Abstract
Pitch pine (Pinus rigida Mill.) is native to North America and has a strong resistance to pine wood nematodes (PWNs). The PWN resistance mechanism of this tree species has yet to be discovered. In this work, we found that the spreading of inoculated PWNs in the branch of P. rigida was significantly suppressed compared to those in the branches of Pinus densiflora (Sieb. et Zucc.) and Pinus koraiensis (Sieb. et Zucc.). Dipping of PWNs in the resins isolated from P. rigida significantly suppressed the PWN mobility and conferred significantly higher PWN mortality compared to those in the resins from P. densiflora and P. koraiensis. All PWNs dipped in P. rigida resin were killed after six days, but more than 50% of the PWNs dipped in the resin from P. densiflora, and P. koraiensis were still alive after six days. The phytochemical analysis of resins revealed that P. rigida resin contained little or no amount of sesquiterpenes compared to those from P. densiflora and P. koraiensis. However, P. rigida resin contained rich amounts of diterpenes, among which dehydroabietic aldehyde, methyl dehydroabietate, and methyl abietate were uniquely detected. Particularly, two pinosylvin stilbenes (trans and cis-3,5-dimethoxystilbene) were accumulated in P. rigida resin, which were not detected in the resins from P. densiflora and P. koraiensis. cis-3,5-Dimethoxystilbene showed high nematicidal activity but not in trans-3,5-dimethoxystilbene. Conclusively, PWN resistance of P. rigida may be due to the toxic chemicals in the resin, in which cis-3,5-dimethoxystilbene may contribute to PWN toxicity. This work is the first demonstration that resin from PWN-resistant P. rigida directly affected PWN mobility and mortality, probably due to toxic phytochemicals in the resin.
1. Introduction
Pinewood nematode (PWN; Bursaphelenchus xylophilus) severely damages various pine species worldwide [1]. The nematodes mainly move through cortical and xylem resin canals in the stem [2]. They proliferate by eating plant tissues such as epithelial cells and parenchyma cells in pine trees, which eventually leads to water deficiency (embolization) by blocking tracheids [3]. It is considered the main factor of pine wilt disease [4]. Several methods have been used to prevent pine wilt disease. The representative control methods for the disease are preventing an insect vector (Monochamus species) by spraying insecticides such as thiacloprid during the hatching season of the vector [5]. Another method is the injection of nematicides such as abamectin into pine trees [6,7]. However, the application of these control methods is not successful in preventing the spread of pine wilt disease (PWD) [8]. A breeding strategy that selects resistant pine trees that survive after PWN infection is one method to induce PWD-resistant lines in several pine species [9,10].
The pinewood nematode is native to North America but was first reported in Japan [11]. Many Pinus species in East Asian countries, including Korea, China, and Japan, have seriously suffered from PWN infection, whereas North American pine species, such as Pinus elliottii Engelm., Pinus rigida Mill., and Pinus taeda L., are highly resistant to PWNs [12]. The detailed mechanism of PWN resistance in American pine species is not yet known. For this reason, it is imperative to investigate the mechanisms of differences in resistance that exist among pine species. In previous studies, researchers suggested that plant defense compounds, pre-existing and/or induced by PWN infection, may contribute to PWN resistance [13,14]. A water-soluble repellent for PWNs was found in the bark of P. taeda [15]. Pinus massoniana Lamb., Pinus strobus L., and Pinus palustris Mill. contain nematicidal substances (mainly water-insoluble pinosylvin stilbenes) in their heartwood [16]. Generally, pinosylvin stilbenes do not accumulate in fresh tissue of pine branches and needles but accumulate mainly in heartwood, which prevents wood tissues from decaying due to fungi [17]. There are various types of stilbenoid compounds in pine species, among which some show strong nematicidal activity [13,14,16].
It is now well accepted that PWNs in susceptible pine species inoculated by the insect vector quickly migrate downwards to the main stem and colonize the whole tree through the resin canal system [2,18], particularly through the thicker resin canals of the phloem and cortex [19]. In contrast, PWN migration has been found to be slower or even completely blocked not only in PWN-resistant conifer species [20,21] but also in PWN-resistant genetic variants [22]. Oku et al. [20] reported that PWN migration was highly inhibited in PWN-inoculated stems of a P. rigida × P. taeda hybrid, which is also categorized as a highly resistant pine species against PWNs. Son et al. [23] compared PWN migrations with PWN-susceptible (Pinus thunbergii Parl.) and PWN-resistant (P. strobus and P. rigida) pine trees. PWNs inoculated on the stem top actively migrated downwards through both cortical resin canals and xylem resin canals in PWN-susceptible pine (P. thunbergii). In contrast, PWN migration was highly inhibited, and the migration of PWNs was particularly restricted in xylem resin canals in resistant pine species (P. strobus and P. rigida). However, further investigation to understand the mechanism of resin in PWN resistance has not been clearly elucidated.
Pitch pine (P. rigida) is native to eastern North America and was introduced for forest restoration in South Korea in 1907. Since then, there have been no reports of PWD infection of pitch pine in South Korea. PWNs inoculated on branches failed to migrate in P. rigida [23]. However, it is unknown what kind of resistance mechanism would affect resistance against PWNs in P. rigida plants. In general, resin is a representative defense substance in conifers [24], and PWN infection highly affects the resin secretion of pine trees [25].
The role of resin in defending against PWNs in PWN-resistant pine species has not been investigated in detail. We postulated that PWN resistance in P. rigida may be due to the nematicidal properties of resin. To address this question, we investigated the role of P. rigida resin in PWN mobility and mortality. Moreover, we investigated the difference in chemical compositions between the resins from PWN-susceptible pine (P. densiflora and P. koraiensis) and PWN-resistant pine (P. rigida), and investigated the nematicidal activity of phytochemicals (trans and cis-3,5-dimethoxystilbene) accumulated only in the resin of P. rigida.
2. Materials and Methods
2.1. PWN Propagation
PWNs were cultured on Botrytis cinerea grown on potato dextrose agar (PDA) medium for 7 days [26]. PWNs were subcultured at 25 °C in darkness for 10 days periodically. Proliferating PWNs were isolated by the Baermann funnel method [27].
2.2. PWN Migration on PWN-Inoculated Stems
Freshly taken stem segments (10 cm long and 9 mm diameter) of P. densiflora, P. koriensis, and P. rigida were inoculated with PWNs (~200 nematodes) to estimate PWN distribution after PWN infection. The PWNs were inoculated in small drilled holes (4 mm) at the base of branches. In order to prevent the drying of samples, PWN-inoculated stem segments were placed in 15 mL Falcon tubes. The inoculation portions, including the proximal excised portion of stem segments, were sealed with Parafilm. After one day, the stems were divided into 2 cm pieces. The nematodes were separated from each stem segment by the Baermann funnel method, and the number of nematodes was counted by observation with a microscope (40×).
2.3. Resin Toxicity to PWNs
In order to examine the resin toxicity to PWNs, resins were collected from surfaces of excised branches of three-year-old pine saplings (P. densiflora, P. koraiensis, and P. rigida) obtained by germination of seeds. Fifty microliters of freshly collected resins were placed into a PCR tube to prevent water evaporation. After centrifugation of the PCR tube, approximately 50 nematodes were immersed into resin. After 3 days, the escape of PWNs from the resin was observed by microscopy.
2.4. Mortality of PWNs by Resin Treatment
Because PWNs escaped from the resin in the PCR tube, PWNs were cultured in resin that was inoculated onto a cell culture slide with a cover (Nunc® Lab-Tek® II—CC2™ Chamber Slide™ system 8 wells, glass slide, 0.7 cm2/well, and sterile) (Sigma–Aldrich Korea, Ltd., Seoul, Korea) to prevent escape from the resin. Approximately 50 nematodes were dipped into resin. The survival rate and migration distance of nematodes were observed by microscopy during 6 days of culture.
2.5. GC–MS Analysis of Resin Compositions in Pinus Species
The resin drops collected from needle tips from three Pinus species (P. densiflora, P. koraiensis, and P. rigida) were extracted by sonication in 100% methanol for 30 min. After centrifugation, the supernatant was filtered through a 0.45 μm membrane. An aliquot (5 µL) of each extraction was analyzed by GC (Agilent 7890A, Agilent Technologies Inc., Santa Clara, CA, USA) equipped with an HP-5MS capillary column (30 m × 0.25 mm, film thickness 0.25 mm) and MSD system (Agilent 5975C) linked to a Triple-Axis detector; carrier gas: He (1.2 mL min−1) and column temperature: 70–220 °C (5 °C min−1), 220–320 °C (4 °C min−1), and 320 °C (5 min hold). The peaks were identified by matching retention times and fragmentation of mass spectra with authentic standards and the mass spectral library of the GC–MS. The analyses were repeated three times, and GC chromatogram data were selected from one representative dataset. The percentage peak area method was used to analyze the area of the peak as a proportion of the total area of all detected peaks to analyze quantity.
2.6. PWN Toxicity of Two Pinosylvin Stilbenes
The trans-3,5-dimethoxystilbene standard for GC–MS analysis was purchased from Tokyo Chemical Industry Co., Ltd. (Toshima, Tokyo, Japan). Methyl dehydroabietate was purchased from Toronto Research Chemicals (TRC, Toronto, ON, Canada). cis-3,5-Dimethoxystilbene was isolated by the n-hexane-ethyl acetate (9:1) fraction of resin and identified by GC/MS.
Trans- and cis-3,5-dimethoxystilbene (100 µg/mL) and methyl dehydroabietate were dissolved in ethanol and diluted to 100 µg/mL by dilution using a 10 mg mL−1 concentration of 2-hydroxypropyl-b-cyclodextrin (HP-β-CD) solution, which was used as an emulsifier by He et al. [28]. Approximately 50 PWNs mixed with adults and J2 stage (2:3) were inoculated in each test solution in ibidi μ-Slide angiogenesis dishes (ibidi, Munich, Germany) and incubated for 2 days at 25 °C. HP-β-CD solution without the tested chemicals was used as a control. The immobilization of PWNs was determined by light microscopy. The experiment was performed in triplicate and repeated three times.
2.7. Statistics
All experiments were repeated in triplicate. Values in all data are presented as the average relative quantities ± standard error (SE). Statistical significance was measured according to one-way ANOVA followed by Duncan’s post hoc analysis at the 5% significance level.
3. Results
3.1. Migration of PWNs in Stems of P. densiflora, P. koraiensis and P. rigida
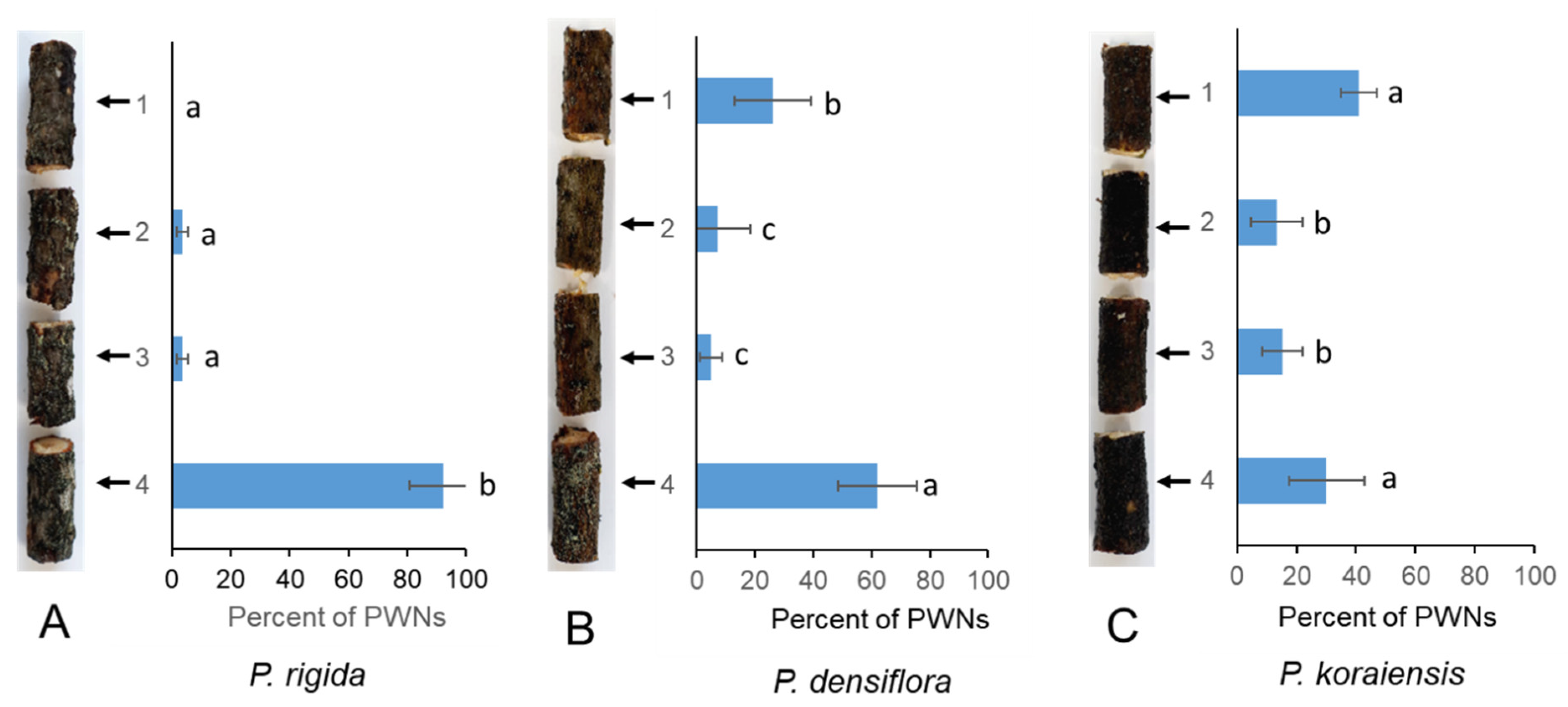
We investigated the distribution of PWNs in stems of PWN-susceptible (P. densiflora and P. koraiensis) and PWN-resistant (P. rigida) pine plants after inoculation with PWNs. Migration of inoculated PWNs in the branch of P. rigida was significantly suppressed compared to those in the branches of P. densiflora and P. koraiensis. PWNs rapidly spread upwards from the inoculated portion in susceptible P. densiflora and P. koraiensis after one day of inoculation (Figure 1). However, in P. rigida plants, most of the PWNs (92 ± 11.4%) remained near the inoculation point (Figure 1).
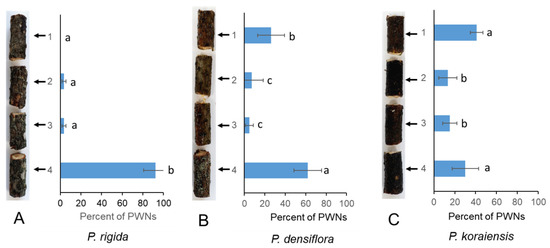
Figure 1.
PWN migration in the stems of three Pinus species ((A) P. rigida Mill.), (B) P. densiflora (Sieb. et Zucc.), and (C) P. koraiensis (Sieb. et Zucc.) after one day of PWN inoculation. PWNs were inoculated into the small, drilled hole located at the base of the branch (number 4 portion). The experiment was repeated in triplicate. Error bars indicate the standard error of the mean (±SE) of three replicate measurements. Different letters above the bars indicate significantly different values (p < 0.05), calculated using one-way ANOVA followed by Duncan’s post hoc analysis.
3.2. PWN Mobility in Resins from P. densiflora, P. koraiensis, and P. rigida
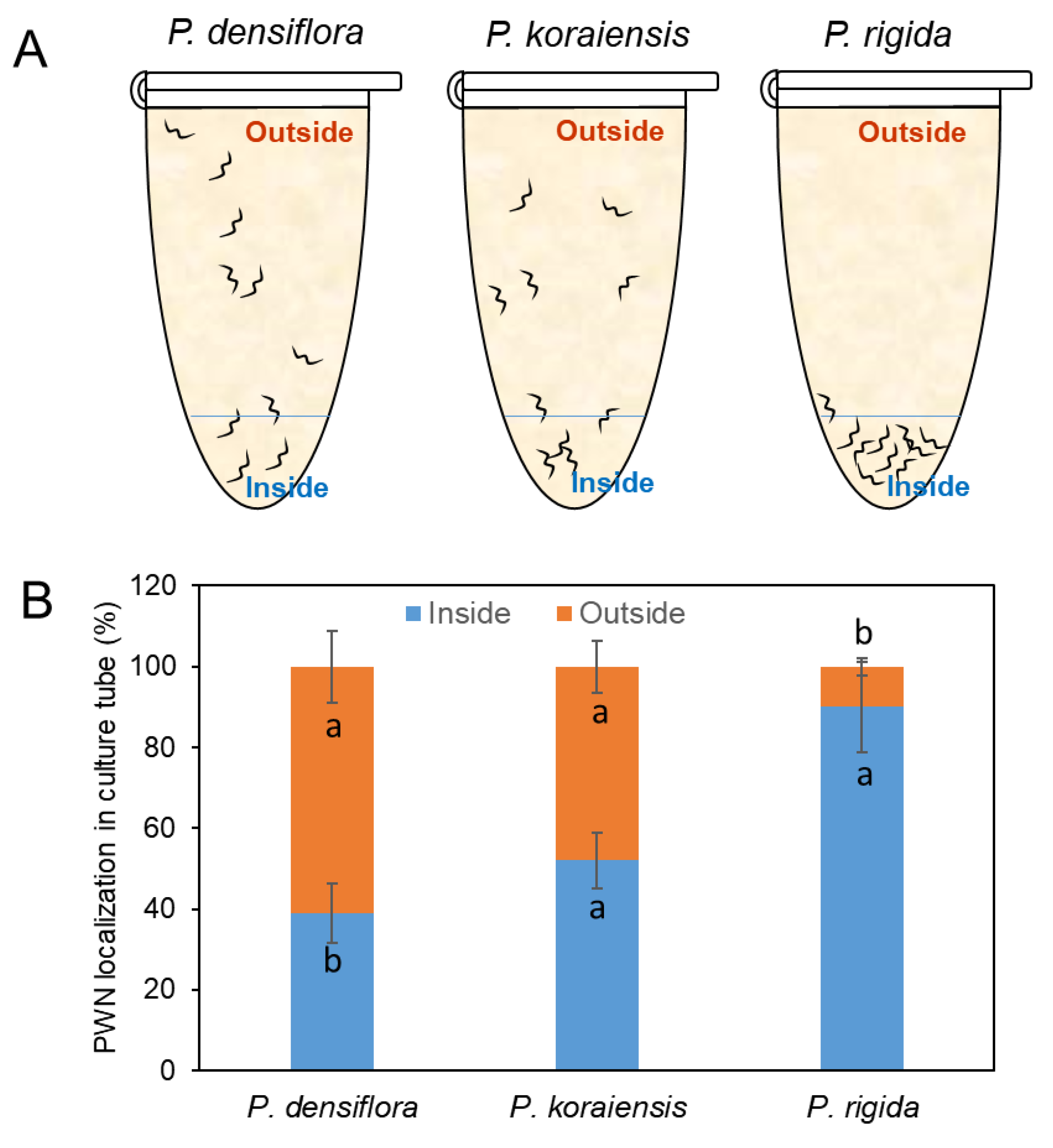
To examine the role of resin on PWN mobility, PWNs were immersed in the isolated resins from P. densiflora, P. koraiensis, and P. rigida. PWN mobility showed a significant reduction in resin from P. rigida compared to those in resins from P. densiflora and P. koraiensis (Figure 2). PWNs at 61 ± 8.9% and 48 ± 6.4% were located on the surfaces of the tube after escape from the resin after three days of culture, respectively (Figure 2A,B). However, more than 90.5 ± 11.2% of the nematodes inoculated in the resin from P. rigida failed to escape from the resin (Figure 2A,B). These results indicate that P. rigida resin contains toxic compounds immobilizing PWNs.
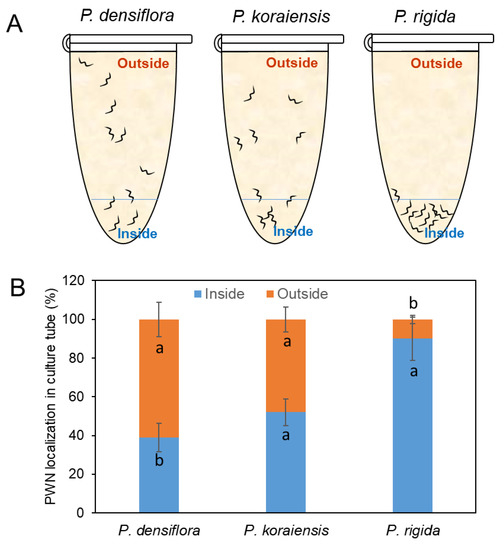
Figure 2.
Movement of PWNs inside and outside of resin from three Pinus species (P. densiflora, P. koraiensis, and P. rigida) during 3 days of culture. (A) Location of PWNs in resin or outside of resin in a PCR tube. (B) Number of PWNs located in resin (inside) or outside of resin after 3 days. Experiment was repeated in triplicate. Error bars indicate the standard error of the mean (±SE) of three replicate measurements. Different letters above the bars indicate significantly different values (p < 0.05), calculated using one-way ANOVA followed by Duncan’s post hoc analysis.
3.3. Mortality of PWNs in Resins from P. densiflora, P. koraiensis, and P. rigida
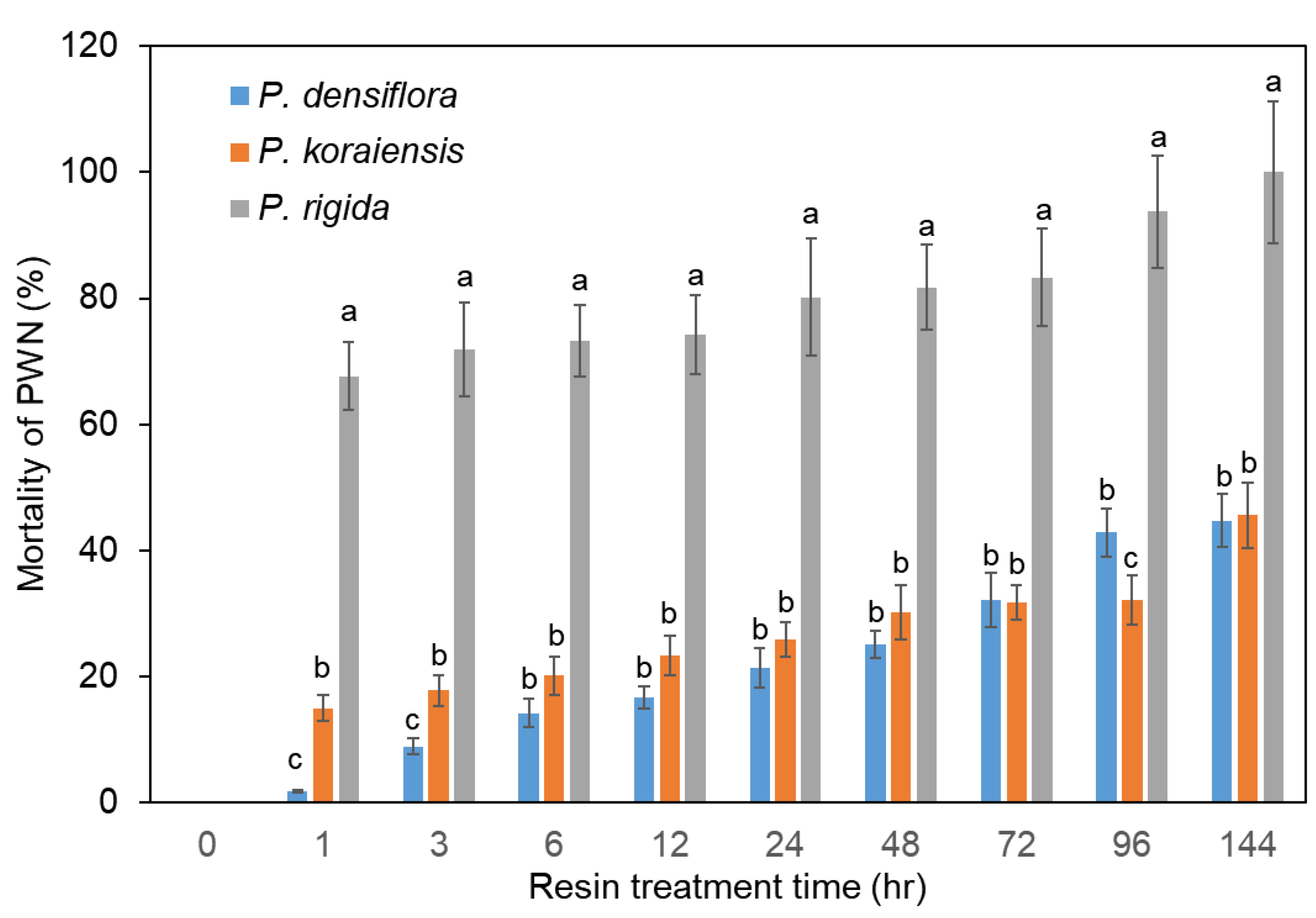
PWNs were directly inoculated into resins from P. densiflora, P. koraiensis, and P. rigida plants in cell culture chamber slides with covers to prevent free movement of PWNs outside of resin. Analysis of PWN mortality revealed that P. rigida resin showed significantly higher nematicidal activity than P. densiflora and P. koraiensis resins (Figure 3). There is no significant difference in resin toxicity between P. densiflora and P. koraiensis (Figure 3). The PWN mortalities in the resin from P. densiflora and P. koraiensis were 42.9% and 45.6%, respectively, after six days of treatment (Figure 3). On the other hand, 68% of the nematodes treated with resin from P. rigida showed no movement after one hour (Figure 3). Subsequently, all nematodes in P. rigida resin were killed after six days (Figure 3).
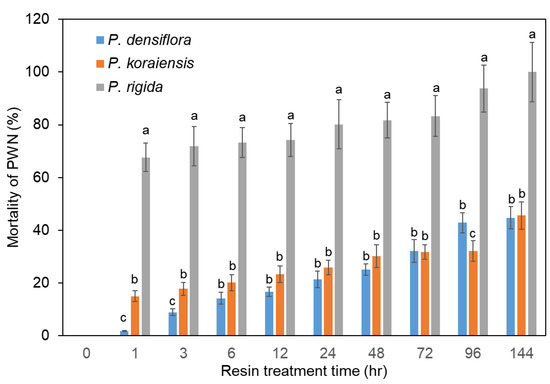
Figure 3.
Mortality of PWNs in resin from three Pinus species. Mortality of PWNs in P. densiflora resin. Mortality of PWNs in P. koraiensis resin. Mortality of PWNs in P. rigida resin. Experiment was repeated in triplicate. Error bars indicate the standard error of the mean (±SE) of three replicate measurements. Different letters above the bars indicate significantly different values (p < 0.05), calculated using one-way ANOVA followed by Duncan’s post hoc analysis.
3.4. Chemical Composition of Resins from P. densiflora, P. koraiensis, and P. rigida
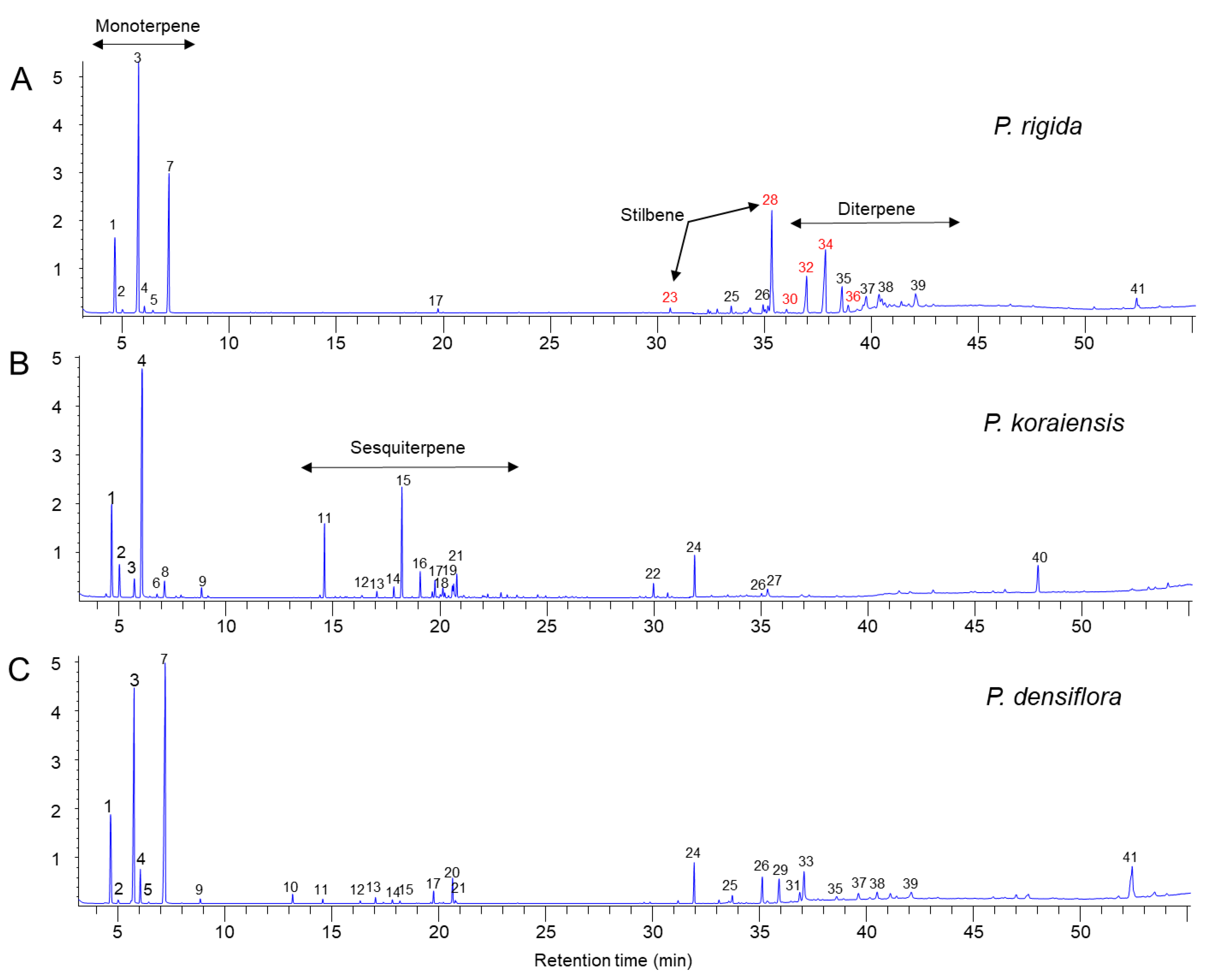
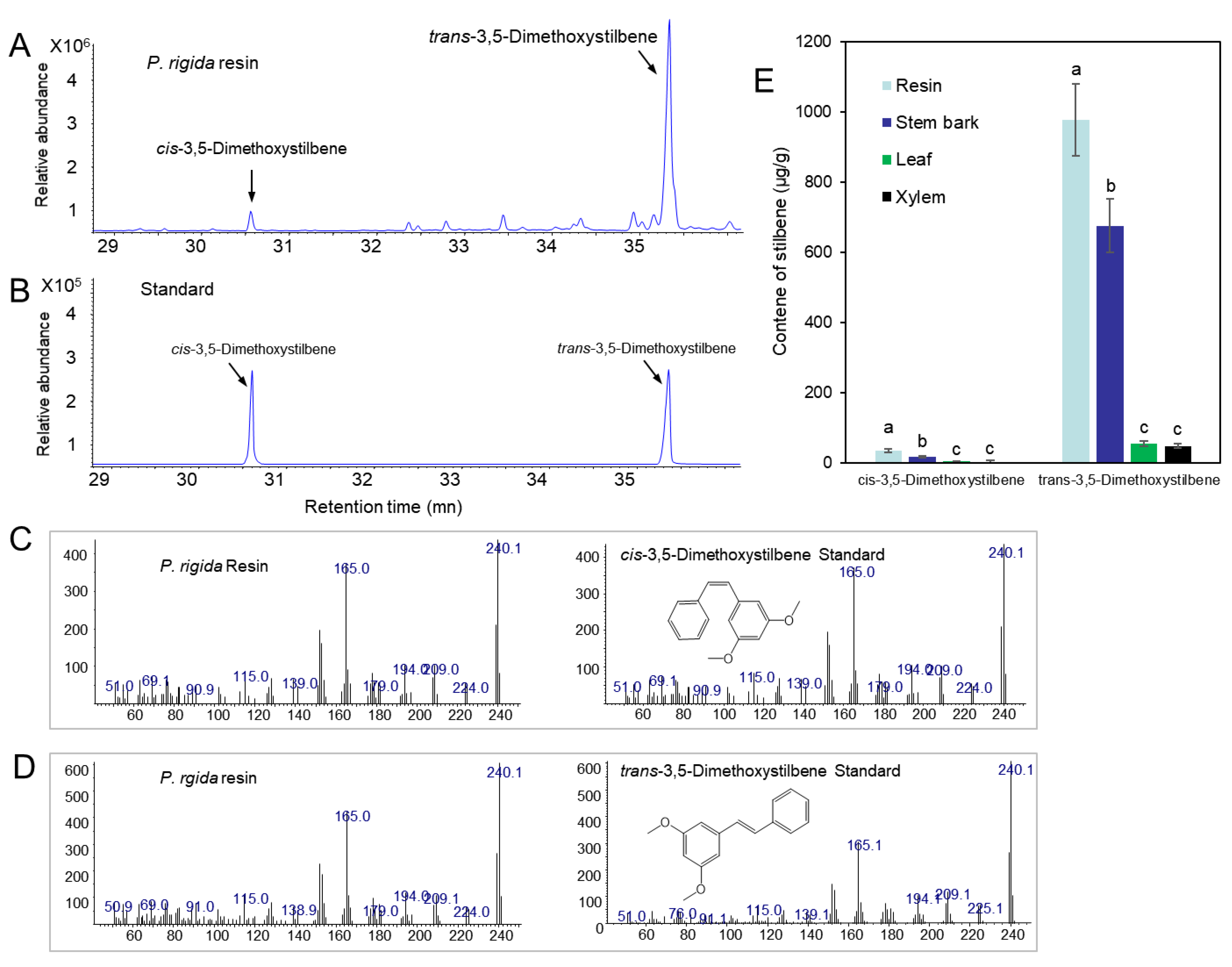
We analyzed the resin components extracted from PWN-susceptible (P. densiflora and P. koraiensis) and PWN-resistant (P. rigida) pine plants by GC–MS. A total of 41 compounds were identified by comparison with the GC/MS library (Figure 4), of which 10 monoterpenoids, 12 sesquiterpenoids, 2 stilbenoids, and several abietane diterpenoids were detected (Table 1). Pinus rigida resin contained little to no sesquiterpenes compared to those of P. densiflora and P. koraiensis. Only β-cubebene was detected as a small peak in the GC chromatogram (Figure 4A). However, P. rigida resin contained a rich amount of diterpenes compared to P. densiflora and P. koraiensis resins, among which dehydroabietic aldehyde, methyl dehydroabietate, and methyl abietate were uniquely detected in P. rigida resin. In particular, methyl dehydroabietate showed the highest peak area among the diterpenoids (Table 1 and Figure 4). Two pinosylvin stilbenes (peak numbers 23 and 28 in Figure 4) were uniquely found in P. rigida resin. The stilbene peaks were identified as trans- and cis-3,5-dimethoxystilbene by comparison of retention time and mass spectra of standard compounds (Figure 5A–D). The two pinosylvin stilbenes had the same mass spectra (Figure 5C,D), but the retention times of the two compounds were different (Figure 5A). Trans-3,5-dimethoxystilbene (pinosylvin dimethyl ether) was determined to be one of the major substances in the resin from P. rigida.
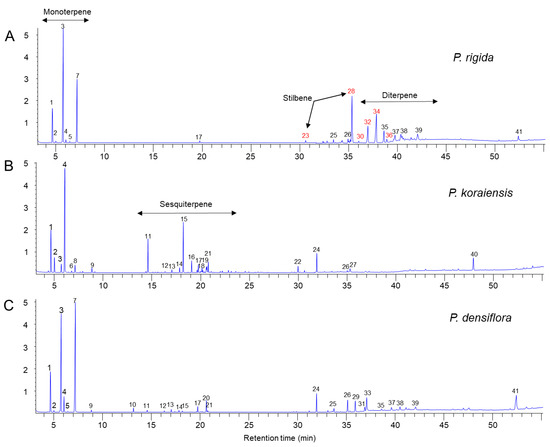
Figure 4.
Chemical composition in resin extracts of three Pinus species (P. rigida, P. koraiensis, and P. densiflora) by GC–MS analysis. (A) GC chromatogram of P. rigida resin. (B) GC chromatogram of P. koraiensis resin. (C) GC chromatogram of P. densiflora resin.

Table 1.
Identification of compounds was achieved using computer matching of the mass spectra with the NIST library or with mass spectra obtained from standard compounds.Compounds presented as dashed lines with amounts less than 0.05% are indicated. The dashed line indicates not detected and/or trace amounts. The bold characters/numbers indicate P. rigida Mill. resin-specific compounds. The values of peak area are mean of three replicates.
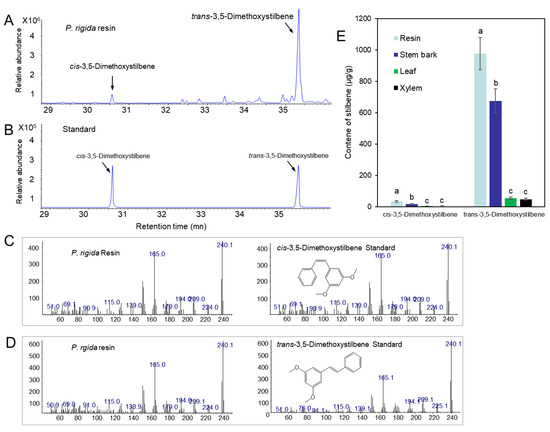
Figure 5.
Identification of trans-3,5-dimethoxystilbene and cis-3,5-dimethoxystilbene and analysis of trans-3,5-dimethoxystilbene and cis-3,5-dimethoxystilbene content in different tissues of P. rigida plants. (A) GC chromatogram of trans-3,5-dimethoxystilbene and cis-3,5-dimethoxystilbene in resin from P. rigida. (B) GC chromatogram of standard trans-3,5-dimethoxystilbene and cis-3,5-dimethoxystilbene. (C) Mass spectra of a peak (30.58 min) detected in resin and cis-3,5-dimethoxystilbene standard. (D) Mass spectra of a peak (35.36 min) detected in resin and trans-3,5-dimethoxystilbene standard. (E) Content of trans-3,5-dimethoxystilbene and cis-3,5-dimethoxystilbene in leaf, resin, bark, and xylem. Error bars indicate the standard error of the mean (±SE) of three replicate measurements. Different letters above the bars indicate significantly different values (p < 0.05), calculated using one-way ANOVA followed by Duncan’s post hoc analysis.
The concentrations of trans- and cis-3,5-dimethoxystilbene were evaluated in different samples (leaf, stem bark, resin, and xylem). Both stem bark and resin showed higher amounts of trans- and cis-3,5-dimethoxystilbene than leaf and xylem tissue (Figure 5E).
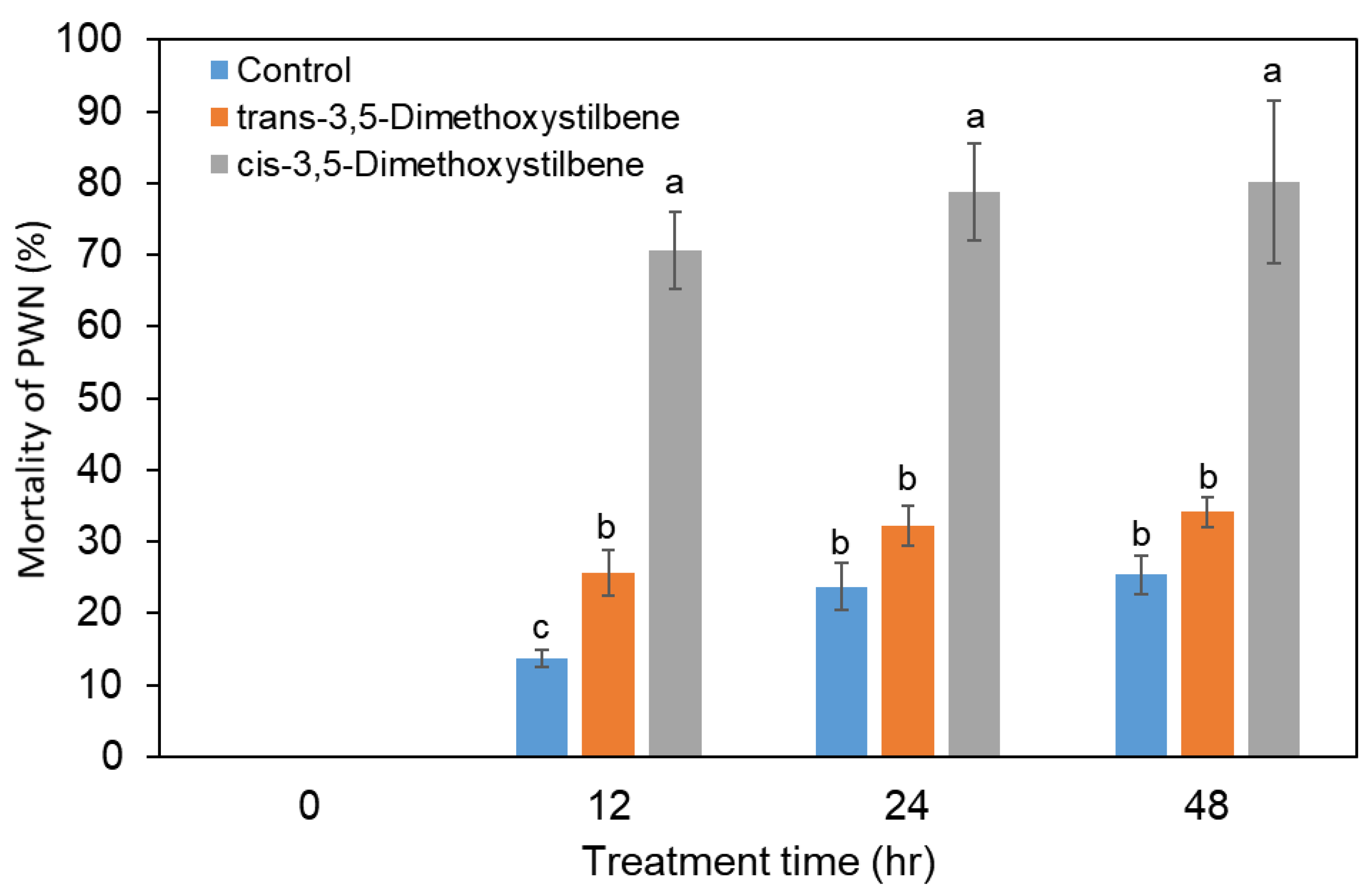
3.5. Nematicidal Activity of 3,5-Dimethoxystilbene against PWNs
In order to investigate the PWN toxicity of the two pinosylvin stilbenes, PWNs were inoculated in 100 µg/mL trans- and cis-3,5-dimethoxystilbene. In PWNs treated with trans-3,5-dimethoxystilbene, there was no significant difference in the nematicidal activity compared to the control group (Figure 6), indicating no nematicidal activity in trans-3,5-dimethoxystilbene. In contrast, cis-3,5-dimethoxystilbene showed significantly high nematicidal activity against PWNs in comparison to the control and trans-3,5-dimethoxystilbene treatment. PWN mortality in cis-3,5-dimethoxystilbene was 78% after 24 h (Figure 6).
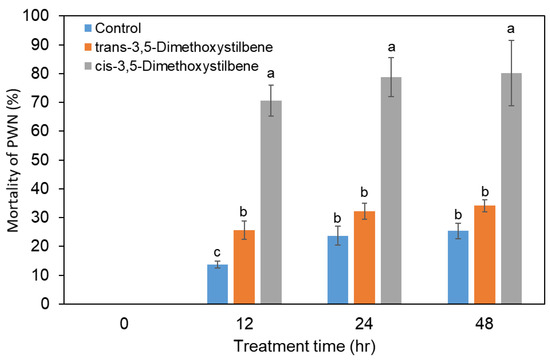
Figure 6.
Nematicidal activity of trans-3,5-dimethoxystilbene and cis-3,5-dimethoxystilbene against Bursaphelenchus xylophilus during 48 h of treatment. Experiment was repeated in triplicate. Error bars indicate the standard error of the mean (±SE) of three replicate measurements. Different letters above the bars indicate significantly different values (p < 0.05), calculated using one-way ANOVA followed by Duncan’s post hoc analysis.
4. Discussion
4.1. Inhibition of PWN Migration in Pine Stems
There were significant differences in the migration of PWNs in the branches of P. densiflora, P. koraiensis and P. rigida after inoculation with PWNs. PWNs were actively dispersed in the branches after PWN inoculation in PWN-susceptible P. densiflora and P. koraiensis. However, in the branches of P. rigida, most inoculated PWNs failed to migrate and remained at the inoculation point of PWNs. Similarly, Oku et al. [20] reported that PWN migration was highly inhibited in PWN-inoculated stems of a P. rigida × P. taeda hybrid, which is also categorized as a highly resistant pine species against PWNs. Son et al. [23] compared PWN migrations with PWN-susceptible (P. thunbergii) and PWN-resistant (P. strobus and P. rigida) pine trees. PWNs inoculated on the stem top actively migrated downwards through both cortical resin canals and xylem resin canals in PWN-susceptible pine (P. thunbergii). In contrast, PWN migration was highly inhibited, and the migration of PWNs was particularly restricted in xylem resin canals in resistant pine species (P. strobus and P. rigida).
The inhibition of PWN migration in resin canals in PWD-resistant pines can be explained by two possible factors. One is the presence of inhibitory compounds that affect the PWN movement [13,16,20,29]. Another possible interpretation has been proposed: anatomical differences in the lumen area of resin canals between susceptible and resistant pines. Kawaguchi [19] reported that the smaller lumen area in resistant P. thunbergii caused the restriction in PWN migration. However, Son et al. [23] and Mori et al. [30] did not see a relationship between anatomical differences in resin canals between PWD-resistant and PWD-susceptible pines. They suggested that the structures of cortical and xylem axial resin canals may not be a general and critical factor preventing PWN migration in resistant pines.
4.2. Resin Toxicity against PWNs
Resin plays an important role as a defense substance for preventing insect invasion or pathogens in conifers [3]. We suppose that suppression of PWN migration in PWN-resistant pine species might be caused by toxic phytochemicals in resins. However, the role of resin in defending against PWNs in PWN-resistant pine species has not been investigated in detail. To investigate the resin toxicity against PWNs, we collected the resins from PWN-susceptible (P. densiflora and P. koraiensis) and -resistant (P. rigida) pine trees and observed the mobility and mortality of PWNs by directly immersing PWNs in resins. We found that the mobility of PWNs was strongly suppressed in the resin from P. rigida. Moreover, P. rigida resin showed a significantly higher PWN mortality rate than P. densiflora and P. koraiensis resins. All the PWNs dipped in P. rigida resin were killed after six days. In contrast, more than 50% of PWNs dipped in the resins from P. densiflora and P. koraiensis were still alive until after six days. These results indicate that the resins from P. rigida directly affected the mobility and mortality of PWNs, probably due to the toxic nematicidal compounds in resin, by which P. rigida plants might be able to attain PWN resistance.
4.3. Chemical Composition of Resins Extracted from P. densiflora, P. koraiensis, and P. rigida
Resin is composed of various phytochemicals, including mainly terpenoids, which are basic substances that constitute or induce defense mechanisms [31]. In order to uncover the nematicidal compounds in P. rigida resin, phytochemical components extracted from resin from PWN-susceptible (P. densiflora and P. koraiensis) and PWN-resistant (P. rigida) pine plants were analyzed by GC/MS. Resins sampled from the three pine species mainly contained monoterpenes, sesquiterpenes, and diterpenes. Pinus rigida resin contained small amounts of sesquiterpenes compared to P. densiflora and P. koraiensis. Pinus rigida resin contained rich and various kinds of diterpenoids, among which dehydroabietic aldehyde, methyl dehydroabietate, methyl abietate, and one unknown compound were uniquely detected in P. rigida resin. Interestingly, P. rigida resin contained two stilbenoid compounds (cis- and trans-3,5-dimethoxystilbene), which are not found in the resin from P. densiflora and P. koraiensis. The above results indicate that the toxicity of P. rigida resin against PWNs might be caused by the different chemical compositions in the resin.
4.4. Toxicity of 3,5-Dimethoxystilbene against PWNs
Generally, pinosylvin stilbenes are particularly rich in heartwood extracts preventing wood tissues from decaying by fungi [17]. In sapwood and needles, pinosylvin stilbenes seem to function as phytoalexins because these compounds are accumulated under abiotic and biotic stresses [32,33,34,35]. Suga [16] firstly reported that some pine stilbenoid compounds have strong nematicidal activity. Recently, the two pinosylvin stilbenes (pinosylvin monomethyl ether and dihydropinosylvin monomethyl ether) in P. strobus had strong nematicidal activity against PWNs and the accumulation of these stilbenes highly enhanced by PWN infection [14]. In this work, we found that Pinus rigida resin contained trans- and cis-3,5-dimethoxystilbene (pinosylvin dimethyl ether and cis-pinosylvin dimethyl ether) in resin even under normal growth conditions without PWN infection. The occurrence of the two stilbenes was also reported in the bark extract of P. banksiana [36], and P. banksiana is moderately resistant to PWNs [3].
We investigated the biological activity of 3,5-dimethoxystilbene against PWNs. PWNs were treated with the two isoforms of 3,5-dimethoxystilbene (cis- and trans-3,5-dimethoxystilbene). cis-3,5-Dimethoxystilbene showed significantly higher nematicidal activity compared to trans-3,5-dimethoxystilbene, which is similar to the result of Suga et al. [16]. They reported that cis-3,5-dimethoxystilbene at 100 μg mL−1 showed strong nematicidal activity against PWNs, but trans-3,5-dimethoxystilbene did not. Although cis-3,5-dimethoxystilbene showed PWN toxicity in an in vitro test, the presence of cis-3,5-dimethoxystilbene (67 μg/g) in P. rigida resin may partially contribute to achieving PWN resistance due to insufficient concentrations.
In addition to the occurrence of 3,5-dimethoxystilbene in P. rigida resin, Pinus rigida resin contained rich and various abietane diterpenes together with several unknown diterpenes. There is no report on the nematicidal activity of pine diterpenoids against PWNs. However, some diterpenoids isolated from coniferous plants were reported to have strong nematicidal activity in some nematode species. Abieta-7,13-diene, ferruginol (abieta-8,11,13-trien-12-ol), and sugiol (12-Hydroxyabieta-8,11,13-trien-7-one) isolated from Juniperus berries have strong nematicidal activity against malarial nematodes (Plasmodium falciparum), and totarol (14-isopropyl podocarpa-8,11,13-trien-13-ol) has nematicidal activity against Caenorhabditis elegans [37]. Although we did not analyze the PWN toxicity of diterpenoids that were uniquely detected in P. rigida resin, the occurrence of various abietane diterpenoids accumulated in P. rigida resin may be involved in additional PWN resistance together with cis-3,5-dimethoxystilbene.
5. Conclusions
In this study, we found that dipping in the resin isolated from P. rigida strongly suppressed PWN mobility and conferred high PWN mortality. In GC–MS analysis of resin extracts from three pine species (P. densiflora, P. koraiensis, and P. rigida), two types of pinosylvin stilbenes (cis- and trans-3,5-dimethoxystilbene) were detected only in P. rigida resin, and several diterpenoids were also particularly rich in P. rigida resin. In the test of PWN toxicity of the two isoforms of 3,5-dimethoxystilbene (cis- and trans-3,5-dimethoxystilbene), cis-3,5-dimethoxystilbene showed higher nematicidal activity than trans-3,5-dimethoxystilbene. From our results, we suggest that toxic phytochemicals accumulated in the resin from P. rigida, such as cis-3,5-dimethoxystilbene, may create PWN resistance in P. rigida pine.
Author Contributions
Y.-E.C. designed the research, and both H.-S.H. and Y.-E.C. wrote the paper. H.-S.H. and J.-Y.H. performed the analysis of secondary compounds by GC/MS. Y.-R.K. determined the PWN toxicity of the resin. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Korea Forest Service (Korea Forestry Promotion Institute), Republic of Korea (Grant No. 2021339A00-2123-CD02).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to privacy restrictions.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Jones, J.T.; Haegeman, A.; Danchin, E.G.J.; Gaur, H.S.; Helder, J.; Jones, M.J.K.; Kikuchi, T.; Manzanilla-López, R.; Palomares-Rius, J.E.; Wesemael, W.M.L.; et al. Top 10 plant-parasitic nematodes in molecular plant pathology. Mol. Plant Pathol. 2013, 14, 946–961. [Google Scholar] [CrossRef] [PubMed]
- Ichihara, Y.; Fukuda, K.; Suzuki, K. Early symptom development and histological changes associated with migration of Bursaphelenchus xylophilus in seedling tissues of Pinus thunbergii. Plant Dis. 2000, 84, 675–680. [Google Scholar] [CrossRef] [PubMed]
- Futai, K. Pine Wood Nematode, Bursaphelenchus Xylophilus. Annu. Rev. Phytopathol. 2013, 51, 61–83. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, T. Responses of water-stressed Pinus thunbergii to inoculation with avirulent pine wood nematode (Bursaphelenchus xylophilus): Water relations and xylem histology. J. For. Res. 1996, 1, 223–226. [Google Scholar] [CrossRef]
- Linit, M.J. Nematode-vector relationships in the pine wilt disease system. J. Nematol. 1988, 20, 227–235. [Google Scholar]
- James, R.; Tisserat, N.; Todd, T. Prevention of pine wilt of scots pine (Pinus sylvestris) with systemic abamectin injections. Arboric. Urban For. 2006, 32, 195–201. [Google Scholar] [CrossRef]
- Sousa, E.; Naves, P.; Vieira, M. Prevention of pine wilt disease induced by Bursaphelenchus xylophilus and Monochamus galloprovincialis by trunk injection of emamectin benzoate. Phytoparasitica 2013, 41, 143–148. [Google Scholar] [CrossRef]
- Kim, B.-N.; Kim, J.H.; Ahn, J.-Y.; Kim, S.; Cho, B.-K.; Kim, Y.-H.; Min, J. A short review of the pinewood nematode, Bursaphelenchus xylophilus. Toxicol. Environ. Health Sci. 2020, 12, 297–304. [Google Scholar] [CrossRef]
- Kurinobu, S. Current status of resistance breeding of Japanese pine species to pine wilt disease. For. Sci. Technol. 2008, 4, 51–57. [Google Scholar] [CrossRef]
- Ribeiro, B.; Espada, M.; Vu, T.; Nóbrega, F.; Mota, M.; Carrasquinho, I. Pine wilt disease: Detection of the pinewood nematode (Bursaphelenchus xylophilus) as a tool for a pine breeding programme. For. Pathol. 2012, 42, 521–525. [Google Scholar] [CrossRef]
- Yano, S. Investigation on pine death in Nagasaki prefecture. Sanrin-Kouhou 1913, 4, 1–14. [Google Scholar]
- Mamiya, Y. Pathology of the pine wilt disease caused by Bursaphelenchus xylophilus. Annu. Rev. Phytopathol. 1983, 21, 201–220. [Google Scholar] [CrossRef] [PubMed]
- Hanawa, F.; Yamada, T.; Nakashima, T. Phytoalexins from Pinus strobus bark infected with pinewood nematode, Bursaphelenchus xylophilus. Phytochemistry 2001, 57, 223–228. [Google Scholar] [CrossRef]
- Hwang, H.S.; Han, J.Y.; Choi, Y.E. Enhanced accumulation of pinosylvin stilbenes and related gene expression in Pinus strobus after infection of pine wood nematode. Tree Physiol. 2021, 41, 1972–1987. [Google Scholar] [CrossRef] [PubMed]
- Futai, K. Responses of two species of Bursaphelenchus to the extracts from pine segments and to the segments immersed in different solvents. Jpn. J. Nematol. 1979, 9, 54–59. [Google Scholar]
- Suga, T.; Ohta, S.; Munesada, K.; Ide, N.; Kurokawa, M.; Shimizu, M.; Ohta, E. Endogenous pine wood nematicidal substances in Pines, Pinus Massoniana, P. Strobus and P. Palustris. Phytochemistry 1993, 33, 1395–1401. [Google Scholar] [CrossRef]
- Harju, A.M.; Venäläinen, M. Measuring the decay resistance of scots pine heartwood indirectly by the Folin-Ciocalteu assay. Can. J. For. Res. 2006, 36, 1797–1804. [Google Scholar] [CrossRef]
- Son, J.A.; Komatsu, M.; Matsushita, N.; Hogetsu, T. Migration of pine wood nematodes in the tissues of Pinus thunbergii. J. For. Res. 2010, 15, 186–193. [Google Scholar] [CrossRef]
- Kawaguchi, E. Relationship between the anatomical characteristics of cortical resin canals and migration of Bursaphelenchus xylophilus in stem cuttings of Pinus thunbergii seedlings. J. Jpn. For. Soc. 2006, 88, 240–244. [Google Scholar] [CrossRef][Green Version]
- Oku, H.; Shiraishi, T.; Chikamatsu, K. Active defense as a mechanism of resistance in pine against pine wilt disease. Jpn. J. Phytopathol. 1989, 55, 603–608. [Google Scholar] [CrossRef]
- Nunes da Silva, M.; Lima, M.R.M.; Vasconcelos, M.W. Susceptibility evaluation of Picea abies and Cupressus lusitanica to the pine wood nematode (Bursaphelenchus xylophilus). Plant Pathol. 2013, 62, 1398–1406. [Google Scholar] [CrossRef]
- Kuroda, K. Inhibiting factors of symptom development in several Japanese red pine (Pinus densiflora) families selected as resistant to pine wilt. J. For. Res. 2004, 9, 217–224. [Google Scholar] [CrossRef]
- Son, J.A.; Matsushita, N.; Hogetsu, T. Migration of Bursaphelenchus xylophilus in cortical and xylem axial resin canals of resistant pines. For. Pathol. 2014, 45, 246–253. [Google Scholar] [CrossRef]
- Martin, D.; Tholl, D.; Gershenzon, J.; Bohlmann, J. Methyl jasmonate induces traumatic resin ducts, terpenoid resin biosynthesis, and terpenoid accumulation in developing xylem of Norway spruce stems. Plant Physiol. 2002, 129, 1003–1018. [Google Scholar] [CrossRef]
- Bolla, R.I.; Nosser, C.; Tamura, H. Chemistry of response of pines to Bursaphelenchus xvlophilus: Resin acids. Jpn. J. Nematol. 1989, 19, 1–6. [Google Scholar]
- Zhu, L.-H.; Ye, J.; Negi, S.; Xu, X.-L.; Wang, Z.-L.; Ji, J.-Y. Pathogenicity of aseptic Bursaphelenchus xylophilus. PLoS ONE 2012, 7, e38095. [Google Scholar] [CrossRef]
- Hooper, D.J. Extraction of nematodes from plant material. In Laboratory Methods for Work with Plant and Soil Nematodes; Reference Book No. 402; Southey, J.F., Ed.; Ministry of Agriculture, Fisheries and Food, Her Majesty’s Stationery Office: London, UK, 1986; pp. 51–58. [Google Scholar]
- He, J.; Zheng, Z.P.; Zhu, Q.; Guo, F.; Chen, J. Encapsulation mechanism of oxyresveratrol by β-cyclodextrin and hydroxypropyl-β-cyclodextrin and computational analysis. Molecules 2017, 22, 1801. [Google Scholar] [CrossRef]
- Yamada, T.; Ito, S.I. Chemical defense responses of wilt resistant pine species, Pinus strobus and P. taeda, against Bursaphelenchus xylophilus infection. Nippon Shokubutsu Byori Gakkaiho 1993, 59, 666–672. [Google Scholar] [CrossRef]
- Mori, Y.; Miyahara, F.; Tsutsumi, Y.; Kondo, R. Relationship between resistance to pine wilt disease and the migration or proliferation of pine wood nematodes. Eur. J. Plant Pathol. 2008, 122, 529–538. [Google Scholar] [CrossRef]
- Phillips, M.A.; Croteau, R.B. Resin-based defenses in conifers. Trends Plant Sci. 1999, 4, 184–190. [Google Scholar] [CrossRef]
- Harju, A.M.; Venäläinen, M.; Laakso, T.; Saranpää, P. Wounding response in xylem of Scots pine seedlings shows wide genetic variation and connection with the constitutive defence of heartwood. Tree Physiol. 2009, 29, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Schoeppner, A.; Kindl, H. Stilbene synthase (pinosylvine synthase) and its induction by ultraviolet light. FEBS Lett. 1979, 108, 349–352. [Google Scholar] [CrossRef]
- Gehlert, R.; Schöppner, A.; Kindl, H. Stilbene synthase from seedlings of Pinus sylvestris: Purification and induction in response to fungal infection. Mol. Plant Microbe. Interact. 1990, 3, 444–449. [Google Scholar] [CrossRef]
- Rosemann, D.; Heller, W.; Sandermann, H. Biochemical plant responses to ozone: II. Induction of stilbene biosynthesis in Scots pine (Pinus sylvestris L.) seedlings. Plant Physiol. 1991, 97, 1280–1286. [Google Scholar] [CrossRef]
- Rowe, J.W.; Bower, C.L.; Wagner, E.R. Extractives of jack pine bark: Occurrence of cis- and trans-pinosylvin dimethyl ether and ferulic acid esters. Phytochemistry 1969, 8, 235–241. [Google Scholar] [CrossRef]
- Samoylenko, V.; Dunbar, D.C.; Gafur, M.A.; Khan, S.I.; Ross, S.A.; Mossa, J.S.; El-Feraly, F.S.; Tekwani, B.L.; Bosselaers, J.; Muhammad, I. Antiparasitic, nematicidal and antifouling constituents from Juniperus berries. Phytother. Res. 2008, 22, 1570–1576. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).