δ15N in Birch and Pine Leaves in the Vicinity of a Large Copper Smelter Indicating a Change in the Conditions of Their Soil Nutrition
Abstract
:1. Introduction
2. Materials and Methods
2.1. The Area and Source of Anthropogenic Impact
2.2. Sample Plots
2.3. The Determination of Cu, Zn, Pb, and Cd in Litter
2.4. Litter Thickness and Root Occurrence in the Litter
2.5. The Collection of Leaves, Litter and Soil to Determine δ13C and δ15N
2.6. Isotopic Analyses
2.7. Data Analysis
3. Results
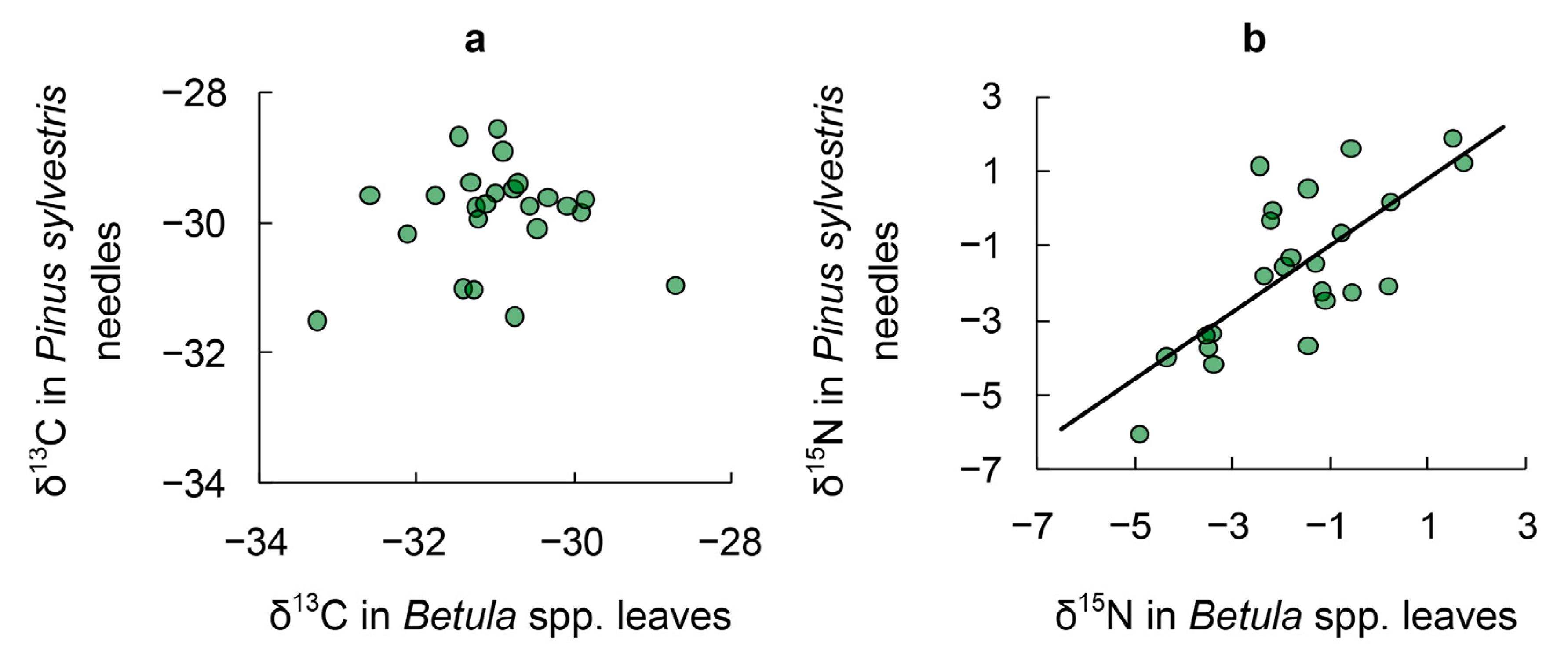
3.1. δ13C in Tree Leaves
3.2. δ15N in Tree Leaves
3.3. δ13C in Litter and Soil
3.4. δ15N in Litter and Soil
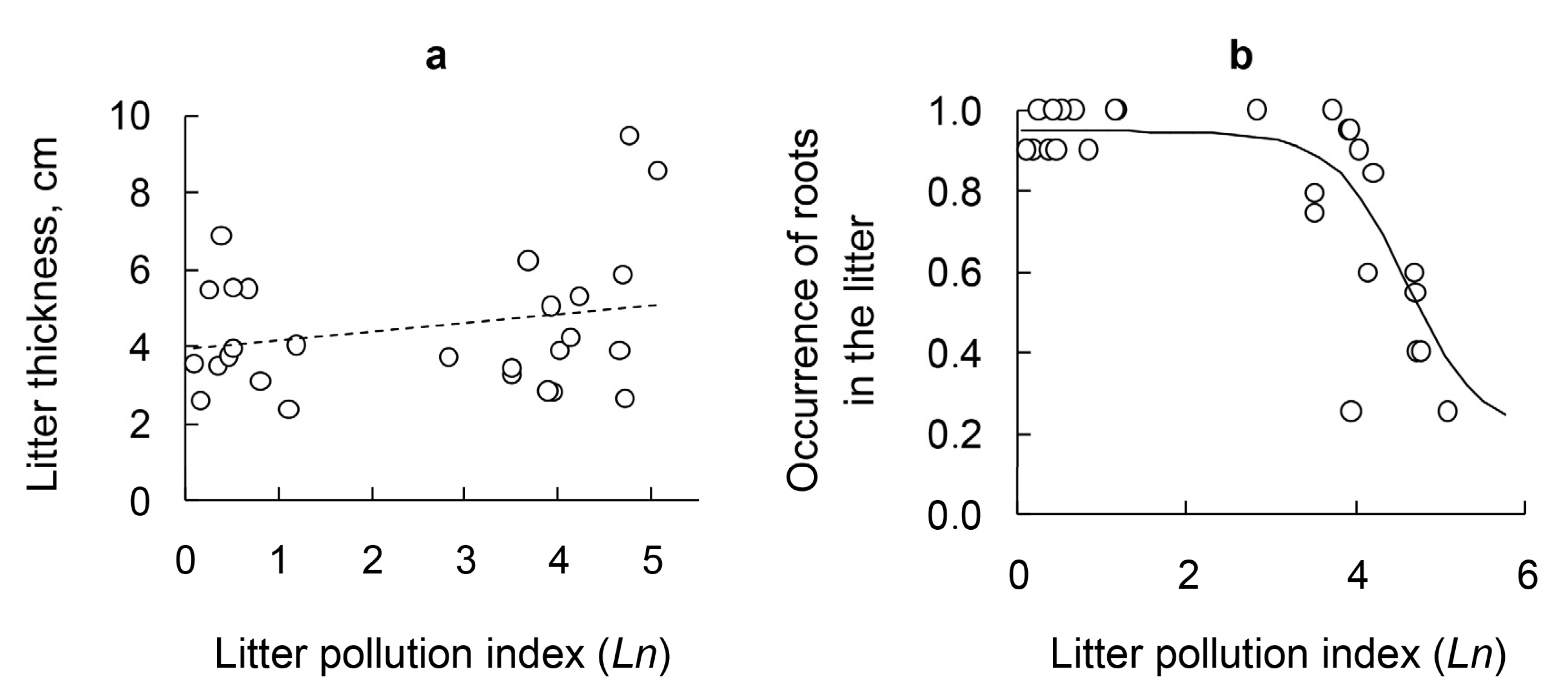
3.5. Litter Thickness and Occurrence of Roots in the Litter
3.6. δ15. N in Tree Leaves versus δ15N in Soil and Root Depth
3.7. Other Possible Explanations for δ15N in Tree Leaves
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Craine, J.M.; Elmore, A.J.; Aidar, M.P.M.; Bustamante, M.; Dawson, T.E.; Hobbie, E.A.; Kahmen, A.; Mack, M.C.; McLauchlan, K.K.; Michelsen, A.; et al. Global patterns of foliar nitrogen isotopes and their relationships with climate, mycorrhizal fungi, foliar nutrient concentrations, and nitrogen availability. New Phytol. 2009, 183, 980–992. [Google Scholar] [CrossRef]
- Dawson, T.E.; Mambelli, S.; Plamboeck, A.H.; Templer, P.H.; Tu, K.P. Stable isotopes in plant ecology. Annu. Rev. Ecol. Syst. 2002, 33, 507–559. [Google Scholar] [CrossRef]
- Lavergne, A.; Sandoval, D.; Hare, V.J.; Graven, H.; Prentice, I.C. Impacts of soil water stress on the acclimated stomatal limitation of photosynthesis: Insights from stable carbon isotope data. Glob. Chang. Biol. 2020, 26, 7158–7172. [Google Scholar] [CrossRef] [PubMed]
- Savard, M.M. Tree-ring stable isotopes and historical perspectives on pollution—An overview. Environ. Pollut. 2010, 158, 2007–2013. [Google Scholar] [CrossRef] [PubMed]
- Tiunov, A.V. Stable carbon and nitrogen isotopes in soilecological studies. Izv. Ross. Akad. Nauk Ser. Biol. 2007, 4, 475–489. [Google Scholar]
- Brooks, J.R.; Flanagan, L.B.; Buchmann, N.; Ehleringer, J.R. Carbon isotope composition of boreal plants: Functional grouping of life forms. Oecologia 1997, 110, 301–311. [Google Scholar] [CrossRef] [PubMed]
- Waigwa, A.N.; Mwangi, B.N.; Gituru, R.W.; Omengo, F.; Zhou, Y.; Wang, Q. Altitudinal variation of leaf carbon isotope for Dendrosenecio keniensis and Lobelia gregoriana in Mount Kenya alpine zone. Biotropica 2021, 53, 1394–1405. [Google Scholar] [CrossRef]
- Craine, J.M.; Brookshire, E.N.J.; Cramer, M.D.; Hasselquist, N.J.; Koba, K.; Marin-Spiotta, E.; Wang, L.X. Ecological interpretations of nitrogen isotope ratios of terrestrial plants and soils. Plant Soil 2015, 369, 1–26. [Google Scholar] [CrossRef]
- Michelsen, A.; Quarmby, C.; Sleep, D.; Jonasson, S. Vascular plant 15N natural abundance in heath and forest tundra ecosystems is closely correlated with presence and type of mycorrhizal fungi in roots. Oecologia 1998, 115, 406–418. [Google Scholar] [CrossRef]
- Peterson, B.J.; Fry, B. Stable isotopes in ecosystem studies. Annu. Rev. Ecol. Syst. 1987, 18, 293–320. [Google Scholar] [CrossRef]
- Makarov, M.I. The nitrogen isotopic composition in soils and plants: Its use in environmental studies (a review). Eurasian Soil. Sci. 2009, 42, 1335–1347. [Google Scholar] [CrossRef]
- Martinelli, L.A.; Piccolo, M.C.; Townsend, A.R.; Vitousek, P.M.; Cuevas, E.; McDowell, W.; Robertson, G.P.; Santos, O.C.; Treseder, K. Nitrogen stable isotopic composition of leaves and soil: Tropical versus temperate forests. Biogeochemistry 1999, 46, 45–65. [Google Scholar] [CrossRef]
- Menge, D.; Baisden, W.; Richardson, S.; Peltzer, D.A.; Barbour, M.M. Declining foliar and litter δ15N diverge from soil, epiphyte and input d15N along a 120000 yr temperate rainforest chronosequence. New Phytol. 2011, 190, 941–952. [Google Scholar] [CrossRef] [PubMed]
- Robinson, D. δ15N as an integrator of the nitrogen cycle. Trends Ecol. Evol. 2001, 16, 153–162. [Google Scholar] [CrossRef]
- Compton, J.E.; Hooker, T.D.; Perakis, S.S. Ecosystem nitrogen distribution and δ15N during a century of forest regrowth after agricultural abandonment. Ecosystems 2007, 10, 1197–1208. [Google Scholar] [CrossRef]
- Hobbie, E.A.; Jumpponen, A.; Trappe, J. Foliar and fungal 15N:14N ratios reflect development of mycorrhizae and nitrogen supply during primary succession: Testing analytical models. Oecologia 2005, 146, 258–268. [Google Scholar] [CrossRef]
- Vitousek, P.M.; Shearer, G.; Kohl, D.H. Foliar15N natural abundance in Hawaiian rainforest: Patterns and possible mechanisms. Oecologia 1989, 78, 383–388. [Google Scholar] [CrossRef]
- Hyodo, F.; Kusaka, S.; Wardle, D.A.; Nilsson, M.C. Changes in stable nitrogen and carbon isotope ratios of plants and soil across a boreal forest fire chronosequence. Plant Soil 2013, 364, 315–323. [Google Scholar] [CrossRef]
- Tu, Y.; Wang, A.; Zhu, F.; Gurmesa, G.A.; Hobbie, E.A.; Zhu, W.; Fang, Y. Trajectories in nitrogen availability during forest secondary succession: Illustrated by foliar δ15N. Ecol. Process. 2022, 11, 31. [Google Scholar] [CrossRef]
- Niemelä, P.; Lumme, I.; Mattson, W.; Arkhipov, V. 13C in tree rings along an air pollution gradient in the Karelian Isthmus, northwest Russia and southeast Finland. Can. J. For. Res. 1997, 27, 609–612. [Google Scholar] [CrossRef]
- Savard, M.M.; Begin, C.; Parent, M. Effects of smelter sulfur dioxide emissions: A spatiotemporal perspective using carbon isotopes in tree rings. J. Environ. Qual. 2004, 33, 13–26. [Google Scholar] [CrossRef] [PubMed]
- Cada, V.; Santruckova, H.; Santrucek, J.; Kubistova, L.; Seedre, M.; Svoboda, M. Complex physiological response of Norway spruce to atmospheric pollution—Decreased carbon isotope discrimination and unchanged tree biomass increment. Front. Plant Sci. 2016, 7, 805. [Google Scholar] [CrossRef] [PubMed]
- Korontzi, S.; Macko, S.A.; Anderson, I.C.; Poth, M.A. A stable isotopic study to determine carbon and nitrogen cycling in a disturbed southern Californian forest ecosystem. Glob. Biogeochem. Cycles 2000, 14, 177–188. [Google Scholar] [CrossRef]
- Kwak, J.H.; Choi, W.J.; Lim, S.S.; Arshad, M.A. Delta C-13, delta N-15, N concentration, and Ca-to-Al ratios of forest samples from Pinus densiflora stands in rural and industrial areas. Chem. Geol. 2009, 264, 385–393. [Google Scholar] [CrossRef]
- Manninen, S.; Zverev, V.E.; Kozlov, M.V. Foliar stable isotope ratios of carbon and nitrogen in boreal forest plants exposed to long-term pollution from the nickel-copper smelter at Monchegorsk, Russia. Environ. Sci. Pollut. Res. 2022, 29, 48880–48892. [Google Scholar] [CrossRef] [PubMed]
- Gebauer, G.; Giesemann, A.; Schulze, E.D.; Jager, H.J. Isotope ratios and concentrations of sulfur and nitrogen in needles and soils of Picea abies stands as influenced by atmospheric deposition of sulfur and nitrogen-compounds. Plant Soil 1994, 164, 267–281. [Google Scholar] [CrossRef]
- Hofmann, D.; Jung, K.; Bender, J.; Gehre, M.; Schuurmann, G. Using natural isotope variations of nitrogen in plants as an early indicator of air pollution stress. J. Mass Spectrom. 1997, 32, 855–863. [Google Scholar] [CrossRef]
- Pearson, J.; Wells, D.M.; Seller, K.J.; Bennett, A.; Soares, A.; Woodall, J.; Ingrouille, M.J. Traffic exposure increases natural N-15 and heavy metal concentrations in mosses. New Phytol. 2000, 147, 317–326. [Google Scholar] [CrossRef]
- Wagner, W.; Wagner, E. Influence of air pollution and site conditions on trends of carbon and oxygen isotope ratios in tree ring cellulose. Isot. Environ. Health Stud. 2006, 42, 353–365. [Google Scholar] [CrossRef]
- Chashchina, O.E.; Chibilev, A.A.; Veselkin, D.V.; Kuyantseva, N.B.; Mumber, A.G. The natural abundance of heavy nitrogen isotope (15N) in plants increases near a large copper smelter. Dokl. Biol. Sci. 2018, 482, 198–201. [Google Scholar] [CrossRef]
- Veselkin, D.V.; Chashchina, O.E.; Kuyantseva, N.B.; Mumber, A.G. Stable carbon and nitrogen isotopes in woody plants and herbs near the large copper smelting plant. Geochem. Int. 2019, 57, 575–582. [Google Scholar] [CrossRef]
- Menyailo, O.V.; Hungate, B.A. Stable and nitrogen stable isotopes in forest soils of Siberia. Dokl. Earth Sci. 2006, 409, 747–749. [Google Scholar] [CrossRef]
- Kozlov, M.V.; Zvereva, E.L.; Zverev, V.E. Impacts of Point Polluters on Terrestrial Biota; Springer: Dordrecht, The Netherlands; Heidelberg, Germany; London, UK; New York, NY, USA, 2009. [Google Scholar]
- Comprehensive Report on the State of the Environment of the Chelyabinsk Region in 2008; Ministry of Radiation and Environmental Safety of the Chelyabinsk Region: Chelyabinsk, Russia, 2009.
- Smorkalov, I.A.; Vorobeichik, E.L. Does long-term industrial pollution affect the fine and coarse root mass in forests? Preliminary investigation of two copper smelter contaminated areas. Water Air Soil Pollut. 2022, 233, 55. [Google Scholar] [CrossRef]
- Koroteeva, E.V.; Veselkin, D.V.; Kuyantseva, N.B.; Chashchina, O.E. The size, but not the fluctuating asymmetry of the leaf, of silver birch changes under the gradient influence of emissions of the Karabash Copper Smelter Plant. Dokl. Biol. Sci. 2015, 460, 36–39. [Google Scholar] [CrossRef] [PubMed]
- Koroteeva, E.V.; Veselkin, D.V.; Kuyantseva, N.B.; Chashchina, O.E. Approach to the industrially polluted area zoning based on heavy metals concentrations in the common pine organs (example of the Karabash copper smelter area). Bull. North-East Sci. Cent. FEB RAS 2015, 3, 86–93. [Google Scholar]
- Koroteeva, E.V.; Veselkin, D.V.; Kuyantseva, N.B.; Mumber, A.G.; Chashchina, O.E. Accumulation of heavy metals in the different Betula pendula Roth organs near the Karabash copper smelter. Agrohimia 2015, 3, 88–96. [Google Scholar]
- Chashchina, O.E.; Kuyantseva, N.B.; Mumber, A.G.; Potapkin, A.B.; Veselkin, D.V. Ground vegetation of the pine forest affected by forest fires in the gradient of emissions of the Karabash Copper Smelter. Bull. Orenbg. State Pedagog. Univ. Electron. Sci. J. 2017, 4, 44–53. [Google Scholar]
- Burnham, K.P.; Anderson, D.R. Model Selection and Multimodel Inference: A Practical Information—Theoretical Approach; Springer: New York, NY, USA, 2002. [Google Scholar]
- Morgun, E.G.; Kovda, I.V.; Ryskov, Y.G.; Oleinik, S.A. Prospects and problems of using the methods of geochemistry of stable carbon isotopes in soil studies. Eurasian Soil. Sci. 2008, 41, 265–275. [Google Scholar] [CrossRef]
- Freedman, B.; Hutchinson, T.C. Effects of smelter pollutants on forest leaf litter decomposition near a nickel–copper smelter at Sudbury, Ontario. Can. J. Bot. 1980, 58, 1722–1736. [Google Scholar] [CrossRef]
- Lukina, N.V.; Orlova, M.A.; Steinnes, E.; Artemkina, N.A.; Gorbacheva, T.T.; Smirnov, V.E.; Belova, E.A. Mass-loss rates from decomposition of plant residues in spruce forests near the northern tree line subject to strong air pollution. Environ. Sci. Pollut. Res. 2017, 24, 19874–19887. [Google Scholar] [CrossRef]
- Mikryukov, V.S.; Dulya, O.V.; Vorobeichik, E.L. Diversity and spatial structure of soil fungi and arbuscular mycorrhizal fungi in forest litter contaminated with copper smelter emissions. Water Air Soil Pollut. 2015, 226, 114. [Google Scholar] [CrossRef]
- Mikryukov, V.S.; Dulya, O.V. Contamination induced transformation of bacterial and fungal communities in spruce-fir and birch forest litter. Appl. Soil Ecol. 2017, 114, 111–122. [Google Scholar] [CrossRef]
- Mikryukov, V.S.; Dulya, O.V.; Modorov, M.V. Phylogenetic signature of fungal response to long-term chemical pollution. Soil Biol. Biochem. 2020, 140, 107644. [Google Scholar] [CrossRef]
- Mathias, J.M.; Thomas, R.B. Disentangling the effects of acidic air pollution, atmospheric CO2, and climate change on recent growth of red spruce trees in the Central Appalachian Mountains. Glob. Chang. Biol. 2018, 24, 3938–3953. [Google Scholar] [CrossRef]
- Savard, M.M.; Martineau, C.; Laganièreb, J.; Bégin, C.; Mariona, J.; Smirnoff, A.; Stefani, F.; Bergeron, J.; Rheault, K.; Paré, D.; et al. Nitrogen isotopes in the soil-to-tree continuum—Tree rings express the soil biogeochemistry of boreal forests exposed to moderate airborne emissions. Sci. Total Environ. 2021, 780, 146581. [Google Scholar] [CrossRef]
- Veselkin, D.V. Distribution of fine roots of coniferous trees over the soil profile under conditions of pollution by emissions from a copper-smelting plant. Russ. J. Ecol. 2002, 33, 231–234. [Google Scholar] [CrossRef]
- Veselkin, D.V. Reduction of absorbing root length in siberian fir and siberian spruce under heavy metal pollution. Russ. J. For. Sci. 2003, 3, 65–68. [Google Scholar]
- Hyodo, F.; Takebayashi, Y.; Makabe, A.; Wardle, D.A.; Koba, K. Changes in stable nitrogen isotopes of plants, bulk soil and soil dissolved N during ecosystem retrogression in boreal forest. Ecol. Res. 2021, 36, 420–429. [Google Scholar] [CrossRef]
- Oulehle, F.; Tahovska, K.; Ač, A.; Kolař, T.; Rybníček, M.; Čermak, P.; Stěpanek, P.; Trnka, M.; Urban, O.; Hruška, J. Changes in forest nitrogen cycling across deposition gradient revealed by δ15N in tree rings. Environ. Pollut. 2022, 304, 119104. [Google Scholar] [CrossRef]
- Nordin, A.; Näsholm, T.; Ericson, L. Effects of simulated N deposition on understorey vegetation of boreal coniferous forest. Funct. Ecol. 1998, 12, 691–699. [Google Scholar] [CrossRef]
- Xu, Y.; Xiao, H. Concentrations and nitrogen isotope compositions of free amino acids in Pinus massoniana (Lamb.) needles of different ages as indicators of atmospheric nitrogen pollution. Atmos. Environ. 2017, 164, 348–359. [Google Scholar] [CrossRef]
- Ammann, M.; Siegwolf, R.; Pichlmayer, F.; Suter, M.; Saurer, M.; Brunold, C. Estimating the uptake of traffic-derived NO2 from 15N abundance in Norway spruce needles. Oecologia 1999, 118, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Siegwolf, R.T.W.; Matyssek, R.; Saurer, M.; Maurer, S.; Günthardt-Goerg, M.; Schmutz, P.; Bucher, J.B. Stable isotope analysis reveals differential effects of soil nitrogen and nitrogen dioxide on the water use efficiency in hybrid poplar. New Phytol. 2001, 149, 33–246. [Google Scholar] [CrossRef] [PubMed]
- Michelsen, A.; Schmidt, I.K.; Jonasson, S.; Quarmby, C.; Sleep, D. Leaf 15N abundance of subarctic plants provides field evidence that ericoid, ectomycorrhizal and non- and arbuscular mycorrhizal species access different sources of soil nitrogen. Oecologia 1996, 105, 53–63. [Google Scholar] [CrossRef]
- Betekhtina, A.A.; Veselkin, D.V. Prevalence and intensity of mycorrhiza formation in herbaceous plants with different types of ecological strategies in the Middle Urals. Russ. J. Ecol. 2011, 3, 192–198. [Google Scholar] [CrossRef]
- Veselkin, D.V. Influence if different types of industrial pollution on diversity of pinus sylvestris ectomycorrhizae. Mycol. Phytopathol. 2006, 40, 122–132. [Google Scholar]
- Veselkin, D.V. Reaction of ectomycorrhizae of Pinus sylvestris to man-made contamination of various types. Sib. J. Ecol. 2005, 4, 753–761. [Google Scholar]



| Plot Number | Distance from the KCS, km | Heavy Metal Concentration in Litter, mg/kg | Litter Pollution Index, Times | Forest Stand Composition | Age of Major Tree Generation, Years | Canopy Cover, % | Grass- Shrub Layer Cover, % | Litter Thickness, cm | Root Occurrence in Litter, % | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cu | Zn | Pb | Cd | |||||||||
| Ilmensky State Reserve | ||||||||||||
| 31 | 37.2 | 29.9 | 244.0 | 64.3 | 1.91 | 3.3 | 10 P.s. | 170 | 10 | 50–60 | 4.0 | 100 |
| 37K | 37.5 | 26.5 | 96.4 | 45.9 | 0.86 | 2.0 | 10 P.s. + B.spp. | 170 | 50–60 | 70–80 | 5.5 | 100 |
| 221\37 | 50.0 | 12.4 | 121.0 | 15.2 | 0.68 | 1.2 | 9 B.spp. 1 P.s. | 120 | 50–60 | 80–90 | 2.6 | 90 |
| 221\28 | 50.0 | 13.7 | 78.0 | 18.6 | 0.60 | 1.1 | 8 P.s. 2 B.spp. | 185 | 50 | 30–40 | 3.6 | 90 |
| 207\18 | 48.4 | 14.9 | 108.0 | 19.9 | 0.80 | 1.3 | 9 P.s. 1 L.s. | 115 | 30–40 | 50–60 | 5.5 | 100 |
| 204\36 | 48.5 | 16.0 | 118.0 | 25.1 | 0.70 | 1.4 | 10 P.s. | 185 | 50–60 | 50–60 | 3.5 | 90 |
| 204\7 | 48.1 | 15.9 | 117.0 | 31.8 | 0.88 | 1.6 | 8 P.s. 2 B.spp. | 85 | 50–60 | 50–60 | 3.7 | 90 |
| 199\26 | 47.7 | 28.4 | 157.0 | 20.8 | 0.59 | 1.7 | 9 B.spp. 1 P.s. | 175 | 70–80 | 60–70 | 5.6 | 100 |
| 199\12 | 47.5 | 19.8 | 137.0 | 29.3 | 0.82 | 1.7 | 5 P.s.1 L.s.4B.sp | 105 | 60–70 | 50–60 | 3.9 | 100 |
| 198\22 | 48.5 | 18.8 | 127.0 | 26.7 | 0.60 | 1.5 | 9 P.s. 2 L.s. | 175 | 50–60 | 70–80 | 6.9 | 100 |
| 78\13 | 32.8 | 25.4 | 201.5 | 39.7 | 1.10 | 2.3 | 10 B.spp. | 115 | 40 | 60–70 | 3.1 | 90 |
| 77\20 | 33.0 | 33.7 | 339.0 | 44.0 | 1.41 | 3.1 | 10 B.spp. | 115 | 40 | 70–80 | 2.4 | 100 |
| The vicinity of the Karabash copper smelter | ||||||||||||
| 186\1 | 6.6 | 1255.0 | 1539.0 | 1141.0 | 15.5 | 55.6 | 7 P.s. 3 B.spp. | 110 | 50–60 | 10–20 | 3.9 | 90 |
| 186\4 | 7.0 | 1219.0 | 1956.0 | 1482.0 | 16.2 | 62.1 | 8 P.s. 2 B.spp. | 120 | 50–60 | 5–10 | 4.3 | 60 |
| 186\4K | 7.0 | 2175.0 | 3177.0 | 2580.0 | 25.3 | 107.2 | 8 P.s. 2 B.spp. | 120 | 50–60 | 5–10 | 3.9 | 60 |
| 186\16 | 7.1 | 896.0 | 2491.0 | 987.0 | 22.7 | 51.9 | 9 B.spp1P.t. + P.s | 110 | 30–40 | <5 | 2.9 | 25 |
| 186\31 | 6.4 | 3436.0 | 2600.0 | 1353.0 | 22.00 | 109.2 | 10 P.s. | 110 | 40 | <10 | 5.9 | 40 |
| 186\35 | 6.5 | 3061.0 | 1987.0 | 2528.0 | 16.80 | 116.8 | 10 P.s. + B.spp. | 110 | 30–40 | <5 | 9.5 | 40 |
| 186\37 | 6.1 | 5372.0 | 2238.0 | 2274.0 | 14.70 | 159.1 | 10 P.s. + B.spp. | 100 | 30–40 | <5 | 8.6 | 25 |
| 185\39 | 5.6 | 1968.0 | 4514.0 | 2489.0 | 37.70 | 111.1 | 10 B.spp. | 100 | 40–50 | <5 | 2.7 | 55 |
| 175\57 | 8.8 | 640.0 | 1733.0 | 594.0 | 11.30 | 33.0 | 9 B.spp. 1 P.s. | 90 | 40 | 40–50 | 3.3 | 75 |
| 175\56 | 8.6 | 1210.0 | 2408.0 | 1633.0 | 21.20 | 68.0 | 5 P.s. 5 B.spp. | 90 | 50–60 | 30–4 | 5.3 | 85 |
| 175\40 | 8.6 | 1090.0 | 1548.0 | 1073.0 | 14.40 | 50.7 | 9 P.s. 1 B.spp. | 120 | 40 | 30 | 5.0 | 95 |
| 175\39 | 8.5 | 629.0 | 947.0 | 807.0 | 8.98 | 32.8 | 8 P.s. 2 B.spp. | 110 | 40 | 20–30 | 3.5 | 80 |
| 175\37 | 9.1 | 790.0 | 2278.0 | 1176.0 | 17.30 | 49.9 | 8 B.spp. 2 P.s. | 95 | 50 | 30–40 | 2.8 | 95 |
| 166\50 | 9.1 | 293.0 | 666.0 | 385.0 | 5.48 | 16.7 | 10 P.s. + B.spp. | 120 | 40 | 50–60 | 3.8 | 100 |
| 166\49 | 9.5 | 893.0 | 1632.0 | 748.0 | 11.60 | 40.5 | 10 P.s. + B.spp. | 140 | 50–60 | 30–40 | 6.2 | 100 |
| № | Predictor Combination | dF | AICc | R2 |
|---|---|---|---|---|
| 1 | δ13C in litter + δ15N in litter | 2 | 186.03 | 0.43 |
| 2 | Occurrence of roots in the litter + δ13C in litter | 2 | 188.65 | 0.40 |
| 3 | δ13C in litter + δ15N in soil | 2 | 189.60 | 0.39 |
| 4 | Litter pollution index (Ln) + δ15N in litter | 2 | 189.89 | 0.38 |
| 5 | δ13C in litter | 1 | 192.14 | 0.33 |
| 6 | Litter pollution index (Ln) + δ15N in soil | 2 | 193.32 | 0.34 |
| 7 | Occurrence of roots in the litter + δ15N in soil | 2 | 194.04 | 0.33 |
| 8 | Litter pollution index (Ln) | 1 | 194.49 | 0.29 |
| 9 | δ13C in litter + δ13C in soil | 2 | 194.50 | 0.33 |
| 10 | Litter pollution index (Ln) + δ13C in litter | 2 | 194.97 | 0.32 |
| 11 | Litter pollution index (Ln) + Occurrence of roots in the litter | 2 | 195.05 | 0.32 |
| 12 | Occurrence of roots in the litter + δ15N in litter | 2 | 195.57 | 0.31 |
| 13 | Litter pollution index (Ln) + δ13C in soil | 2 | 196.02 | 0.30 |
| 14 | Occurrence of roots in the litter | 1 | 198.98 | 0.23 |
| 15 | Occurrence of roots in the litter + δ13C in soil | 2 | 201.33 | 0.23 |
| 16 | δ13C in soil + δ15N in soil | 2 | 202.46 | 0.21 |
| 17 | δ15N in litter + δ13C in soil | 2 | 202.95 | 0.20 |
| 18 | δ15N in soil | 1 | 204.71 | 0.14 |
| 19 | δ15N in litter + δ15N in soil | 2 | 206.95 | 0.14 |
| 20 | δ15N in litter | 1 | 207.51 | 0.09 |
| 21 | δ13C in soil | 1 | 211.46 | 0.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Veselkin, D.; Kuyantseva, N.; Mumber, A.; Molchanova, D.; Kiseleva, D. δ15N in Birch and Pine Leaves in the Vicinity of a Large Copper Smelter Indicating a Change in the Conditions of Their Soil Nutrition. Forests 2022, 13, 1299. https://doi.org/10.3390/f13081299
Veselkin D, Kuyantseva N, Mumber A, Molchanova D, Kiseleva D. δ15N in Birch and Pine Leaves in the Vicinity of a Large Copper Smelter Indicating a Change in the Conditions of Their Soil Nutrition. Forests. 2022; 13(8):1299. https://doi.org/10.3390/f13081299
Chicago/Turabian StyleVeselkin, Denis, Nadezhda Kuyantseva, Aleksandr Mumber, Darya Molchanova, and Daria Kiseleva. 2022. "δ15N in Birch and Pine Leaves in the Vicinity of a Large Copper Smelter Indicating a Change in the Conditions of Their Soil Nutrition" Forests 13, no. 8: 1299. https://doi.org/10.3390/f13081299
APA StyleVeselkin, D., Kuyantseva, N., Mumber, A., Molchanova, D., & Kiseleva, D. (2022). δ15N in Birch and Pine Leaves in the Vicinity of a Large Copper Smelter Indicating a Change in the Conditions of Their Soil Nutrition. Forests, 13(8), 1299. https://doi.org/10.3390/f13081299






