Improving a Process-Based Model to Simulate Forest Carbon Allocation under Varied Stand Density
Abstract
:1. Introduction
2. Materials and Methods
2.1. The Model
2.1.1. Model Description
2.1.2. Modification of the Carbon Allocation Module
2.1.3. Calculation of the Water, Light, and Nitrogen Availability Factors
2.2. Study Sites
2.3. Simulations
2.3.1. Parameterization and Initialization
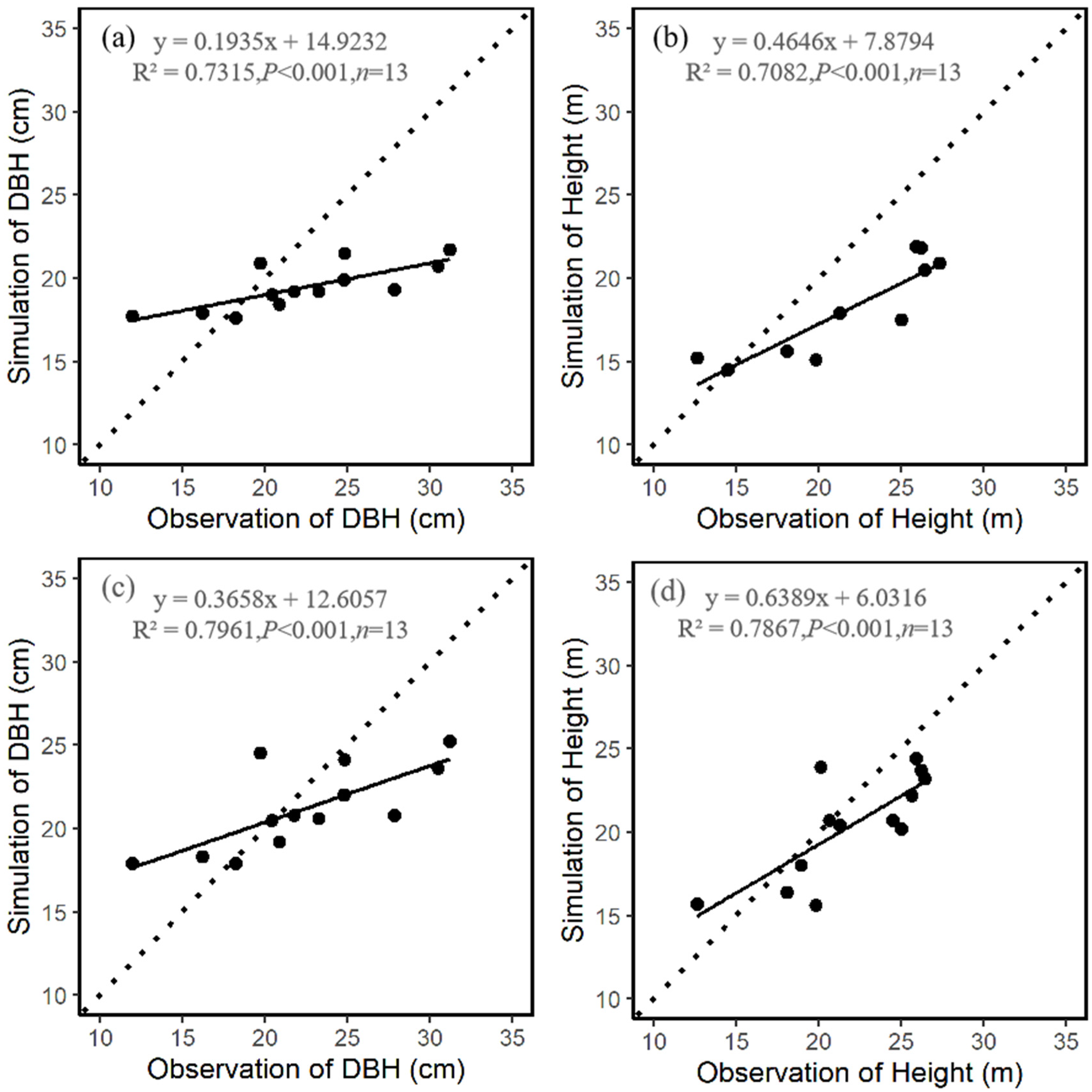
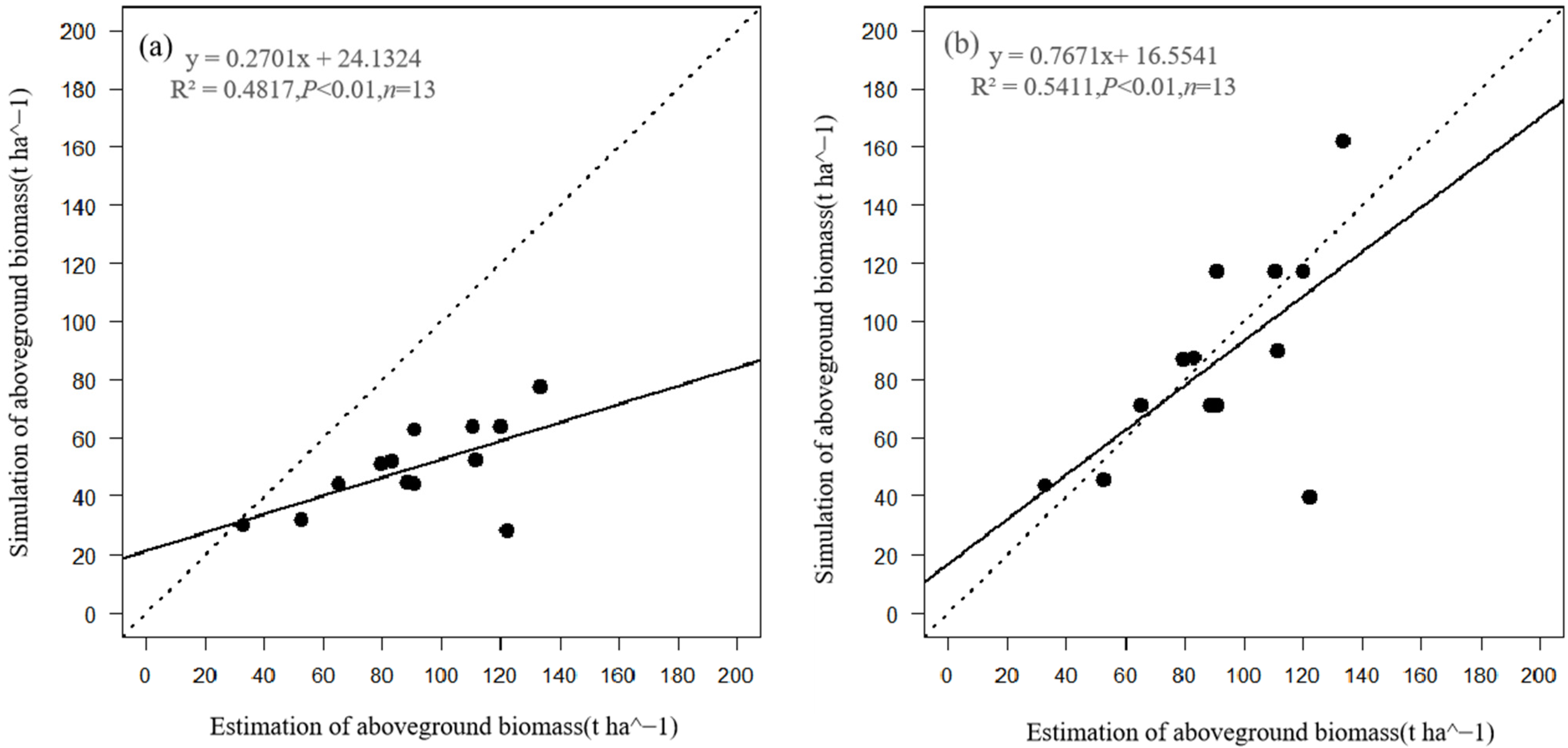
2.3.2. Model Evaluation
2.3.3. Simulation Experiments
3. Results
3.1. Comparison of Stand Characteristics
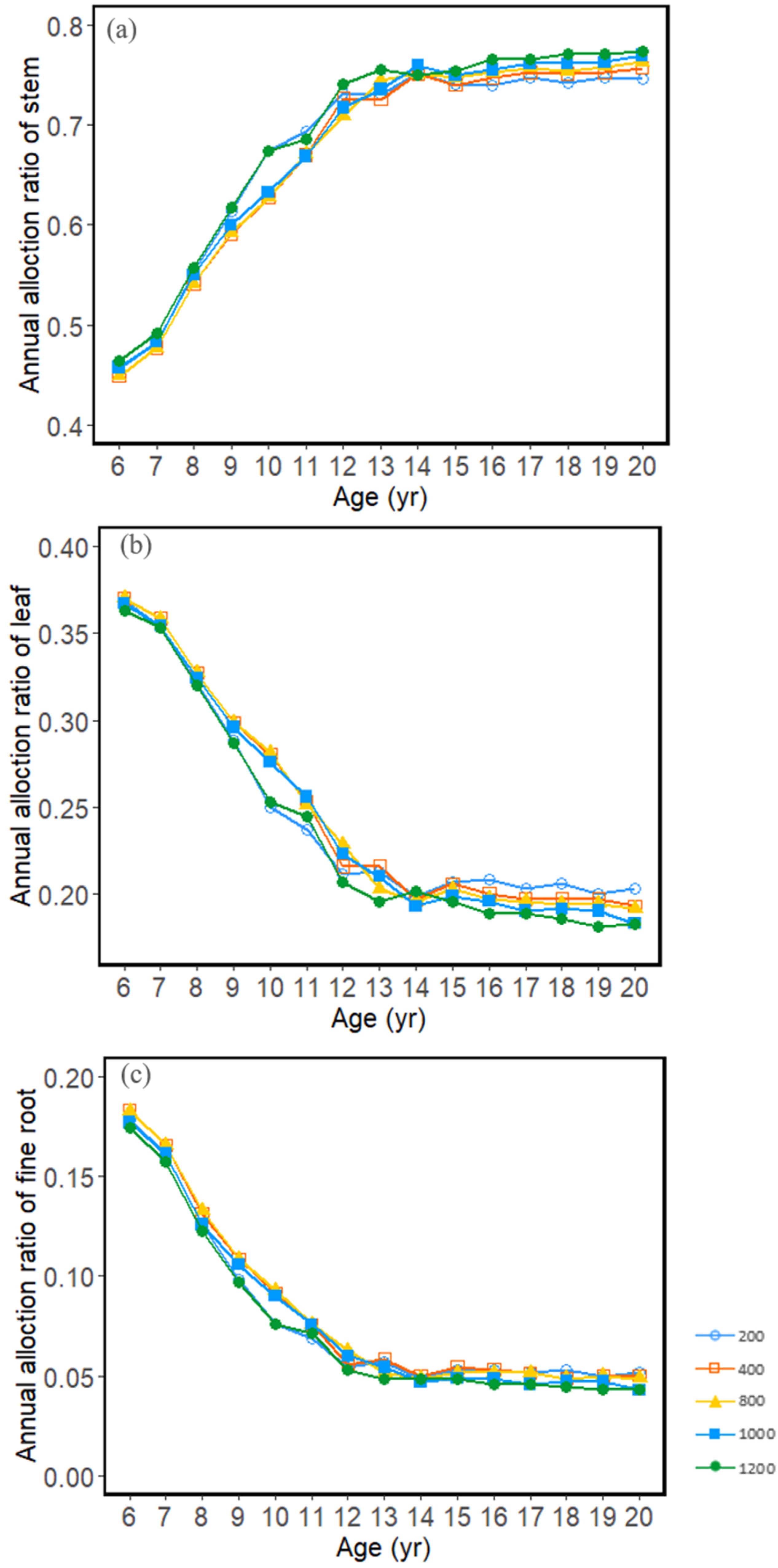
3.2. Carbon Allocation and Growth Change with Stand Density
3.3. Seasonal Dynamics of Carbon Allocation
4. Discussion
4.1. Module Improvement
4.2. Effects of Stand Density on Carbon Allocation and Stand Growth
4.3. Uncertainties of Model Simulation and Future Work
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lin, B.; Ge, J. To harvest or not to harvest? Forest management as a trade-off between bioenergy production and carbon sink. J. Clean. Prod. 2020, 268, 122219. [Google Scholar] [CrossRef]
- Liu, Q.; Cai, H.; Jin, G. Effects of selective cutting on the carbon density and net primary productivity of a mixed broadleaved-Korean pine forest in Northeast China. Chin. J. Appl. Ecol. 2013, 24, 2709–2716, (In Chinese with English Abstract). [Google Scholar]
- Wertz, B.; Bembenek, M.; Karaszewski, Z.; Ochal, W.; Skorupski, M.; Strzelinski, P.; Wegiel, A.; Mederski, P.S. Impact of stand density and tree social status on aboveground biomass allocation of Scots Pine Pinus sylvestris L. Forests 2020, 11, 765. [Google Scholar] [CrossRef]
- Xia, J.Z.; Yuan, W.P.; Lienert, S.; Joos, F.; Ciais, P.; Viovy, N.; Wang, Y.P.; Wang, X.F.; Zhang, H.C.; Chen, Y.; et al. Global patterns in net primary production allocation regulated by environmental conditions and forest stand age: A model-data comparison. J. Geophys. Res. Biogeosci. 2019, 124, 2039–2059. [Google Scholar] [CrossRef] [Green Version]
- Poorter, H.; Niklas, K.J.; Reich, P.B.; Oleksyn, J.; Poot, P.; Mommer, L. Biomass allocation to leaves, stems and roots: Meta-analyses of interspecific variation and environmental control. New Phytol. 2012, 193, 30–50. [Google Scholar] [CrossRef] [PubMed]
- Postma, J.A.; Hecht, V.L.; Hikosaka, K.; Nord, E.A.; Pons, T.L.; Poorter, H. Dividing the pie:a quantitative review on plant density responses. Plant Cell Environ. 2021, 44, 1072–1094. [Google Scholar] [CrossRef]
- De Kauwe, M.G.; Medlyn, B.E.; Zaehle, S.; Walker, A.P.; Dietze, M.C.; Wang, Y.P.; Luo, Y.; Jain, A.K.; El-Masri, B.; Hickler, T.; et al. Where does the carbon go? A model-data intercomparison of vegetation carbon allocation and turnover processes at two temperate forest free-air CO2 enrichment sites. New Phytol. 2014, 203, 883–899. [Google Scholar] [CrossRef] [Green Version]
- Merganicova, K.; Merganic, J.; Lehtonen, A.; Vacchiano, G.; Sever, M.Z.O.; Augustynczik, A.L.D.; Grote, R.; Kyselova, I.; Makela, A.; Yousefpour, R.; et al. Forest carbon allocation modelling under climate change. Tree Physiol. 2019, 39, 1937–1960. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.P.; Law, R.M.; Pak, B. A global model of carbon, nitrogen and phosphorus cycles for the terrestrial biosphere. Biogeosci. 2010, 7, 2261–2282. [Google Scholar] [CrossRef] [Green Version]
- Thornton, P.E.; Lamarque, J.F.; Rosenbloom, N.A.; Mahowald, N.M. Influence of carbon-nitrogen cycle coupling on land model response to CO2 fertilization and climate variability. Glob. Biogeochem. Cycles 2007, 21, Gb4018. [Google Scholar] [CrossRef]
- Medvigy, D.; Wofsy, S.C.; Munger, J.W.; Hollinger, D.Y.; Moorcroft, P.R. Mechanistic scaling of ecosystem function and dynamics in space and time: Ecosystem Demography model version 2. J. Geophys. Res. Biogeosci. 2009, 114, G01002. [Google Scholar] [CrossRef] [Green Version]
- Smith, B.; Warlind, D.; Arneth, A.; Hickler, T.; Leadley, P.; Siltberg, J.; Zaehle, S. Implications of incorporating N cycling and N limitations on primary production in an individual-based dynamic vegetation model. Biogeosciences 2014, 11, 2027–2054. [Google Scholar] [CrossRef] [Green Version]
- Song, Y.; Jain, A.K.; McIsaac, G.F. Implementation of dynamic crop growth processes into a land surface model: Evaluation of energy, water and carbon fluxes under corn and soybean rotation. Biogeosciences 2013, 10, 8039–8066. [Google Scholar] [CrossRef] [Green Version]
- Trugman, A.T.; Anderegg, L.D.L.; Wolfe, B.T.; Birami, B.; Ruehr, N.K.; Detto, M.; Bartlete, M.K.; Anderegg, W.R.L. Climate and plant trait strategies determine tree carbon allocation to leaves and mediate future forest productivity. Glob. Chang. Biol. 2019, 25, 3395–3405. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prilepova, O.; Hart, Q.; Merz, J.; Parker, N.; Bandaru, V.; Jenkins, B. Design of a GIS-based web application for simulating biofuel feedstock yields. ISPRS Int. J. Geo-Inf. 2014, 3, 929–941. [Google Scholar] [CrossRef] [Green Version]
- Guillemot, J.; Francois, C.; Hmimina, G.; Dufrene, E.; Martin-StPaul, N.K.; Soudani, K.; Marie, G.; Ourcival, J.M.; Delpierre, N. Environmental control of carbon allocation matters for modelling forest growth. New Phytol. 2017, 214, 180–193. [Google Scholar] [CrossRef] [Green Version]
- Garcia, E.S.; Tague, C.L.; Choate, J.S. Uncertainty in carbon allocation strategy and ecophysiological parameterization influences on carbon and streamflow estimates for two western US forested watersheds. Ecol. Model. 2016, 342, 19–33. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Peng, C.; Zhang, S.Y.; Zhou, X.; Larocque, G.R.; Kneeshaw, D.D.; Lei, X. Development of TRIPLEX-Management model for simulating the response of forest growth to pre-commercial thinning. Ecol. Model. 2011, 222, 2249–2261. [Google Scholar] [CrossRef]
- Peng, C.H.; Liu, J.X.; Dang, Q.L.; Apps, M.J.; Jiang, H. TRIPLEX: A generic hybrid model for predicting forest growth and carbon and nitrogen dynamics. Ecol. Model. 2002, 153, 109–130. [Google Scholar] [CrossRef]
- Landsberg, J.J.; Waring, R.H. A generalised model of forest productivity using simplified concepts of radiation-use efficiency, carbon balance and partitioning. For. Ecol. Manag. 1997, 95, 209–228. [Google Scholar] [CrossRef]
- Bossel, H. Treedyn3 forest simulation model. Ecol. Model. 1996, 90, 187–227. [Google Scholar] [CrossRef]
- Parton, W.J.; Scurlock, J.M.O.; Ojima, D.S.; Gilmanov, T.G.; Scholes, R.J.; Schimel, D.S.; Kirchner, T.; Menaut, J.C.; Seastedt, T.; Garcia Moya, E.; et al. Observations and modeling of biomass and soil organic matter dynamics for the grassland biome worldwide. Glob. Biogeochem. Cycles 1993, 7, 785–809. [Google Scholar] [CrossRef] [Green Version]
- Meyer, N.; Welp, G.; Amelung, W. The temperature sensitivity (Q10) of soil respiration: Controlling factors and spatial prediction at regional scale based on environmental soil classes. Glob. Biogeochem. Cycles 2018, 32, 306–323. [Google Scholar] [CrossRef]
- Peng, C.; Zhou, X.; Zhao, S.; Wang, X.; Zhu, B.; Piao, S.; Fang, J. Quantifying the response of forest carbon balance to future climate change in Northeastern China: Model validation and prediction. Glob. Planet. Chang. 2009, 66, 179–194. [Google Scholar] [CrossRef]
- Xia, J.; Chen, Y.; Liang, S.; Liu, D.; Yuan, W. Global simulations of carbon allocation coefficients for deciduous vegetation types. Tellus B 2015, 67, 28016. [Google Scholar] [CrossRef] [Green Version]
- Malhi, Y.; Doughty, C.; Galbraith, D. The allocation of ecosystem net primary productivity in tropical forests. Philos. Trans. R. Soc. B Biol. Sci. 2011, 366, 3225–3245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xia, J.; Yuan, W.; Wang, Y.-P.; Zhang, Q. Adaptive carbon allocation by plants enhances the terrestrial carbon sink. Sci. Rep. 2017, 7, 3341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, W.; Yang, B.; Dong, K.; Zhang, Y.; Peng, S.; Ruan, H.; Zheng, A.; Cao, G. Effect of soil fauna on organic nitrogen mineralization under different land use patterns in a coastal area of northern Jiangsu Province, China. J. Nanjing For. Univ. 2016, 40, 1–9. [Google Scholar]
- Xu, W.; Zhang, Y.; Wang, G.; Ruan, H. Response of carbon metabolism by soil microbes to different fertilization regimes in a poplar plantation in coastal area of northern Jiangsu, China. Chin. J. Ecol. 2015, 34, 1791–1797, (In Chinese with English abstract). [Google Scholar]
- Xie, T.; Zhang, A.; Wang, G.; Ruan, H.; Xu, Y.; Xu, C.; Ge, Z. Seasonal variation patterns of soil labile organic carbon in poplar plantations with different ages in northern Jiangsu. Chin. J. Ecol. 2012, 31, 1171–1178. [Google Scholar]
- Guo, J.; Wang, B.; Wang, G.B.; Myo, S.T.Z.; Cao, F.L. Effects of three cropland afforestation practices on the vertical distribution of soil organic carbon pools and nutrients in eastern China. Glob. Ecol. Conserv. 2020, 22, e009913. [Google Scholar] [CrossRef]
- Xue, L.; Jacobs, D.F.; Zeng, S.; Yang, Z.; Guo, S.; Liu, B. Relationship between above-ground biomass allocation and stand density index in Populus x euramericana stands. Forestry 2012, 85, 611–619. [Google Scholar] [CrossRef] [Green Version]
- Ge, X.; Tian, Y.; Tang, L. Nutrient distribution indicated whole-tree harvesting as a possible factor restricting the sustainable productivity of a poplar plantation system in China. PLoS ONE 2015, 10, e0125303. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Tian, Y.; Ding, S.; Lv, Y.; Samjhana, W.; Fang, S. Growth, carbon storage, and optimal rotation in poplar plantations: A case study on clone and planting spacing effects. Forests 2020, 11, 842. [Google Scholar] [CrossRef]
- Ai, P.; Hu, H.; Lu, X.; Yang, J.; Zhao, Y.; Wang, L.; Wang, D. Soil characteristics of shelter forests in Lianyungang coastal sandy land. J. Northeast. For. Univ. 2012, 40, 65–69, (In Chinese with English abstract). [Google Scholar] [CrossRef]
- Zhang, J.; Ge, Y.; Chang, J.; Jiang, B.; Jiang, H.; Peng, C.H.; Zhu, J.R.; Yuan, W.G.; Qi, L.Z.; Yu, S.Q. Carbon storage by ecological service forests in Zhejiang Province, subtropical China. For. Ecol. Manag. 2007, 245, 64–75. [Google Scholar] [CrossRef]
- Zhang, J.; Chu, Z.; Ge, Y.; Zhou, X.; Jiang, H.; Chang, J.; Peng, C.; Zheng, J.; Jiang, B.; Zhu, J.; et al. TRIPLEX model testing and application for predicting forest growth and biomass production in the subtropical forest zone of China’s Zhejiang Province. Ecol. Model. 2008, 219, 264–275. [Google Scholar] [CrossRef]
- Zhao, M.; Xiang, W.; Deng, X.; Tian, D.; Huang, Z.; Zhou, X.; Yu, G.; He, H.; Peng, C. Application of TRIPLEX model for predicting Cunninghamia lanceolata and Pinus massoniana forest stand production in Hunan Province, southern China. Ecol. Model. 2013, 250, 58–71. [Google Scholar] [CrossRef]
- Zhang, X.; Zang, R.; Li, C. Population differences in physiological and morphological adaptations of Populus davidiana seedlings in response to progressive drought stress. Plant Sci. 2004, 166, 791–797. [Google Scholar] [CrossRef]
- Zhou, X.L.; Peng, C.H.; Dang, Q.L.; Chen, J.X.; Parton, S. Predicting forest growth and yield in northeastern Ontario using the process-based model of TRIPLEX1.0. Can. J. For. Res. 2005, 35, 2268–2280. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Peng, C.; Kneeshaw, D.D.; Larocque, G.R.; Song, X.; Zhou, X. Quantifying the effects of climate change and harvesting on carbon dynamics of boreal aspen and jack pine forests using the TRIPLEX-Management model. For. Ecol. Manag. 2012, 281, 152–162. [Google Scholar] [CrossRef]
- Luo, Y.; Su, B.; Currie, W.S.; Dukes, J.S.; Finzi, A.C.; Hartwig, U.; Hungate, B.; McMurtrie, R.E.; Oren, R.; Parton, W.J.; et al. Progressive nitrogen limitation of ecosystem responses to rising atmospheric carbon dioxide. Bioscience 2004, 54, 731–739. [Google Scholar] [CrossRef] [Green Version]
- Makela, A.; Valentine, H.T.; Helmisaari, H.S. Optimal co-allocation of carbon and nitrogen in a forest stand at steady state. New Phytol. 2008, 180, 114–123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Friedlingstein, P.; Joel, G.; Field, C.B.; Fung, I.Y. Toward an allocation scheme for global terrestrial carbon models. Glob. Chang. Biol. 1999, 5, 755–770. [Google Scholar] [CrossRef]
- Ise, T.; Litton, C.M.; Giardina, C.P.; Ito, A. Comparison of modeling approaches for carbon partitioning: Impact on estimates of global net primary production and equilibrium biomass of woody vegetation from MODIS GPP. J. Geophys. Res. Biogeosci. 2010, 115, 11. [Google Scholar] [CrossRef]
- Liu, X.; Wang, Y.; Xu, B.; Zhang, Q. Effects on growth factors and wood physical and mechanical properties of populus × xiaohei in different planting densities. J. Anhui Agric. Univ. 2007, 34, 226–231. [Google Scholar]
- Wang, Y.; Wu, Q. Study on density configuration of poplar afforestation in Barren Site. Beijing Agric. 2013, 6, 69–70, (In Chinese with English Abstract). [Google Scholar]
- Zhang, Q.; Zhi, E.; Gu, X.; Li, X.; Wang, H. Effects of afforestation density on poplar growth. J. Hebei For. Sci. Technol. 2010, 5, 1–3, (In Chinese with English Abstract). [Google Scholar]
- Mäkinen, H.; Isomäki, A. Thinning intensity and long-term changes in increment and stem form of Scots pine trees. For. Ecol. Manage. 2004, 203, 21–34. [Google Scholar] [CrossRef]
- Xue, L.; Pan, L.; Zhang, R.; Xu, P.-b. Density effects on the growth of self-thinning Eucalyptus urophylla stands. Trees 2011, 25, 1021–1031. [Google Scholar] [CrossRef]
- Wu, M.; Wu, L.; Tang, Y.; Xu, S.; Peng, L.; Huang, F. Effects of density on growth of poplar and its quantitative maturity research. Hunan For. Acad. 2010, 37, 36–39, (In Chinese with English Abstract). [Google Scholar]
- Alatan, H. The effect of different planting density to growth of poplar plantation in Horqin Sand Land. For. Sci. Technol. 2015, 40, 34–37. [Google Scholar]
- Allen, C.D.; Macalady, A.K.; Chenchouni, H.; Bachelet, D.; McDowell, N.; Vennetier, M.; Kitzberger, T.; Rigling, A.; Breshears, D.D.; Hogg, E.H.; et al. A global overview of drought and heat-induced tree mortality reveals emerging climate change risks for forests. For. Ecol. Manag. 2010, 259, 660–684. [Google Scholar] [CrossRef] [Green Version]


| Site | Location | Planting Year | MAT | MAP | Soil Type | Soil Texture | Age | SD | DBH | H | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Dongtai1 | 120°49′ E, 33°05′ N | 2006 | 14.6 | 1069 | Desalted meadow | Sandy loam | 9 | 333 | 23.23 | 21.28 | [28] |
| Dongtai2 | 120°49′ E, 33°05′ N | 2006 | 14.6 | 1069 | Desalted meadow | Sandy loam | 7 | 667 | 16.21 | 18.08 | [29] |
| Dongtai3 | 120°49′ E, 33°05′ N | 1996 | 14.6 | 1069 | Desalted meadow | Sandy loam | 15 | 333 | 33.2 | 27.3 | [30] |
| Dongtai4 | 120°49′ E, 33°05′ N | 2006 | 14.6 | 1069 | Desalted meadow | Sandy loam | 10 | 607 | 20.85 | 18.91 | [31] |
| Dongtai5 | 120°49′ E, 33°05′ N | 2006 | 14.6 | 1069 | Desalted meadow | Sandy loam | 10 | 312 | 27.82 | 20.63 | [31] |
| Suining1 | 118°04′ E, 33°53′ N | 1998 | 16 | 1300 | Alluvial sandy | Sandy loam | 9 | 204 | 31.2 | 25.9 | [32] |
| Suining2 | 118°04′ E, 33°53′ N | 1998 | 16 | 1300 | Alluvial sandy | Sandy loam | 9 | 278 | 30.5 | 26.4 | [32] |
| Suining3 | 118°04′ E, 33°53′ N | 1998 | 16 | 1300 | Alluvial sandy | Sandy loam | 9 | 400 | 24.8 | 26.2 | [32] |
| Suining4 | 118°04′ E, 33°53′ N | 1998 | 16 | 1300 | Alluvial sandy | Sandy loam | 9 | 625 | 20.4 | 25 | [32] |
| Zhoushan | 119°40′ E, 32°20′ N | 2002 | 14.5 | 1000 | Lacustrine sediments | Clay texture | 10 | 1111 | 19.8 | 18.2 | [33] |
| Sihong1 | 118°36′ E, 33°28′ N | 2007 | 14.4 | 972.5 | Deposited soil | Sandy black | 13 | 400 | 21.75 | 24.48 | [34] |
| Sihong2 | 118°36′ E, 33°28′ N | 2007 | 14.4 | 972.5 | Deposited soil | Sandy black | 13 | 278 | 24.79 | 25.62 | [34] |
| Ganyu | 119°07′ E, 34°50′ N | 2002 | 13.2 | 976.4 | Tibba sandy | Sandy loam | 8 | 1111 | 11.96 | 12.6 | [35] |
| Parameter | Description | Note |
|---|---|---|
| Absorb = 0.15 | Atmospheric absorption factor | [37] |
| Cloud = 0.45 | Time fraction of cloudy days | This study |
| PAR factor = 0.65 | Solar radiation fraction | This study |
| BlCond = 0.12 | Canopy boundary layer conductance (mL m−2 s−1) | This study |
| MaxCond = 0.26 | Max. canopy conductance (mL m−2 s−1) | This study |
| StomCond = 0.012 | Stomata conductance (mL m−2 s−1) | This study |
| ExtCoef = 0.46 | Radiation extinction coefficient | [38] |
| TaMin = 7 | Min. temperature for growth (°C) | This study |
| TaMax = 40 | Max. temperature for growth (°C) | This study |
| Topt = 25 | Optimum temperature for growth (°C) | This study |
| N factor = 0.2 | N factor for tree growth | [38] |
| Na = 3 | Effects of age on GPP | [38] |
| Sla = 13 | Specific leaf area (m2 kg−1) | [39] |
| GamaF = 1 | Leaf turnover per year | This study |
| GamaR = 0.21 | Fine root turnover per year | This study |
| Lnr = 26 | Lignin–N ratio | [38] |
| Ls = 0.215, 0.215, 0.2351, 0.255, 0.255 | Lignin in leaf, fine root, coarse root, branch, and wood | [38] |
| A1, A2, A3 = 15, 45, 125 | Soil water depth of layer 1, 2, and 3 (cm) | [38] |
| AWL1, 2, 3 = 0.5, 0.3, 0.2 | Relative root density for layer 1, 2, and 3 | [38] |
| KF = 0.5 | Fraction of H2O flow to stream | [40] |
| KD = 0.5 | Fraction of H2O flow to deep storage | [40] |
| KX = 0.3 | Fraction of deep storage water to stream | [40] |
| AWater = 250 | Max. soil water (mm) | [40] |
| MiuNorm = 0 | Normal mortality | This study |
| CSP = 0.26 | Wood C density (t C m−3) | This study |
| CD = 20 | Crown to stem diameter ratio | This study |
| AlphaC = 0.08 | Canopy quantum efficiency | This study |
| MaxHeight = 30 | Max. height (m) | This study |
| AgeMax = 100 | Max. stand age (year) | This study |
| Variable | MAE | NRMSE | d |
| Original model | |||
| Mean DBH (cm) | 24.8 | 0.3 | 0.6 |
| Mean height (m) | 24.4 | 0.4 | 0.7 |
| Aboveground biomass (t ha−1) | 60.5 | 0.6 | 0.5 |
| Modified model | |||
| Mean DBH (cm) | 18.7 | 0.2 | 0.7 |
| Mean height (m) | 15.4 | 0.2 | 0.8 |
| Aboveground biomass (t ha−1) | 26.6 | 0.3 | 0.8 |
| Poplar | Density (Stems ha−1) | |||||
|---|---|---|---|---|---|---|
| 200 | 400 | 600 | 800 | 1000 | 1200 | |
| Site 9 (Age = 20 years) | ||||||
| 31.7 | 26.6 | 24 | 22.7 | 22 | 21.5 | |
| 26.9 | 25.3 | 23.6 | 22.7 | 22.3 | 21.9 | |
| AGB | 246.9 | 261.2 | 252.9 | 231.9 | 221.9 | 217.1 |
| SC | 124.5 | 124.7 | 124.8 | 126.4 | 127.3 | 127.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiao, W.; Wang, W.; Peng, C.; Lei, X.; Ruan, H.; Li, H.; Yang, Y.; Grabarnik, P.; Shanin, V. Improving a Process-Based Model to Simulate Forest Carbon Allocation under Varied Stand Density. Forests 2022, 13, 1212. https://doi.org/10.3390/f13081212
Jiao W, Wang W, Peng C, Lei X, Ruan H, Li H, Yang Y, Grabarnik P, Shanin V. Improving a Process-Based Model to Simulate Forest Carbon Allocation under Varied Stand Density. Forests. 2022; 13(8):1212. https://doi.org/10.3390/f13081212
Chicago/Turabian StyleJiao, Wenxing, Weifeng Wang, Changhui Peng, Xiangdong Lei, Honghua Ruan, Haikui Li, Yanrong Yang, Pavel Grabarnik, and Vladimir Shanin. 2022. "Improving a Process-Based Model to Simulate Forest Carbon Allocation under Varied Stand Density" Forests 13, no. 8: 1212. https://doi.org/10.3390/f13081212
APA StyleJiao, W., Wang, W., Peng, C., Lei, X., Ruan, H., Li, H., Yang, Y., Grabarnik, P., & Shanin, V. (2022). Improving a Process-Based Model to Simulate Forest Carbon Allocation under Varied Stand Density. Forests, 13(8), 1212. https://doi.org/10.3390/f13081212






