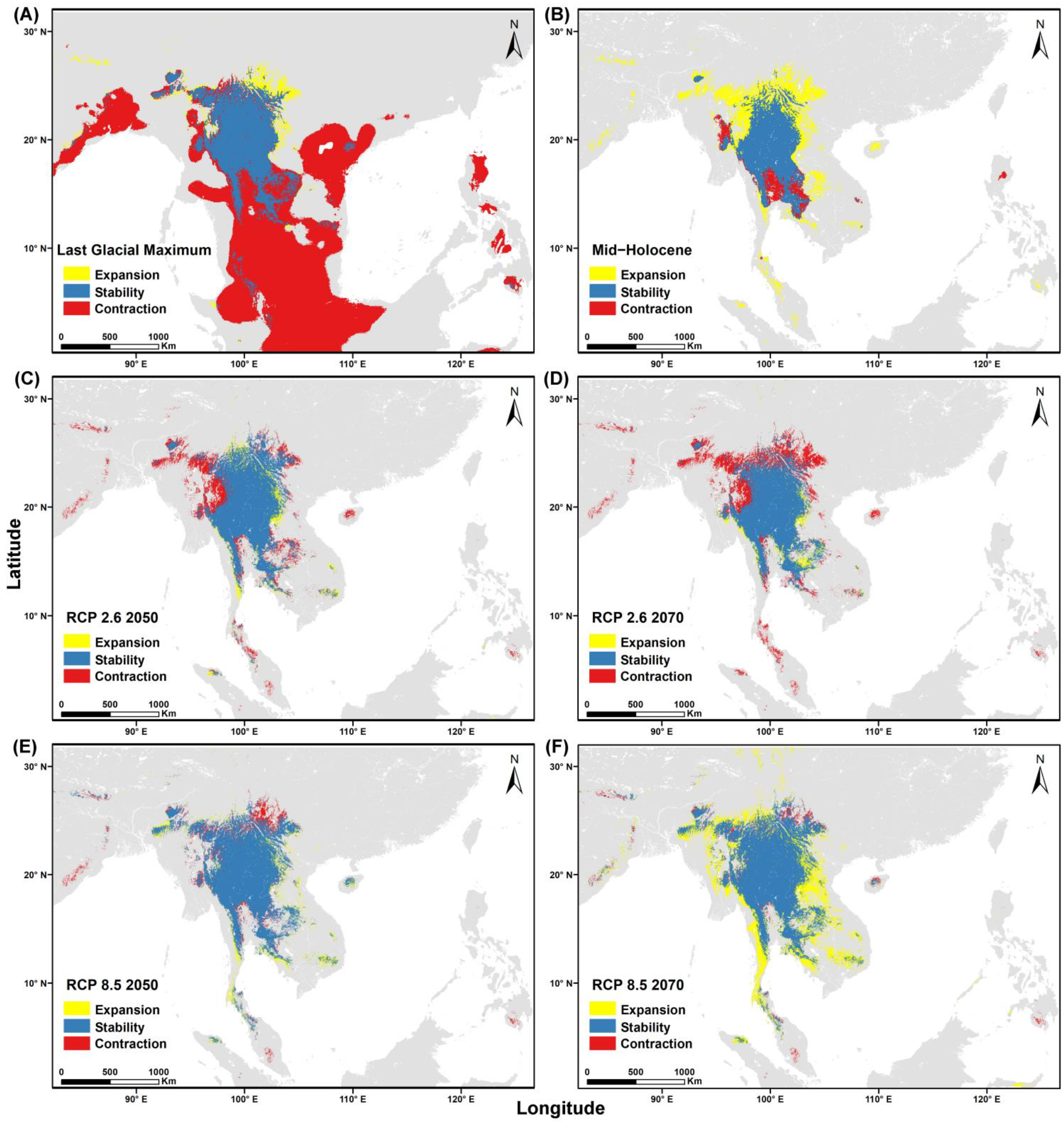
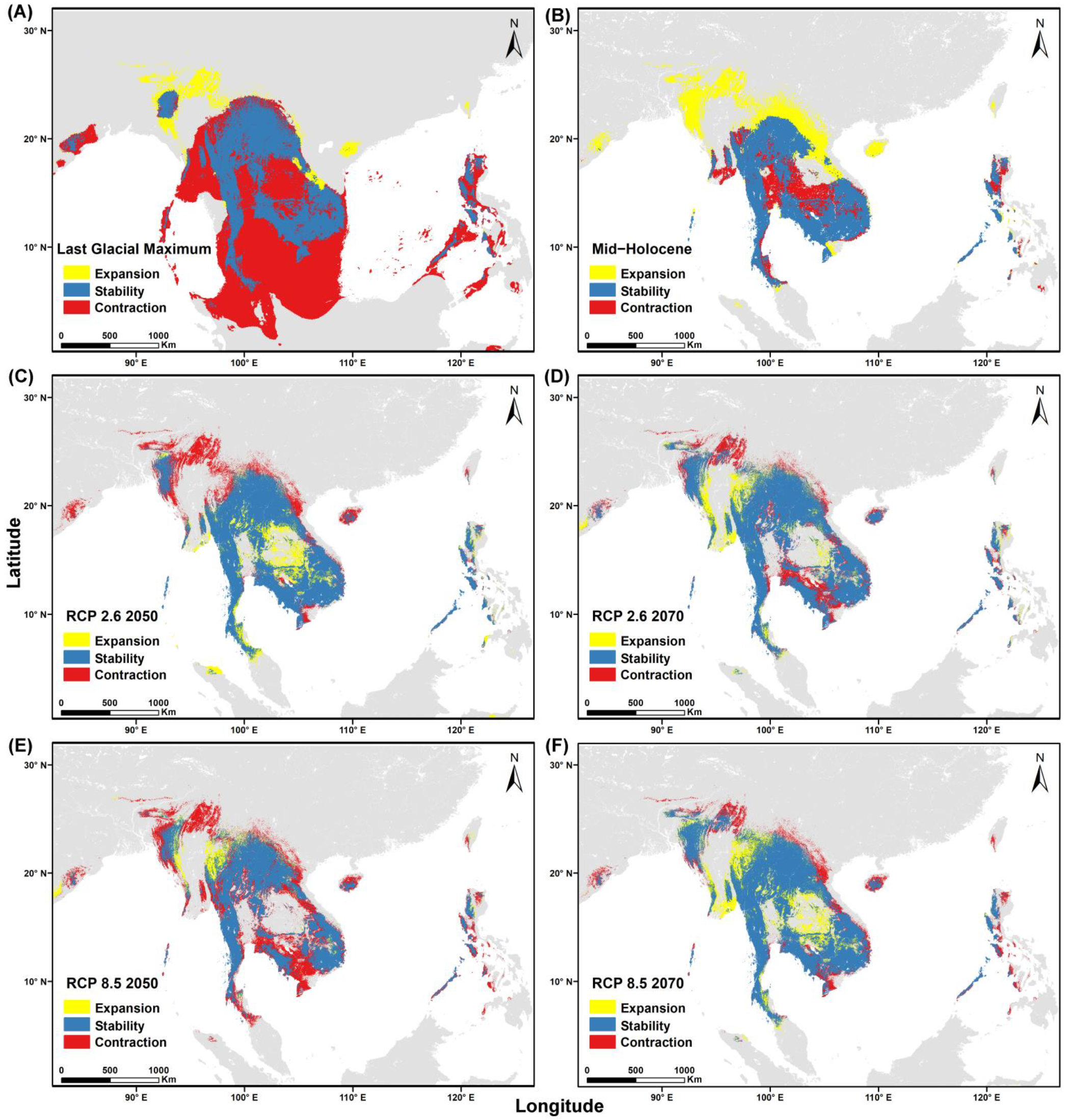
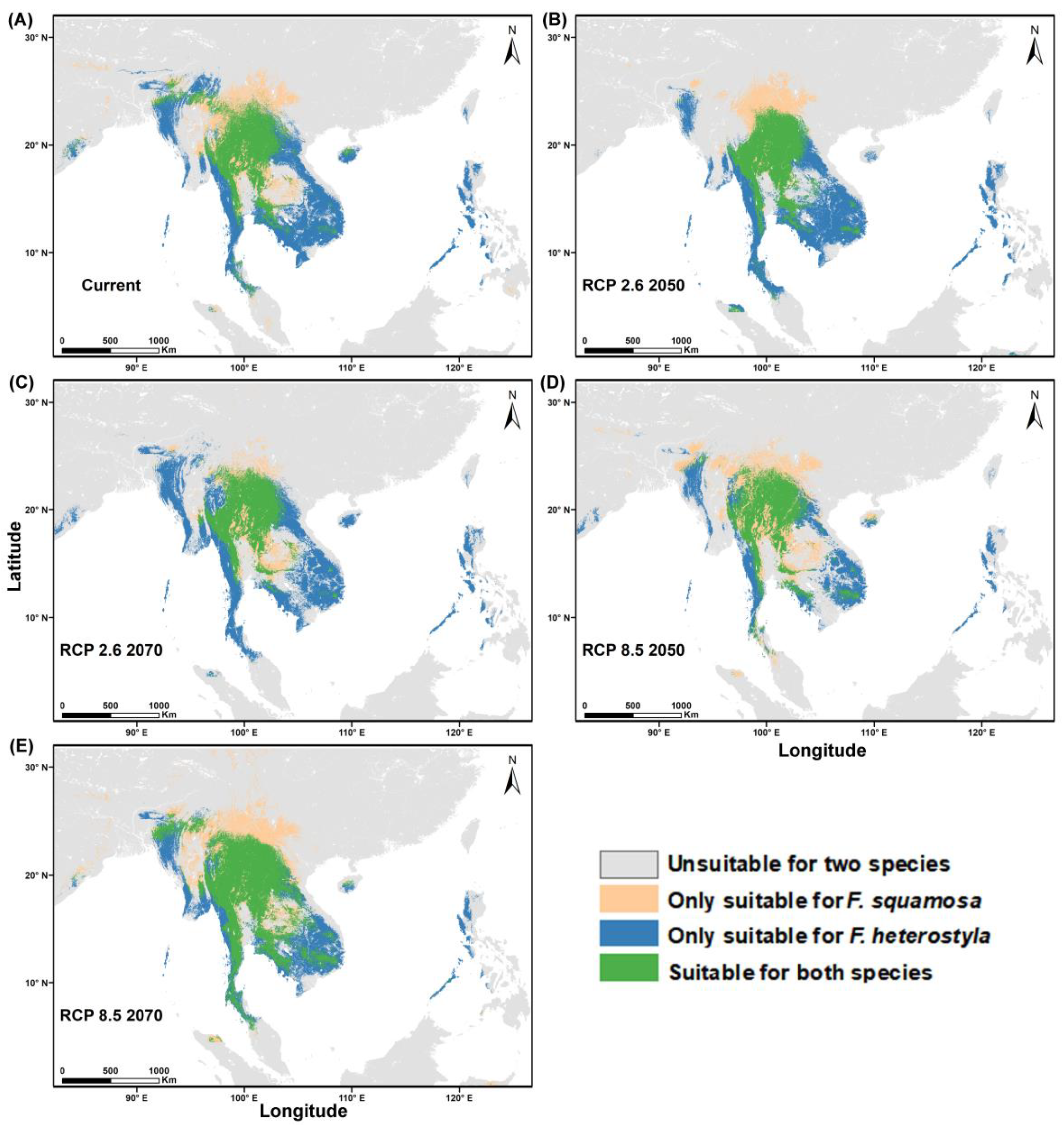
3.3. Predicting Suitable Habitats
3.4. Niche Overlap and Distribution Area for Both Species
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jump, A.S.; Peñuelas, J. Genetic effects of chronic habitat fragmentation in a wind-pollinated tree. Proc. Natl. Acad. Sci. USA 2006, 103, 8096–8100. [Google Scholar] [CrossRef] [PubMed]
- Thornton, P.K.; Herrero, M.; Freeman, H.A.; Okeyo, A.M.; Rege, E.; Jones, P.G.; McDermott, J. Vulnerability, climate change and livestock-opportunities and challenges for the poor. J. Semi-Arid. Trop. Agric. Res. 2007, 4, 1–23. [Google Scholar]
- Bacles, C.F.E.; Jump, A.S. Taking a tree’s perspective on forest fragmentation genetic. Trends Plant Sci. 2011, 16, 13–18. [Google Scholar] [CrossRef]
- García-Valdés, R.; Svennig, J.-C.; Zavala, M.A.; Purves, D.W.; Araújo, M.B. Evaluating the combined effects of climate and land-use change on tree species distribution. J. Appl. Ecol. 2015, 52, 902–912. [Google Scholar] [CrossRef]
- Zambrano, J.; Garzon-Lopez, C.X.; Yeager, L.; Fortunel, C.; Cordeiro, N.J.; Beckman, N.G. The effects of habitat loss and fragmentation on plant functional traits and functional diversity: What do we know so far? Oecologia 2019, 191, 505–518. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Xai, W.; Luan, X.; Zhuang, H.; Khan, T.U.; Zhang, G.; Wu, S. Use of historical data to assess the impact of climate change and anthropogenic disturbance on the black-billed capercaillie (Tetrao urogalloides) in northeast China. Glob. Ecol. Conserv. 2020, 22, e00972. [Google Scholar] [CrossRef]
- Dibattista, J.D. Patterns of genetic variation in anthropogenically impacted populations. Conserv. Genet. 2008, 9, 141–156. [Google Scholar] [CrossRef]
- Ikeda, D.; Max, T.L.; Allan, G.J.; Lau, M.K.; Shuster, S.M.; Whitham, T.G. Genetically informed ecological niche models improve climate change predictions. Glob. Chang. Biol. 2017, 23, 164–176. [Google Scholar] [CrossRef]
- Culshaw, V.; Mairal, M.; Sanmartin, I. Biogeography meets niche modeling: Interring the role of deep time climate change when data is limited. Front. Ecol. Evol. 2021, 9, 662092. [Google Scholar] [CrossRef]
- Williams, J.E. The biodiversity crisis and adaptation to climate change: A case study from Australia’s forests. Environ. Monit. Assess. 2000, 61, 65–74. [Google Scholar] [CrossRef]
- Lewis, O.T. Climate change, species-area curves and the extinction crisis. R. Soc. 2006, 361, 1465. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rinawati, F.; Stein, K.; Lindner, A. Climate change impacts om biodiversity-the setting of a lingering global crisis. Diversity 2013, 5, 114–123. [Google Scholar] [CrossRef]
- Tordoff, A.W.; Bezuijen, M.R.; Duckworth, J.W.; Fellowes, J.R.; Koenig, K.; Pollard, E.H.B.; Royo, A.G. Ecosystem Profile Indo-Burma Biodiversity Hotspot 2011 Update; Critical Ecosystem Partnership Fund: Washington, DC, USA, 2012. [Google Scholar]
- Davis, S.D.; Droop, S.J.; Gregerson, P.; Henson, L.; Leon, C.J.; Synge, H.; Villa-Lobos, J.L.; Zantovska, J. Plants in Danger, What Do We Know; IUCN: Gland, Switzerland; Cambridge, UK, 1986. [Google Scholar]
- Davis, S.D.; Heywood, V.H.; Hamilton, A.C. Centres of Plant Diversity: A Guide and Strategy for Their Conservation: Asia, Australasia and the Pacific; IUCN Publications Unit: Cambridge, UK, 1995; Volume 2. [Google Scholar]
- Mittermeier, R.A.; Robles Gil, P.; Hoffmann, M.; Pilgrim, J.D.; Brooks, T.M.; Mittermeier, C.G.; Lamoreux, J.; da Fonseca, G.A.B. Hotspots Revisited: Earth’s Biologically Richest and Most Endangered Ecoregions; CEMEX: Mexico City, Mexico, 2004. [Google Scholar]
- Malcolm, J.R.; Liu, C.; Neilson, R.P.; Hansen, L.; Hannah, L. Global warming and extinctions of endemic species from biodiversity hotspots. Conserv. Biol. 2006, 20, 538–548. [Google Scholar] [CrossRef] [PubMed]
- Herre, E.; Jandér, K.; Machado, C. Evolutionary ecology of figs and their associates: Recent progress and outstanding puzzles. Annu. Rev. Ecol. Evol. Syst. 2008, 39, 439–458. [Google Scholar] [CrossRef]
- Jenzen, D.H. How to be a Fig. Annu. Rev. Ecol. Evol. Syst. 1979, 10, 13–51. [Google Scholar] [CrossRef]
- Wiebes, J.T. Co-evolution if figs and their insect pollinators. Annu. Rev. Ecol. Evol. Syst. 1979, 10, 1–12. [Google Scholar] [CrossRef]
- Cruaud, A.; Rønsted, N.; Chantarasuwan, B.; Chou, L.S.; Clement, W.L.; Couloux, A.; Cousins, B.; Genson, G.; Harrison, R.H.; Hanson, P.E.; et al. An extreme case of plant-insect co-diversification: Figs and fig-pollinating wasps. Syst. Biol. 2012, 61, 1029–1047. [Google Scholar] [CrossRef]
- Michaloud, G.; Carriere, S.; Kobbi, M. Exceptions to the one: One relationship between African fig trees and their fig wasp pollinators: Possible evolutionary scenarios. J. Biogeogr. 1996, 23, 513–520. [Google Scholar] [CrossRef]
- Rasplus, J.Y. The one-to-one species-specificity of the Ficus-Agaoninae mutualism: How casual? In The Biodiversity of African Plants; van der Maesen, L.J.G., van der Burgt, X.M., van Medenbach de Rooy, J.M., Eds.; Kluwer Academic Publishers: Wageningen, The Netherlands, 1996; pp. 639–649. [Google Scholar]
- Moe, A.M.; Rossi, D.R.; Weiblen, G.D. Pollinator sharing in dioecious figs (Ficus: Moraceae). Biol. J. Linn. Soc. 2011, 103, 546–558. [Google Scholar] [CrossRef]
- Darwell, C.T.; Al-Beidh, S.; Cook, J.M. Molecular species delimitation of a symbiotic fig-pollinating wasp species complex reveals extreme deviation from reciprocal partner specificity. BMC Evol. Biol. 2014, 14, 189. [Google Scholar] [CrossRef]
- Yu, H.; Tian, E.; Zheng, L.; Deng, X.; Cheng, Y.; Chen, L.; Wu, W.; Tanming, W.; Zhang, D.; Compton, S.G.; et al. Multiple parapatric pollinators have radiated across a continental fig tree displaying clinal genetic variation. Mol. Ecol. 2019, 28, 2391–2405. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Jandér, K.C.; Huang, J.F.; Wang, B.; Zhao, J.B.; Miao, B.G.; Peng, Y.Q.; Herre, E.A. The evolution of parasitism from mutualism in wasps pollinating the fig, Ficus microcarpa, in Yunnan Province, China. Proc. Natl. Acad. Sci. USA 2021, 188, e2021148118. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.X.; Yang, D.R.; Peng, Y.Q.; Compton, S.G. Complementary fruiting phenologies facilitate sharing of one pollinator fig wasps by two fig trees. J. Plant Ecol. 2015, 8, 197–206. [Google Scholar] [CrossRef]
- Fungjanthuek, J.; Zhang, Z.R.; Peng, Y.Q.; Gao, J. The complete chloroplast genome of two related fig species Ficus squamosa and Ficus heterostyla. Mitochondrial DNA Part B 2022, 7, 236–238. [Google Scholar] [CrossRef]
- Pothasin, P.; Compton, S.G.; Wangpakapattanawong, P. Seasonality of leaf and fig production in Ficus squamosa, a fig tree with seeds dispersed by water. PLoS ONE 2016, 11, e0152380. [Google Scholar] [CrossRef] [PubMed]
- Shanahan, M.; So, S.; Gompton, S.G.; Corlett, R.T. Fig-eating by vertebrate frugivores: A global review. Biol. Rev. 2001, 76, 529–572. [Google Scholar] [CrossRef]
- Stock, C.A.; Alexander, M.A.; Bond, N.A.; Brander, K.M.; Cheung, W.W.; Curchitser, E.N.; Delworth, T.L.; Dunne, J.P.; Griffies, S.M.; Haltuch, M.A.; et al. On the use of IPCC-class models to assess the impact of climate on living marine resources. Prog. Oceanogr. 2011, 88, 1–27. [Google Scholar] [CrossRef]
- Bojinski, S.; Verstraete, M.; Peterson, T.C.; Richter, C.; Simmons, A.; Zemp, M. The concept of essential climate variables in support of climate research, applications, and policy. Bull. Am. Meteorol. Soc. 2014, 95, 1431–1443. [Google Scholar] [CrossRef]
- Harris, R.M.B.; Grose, M.R.; Lee, G.; Bindoff, N.L.; Porfirio, L.L.; Fox-Hughes, P. Climate projections for ecologists: Climate projections for ecologists. Wiley Interdiscip. Rev. Clim. Chang. 2014, 5, 621–637. [Google Scholar] [CrossRef]
- Cavanagh, R.D.; Murphy, E.J.; Bracegirdle, T.J.; John, T.; Knowland, C.A.; Corney, S.P.; Smith, W.O.; Waluda, C.M.; Johnston, N.M.; Bellberby, R.G.J. A synergistic approach for evaluating climate model output for ecological applications. Front. Mar. Sci. 2017, 4, 308. [Google Scholar] [CrossRef]
- Li, Y.; Li, M.; Li, C.; Liu, Z. Optimized Maxent model predictions of climate change impacts on the suitable distribution of Cunninghamia lanceolata in China. Forests 2020, 11, 302. [Google Scholar] [CrossRef] [Green Version]
- Berg, C.C.; Pattharahirantrlcin, N.; Chantarasuwan, B. Cecropiaceae and Moraceae. In Flora of Thailand; Santisuk, T., Larsen, K., Eds.; Forest Herbarium, Royal Forest Department: New York, NY, USA, 2011; Volume 10, pp. 475–675. [Google Scholar]
- Zhou, Z.; Gilbert, M.G. Flora of China. 2003, 5, 37–71. Available online: http://www.efloras.org/flora_page.aspx?flora_id=2 (accessed on 21 August 2022).
- Warren, D.L.; Seifert, S.N. Ecological niche modeling in Maxent: The importance of model complexity and the performance of model selection criteria. Ecol. Appl. 2011, 21, 335–342. [Google Scholar] [CrossRef] [PubMed]
- Crowther, T.W.; Glick, H.B.; Covey, K.R.; Bettigole, C.; Maynard, D.S.; Thomas, S.M.; Smith, J.R.; Hintler, G.; Duguid, M.C.; Amatulli, G.; et al. Mapping tree density at a global scale. Nature 2015, 525, 201–205. [Google Scholar] [CrossRef]
- Simard, M.; Pinto, N.; Fisher, J.B.; Baccini, A. Mapping forest canopy height globally with spaceborne lidar. J. Geophys. Res. Biogeosciences 2011, 116, G04021. [Google Scholar] [CrossRef]
- Hughes, A.C.; Satasook, C.; Bates, P.J.; Bumrungsri, S.; Jones, G. The projected effects of climatic and vegetation changes on the distribution and diversity of Southeast Asian bats. Glob. Chang. Biol. 2012, 18, 1854–1865. [Google Scholar] [CrossRef]
- Meinshausen, M.; Smith, S.J.; Calvin, K.; Daniel, J.S.; Kainuma, M.L.T.; Lamarque, J.F.; Matsumoto, K.; Montzka, S.A.; Raper, S.C.B.; Riahi, K. The RCP greenhouse gas concentrations and their extensions from 1765 to 2300. Clim. Chang. 2011, 109, 213–241. [Google Scholar] [CrossRef]
- Su, H.; Bista, M.; Li, M. Mapping habitat suitability for Asiatic black bear and red panda in Makalu Barun National Park of Nepal from Maxent and GARP models. Sci. Rep. 2021, 11, 14135. [Google Scholar] [CrossRef]
- Warren, D.L.; Glor, R.E.; Turelli, M. Environmental niche equivalency versus conservatism: Quantitative approaches to niche evolution. Evolution 2008, 62, 2868–2883. [Google Scholar] [CrossRef]
- Noce, S.; Caposaso, L.; Santini, M. A new global dataset of bioclimatic indicators. Sci. Data 2020, 7, 1–12. [Google Scholar] [CrossRef]
- Elith, J.; Phillips, S.J.; Hastie, T.; Dudík, M.; Chee, Y.E.; Yates, C.J. A statistical explanation of MaxEnt for ecologists. Divers. Distrib. 2011, 17, 43–57. [Google Scholar] [CrossRef]
- Shao, H.; Tian, J.Q.; Guo, K.; Sun, J.X. Effects of sample size and species traits on performance of Bioclim in predicting geographical distribution of tree species-a case study with 12 deciduous Quercus species indigenous to China. Chin. J. Plant Ecol. 2009, 33, 870–877. [Google Scholar]
- Suárez-Mota, M.E.; Villaseñor, J.L. Ecological niche overlap among species of the genus Zaluzania (Asteraceae) from the dry regions of Mexico. Plant Ecol. Evol. 2020, 153, 337–347. [Google Scholar] [CrossRef]
- Chown, S.L.; Hoffmann, A.A.; Kristensen, T.N.; Angilletta, M.J.J.; Stenseth, N.C.; Pertaldi, C. Adapting to climate change: A perspective from evolutionary physiology. Clim. Res. 2010, 43, 3–15. [Google Scholar] [CrossRef]
- Jensen, A.M.; Schamp, B.S.; Belleau, A. Evidence of temporal niche separation via low flowering time overlap in an old-feld plant community. Oecologia 2019, 189, 1071–1082. [Google Scholar] [CrossRef]
- Seavy, N.E.; Gardali, T.; Golet, G.H.; Griggs, F.O.; Howell, C.A.; Kelsey, R.; Small-Lorenz, S.; Viers, J.H.; Weigand, J. Why climate change makes riparian restoration more important than ever: Recommendations for practice and research. Ecol. Restor. 2009, 27, 330–338. [Google Scholar] [CrossRef]
- Capon, S.J.; Chambers, L.E.; Nally, R.M.; Naiman, R.J.; Davies, P.; Marshall, N.; Pittock, J.; Reid, M.; Capon, T.; Douglas, M.; et al. Riparian ecosystems in the 21st century: Hotspots for climate change adaptation? Ecosystems 2013, 16, 359–381. [Google Scholar] [CrossRef]
- Kominoski, J.S.; Shah, J.J.F.; Canhoto, C.; Fischer, D.G.; Giling, D.P.; González, E.; Griffiths, N.A.; Larrãnaga, A.; LeRoy, C.J.; Mineau, M.M.; et al. Forecasting functional implications of global changes in riparian plant communities. Front. Ecol. Environ. 2013, 11, 423–432. [Google Scholar] [CrossRef]
- Flanagan, N.E.; Richardson, C.J.; Ho, M. Connecting differential responses of native and invasive riparian plants to climate change and environmental alteration. Ecol. Appl. 2015, 25, 753–767. [Google Scholar] [CrossRef]
- Pearson, R.G.; Dawson, T.P. Predicting the impacts of climate change on the distribution of species: Are bioclimate envelope models useful? Glob. Ecol. Biogeogr. 2003, 12, 361–371. [Google Scholar] [CrossRef]
- Braunisch, L.; Coppes, J.; Arlettaz, R.; Suchant, R.; Schmid, H.; Bollmann, K. Selecting from correlated climate variables: A major source of uncertainty for predicting species distributions under climate change. Ecography 2013, 36, 971–983. [Google Scholar] [CrossRef]
- Proffit, M.; Lapeyre, B.; Buatois, B.; Deng, X.X.; Arnal, P.; Gouzerh, F.; Carrasco, D.; Hossaert-McKey, M. Chemical signal is in the blend: Bases of plant-pollinator encounter in a highly specialized interaction. Sci. Rep. 2020, 10, 10071. [Google Scholar] [CrossRef] [PubMed]
- Qin, A.; Liu, B.; Guo, Q.; Bussmann, R.W.; Ma, F.; Jian, Z.; Xu, G.; Pei, S. Maxent modeling for predicting impacts of climate change on the potential distribution of Thuja sutchuensis Franch., an extremely endangered conifer from southwestern China. Glob. Ecol. Conserv. 2017, 10, 139–146. [Google Scholar] [CrossRef]
- Qin, A.; Jin, K.; Batsaikhun, M.E.; Nyamjav, J.; Li, G.; Li, J.; Xue, Y.; Sun, G.; Wu, L.; Indree, T.; et al. Predicting the current and future suitable habitats of the main dietary plants of the Gobi Bear using Maxent modeling. Glob. Ecol. Conserv. 2020, 22, e01032. [Google Scholar] [CrossRef]
- Prayoon, U.; Suksavate, W.; Chaiyes, A.; Winitpornsawan, S.; Tunhikorn, S.; Faengbubpha, K.; Angkaew, C.; Pattanakiat, S.; Duengkae, P. Past, present and future habitat suitable for gaur (Bos gaurus) in Thailand. Agric. Nat. Resour. 2021, 55, 743–756. [Google Scholar]
- Valencia-Rodríguez, D.; Jiménez-Segura, L.; Rogéliz, C.A.; Parra, J.L. Ecological niche modeling as an effective tool to predict the distribution of freshwater organisms: The case of the Sabaleta Brycon henni (Eigenmann, 1913). PLoS ONE 2021, 16, e0247876. [Google Scholar] [CrossRef] [PubMed]

| Category | Variable | Abbreviation | Source |
|---|---|---|---|
| Soil variables | Bulk density * | bdod * | SoilGrids250m |
| Cation exchange capacity (at pH 7) | cec | ||
| Coarse fragments | cfvo | ||
| Clay content * | clay * | ||
| Nitrogen | nitrogen | ||
| Organic carbon density * | ocd * | ||
| Soil organic carbon stock | ocs | ||
| Soil pH | ph | ||
| Sand * | sand * | ||
| Silt * | silt * | ||
| Soil organic carbon | soc | ||
| Habitat variables | Tree height * | canopy * | [40,41] |
| Forest cover | cover | ||
| Bioclimatic variables | Annual mean temperature * | bio1 * | WORLDCLIM |
| Mean diurnal range (mean of monthly (max temp − min temp)) | bio2 | ||
| Isothermality (BIO2/BIO7) (×100) | bio3 | ||
| Temperature seasonality (standard deviation ×100) | bio4 | ||
| Max temperature of warmest month * | bio5 * | ||
| Min temperature of coldest month * | bio6 * | ||
| Temperature annual range (BIO5–BIO6) | bio7 | ||
| Mean temperature of wettest quarter | bio8 | ||
| Mean temperature of driest quarter | bio9 | ||
| Mean temperature of warmest quarter * | bio10 * | ||
| Mean temperature of coldest quarter * | bio11 * | ||
| Annual precipitation | bio12 | ||
| Precipitation of wettest month * | bio13 * | ||
| Precipitation of driest month * | bio14 * | ||
| Precipitation seasonality (coefficient of variation) | bio15 | ||
| Precipitation of wettest quarter * | bio16 * | ||
| Precipitation of driest quarter | bio17 | ||
| Precipitation of warmest quarter | bio18 * | ||
| Precipitation of coldest quarter * | bio19 * |
| Abbreviation | Variable Definition | Ficus squamosa | Ficus heterostyla | ||
|---|---|---|---|---|---|
| Contribution (%) | Permutation | Contribution (%) | Permutation | ||
| cec | Cation exchange capacity (at pH 7) | 10.9 | 13.4 | 1.3 | 1.3 |
| cfvo | Coarse fragments | 4.9 | 3 | 3.1 | 2.4 |
| nitrogen | Nitrogen | 0.2 | 0.5 | 4.8 | 4.7 |
| ocs | Soil organic carbon stock | 0.7 | 3.5 | 1.3 | 2.2 |
| ph | Soil pH | 3.5 | 2.9 | 1 | 0.7 |
| soc | Soil organic carbon | 0.8 | 0.9 | 1.9 | 1.4 |
| cover | Forest cover | 0.3 | 0.6 | 7.3 | 3.8 |
| bio2 | Mean diurnal range (mean of monthly (max temp − min temp)) | 33.9 | 40.5 | 1.2 | 1.6 |
| bio3 | Isothermality (BIO2/BIO7) (×100) | 17.4 | 0 | 7.6 | 1 |
| bio4 | Temperature seasonality (standard deviation ×100) | 5.4 | 9.6 | 23.6 | 40.9 |
| bio7 | Temperature annual range (BIO5–BIO6) | 5.3 | 0.5 | 17.8 | 0.6 |
| bio8 | Mean temperature of wettest quarter | 3.2 | 4.2 | 0.1 | 0.8 |
| bio9 | Mean temperature of driest quarter | 1.3 | 1.9 | 3.9 | 0.6 |
| bio12 | Annual precipitation | 2.8 | 11 | 0.3 | 0.1 |
| bio15 | Precipitation seasonality (coefficient of variation) | 3.6 | 2.2 | 8.9 | 0.7 |
| bio17 | Precipitation of driest quarter | 5.8 | 5.4 | 15.8 | 37.5 |
| Environmental Variable | F. squamosa | F. heterostyla | ||
|---|---|---|---|---|
| Suitable Ranges | Most Suitable Value | Suitable Ranges | Most Suitable Value | |
| Mean diurnal range (°C) | >9.69 | 12.04 | >3.53 | 12.04 |
| Isothermality | 43.47–74.97 | 51.48 | 46.97–78.92 | 58.40 |
| Temperature seasonality (C of V) | 10.29–48.82 | 24.68 | 6.24–37.58 | 14.56 |
| Temperature annual range (°C) | 14.56–27.50 | 21.99 | 9.92–23.43 | 15.30 |
| Mean temperature of wettest quarter (°C) | 14.29–29.43 | 25.64 | >18.85 | 25.86 |
| Mean temperature of driest quarter (°C) | 3.90–28.46 | 22.07 | >15.05 | 21.70 |
| Annual precipitation (mm) | <2525.67 | 1 129.60 | >905.74 | \ |
| Precipitation seasonality (C of V) | 0.49–1.19 | 0.78 | 0.57–1.22 | 0.76 |
| Precipitation of driest quarter (mm) | 8.06–161.52 | 37.06 | 20.46–132.17 | 37.65 |
| Niche Overlap | Schoener’s Parameter (D) | Hellinger’s-Based Parameter (I) |
|---|---|---|
| Current epoch | 0.7030 | 0.9253 |
| RCP2.6 2050 | 0.7168 | 0.9332 |
| RCP2.6 2070 | 0.6979 | 0.9259 |
| RCP8.5 2050 | 0.7336 | 0.9376 |
| RCP8.5 2070 | 0.7941 | 0.9625 |
| Species | Area (%) | ||||||
|---|---|---|---|---|---|---|---|
| LGM | Mid | Current | RCP 2.6 2050 | RCP 2.6 2070 | RCP 8.5 2050 | RCP 8.5 2070 | |
| F. squamosa | 31.76% | 7.80% | 12.49% | 10.28% | 9.10% | 12.34% | 17.69% |
| F. heterostyla | 29.97% | 16.54% | 18.91% | 17.37% | 17.49% | 12.71% | 19.11% |
| Overlap | N.D. | N.D. | 7.57% | 7.09% | 6.43% | 6.55% | 12.05% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fungjanthuek, J.; Huang, M.-J.; Hughes, A.C.; Huang, J.-F.; Chen, H.-H.; Gao, J.; Peng, Y.-Q. Ecological Niche Overlap and Prediction of the Potential Distribution of Two Sympatric Ficus (Moraceae) Species in the Indo-Burma Region. Forests 2022, 13, 1420. https://doi.org/10.3390/f13091420
Fungjanthuek J, Huang M-J, Hughes AC, Huang J-F, Chen H-H, Gao J, Peng Y-Q. Ecological Niche Overlap and Prediction of the Potential Distribution of Two Sympatric Ficus (Moraceae) Species in the Indo-Burma Region. Forests. 2022; 13(9):1420. https://doi.org/10.3390/f13091420
Chicago/Turabian StyleFungjanthuek, Jenjira, Man-Juan Huang, Alice C. Hughes, Jian-Feng Huang, Huan-Huan Chen, Jie Gao, and Yan-Qiong Peng. 2022. "Ecological Niche Overlap and Prediction of the Potential Distribution of Two Sympatric Ficus (Moraceae) Species in the Indo-Burma Region" Forests 13, no. 9: 1420. https://doi.org/10.3390/f13091420
APA StyleFungjanthuek, J., Huang, M.-J., Hughes, A. C., Huang, J.-F., Chen, H.-H., Gao, J., & Peng, Y.-Q. (2022). Ecological Niche Overlap and Prediction of the Potential Distribution of Two Sympatric Ficus (Moraceae) Species in the Indo-Burma Region. Forests, 13(9), 1420. https://doi.org/10.3390/f13091420






