The Dynamics of Non-Structural Carbohydrates in Different Types of Bamboo in Response to Their Phenological Variations: Implications for Managing Bamboo Plantations
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Methods
2.2.1. Moisture Content Determination
2.2.2. Soluble Sugar, Starch, and NSC Content Determination
2.2.3. Starch Granule Localization
2.2.4. Statistical Analysis
3. Results
3.1. Phenology of Three Types of Bamboo
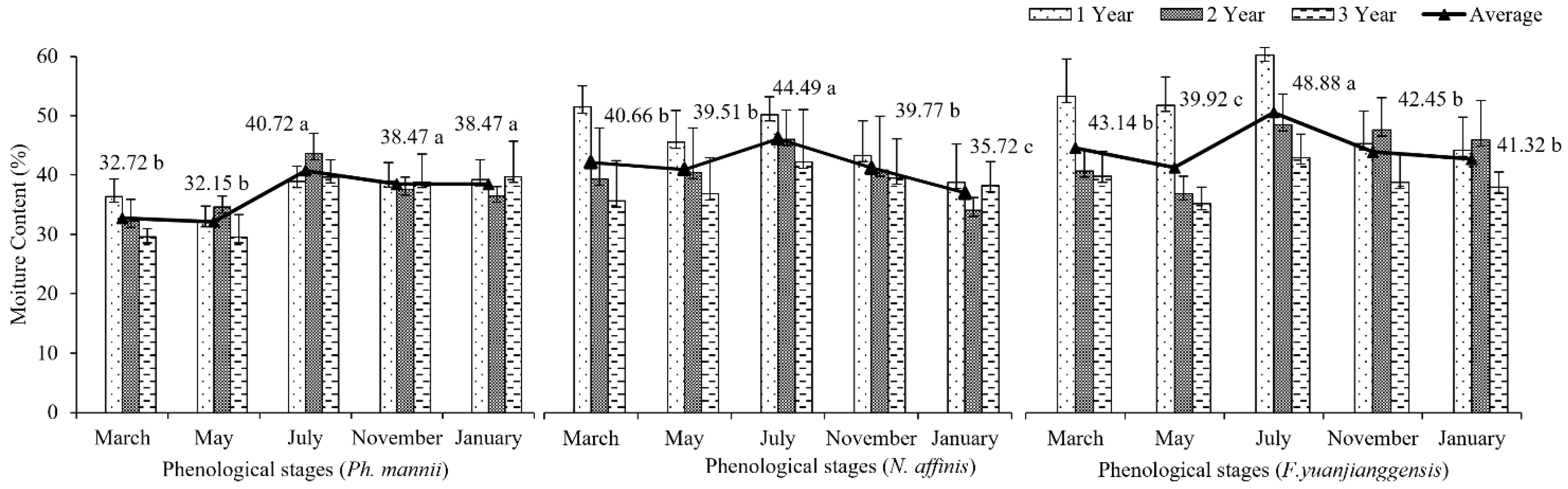
3.2. Phenological Patterns of Moisture Content in Three Types of Bamboo
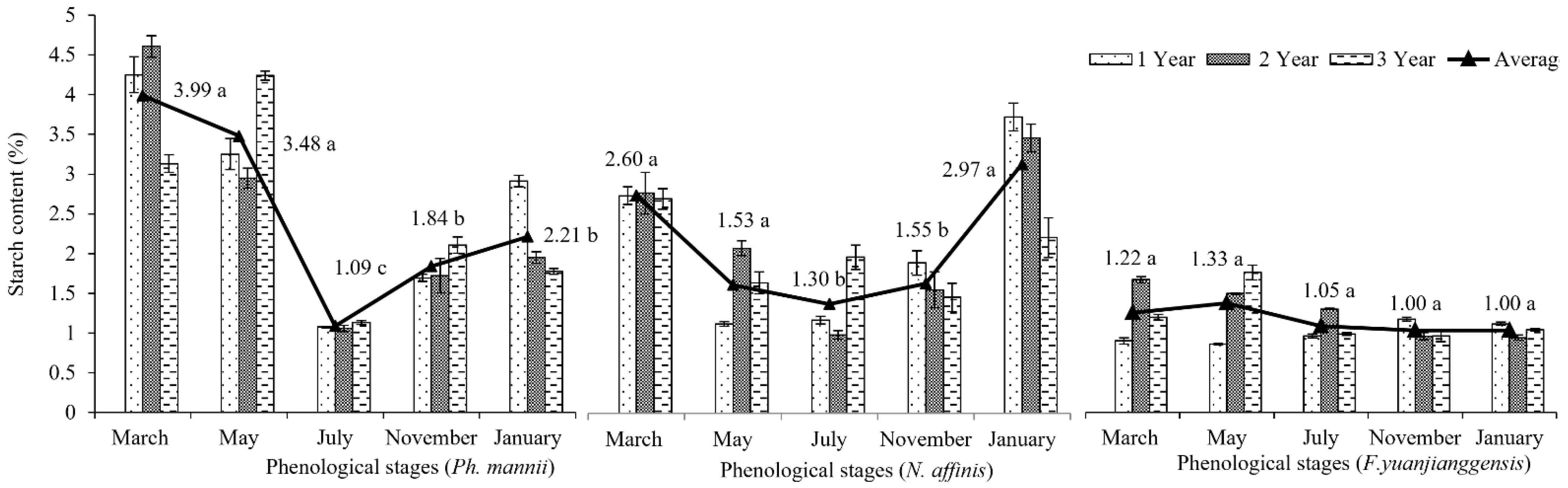
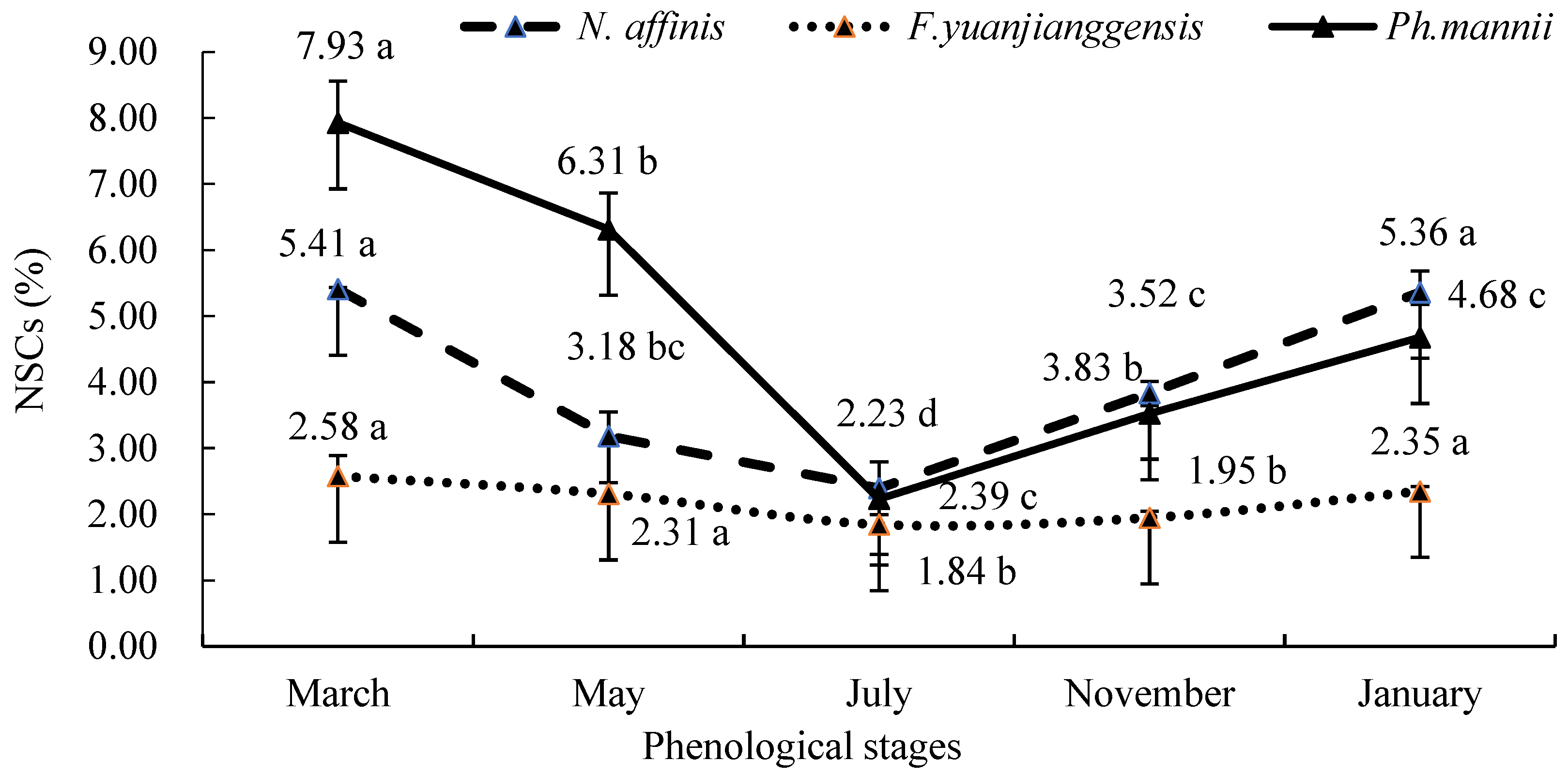
3.3. Phenological Patterns of NSCs in Three Types of Bamboo
4. Discussion
4.1. Phenology of Different Types of Bamboo
4.2. Moisture Content Variation in Different Types of Bamboo
4.3. NSCs Variations in Different Types of Bamboo
5. Implications for Managing Bamboo Plantations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Yang, Y.; Wang, K.; Wang, J. Bamboo Flora of Yunnan; Yunnan Press: Kunming, China, 2018; pp. 1–11. [Google Scholar]
- Okahisa, Y.; Yoshimura, T.; Imamura, Y. Seasonal and height-dependent fluctuation of starch and free glucose contents in moso bamboo (Phyllostachys pubescens) and its relation to attack by termites and decay fungi. J. Wood Sci. 2006, 52, 445–451. [Google Scholar] [CrossRef]
- Fan, Y. Seasonal Variation of Starch Content in Dendrocalamus giganteus and Phyllostachys edulis and Its Relationship to Insect Damage. Master’s Thesis, Southwest Forestry University, Kunming, China, 2010; pp. 21–36. [Google Scholar]
- Troya Mera, F.A.; Xu, C. Plantation management and resource economics of bamboo in China. Cienc. Tecnol. 2014, 7, 1–12. [Google Scholar] [CrossRef]
- Kramer, P.J.; Kozlowski, T.T. Physiology of Woody Plants; Academic Press: New York, NY, USA, 1979. [Google Scholar]
- Wong, B.L.; Baggett, K.L.; Rye, A.H. Seasonal patterns of reserve and soluble carbohydrates in mature sugar maple (Acer saccharum). Can. J. Bot. 2003, 81, 780–788. [Google Scholar] [CrossRef] [Green Version]
- Scofield, G.N.; Ruuska, S.A.; Aoki, N.; Lewis, D.C.; Tabe, L.M.; Jenkins, C.L.D. Starch storage in the stems of wheat plants: Localization and temporal changes. Ann. Bot. 2009, 103, 859–868. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galvez, D.A.; Landhäusser, S.M.; Tyree, M.T. Low root reserve accumulation during drought may lead to winter mortality in poplar seedlings. New Phytol. 2013, 198, 139–148. [Google Scholar] [CrossRef]
- Hartmann, H.; Trumbore, S. Understanding the roles of nonstructural carbohydrates in forest trees-from what we can measure to what we want to know. New Phytol. 2016, 211, 386–403. [Google Scholar] [CrossRef] [Green Version]
- Kozlowski, T.T. Carbohydrate sources and sinks in woody plants. Bot. Rev. 1992, 58, 107–222. [Google Scholar] [CrossRef]
- Wang, S.; Ding, Y.; Lin, S.; Ji, X.; Zhan, H. Seasonal changes of endogenous soluble sugar and starch in different developmental stages of Fargesia yunnanensis. J. Wood Sci. 2016, 62, 1–11. [Google Scholar] [CrossRef]
- Hirano, Y.; Shida, S.; Arima, T. Effect of cutting season on biodeterioration in Madake (Phyllostachys bambusoides). Mokuzai Gakkaishi 2003, 49, 437–445. (In Japanese) [Google Scholar]
- National Meteorological Science Data Center of China. Available online: http://data.cma.cn/analysis/yearbooks.html (accessed on 23 January 2019).
- Reeb, J.; Milota, M. Moisture content by the oven-dry method for industry testing. In Proceedings of the 50th Annual Dubois Meeting of the Western Dry Kiln Association, Portland, OR, USA, May 1999; pp. 66–74. Available online: https://ir.library.oregonstate.edu/concern/conference_proceedings_or_journals/fq977v782 (accessed on 23 January 2019).
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Franklin, D.C. Vegetative phenology and growth of a facultatively deciduous bamboo in a monsoonal climate. Biotropica 2005, 37, 343–350. [Google Scholar] [CrossRef]
- Zhou, F. Silviculture of Bamboo; China Forestry Publishing House: Beijing, China, 1998; pp. 93–101. [Google Scholar]
- Bhat, D.M.; Murali, K.S. Phenology of understorey species of tropical moist forest of Western Ghats region of Uttara Kannada district in South India. Curr. Sci. 2001, 81, 799–805. [Google Scholar]
- Yaron, B.; Danfors, E.; Vaadia, Y. Arid Zone Irrigation; Springer: New York, NY, USA, 1973; p. 200. [Google Scholar]
- Liese, W. The Anatomy of Bamboo Culms; INBAR Technical Report, No. 18; International Network for Bamboo and Rattan: Beijing, China, 1998; pp. 90–93. [Google Scholar]
- Zheng, Y.; Hong, W. Silviculture of Moso Plantation; Xiameng University Press: Xiameng, China, 1999; pp. 34–58. [Google Scholar]
- Luo, X.; Huang, Q. Relationships between Leaf and Stem Soluble Sugar Content and Tuberous Root Starch Accumulation in Cassava. J. Agric. Sci. 2011, 3, 64–72. [Google Scholar] [CrossRef]
- Bodelón, O.G.; Blanch, M.; Sanchez-Ballesta, M.T.; Escribano, M.I.; Merodio, C. The effects of high CO2 levels on anthocyanin composition, antioxidant activity and soluble sugar content of strawberries stored at low non-freezing temperature. Food Chem. 2010, 122, 673–678. [Google Scholar] [CrossRef]
- Aoki, T. Studies on Major Bamboo Specie in Japan; Ashishobo: Fukuoka, Japan, 1987; pp. 267–270. (In Japanese) [Google Scholar]
- Richardson, A.D.; Carbone, M.S.; Keenan, T.F.; Czimczik, C.I.; Hollinger, D.Y.; Murakami, P.; Schaberg, P.G.; Xu, C. Seasonal dynamics and age of stemwood nonstructural carbohydrates in temperate forest trees. New Phytol. 2013, 197, 850–861. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, B.; Yang, Y.; Zhang, G.; Sun, M.; Shi, M. A Study on Cultivation and Integrated Utilization of Large-size Cluster Bamboo. J. West China For. Sci. 2007, 36, 1–9, (In Chinese with English Abstract). [Google Scholar]
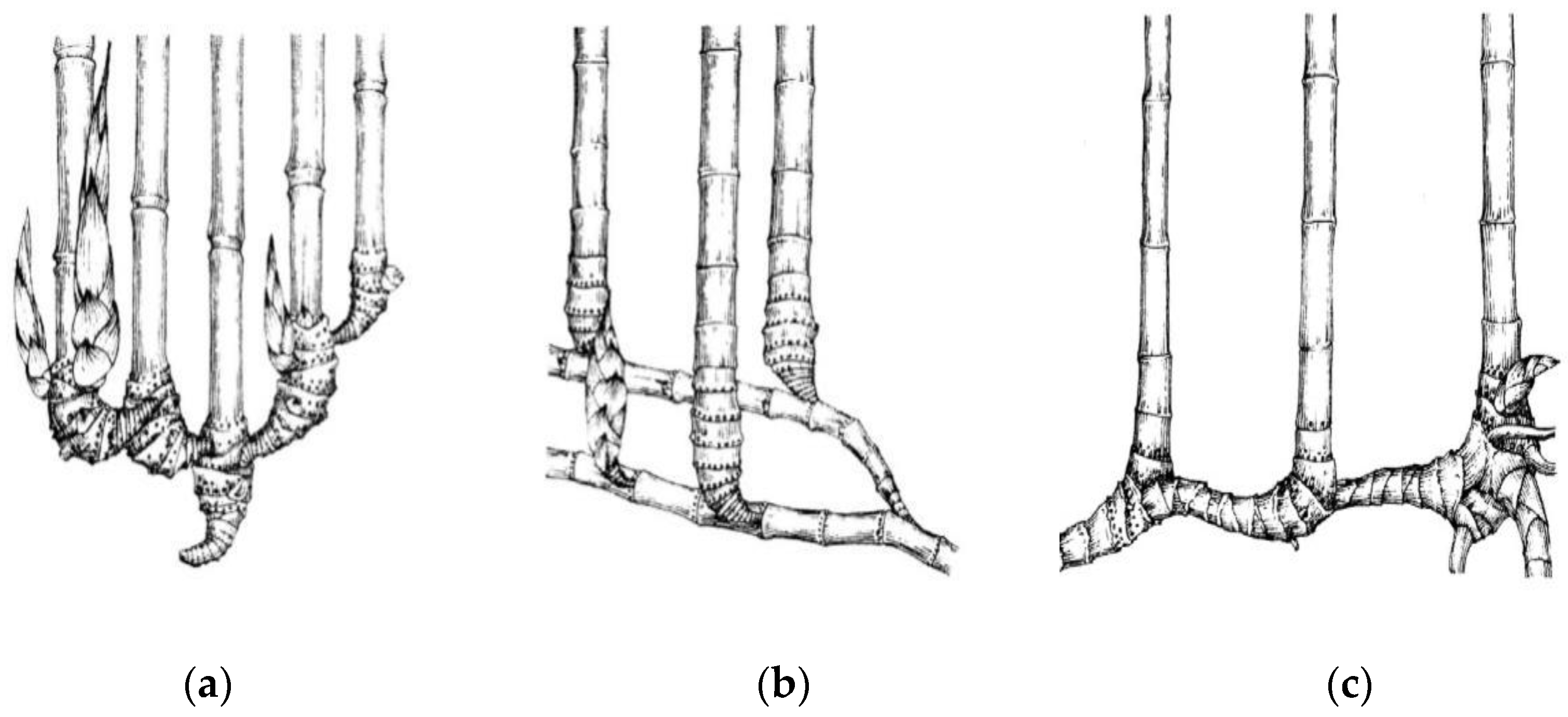
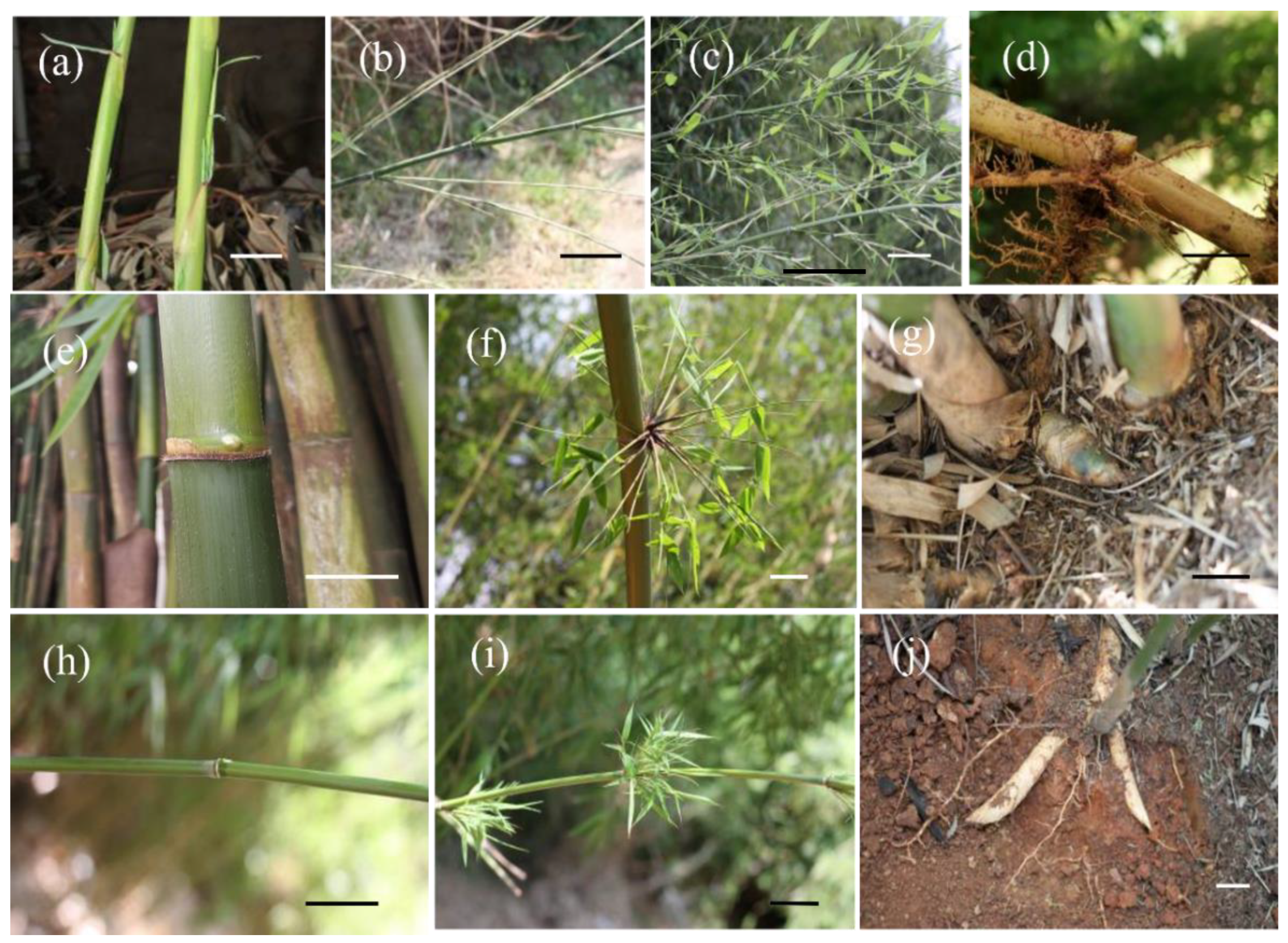

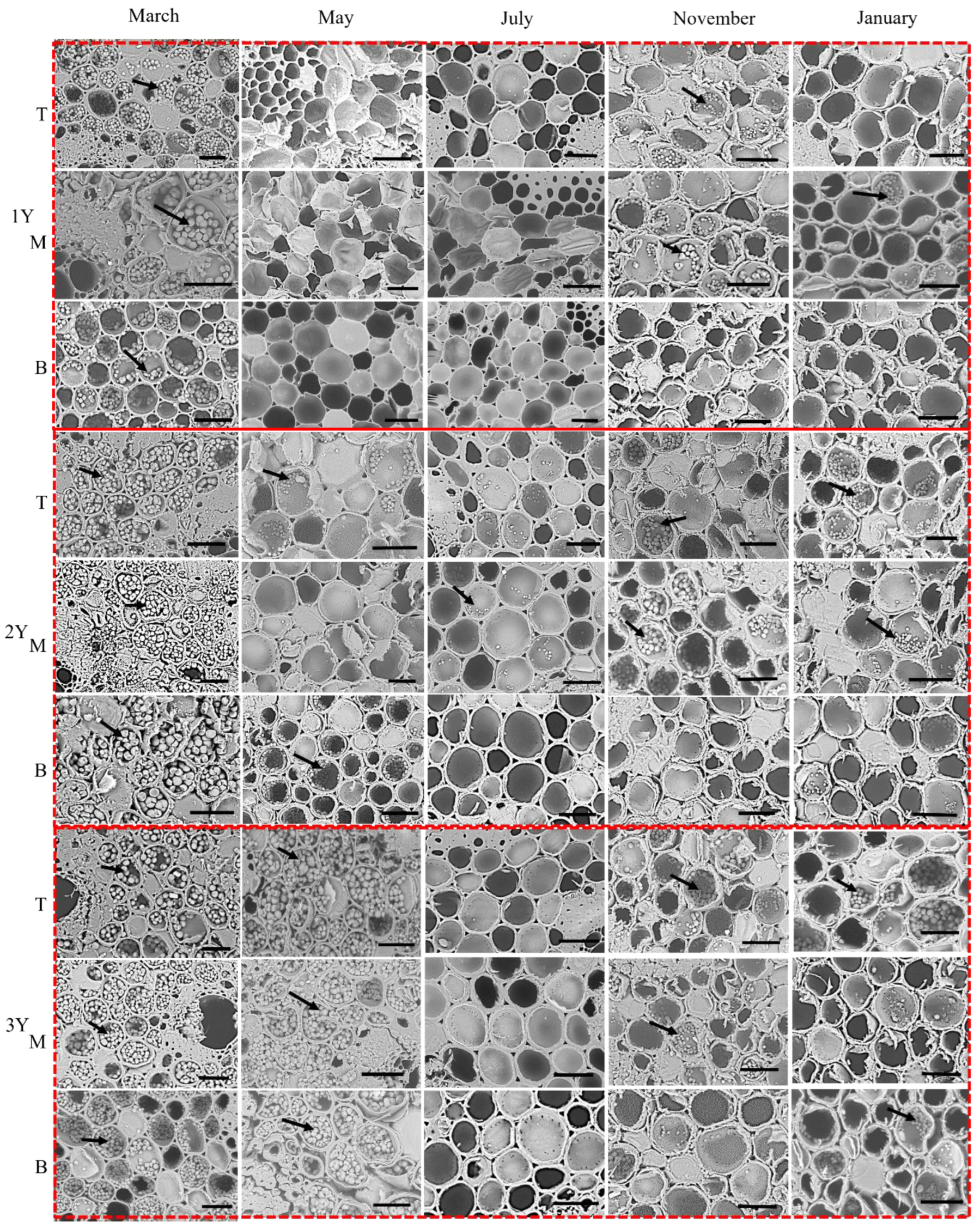
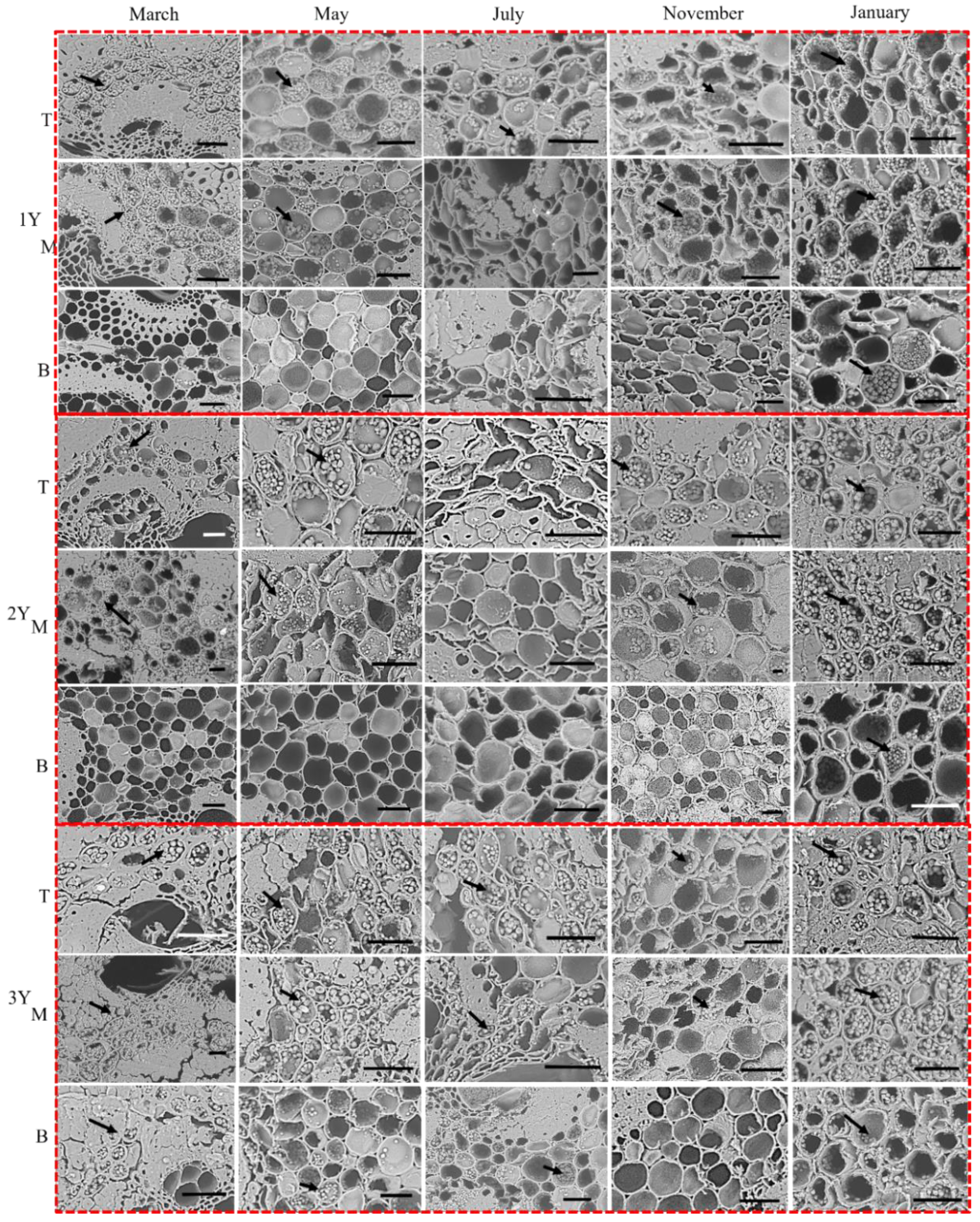
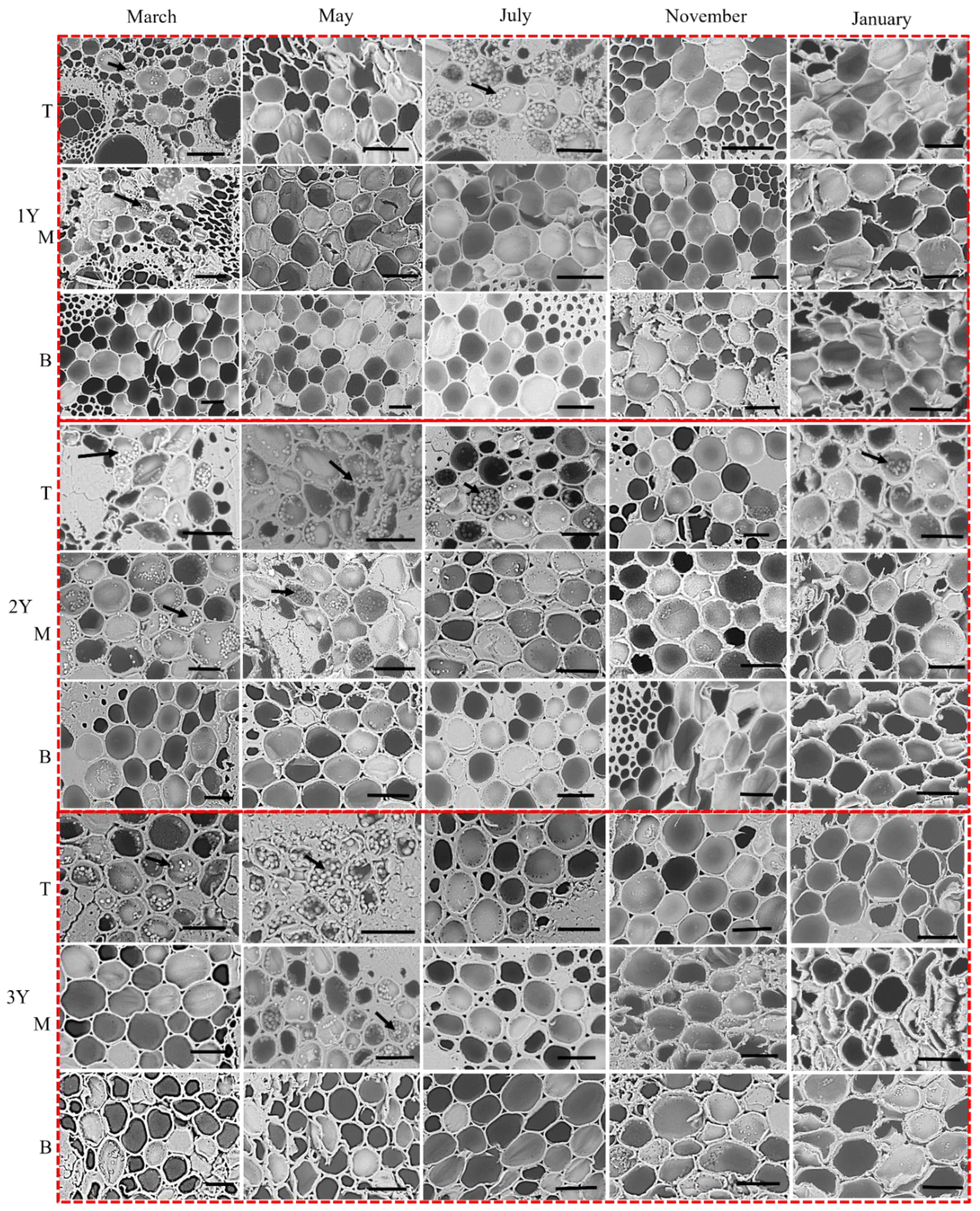
| Sample Timing | Phyllostachys mannii (Monopodial Bamboo with Scattered Culms) | Neosinocalamus affinis (Sympodial Bamboo with Caespitose Culms) | Fargesia yuanjiangensis (Sympodial Bamboo with Scattered Culms) |
|---|---|---|---|
| March, 2016 Spring/dry season | Shooting started. | Branching and leafing started and few nodal buds on 1-year culms sprouted. | Branching and leafing started and a few nodal buds on 1-year culms sprouted. |
| Shoot buds at the base of 1 and 2-year culms started differentiating and formed pseudorhizomes. | |||
| May, 2016 Spring/dry season | Most shoots completed height growth and started to extend branches and then sprouted new leaves. | Nodal buds of 1-year culms started sprouting. Branch and leaf extended simultaneously, and shoot buds started sprouting at the bases of 1- and 2-year culms. | Nodal buds of 1-year culms started sprouting. Branch and leaf extended simultaneously, and shoot buds started sprouting at the bases of 1- and 2-year culms. |
| July, 2016 Summer/rainy season | Culm sheaths dried out and eventually fell off. Branching, leafing, and shooting completed. New shoot buds started differentiating at the rhizome node underground. | Shoot buds continued forming and developing. Shoots generated out of the ground constantly. | Shoots started to sprout out of the ground, and August was the peak shooting season. |
| November, 2016 Autumn/dry season | No obvious change in bamboo culm. | Shooting completed, and most finished height growth. | Shooting completed and most finished height growth. |
| January, 2017 Winter/dry season | New culms with branches and leaves were dormant. | New culms enclosed in sheaths without branches and leaves were dormant. | New culms enclosed in sheaths without branches and leaves were dormant. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhan, H.; He, W.; Li, M.; Yu, L.; Li, J.; Wang, C.; Wang, S. The Dynamics of Non-Structural Carbohydrates in Different Types of Bamboo in Response to Their Phenological Variations: Implications for Managing Bamboo Plantations. Forests 2022, 13, 1218. https://doi.org/10.3390/f13081218
Zhan H, He W, Li M, Yu L, Li J, Wang C, Wang S. The Dynamics of Non-Structural Carbohydrates in Different Types of Bamboo in Response to Their Phenological Variations: Implications for Managing Bamboo Plantations. Forests. 2022; 13(8):1218. https://doi.org/10.3390/f13081218
Chicago/Turabian StyleZhan, Hui, Wenzhi He, Maobiao Li, Lixia Yu, Juan Li, Changming Wang, and Shuguang Wang. 2022. "The Dynamics of Non-Structural Carbohydrates in Different Types of Bamboo in Response to Their Phenological Variations: Implications for Managing Bamboo Plantations" Forests 13, no. 8: 1218. https://doi.org/10.3390/f13081218
APA StyleZhan, H., He, W., Li, M., Yu, L., Li, J., Wang, C., & Wang, S. (2022). The Dynamics of Non-Structural Carbohydrates in Different Types of Bamboo in Response to Their Phenological Variations: Implications for Managing Bamboo Plantations. Forests, 13(8), 1218. https://doi.org/10.3390/f13081218






