Plant Growth and Nutrient Composition of Shrub and Arbor Willows Grown in Cu-Contaminated Flooded Soil
Abstract
:1. Introduction
2. Materials and Methods
2.1. Soil Preparation and Plant Cultivation
2.2. Measurement of the Leaf Gas Exchange
2.3. Determination of Cu and Other Nutrients
2.4. Calculations and Statistical Analysis
3. Results
3.1. Plant Growth and Biomass Production
3.2. Leaf Gas Exchange
3.3. Accumulation and Distribution of Cu in Willows
3.4. Plant C, N, and P and Corresponding Stoichiometry
3.5. Other Nutrients and Multi-Element:Cu Stoichiometry
4. Discussion
4.1. Effects of Flooding on Willow Growth and Photosynthesis
4.2. Effect of Flooding on Cu Accumulation and Distribution
4.3. Effects of Flooding on the Stoichiometry Patterns of Plant C, N, and P
4.4. Effects of Flooding on Other Nutrients and Their Correlation with Cu
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Shaheen, S.M.; Antoniadis, V.; Kwon, E.; Song, H.; Wang, S.-L.; Hseu, Z.-Y.; Rinklebe, J. Soil Contamination by Potentially Toxic Elements and the Associated Human Health Risk in Geo- and Anthropogenic Contaminated Soils: A Case Study from the Temperate Region (Germany) and the Arid Region (Egypt). Environ. Pollut. 2020, 262, 114312. [Google Scholar] [CrossRef] [PubMed]
- Adrees, M.; Ali, S.; Rizwan, M.; Ibrahim, M.; Abbas, F.; Farid, M.; Zia-ur-Rehman, M.; Irshad, M.K.; Bharwana, S.A. The Effect of Excess Copper on Growth and Physiology of Important Food Crops: A Review. Environ. Sci. Pollut. Res. 2015, 22, 8148–8162. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zerbe, S.; Han, W.; Thevs, N.; Ji, C. Nitrogen and Phosphorus Stoichiometry of Common Reed (Phragmites australis) and Its Relationship to Nutrient Availability in Northern China. Aquat. Bot. 2014, 112, 84–90. [Google Scholar] [CrossRef]
- Rehman, M.; Maqbool, Z.; Peng, D.; Liu, L. Morpho-Physiological Traits, Antioxidant Capacity and Phytoextraction of Copper by Ramie (Boehmeria nivea L.) Grown as Fodder in Copper-Contaminated Soil. Environ. Sci. Pollut. Res. 2019, 26, 5851–5861. [Google Scholar] [CrossRef] [PubMed]
- Brunetto, G.; Bastos de Melo, G.W.; Terzano, R.; Del Buono, D.; Astolfi, S.; Tomasi, N.; Pii, Y.; Mimmo, T.; Cesco, S. Copper Accumulation in Vineyard Soils: Rhizosphere Processes and Agronomic Practices to Limit Its Toxicity. Chemosphere 2016, 162, 293–307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Milla-Moreno, E.; Guy, R.D. Growth Response, Uptake and Mobilization of Metals in Native Plant Species on Tailings at a Chilean Copper Mine. Int. J. Phytoremediat. 2021, 23, 539–547. [Google Scholar] [CrossRef]
- Oladoye, P.O.; Olowe, O.M.; Asemoloye, M.D. Phytoremediation technology and food security impacts of heavy metal contaminated soils: A review of literature. Chemosphere 2022, 288, 132555. [Google Scholar] [CrossRef]
- Pilipovic, A.; Zalesny, R.; Roncevic, S.; Nikolic, N.; Orlovic, S.; Beljin, J.; Katanic, M. Growth, Physiology, and Phytoextraction Potential of Poplar and Willow Established in Soils Amended with Heavy-Metal Contaminated, Dredged River Sediments. J. Environ. Manag. 2019, 239, 352–365. [Google Scholar] [CrossRef]
- Dos Santos Utmazian, M.N.; Wieshammer, G.; Vega, R.; Wenzel, W.W. Hydroponic Screening for Metal Resistance and Accumulation of Cadmium and Zinc in Twenty Clones of Willows and Poplars. Environ. Pollut. 2007, 148, 155–165. [Google Scholar] [CrossRef]
- Wang, S.F.; Shi, X.; Sun, H.J.; Chen, Y.T.; Pan, H.W.; Yang, X.E.; Rafiq, T. Variations in Metal Tolerance and Accumulation in Three Hydroponically Cultivated Varieties of Salix integra Treated with Lead. PLoS ONE 2014, 9, e108568. [Google Scholar] [CrossRef]
- Kang, W.; Bao, J.G.; Zheng, J.; Xu, F.; Wang, L.M. Phytoremediation of heavy metal contaminated soil potential by woody plants on Tonglushan ancient copper spoil heap in China. Int. J. Phytoremediat. 2018, 20, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Tőzsér, D.; Magura, T.; Simon, E. Heavy Metal Uptake by Plant Parts of Willow Species: A Meta-Analysis. J. Hazard. Mater. 2017, 336, 101–109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bourgeade, P.; Bourioug, M.; Macor, S.; Alaoui-Sossé, L.; Alaoui-Sossé, B.; Aleya, L. Potential Vulnerability of Oak Forests to Climate Change-Induced Flooding: Effects of Mild Oxygen Deficiency on Quercus robur and Quercus petraea Seedling Physiology. Environ. Sci. Pollut. Res. 2018, 25, 5550–5557. [Google Scholar] [CrossRef] [PubMed]
- Viciedo, D.O.; de Mello Prado, R.; Martínez, C.A.; Habermann, E.; de Cássia Piccolo, M. Short-term warming and water stress affect Panicum maximum Jacq. stoichiometric homeostasis and biomass production. Sci. Total Environ. 2019, 681, 267–274. [Google Scholar] [CrossRef]
- Birnbaum, D.; de Kroon, H.; Huber, H.; Zhang, Q.; de Best, S.; Beljaars, S.J.M.; Visser, E.J.W. Benefits of flooding-induced aquatic adventitious roots depend on the duration of submergence: Linking plant performance to root functioning. Ann. Bot. 2017, 120, 171–180. [Google Scholar] [CrossRef]
- Pierce, S.C.; Moore, M.T.; Dan, L.; Pezeshki, S.R. Erratum to: Macronutrient (N, P, K) and Redoximorphic Metal (Fe, Mn) Allocation in Leersia oryzoides (Rice Cutgrass) Grown Under Different Flood Regimes. Water Air Soil Pollut. 2010, 207, 73–84. [Google Scholar] [CrossRef]
- Yang, J.; Zheng, G.; Yang, J.; Wan, X.; Song, B.; Cai, W.; Guo, J. Phytoaccumulation of Heavy Metals (Pb, Zn, and Cd) by 10 Wetland Plant Species under Different Hydrological Regimes. Ecol. Eng. 2017, 107, 56–64. [Google Scholar] [CrossRef]
- Ponting, J.; Kelly, T.J.; Verhoef, A.; Watts, M.J.; Sizmur, T. The impact of increased flooding occurrence on the mobility of potentially toxic elements in floodplain soil—A review. Sci. Total Environ. 2021, 754, 142040. [Google Scholar] [CrossRef]
- Hirabayashi, Y.; Mahendran, R.; Koirala, S.; Konoshima, L.; Yamazaki, D.; Watanabe, S.; Kim, H.; Kanae, S. Global flood risk under climate change. Nat. Clim. Change 2013, 3, 816–821. [Google Scholar] [CrossRef]
- Rinklebe, J.; Shaheen, S.M.; Yu, K. Release of As, Ba, Cd, Cu, Pb, and Sr under Pre-Definite Redox Conditions in Different Rice Paddy Soils Originating from the U.S.A. and Asia. Geoderma 2016, 270, 21–32. [Google Scholar] [CrossRef]
- Shaheen, S.M.; Rinklebe, J.; Frohne, T.; White, J.R.; DeLaune, R.D. Redox Effects on Release Kinetics of Arsenic, Cadmium, Cobalt, and Vanadium in Wax Lake Deltaic Freshwater Marsh Soils. Chemosphere 2016, 150, 740–748. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Ma, C.; Chen, G.; Zhang, J.; Xing, B. Physiological and Biochemical Responses of Salix Integra Thunb. under Copper Stress as Affected by Soil Flooding. Environ. Pollut. 2017, 225, 644–653. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Xiao, J.; Salam, M.M.A.; Ma, C.; Chen, G. Impacts of Bamboo Biochar on the Phytoremediation Potential of Salix psammophila Grown in Multi-Metals Contaminated Soil. Int. J. Phytoremediat. 2021, 23, 387–399. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.M.; Xia, Y.H.; Hu, Y.J.; Chen, X.B.; Rui, Y.C.; Gunina, A.; He, X.Y.; Ge, T.D.; Wu, J.S.; Su, Y.R.; et al. Stoichiometry of carbon, nitrogen, and phosphorus in soil: Effects of agricultural land use and climate at a continental scale. Soil Till. Res. 2021, 209, 104903. [Google Scholar] [CrossRef]
- Wen, J.; Ji, H.; Sun, N.; Tao, H.; Du, B.; Hui, D.; Liu, C. Imbalanced Plant Stoichiometry at Contrasting Geologic-Derived Phosphorus Sites in Subtropics: The Role of Microelements and Plant Functional Group. Plant Soil 2018, 430, 113–125. [Google Scholar] [CrossRef]
- Högberg, P.; Näsholm, T.; Franklin, O.; Högberg, M.N. Tamm Review: On the Nature of the Nitrogen Limitation to Plant Growth in Fennoscandian Boreal Forests. For. Ecol. Manag. 2017, 403, 161–185. [Google Scholar] [CrossRef] [Green Version]
- Hu, M.; Peñuelas, J.; Sardans, J.; Sun, Z.; Wilson, B.J.; Huang, J.; Zhu, Q.; Tong, C. Stoichiometry Patterns of Plant Organ N and P in Coastal Herbaceous Wetlands along the East China Sea: Implications for Biogeochemical Niche. Plant Soil 2018, 431, 273–288. [Google Scholar] [CrossRef]
- Huang, D.; Wang, D.; Ren, Y. Using Leaf Nutrient Stoichiometry as an Indicator of Flood Tolerance and Eutrophication in the Riparian Zone of the Lijang River. Ecol. Indic. 2019, 98, 821–829. [Google Scholar] [CrossRef]
- Li, W.; Cao, T.; Ni, L.; Zhang, X.; Zhu, G.; Xie, P. Effects of Water Depth on Carbon, Nitrogen and Phosphorus Stoichiometry of Five Submersed Macrophytes in an In Situ Experiment. Ecol. Eng. 2013, 61, 358–365. [Google Scholar] [CrossRef]
- Yuan, G.; Cao, T.; Fu, H.; Ni, L.; Zhang, X.; Li, W.; Song, X.; Xie, P.; Jeppesen, E. Linking Carbon and Nitrogen Metabolism to Depth Distribution of Submersed Macrophytes Using High Ammonium Dosing Tests and a Lake Survey. Freshw. Biol. 2013, 58, 2532–2540. [Google Scholar] [CrossRef]
- Chen, G.-C.; Liu, Z.; Zhang, J.; Owens, G. Phytoaccumulation of Copper in Willow Seedlings under Different Hydrological Regimes. Ecol. Eng. 2012, 44, 285–289. [Google Scholar] [CrossRef]
- Cao, Y.; Ma, C.; Zhang, J.; Wang, S.; White, J.C.; Chen, G.; Xing, B. Accumulation and Spatial Distribution of Copper and Nutrients in Willow as Affected by Soil Flooding: A Synchrotron-Based X-ray Fluorescence Study. Environ. Pollut. 2019, 246, 980–989. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Wu, J.; Clark, C.M.; Pan, Q.; Zhang, L.; Chen, S.; Wang, Q.; Han, X. Grazing Alters Ecosystem Functioning and C:N:P Stoichiometry of Grasslands along a Regional Precipitation Gradient. J. Appl. Ecol. 2012, 49, 1204–1215. [Google Scholar] [CrossRef]
- Padmavathiamma, P.K.; Li, L.Y. Phytoremediation Technology: Hyper-Accumulation Metals in Plants. Water Air Soil Pollut. 2007, 184, 105–126. [Google Scholar] [CrossRef]
- Du, K.; Xu, L.; Wu, H.; Tu, B.; Zheng, B. Ecophysiological and Morphological Adaption to Soil Flooding of Two Poplar Clones Differing in Flood-Tolerance. Flora Morphol. Distrib. Funct. Ecol. Plants 2012, 207, 96–106. [Google Scholar] [CrossRef]
- Benimeli, C.S.; Medina, A.; Navarro, C.M.; Medina, R.B.; Amoroso, M.J.; Gómez, M.I. Bioaccumulation of Copper by Zea mays: Impact on Root, Shoot and Leaf Growth. Water Air Soil Pollut. 2010, 210, 365–370. [Google Scholar] [CrossRef]
- Chen, H.; Qualls, R.G.; Miller, G.C. Adaptive Responses of Lepidium latifolium to Soil Flooding: Biomass Allocation, Adventitious Rooting, Aerenchyma Formation and Ethylene Production. Environ. Exp. Bot. 2002, 48, 119–128. [Google Scholar] [CrossRef]
- Borghi, M.; Tognetti, R.; Monteforti, G.; Sebastiani, L. Responses of Populus × euramericana (P. deltoides × P. nigra) Clone Adda to Increasing Copper Concentrations. Environ. Exp. Bot. 2007, 61, 66–73. [Google Scholar] [CrossRef]
- Kabata-Pendias, A. Trace Elements in Soils and Plants, 4th ed.; CRC Press: Boca Raton, FL, USA, 2011; ISBN 978-1-4200-9368-1. [Google Scholar]
- Yu, B.; Zhao, C.Y.; Li, J.; Li, J.Y.; Peng, G. Morphological, Physiological, and Biochemical Responses of Populus euphratica to Soil Flooding. Photosynthetica 2015, 53, 110–117. [Google Scholar] [CrossRef]
- Li, X.; Li, N.; Yang, Y.; Ye, F.; Chen, F.; Chen, F. Morphological and Photosynthetic Responses of Riparian Plant Distylium chinense Seedlings to Simulated Autumn and Winter Flooding in Three Gorges Reservoir Region of the Yangtze River, China. Acta Ecol. Sin. 2011, 31, 31–39. [Google Scholar] [CrossRef]
- Kozlowski, T.T. Responses of Woody Plants to Flooding and Salinity. Tree Physiol. 1997, 17, 490. [Google Scholar] [CrossRef]
- Mielke, M.S.; Almeida, A.; Gomes, F.P.; Aguilar, M.; Mangabeira, P. Leaf Gas Exchange, Chlorophyll Fluorescence and Growth Responses of Genipa americana Seedlings to Soil Flooding. Environ. Exp. Bot. 2003, 50, 221–231. [Google Scholar] [CrossRef]
- Chen, H.; Qualls, R.G.; Blank, R.R. Effect of Soil Flooding on Photosynthesis, Carbohydrate Partitioning and Nutrient Uptake in the Invasive Exotic Lepidium latifolium. Aquat. Bot. 2005, 82, 250–268. [Google Scholar] [CrossRef]
- Farquhar, G.D.; Sharkey, T.D. Stomatal Conductance and Photosynthesis. Annu. Rev. Plant Physiol. 1982, 33, 317–345. [Google Scholar] [CrossRef]
- Vandecasteele, B.; Laing, G.D.; Quataert, P.; Tack, F.M.G. Differences in Cd and Zn Bioaccumulation for the Flood-Tolerant Salix cinerea Rooting in Seasonally Flooded Contaminated Sediments. Sci. Total Environ. 2005, 341, 251–263. [Google Scholar] [CrossRef]
- Zimmer, D. Spatial Distribution of Arsenic and Heavy Metals in Willow Roots from a Contaminated Floodplain Soil Measured by X-ray Fluorescence Spectroscopy. Sci. Total Environ. 2011, 409, 4094–4100. [Google Scholar] [CrossRef]
- Ye, Z.H.; Baker, A.J.M.; Wong, M.H.; Willis, A.J. Zinc, Lead and Cadmium Tolerance, Uptake and Accumulation by Typha latifolia. New Phytol. 1997, 136, 469–480. [Google Scholar] [CrossRef]
- Kreuzwieser, J.; Fürniss, S.; Rennenberg, H. Impact of Waterlogging on the N-Metabolism of Flood Tolerant and Non-Tolerant Tree Species: Impact of Flooding on N-Metabolism. Plant Cell Environ. 2002, 25, 1039–1049. [Google Scholar] [CrossRef]
- Vandecasteele, B.; Du Laing, G.; Lettens, S.; Jordaens, K.; Tack, F.M.G. Influence of Flooding and Metal Immobilising Soil Amendments on Availability of Metals for Willows and Earthworms in Calcareous Dredged Sediment-Derived Soils. Environ. Pollut. 2010, 158, 2181–2188. [Google Scholar] [CrossRef]
- Kissoon, L.T.T.; Jacob, D.L.; Otte, M.L. Multiple Elements in Typha angustifolia Rhizosphere and Plants: Wetland versus Dryland. Environ. Exp. Bot. 2011, 72, 232–241. [Google Scholar] [CrossRef]
- Ekvall, L.; Greger, M. Effects of Environmental Biomass-Producing Factors on Cd Uptake in Two Swedish Ecotypes of Pinus sylvestris. Environ. Pollut. 2003, 121, 401–411. [Google Scholar] [CrossRef]
- Cheng, H.; Wang, M.; Wong, M.H.; Ye, Z. Does Radial Oxygen Loss and Iron Plaque Formation on Roots Alter Cd and Pb Uptake and Distribution in Rice Plant Tissues? Plant Soil 2014, 375, 137–148. [Google Scholar] [CrossRef]
- Chen, Z.; Zhu, Y.; Liu, W.; Meharg, A.A. Direct Evidence Showing the Effect of Root Surface Iron Plaque on Arsenite and Arsenate Uptake into Rice (Oryza sativa) Roots. New Phytol. 2005, 165, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Simpson, S.L.; Rosner, J.; Ellis, J. Competitive Displacement Reactions of Cadmium, Copper, and Zinc Added to a Polluted, Sulfidic Estuarine Sediment. Environ. Toxicol. Chem. 2000, 19, 1992–1999. [Google Scholar] [CrossRef]
- Qiu, X.; Wang, H.; Peng, D.; Liu, X.; Yang, F.; Li, Z.; Cheng, S. Thinning Drives C:N:P Stoichiometry and Nutrient Resorption in Larix principis-rupprechtii Plantations in North China. For. Ecol. Manag. 2020, 462, 117984. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, W.; Xu, M.; Deng, J.; Han, X.; Yang, G.; Feng, Y.; Ren, G. Response of Forest Growth to C:N:P Stoichiometry in Plants and Soils during Robinia pseudoacacia Afforestation on the Loess Plateau, China. Geoderma 2019, 337, 280–289. [Google Scholar] [CrossRef]
- Niu, D.; Zhang, C.; Ma, P.; Fu, H.; Elser, J.J. Responses of Leaf C:N:P Stoichiometry to Water Supply in the Desert Shrub Zygophyllum xanthoxylum. Plant Biol. 2019, 21, 82–88. [Google Scholar] [CrossRef] [Green Version]
- Lizaso, J.I.; Melendez, L.M.; Ramirez, R. Early Flooding of Two Cultivars of Tropical Maize. Ii. Nutritional Responses. J. Plant Nutr. 2001, 24, 997–1011. [Google Scholar] [CrossRef]
- Trought, M.C.T.; Drew, M.C. The Development of Waterlogging Damage in Wheat Seedlings (Triticum aestivum L.). Plant Soil 1980, 54, 77–94. [Google Scholar] [CrossRef]
- Penuelas, J.; Sardans, J.; Llusià, J.; Owen, S.M.; Carnicer, J.; Giambelluca, T.W.; Rezende, E.L.; Waite, M.; Niinemets, Ü. Faster Returns on ‘Leaf Economics’ and Different Biogeochemical Niche in Invasive Compared with Native Plant Species. Glob. Change Biol. 2010, 16, 2171–2185. [Google Scholar] [CrossRef] [Green Version]
- Collins, C.G.; Wright, S.J.; Wurzburger, N. Root and Leaf Traits Reflect Distinct Resource Acquisition Strategies in Tropical Lianas and Trees. Oecologia 2016, 180, 1037–1047. [Google Scholar] [CrossRef] [PubMed]
- Herbert, D.A.; Williams, M.; Rastetter, E.B. A Model Analysis of N and P Limitation on Carbon Accumulation in Amazonian Secondary Forest after Alternate Land-Use Abandonment. Biogeochemistry 2003, 65, 121–150. [Google Scholar] [CrossRef]
- Güsewell, S.; Koerselman, W. Variation in Nitrogen and Phosphorus Concentrations of Wetland Plants. Perspect. Plant Ecol. Evol. Syst. 2002, 5, 37–61. [Google Scholar] [CrossRef]
- Sardans, J.; Peñuelas, J. Trees Increase Their P:N Ratio with Size. Glob. Ecol. Biogeogr. 2015, 24, 147–156. [Google Scholar] [CrossRef] [Green Version]
- Minden, V.; Kleyer, M. Internal and External Regulation of Plant Organ Stoichiometry. Plant Biol. 2015, 16, 897–907. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Song, M.; Zhang, S.; Cai, Z.; Lei, Y. Unravelling Community Assemblages through Multi-Element Stoichiometry in Plant Leaves and Roots across Primary Successional Stages in a Glacier Retreat Area. Plant Soil 2018, 428, 291–305. [Google Scholar] [CrossRef]
- Chrysargyris, A.; Papakyriakou, E.; Petropoulos, S.A.; Tzortzakis, N. The Combined and Single Effect of Salinity and Copper Stress on Growth and Quality of Mentha spicata Plants. J. Hazard. Mater. 2019, 368, 584–593. [Google Scholar] [CrossRef]
- Ducic, T.; Polle, A. Transport and Detoxification of Manganese and Copper in Plants. Braz. J. Plant Physiol. 2005, 17, 103–112. [Google Scholar] [CrossRef] [Green Version]
- Lei, Y.; Zhou, J.; Xiao, H.; Duan, B.; Wu, Y.; Korpelainen, H.; Li, C. Soil Nematode Assemblages as Bioindicators of Primary Succession along a 120-Year-Old Chronosequence on the Hailuogou Glacier Forefield, SW China. Soil Biol. Biochem. 2015, 88, 362–371. [Google Scholar] [CrossRef]
- Yu, Q.; Chen, Q.; Elser, J.J.; He, N.; Wu, H.; Zhang, G.; Wu, J.; Bai, Y.; Han, X. Linking Stoichiometric Homoeostasis with Ecosystem Structure, Functioning and Stability: Homoeostasis Underpins Ecosystem Properties. Ecol. Lett. 2010, 13, 1390–1399. [Google Scholar] [CrossRef]
- Karimi, R.; Folt, C.L. Beyond Macronutrients: Element Variability and Multielement Stoichiometry in Freshwater Invertebrates. Ecol. Lett. 2006, 9, 1273–1283. [Google Scholar] [CrossRef] [PubMed]
- Mckevlin, M.R.; Hook, D.D.; Mckee, W.H.; Wallace, S.U.; Woodruff, J.R. Phosphorus Allocation in Flooded Loblolly Pine Seedlings in Relation to Iron Uptake. Can. J. For. Res. 1987, 17, 1572–1576. [Google Scholar] [CrossRef]
- Solti, Á.; Sárvári, É.; Tóth, B.; Basa, B.; Lévai, L.; Fodor, F. Cd Affects the Translocation of Some Metals Either Fe-like or Ca-like Way in Poplar. Plant Physiol. Biochem. 2011, 49, 494–498. [Google Scholar] [CrossRef] [PubMed]
- Moore, B.C.; Lafer, J.E.; Funk, W.H. Influence of Aquatic Macrophytes on Phosphorus and Sediment Porewater Chemistry in a Freshwater Wetland. Aquat. Bot. 1994, 49, 137–148. [Google Scholar] [CrossRef]
- Zhang, J.; He, N.; Liu, C.; Xu, L.; Chen, Z.; Li, Y.; Wang, R.; Yu, G.; Sun, W.; Xiao, C.; et al. Variation and Evolution of C:N Ratio among Different Organs Enable Plants to Adapt to N-Limited Environments. Glob. Change Biol. 2020, 26, 2534–2543. [Google Scholar] [CrossRef]
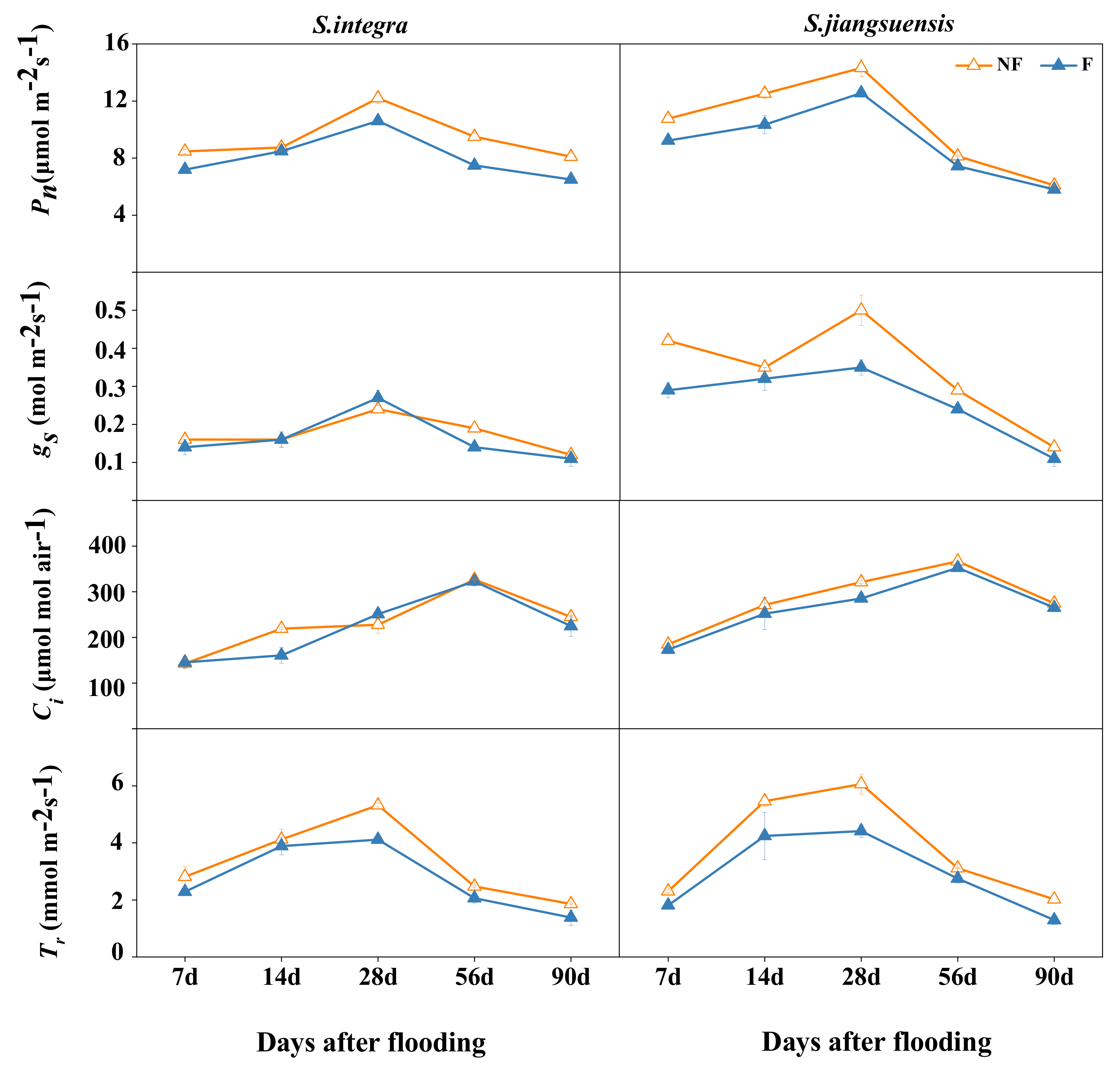
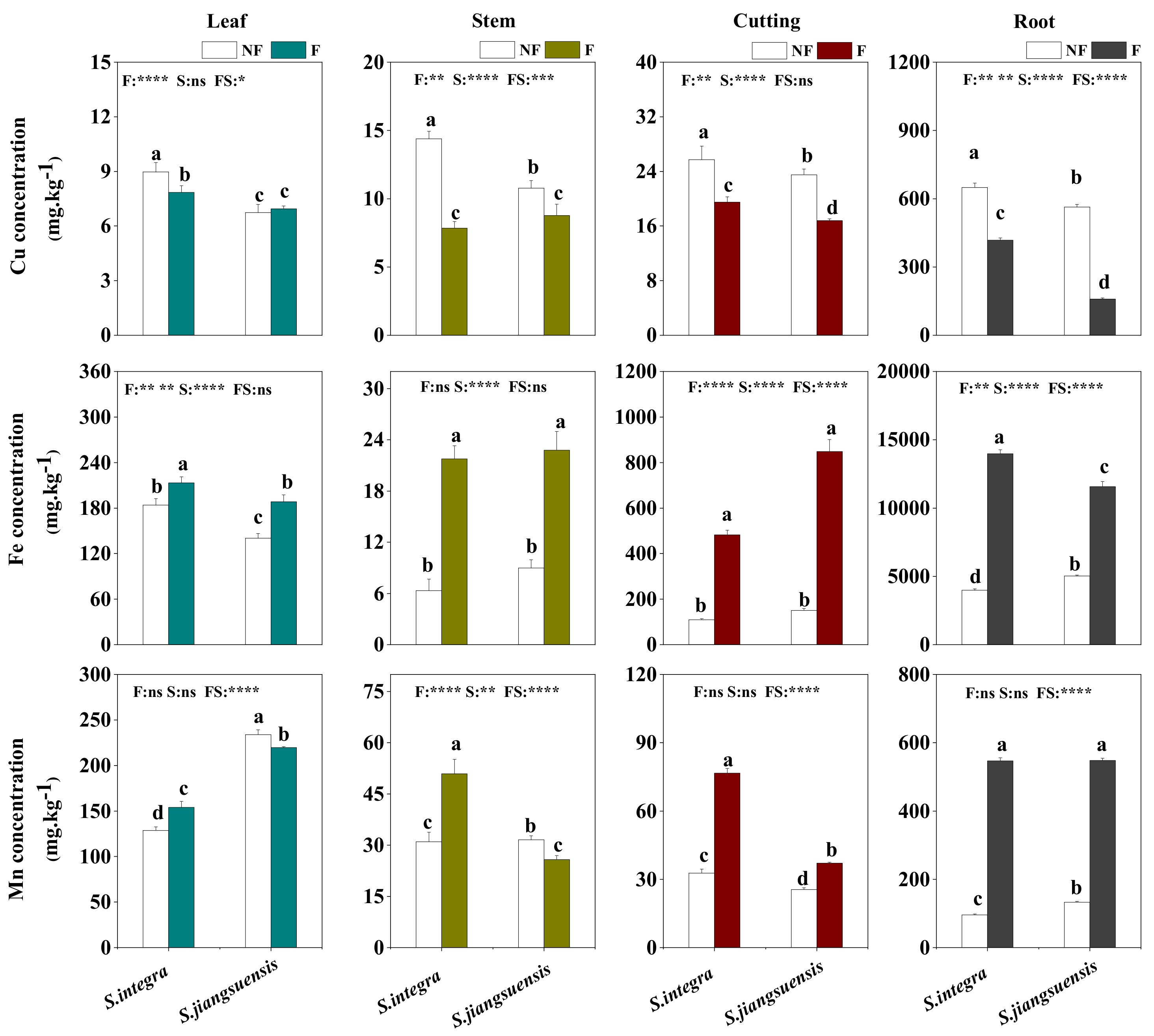
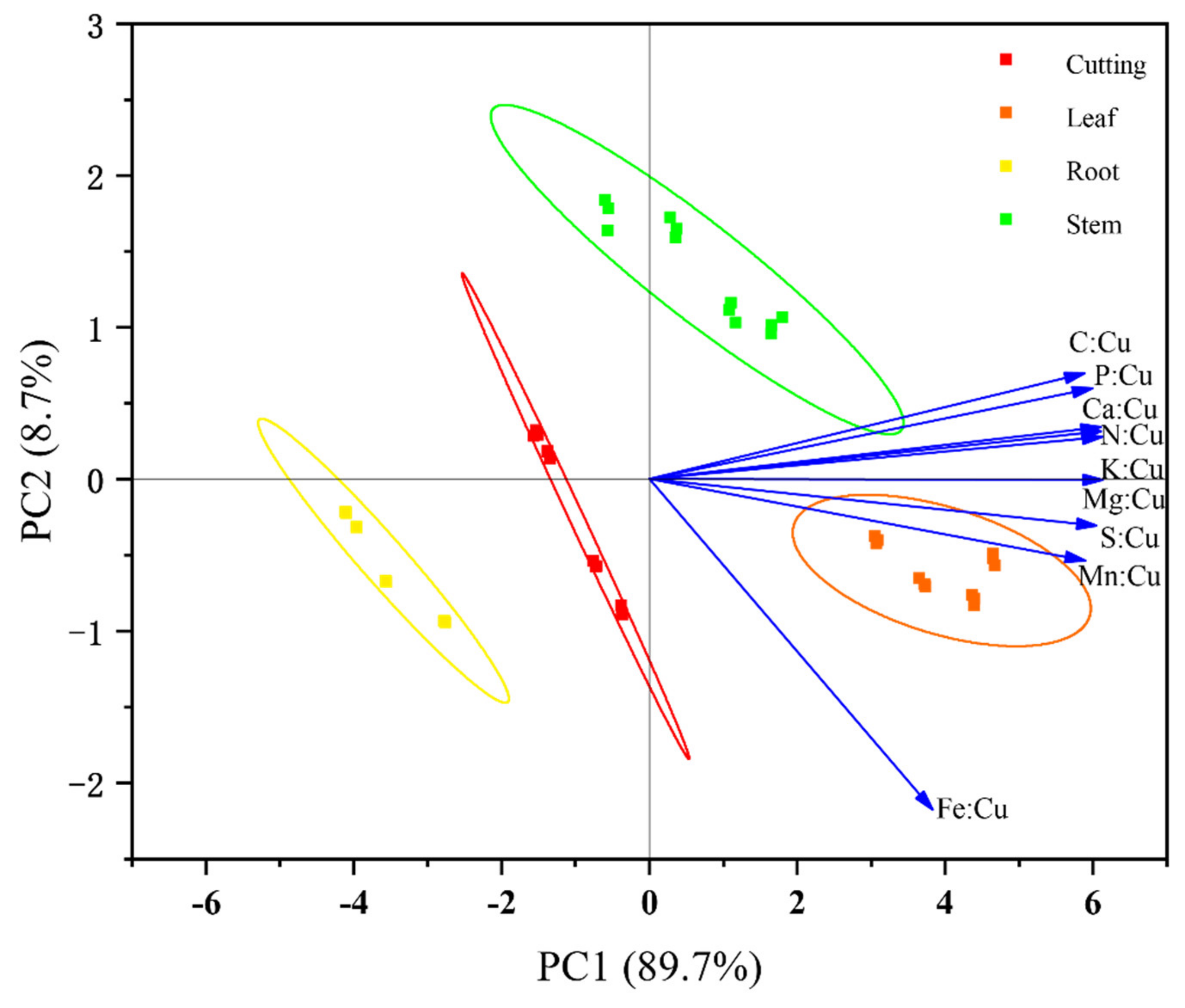
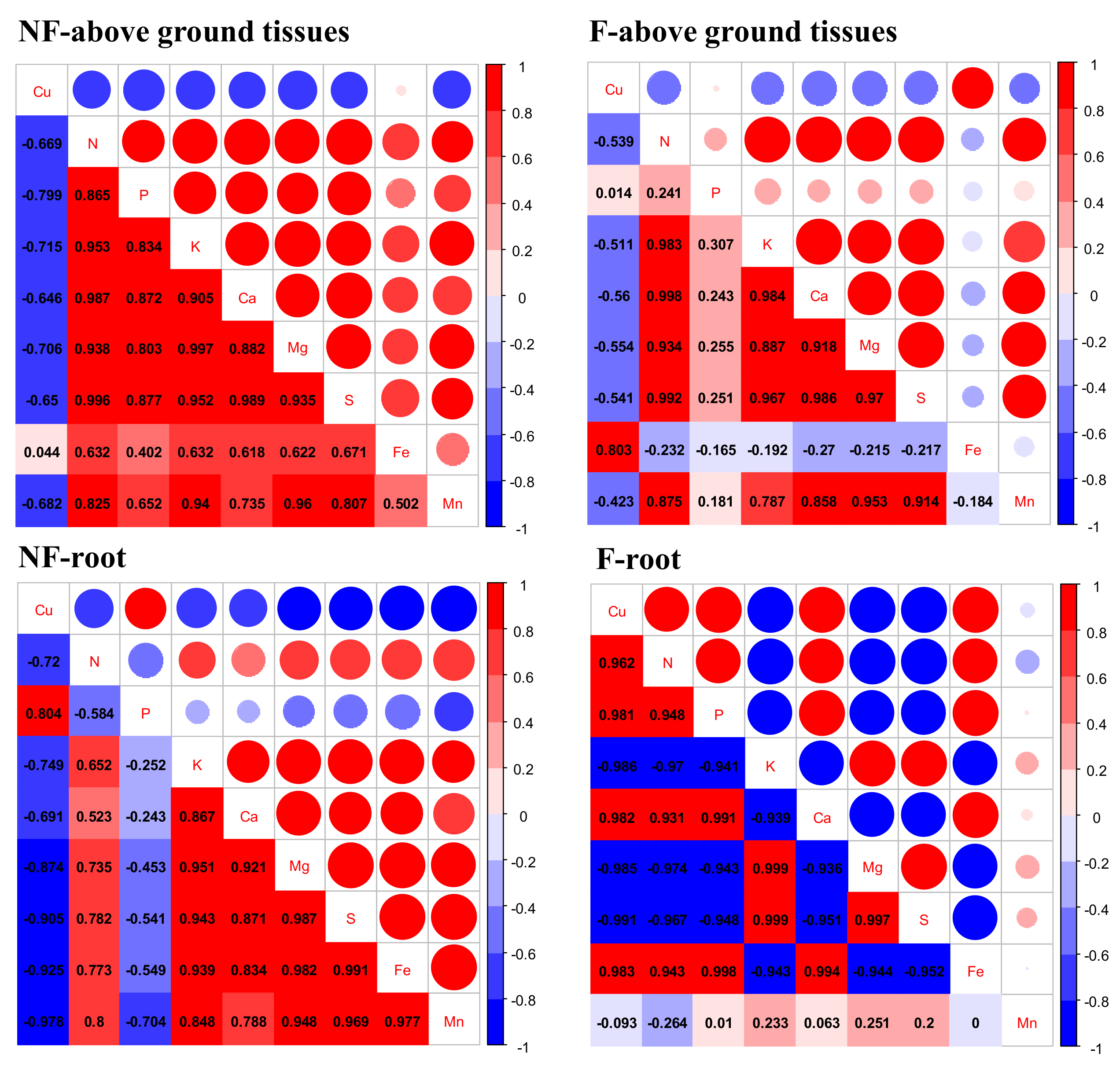
| Willow Species | Treatments | Leaf g, DW | Stem g, DW | Cutting g, DW | Root g, DW | Total g, DW | Plant Height cm | Leaf Area cm2 |
|---|---|---|---|---|---|---|---|---|
| S. integra | Non-flooded | 0.18 b | 0.51 b | 0.34 ab | 0.35 a | 1.1 b | 2.9 a | 0.2 b |
| Flooded | 0.34 c | 0.62 b | 0.45 b | 0.46 c | 1.6 b | 4.9 bc | 0.4 b | |
| S. jiangsuensis | Non-flooded | 0.42 a | 0.47 a | 0.51 a | 0.19 a | 1.3 a | 6.45 b | 0.7 a |
| Flooded | 0.41 a | 0.36 a | 0.65 ab | 0.33 b | 0.8 a | 3.9 c | 0.3 a | |
| Significance | Treatments | ns | ns | ns | **** | **** | **** | ns |
| Species | **** | **** | * | *** | * | ** | **** | |
| Treatments Species | ns | ns | ns | ns | ns | ns | ns |
| S. integra | S. jiangsuensis | Significance | |||||
|---|---|---|---|---|---|---|---|
| Non-Flooded | Flooded | Non-Flooded | Flooded | Treatments | Species | Treatments Species | |
| BCF—root | 0.08 a | 0.04 c | 0.05 b | 0.02 d | **** | **** | **** |
| BCF—aboveground tissues | 0.001 a | 0.001 c | 0.001 b | 0.002 d | **** | **** | *** |
| TF | 0.000 bc | 0.000 b | 0.000 c | 0.003 a | **** | **** | **** |
| Willow Species | Treatments | DCB Fe kg−1) | DCB Mn kg−1) | DCB Cu kg−1) | DCB S kg−1) |
|---|---|---|---|---|---|
| S. integra | Non-flooded | 2.8 d | 0.2 d | 1.3 a | 1.3 b |
| Flooded | 74.8 a | 2.3 a | 0.3 d | 0.4 c | |
| S. jiangsuensis | Non-flooded | 33.1 c | 0.72 c | 0.8 b | 0.2 c |
| Flooded | 199 b | 2.3 b | 1.1 a | 1.1 d | |
| Significance | Treatments | **** | ns | **** | **** |
| Species | **** | ns | ns | ns | |
| Treatments Species | **** | **** | **** | *** |
| Element and Ratios | Plant Species | Plant Tissues | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Leaf | Stem | Cutting | Root | ||||||
| Non-Flooded | Flooded | Non-Flooded | Flooded | Non-Flooded | Flooded | Non-Flooded | Flooded | ||
| C | S. integra | 6.9 a | 9.4 a | 9.2 a | 5.5 a | 8.6 a | 9.4 b | 15.9 a | 18.0 a |
| S. jiangsuensis | 16.0 a | 20.5 a | 10.2 b | 9.5 a | 8.1 ab | 10.4 ab | 8.3 a | 12.9 a | |
| N | S. integra | 0.2 a | 0.1 b | 0.18 a | 0.21 a | 0.05 a | 0.08 b | 0.07 a | 0.17 a |
| S. jiangsuensis | 0.2 | 0.2 d | 0.04 b | 0.08 b | 0.06 c | 0.05 b | 0.08 a | 0.01 b | |
| P | S. integra | 0.05 a | 0.04 c | 0.01 b | 0.11 c | 0.11 b | 0.04 ab | 0.15 c | 0.24 a |
| S. jiangsuensis | 0.1 b | 0.03 c | 0.07 a | 0.05 b | 0.04 a | 0.01 c | 0.02 | 0.18 b | |
| C:N | S. integra | 0.3 b | 1.1 b | 6.8 b | 5.7 b | 1.2 b | 5.6 b | 2.9 b | 4.2 b |
| S. jiangsuensis | 1.9 a | 1.4 a | 4.9 a | 5.3 a | 5.6 a | 2.5 b | 1.5 b | 2.2 a | |
| C:P | S. integra | 1.4 c | 13.1 a | 6.8 b | 29.7 a | 31.2 ab | 14.3 a | 6.3 ab | 4.1 c |
| S. jiangsuensis | 7.9 b | 9.4 a | 12.5 c | 16.0 b | 10.0 b | 10.6 ab | 1.6 a | 6.3 b | |
| N:P | S. integra | 0.01 c | 0.2 a | 0.10 b | 0.11 a | 0.2 a | 0.05 a | 0.06 b | 0.05 d |
| S. jiangsuensis | 0.2 d | 0.08 b | 0.04 d | 0.04 c | 0.06 b | 0.06 a | 0.03 a | 0.05 c | |
| Log Element:Log Cu Ratio | Plant Species | Plant Tissues | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Leaf | Stem | Cutting | Root | ||||||
| Non-Flooded | Flooded | Non-Flooded | Flooded | Non-Flooded | Flooded | Non-Flooded | Flooded | ||
| logC:logCu | S. integra | 0.01 d | 0.01 c | 0.01 d | 0.01 a | 0.01 d | 0.01 b | 2.01 ± 0.01 d | 0.01 b |
| S. jiangsuensis | 0.02 a | 0.02 b | 0.01 c | 0.01 b | 0.01 c | 0.01 a | 0.00 c | 0.01 a | |
| logN:logCu | S. integra | 0.01 d | 0.01 c | 0.02 d | 0.03 a | 0.00 c | 0.01 b | 0.00 d | 0.00 b |
| S. jiangsuensis | 0.01 a | 0.01 b | 0.01 c | 0.01 b | 0.01 d | 0.00 a | 0.00 c | 0.00 a | |
| logP:logCu | S. integra | 0.01 c | 0.01 c | 0.00 d | 0.04 a | 0.02 d | 0.01 b | 0.01 d | 0.01 b |
| S. jiangsuensis | 0.02 a | 0.01 b | 0.01 c | 0.02 b | 0.01 c | 0.00 a | 0.00 c | 0.01 a | |
| logK:logCu | S. integra | 0.00 d | 0.02 c | 0.01 d | 0.04 a | 0.01 d | 0.01 b | 0.01 d | 0.00 b |
| S. jiangsuensis | 0.00 a | 0.00 b | 0.02 c | 0.01 b | 0.01 c | 0.01 a | 0.00 c | 0.00 a | |
| logCa:logCu | S. integra | 0.01 d | 0.02 c | 0.00 d | 0.05 a | 0.01 d | 0.01 b | 0.00 d | 0.00 b |
| S. jiangsuensis | 0.01 a | 0.00 b | 0.02 c | 0.02 b | 0.01 c | 0.02 a | 0.00 c | 0.00 a | |
| logMg:logCu | S. integra | 0.01 d | 0.02 c | 0.00 d | 0.04 b | 0.01 c | 0.01 b | 0.01 d | 0.00 b |
| S. jiangsuensis | 0.01 a | 0.00 b | 0.02 c | 0.02 a | 0.01 b | 0.00 a | 0.00 c | 0.00 a | |
| logS:logCu | S. integra | 0.01 c | 0.02 b | 0.01 d | 0.04 a | 0.01 c | 0.01 b | 0.00 d | 0.01 b |
| S. jiangsuensis | 0.01 a | 0.00 a | 0.02 c | 0.02 b | 0.01 c | 0.00 a | 0.00 c | 0.00 a | |
| logFe:logCu | S. integra | 0.02 c | 0.02 b | 0.08 c | 0.03 a | 0.01 d | 0.01 b | 0.00 d | 0.00 b |
| S. jiangsuensis | 0.02 b | 0.02 a | 0.05 b | 0.04 a | 0.02 c | 0.02 a | 0.00 c | 0.01 a | |
| logMn:logCu | S. integra | 0.01 d | 0.02 c | 0.03 c | 0.04 a | 0.02 c | 0.01 a | 0.00 d | 0.00 b |
| S. jiangsuensis | 0.01 a | 0.00 b | 0.02 b | 0.02 b | 0.01 d | 0.00 b | 0.00 c | 0.00 a | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, Y.; Xiao, J.; Chen, J.; Li, X.; Shi, J.; Chen, G. Plant Growth and Nutrient Composition of Shrub and Arbor Willows Grown in Cu-Contaminated Flooded Soil. Forests 2022, 13, 989. https://doi.org/10.3390/f13070989
Cao Y, Xiao J, Chen J, Li X, Shi J, Chen G. Plant Growth and Nutrient Composition of Shrub and Arbor Willows Grown in Cu-Contaminated Flooded Soil. Forests. 2022; 13(7):989. https://doi.org/10.3390/f13070989
Chicago/Turabian StyleCao, Yini, Jiang Xiao, Jie Chen, Xiaogang Li, Jiuxi Shi, and Guangcai Chen. 2022. "Plant Growth and Nutrient Composition of Shrub and Arbor Willows Grown in Cu-Contaminated Flooded Soil" Forests 13, no. 7: 989. https://doi.org/10.3390/f13070989
APA StyleCao, Y., Xiao, J., Chen, J., Li, X., Shi, J., & Chen, G. (2022). Plant Growth and Nutrient Composition of Shrub and Arbor Willows Grown in Cu-Contaminated Flooded Soil. Forests, 13(7), 989. https://doi.org/10.3390/f13070989






