Tropical Cyclone Disturbances Induce Contrasting Impacts on Forest Structure, Plant Composition, and Soil Properties in Temperate Broadleaf and Coniferous Forests
Abstract
:1. Introduction
2. Materials and Methods
2.1. Site Description
2.2. Vegetation and Soil Sampling
2.3. Statistical Analyses
3. Results
3.1. Changes in Vegetation Composition and Forest Structure
3.2. Changes in Soil and Shifts in Environmental Control of Vegetation Attributes
4. Discussion
4.1. Vegetation Changes after Typhoons
4.2. Edaphic Conditions and Vegetation Responses
4.3. Future Projection of Subalpine Coniferous Forests on Mt. Halla
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Coumou, D.; Rahmstorf, S. A decade of weather extremes. Nat. Clim. Chang. 2012, 2, 491–496. [Google Scholar] [CrossRef]
- Trenberth, K.E.; Fasullo, J.T.; Balmaseda, M.A. Earth’s energy imbalance. J. Climatol. 2014, 27, 3129–3144. [Google Scholar] [CrossRef] [Green Version]
- Mei, W.; Xie, S.P. Intensification of landfalling typhoons over the northwest Pacific since the late 1970s. Nat. Geosci. 2016, 9, 753–757. [Google Scholar] [CrossRef]
- Altman, J.; Ukhvatkina, O.N.; Omelko, A.M.; Macek, M.; Plener, T.; Pejcha, V.; Cerny, T.; Petrik, P.; Srutek, M.; Song, J.S.; et al. Poleward migration of the destructive effects of tropical cyclones during the 20th century. Proc. Natl. Acad. Sci. USA 2018, 115, 11543–11548. [Google Scholar] [CrossRef] [Green Version]
- Lugo, A.E. Visible and invisible effects of hurricanes on forest ecosystems: An international review. Austral Ecol. 2008, 33, 368–398. [Google Scholar] [CrossRef]
- Liu, B.; Pan, L.; Xue, L. A review of the effect of typhoon on forests. Acta Ecol. Sin. 2012, 32, 1596–1605. [Google Scholar] [CrossRef]
- Kosugi, R.; Shibuya, M.; Ishibashi, S. Sixty-year post-windthrow study of stand dynamics in two natural forests differing in pre-disturbance composition. Ecosphere 2016, 7, e01571. [Google Scholar] [CrossRef] [Green Version]
- Zong, S.; He, H.; Liu, K.; Du, H.; Wu, Z.; Zhao, Y.; Jin, H. Typhoon diverged forest succession from natural trajectory in the treeline ecotone of the Changbai Mountains, Northeast China. For. Ecol. Manag. 2018, 407, 75–83. [Google Scholar] [CrossRef]
- Peterson, C.J.; Pickett, S.T. Treefall and resprouting following catastrophic windthrow in an old-growth hemlock hardwoods forest. For. Ecol. Manag. 1991, 42, 205–217. [Google Scholar] [CrossRef]
- Altman, J.; Fibich, P.; Leps, J.; Uemura, S.; Hara, T.; Dolezal, J. Linking spatiotemporal disturbance history with tree regeneration and diversity in an old-growth forest in northern Japan. Perspect. Plant Ecol. Evol. Syst. 2016, 21, 1–13. [Google Scholar] [CrossRef]
- Šamonil, P.; Daněk, P.; Schaetzl, R.J.; Tejnecký, V.; Drábek, O. Converse pathways of soil evolution caused by tree uprooting: A synthesis from three regions with varying soil formation processes. Catena 2018, 161, 122–136. [Google Scholar] [CrossRef]
- Walker, L.R. Seedling and sapling dynamics in treefall pits in Puerto Rico. Biotropica 2000, 32, 262–275. [Google Scholar] [CrossRef]
- Arévalo, J.R.; DeCoster, J.K.; McAlister, S.D.; Palmer, M.W. Changes in two Minnesota forests during 14 years following catastrophic windthrow. J. Veg. Sci. 2000, 11, 833–840. [Google Scholar] [CrossRef]
- Dodet, M.; Collet, C.; Frochot, H.; Wehrlen, L. Tree regeneration and plant species diversity responses to vegetation control following a major windthrow in mixed broadleaved stands. Eur. J. For. Res. 2011, 130, 41–53. [Google Scholar] [CrossRef]
- Curran, T.J.; Brown, R.L.; Edwards, E.; Hopkins, K.; Kelley, C.; McCarthy, E.; Pounds, E.; Solan, R.; Wolf, J. Plant functional traits explain interspecific differences in immediate cyclone damage to trees of an endangered rainforest community in north Queensland. Austral Ecol. 2008, 33, 451–461. [Google Scholar] [CrossRef]
- Zimmerman, J.K.; Willig, M.R.; Walker, L.R.; Silver, W.L. Introduction: Disturbance and Caribbean ecosystems. Biotropica 1996, 28, 414–423. [Google Scholar] [CrossRef]
- Boose, E.R.; Serrano, M.I.; Foster, D.R. Landscape and regional impacts of hurricanes in Puerto Rico. Ecol. Monogr. 2004, 74, 335–352. [Google Scholar] [CrossRef]
- Shiels, A.B.; González, G. Understanding the key mechanisms of tropical forest responses to canopy loss and biomass deposition from experimental hurricane effects. For. Ecol. Manag. 2014, 332, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Shiels, A.B.; González, G.; Willig, M.R. Responses to canopy loss and debris deposition in a tropical forest ecosystem: Synthesis from an experimental manipulation simulating effects of hurricane disturbance. For. Ecol. Manag. 2014, 332, 124–133. [Google Scholar] [CrossRef] [Green Version]
- Shiels, A.B.; González, G.; Lodge, J.; Willig, M.R.; Zimmerman, J.K. Cascading effects of canopy opening and debris deposition from a large-scale hurricane experiment in a tropical rain forest. BioScience 2015, 65, 871–881. [Google Scholar] [CrossRef] [Green Version]
- Gavito, M.E.; Sandoval-Pérez, A.L.; del Castillo, K.; Cohen-Salgado, D.; Colarte-Avilés, M.E.; Mora, F.; Santibáñez-Rentería, A.; Siddique, I.; Urquio-Ramos, C. Resilience of soil nutrient availability and organic matter decomposition to hurricane impact in a tropical dry forest ecosystem. For. Ecol. Manag. 2018, 426, 81–90. [Google Scholar] [CrossRef]
- Jaramillo, V.J.; Martínez-Yrízar, A.; Maass, M.; Nava-Mendoza, M.; Castañeda-Gómez, L.; Ahedo-Hernández, R.; Araiza, S.; Verduzco, A. Hurricane impact on biogeochemical processes in a tropical dry forest in western Mexico. For. Ecol. Manag. 2018, 426, 72–80. [Google Scholar] [CrossRef]
- Alexander, J.; Fielding, C.R.; Wakefield, S.J.; George, M.T.; Cottnam, C.F. Fluvial geochemistry through a short-duration, tropical-cyclone induced discharge event in the Burdekin River and Hann Creek, North Queensland, Australia. Aquat. Geochem. 2001, 7, 275–293. [Google Scholar] [CrossRef]
- McDowell, W.H.; Liptzin, D.A. Linking soils and streams: Response of soil solution chemistry to simulated hurricane disturbance mirrors stream chemistry following a severe hurricane. For. Ecol. Manag. 2014, 332, 56–63. [Google Scholar] [CrossRef]
- Meléndez-Ackerman, E.; Calisto-Pérez, C.; Morales-Vargas, M.; Fumero-Cabán, J. Post-hurricane recovery of a herbaceous understorey plant in a tropical rain forest in Puerto-Rico. J. Trop. Ecol. 2003, 19, 677–684. [Google Scholar] [CrossRef]
- Peterson, C.J.; Pickett, S.T. Forest reorganization: A case study in an old-growth forest catastrophic blowdown. Ecology 1995, 76, 763–774. [Google Scholar] [CrossRef]
- Vandermeer, J.; Mallona, M.A.; Boucher, D.; Yih, K.; Perfecto, I. Three years of ingrowth following catastrophic hurricane damage on the Carribean coast of Nicaragua: Evidence in support of the direct regeneration hypothesis. J. Trop. Ecol. 1995, 11, 465–471. [Google Scholar] [CrossRef]
- Xi, W. Synergistic effects of tropical cyclones on forest ecosystems: A global synthesis. J. For. Res. 2015, 26, 1–21. [Google Scholar] [CrossRef]
- Kim, E.S.; Oh, C.H.; Park, H.C.; Lee, S.H.; Choi, J.; Lee, S.H.; Cho, H.B.; Lim, W.; Kim, H.; Yoon, Y.K. Disturbed regeneration of saplings of Korean fir (Abies koreana Wilson), an endemic tree species, in Hallasan National park, a UNESCO Biosphere Reserve, Jeju Island, Korea. J. Mar. Isl. Cult. 2016, 5, 68–78. [Google Scholar] [CrossRef] [Green Version]
- Boucher, D.H.; Vandermeer, J.H.; Yih, K.; Zamora, N. Contrasting hurricane damage in tropical rain forest and pine forest. Ecology 1990, 71, 2022–2024. [Google Scholar] [CrossRef]
- Gannon, B.M.; Martin, P.H. Reconstructing hurricane disturbance in a tropical montane forest landscape in the Cordillera Central, Dominican Republic: Implications for vegetation patterns and dynamics. Arct. Antarct. Alp. Res. 2014, 46, 767–776. [Google Scholar] [CrossRef] [Green Version]
- Xi, W.; Peet, R.K.; Urban, D.L. Changes in forest structure, species diversity and spatial pattern following hurricane disturbance in a Piedmont North Carolina forest, USA. J. Plant Ecol. 2008, 1, 43–57. [Google Scholar] [CrossRef]
- Romme, W.H.; Everham, E.H.; Frelich, L.E.; Moritz, M.A.; Sparks, R.E. Are large, infrequent disturbances qualitatively different from small, frequent disturbances? Ecosystems 1998, 1, 524–534. [Google Scholar] [CrossRef]
- Turner, M.G.; Baker, W.L.; Peterson, C.J.; Peet, R.K. Factors influencing succession: Lessons from large, infrequent natural disturbances. Ecosystems 1998, 1, 511–523. [Google Scholar] [CrossRef]
- Holeksa, J.; Jaloviar, P.; Kucbel, S.; Saniga, M.; Svoboda, M.; Szewczyk, J.; Szwagrzyk, J.; Zielonka, T.; Żiwiec, M. Models of disturbance driven dynamics in the West Carpathian spruce forests. For. Ecol. Manag. 2017, 388, 79–89. [Google Scholar] [CrossRef]
- Puhe, J.; Ulrich, B. Global Climate Change and Human Impacts on Forest Ecosystems; Springer: Berlin, Germany, 2001. [Google Scholar]
- Bellingham, P.J.; Kohyama, T.; Aiba, S.I. The effects of a typhoon on Japanese warm temperate rainforests. Ecol. Res. 1996, 11, 229–247. [Google Scholar] [CrossRef]
- Harcombe, P.A.; Leipzig, L.E.M.; Elsik, I.S. Effects of hurricane Rita on three long-term forest study plots in East Texas, USA. Wetlands 2009, 29, 88–100. [Google Scholar] [CrossRef]
- Altman, J. Tree-ring-based disturbance reconstruction in interdisciplinary research: Current state and future directions. Dendrochronologia 2020, 63, 125733. [Google Scholar] [CrossRef]
- Collins-Key, S.A.; Altman, J. Detecting tropical cyclones from climate-and oscillation-free tree-ring width chronology of longleaf pine in south-central Georgia. Glob. Planet. Chang. 2021, 201, 103490. [Google Scholar] [CrossRef]
- Fischer, A.; Marshall, P.; Camp, A. Disturbances in deciduous temperate forest ecosystems of the northern hemisphere: Their effects on both recent and future forest development. Biodivers. Conserv. 2013, 22, 1863–1893. [Google Scholar] [CrossRef]
- Ibanez, T.; Keppel, G.; Menkes, C.; Gillespie, T.W.; Lengaigne, M.; Mangeas, M.; Rivas-Torres, G.; Birnbaum, P. Globally consistent impact of tropical cyclones on the structure of tropical and subtropical forests. J. Ecol. 2019, 107, 279–292. [Google Scholar] [CrossRef]
- Lin, T.C.; Hamburg, S.P.; Lin, K.C.; Wang, L.J.; Chang, C.T.; Hsia, Y.J.; Vadeboncoeur, M.A.; Mabry McMullen, C.M.; Liu, C.P. Typhoon Disturbance and Forest Dynamics: Lessons from a Northwest Pacific Subtropical Forest. Ecosystems 2011, 14, 127–143. [Google Scholar] [CrossRef]
- Ishikawa, Y.; Krestov, P.V.; Namikawa, K. Disturbance history and tree establishment in old-growth Pinus koraiensis-hardwood forests in the Russian Far East. J. Veg. Sci. 1999, 10, 439–448. [Google Scholar] [CrossRef]
- Lin, T.C.; Hogan, J.A.; Chang, C.T. Tropical cyclone ecology: A scale-link perspective. Trends Ecol. Evol. 2020, 35, 594–604. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Altman, J.; Saurer, M.; Dolezal, J.; Maredova, N.; Song, J.S.; Ho, C.H.; Treydte, K. Large volcanic eruptions reduce landfalling tropical cyclone activity: Evidence from tree rings. Sci. Total Environ. 2021, 775, 145899. [Google Scholar] [CrossRef]
- IPCC. Climate Change 2021: The Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Masson-Delmotte, V., Zhai, P., Pirani, A., Connors, S.L., Péan, C., Berger, S., Caud, N., Chen, Y., Goldfarb, L., Gomis, M.I., et al., Eds.; Cambridge University Press: Cambridge, UK, 2021. [Google Scholar]
- Janda, P.; Ukhvatkina, O.N.; Vozmishcheva, A.S.; Omelko, A.M.; Doležal, J.; Krestov, P.V.; Zhmerenetzky, A.A.; Song, J.S.; Altman, J. Tree canopy accession strategy changes along the latitudinal gradient of temperate Northeast Asia. Glob. Ecol. Biogeogr. 2021, 30, 738–748. [Google Scholar] [CrossRef]
- Park, D.S.R.; Ho, C.H.; Kim, J.H.; Kim, H.S. Strong landfall typhoons in Korea and Japan in the recent decade. J. Geophys. Res. Atmos. 2011, 116, D07105. [Google Scholar] [CrossRef] [Green Version]
- Guan, S.; Li, S.; Hou, Y.; Hu, P.; Liu, Z.; Feng, J. The increasing threat of landfalling typhoons in the western North Pacific between 1974 and 2013. Int. J. Appl. Earth Obs. Geoinf. 2018, 68, 279–286. [Google Scholar] [CrossRef]
- Černý, T.; Kopecký, M.; Petřík, P.; Song, J.S.; Šrůtek, M.; Valachovič, M.; Altman, J.; Doležal, J. Classification of Korean forests: Patterns along geographic and environmental gradients. Appl. Veg. Sci. 2015, 18, 5–22. [Google Scholar] [CrossRef]
- Imbert, D.; Portecop, J. Hurricane disturbance and forest resilience: Assessing structural vs. functional changes in a Caribbean dry forest. For. Ecol. Manag. 2008, 255, 3494–3501. [Google Scholar] [CrossRef]
- Chi, C.H.; McEwan, R.W.; Chang, C.T.; Zheng, C.; Yang, Z.; Chiang, J.M.; Lin, T.C. Typhoon disturbance mediates elevational patterns of forest structure, but not species diversity, in humid monsoon Asia. Ecosystems 2015, 18, 1410–1423. [Google Scholar] [CrossRef]
- Dolezal, J.; Altman, J.; Kopecky, M.; Cerny, T.; Janecek, S.; Bartos, M.; Petrik, P.; Srutek, M.; Leps, J.; Song, J.S. Plant diversity changes during the postglacial in East Asia: Insights from forest refugia on Halla Volcano, Jeju Island. PLoS ONE 2012, 7, e33065. [Google Scholar] [CrossRef]
- Woo, K.S.; Sohn, Y.K.; Ahn, U.S.; Yoon, S.H. (Eds.) Jeju Island Geopark—A Volcanic Wonder of Korea; Springer: Berlin, Germany, 2013. [Google Scholar]
- Hagedorn, B.; Mair, A.; Tillery, S.; El-Kadi, A.I.; Ha, K.; Koh, G.W. Simple equations for temperature simulations on mid-latitude volcanic islands: A case study from Jeju (Republic of Korea). Geosci. J. 2014, 18, 381–396. [Google Scholar] [CrossRef]
- Anonymous. Data Book of Mt. Halla; Research Institute for Mt. Halla: Jejusi, Korea, 2007. [Google Scholar]
- Černý, T.; Doležal, J.; Janeček, Š.; Šrůtek, M.; Valachovič, M.; Petřík, P.; Altman, J.; Bartoš, M.; Song, J.S. Environmental correlates of plant diversity in Korean temperate forests. Acta Oecologica 2013, 47, 37–45. [Google Scholar] [CrossRef]
- Yim, Y.J.; Kim, J.U.; Lee, N.J.; Kim, Y.B.; Paek, K.S. Phytosociological classification of plant communities on Mt. Halla National Park, Korea. Korean J. Ecol. 1990, 13, 101–130. [Google Scholar]
- Park, C.W. (Ed.) The Genera of Vascular Plants of Korea; Academy Publishing Company: Seoul, Korea, 2007. [Google Scholar]
- Song, J.S. Phytosociology of subalpine coniferous forests in Korea. I. Syntaxonomical interpretation. Ecol. Res. 1991, 6, 1–19. [Google Scholar] [CrossRef]
- Kang, S.J.; Kwak, A.K.; Kikuchi, T. A phytosociological description of the Abies koreana forest on Mt. Halla in Cheju Island, Korea. Korean J. Ecol. 1997, 20, 293–298. [Google Scholar]
- Chae, J.W.; Jeong, W.M.; Jun, K.C.; Choi, J.Y.; Park, W.S.; Park, W.K. Extreme waves generated by typhoon Bolaven (201215) in southern Korean waters. In Proceedings of the 7th International Conference on Asian and Pacific Coasts, Bali, Indonesia, 24 September 2013; pp. 996–1001. [Google Scholar]
- Yuk, J.H.; Park, J.; Joh, M. Modelling of storm-induced seawater flooding in the Suyeong River area, South Korea: A case study due to the storm surge and waves during Typhoon Sanba. J. Coast. Res. 2018, 85, 746–750. [Google Scholar] [CrossRef]
- Won, C.K. Study of Geologic Development and the Volcanic Activity of the Jeju Island. Diploma Thesis, Department of Geography, Kon-Kuk University, Seoul, Korea, 1975. (In Korean with English Summary). [Google Scholar]
- Westhoff, V.; van der Maarel, E. The Braun-Blanquet approach. In Classification of Plant Communities; Whittaker, R.H., Ed.; W. Junk: The Hague, The Netherlands, 1978; pp. 287–399. [Google Scholar]
- ter Braak, C.J.F.; Šmilauer, P. Canoco Reference Manual and User’s Guide: Software for Ordination (Version 5.0); Microcomputer Power: Ithaca, NY, USA, 2012. [Google Scholar]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing (Version 4.1.2); R Foundation for Statistical Computing: Vienna, Austria, 2021; Available online: https://www.R-project.org/ (accessed on 26 June 2022).
- Barry, R.G. Mountain Weather and Climate; Cambridge University Press: Cambridge, UK, 2008. [Google Scholar]
- Quine, C.P.; Gardiner, B.A. Understanding how the interaction of wind and trees result in windthrow, stem breakage, and canopy gap formation. In Plant Disturbance Ecology: The Process and the Response; Johnson, E.A., Miyanishi, K., Eds.; Elsevier: Amsterdam, The Netherlands, 2007; pp. 103–155. [Google Scholar]
- Metcalfe, D.J.; Bradford, M.G.; Ford, A.J. Cyclone damage to tropical rain forest: Species- and community-level impact. Austral Ecol. 2008, 33, 432–441. [Google Scholar] [CrossRef]
- Day, W.R. Soil conditions which determine windthrow in forests. Forestry 1950, 23, 90–95. [Google Scholar] [CrossRef]
- Seo, J.W.; Choi, E.B.; Park, J.H.; Kim, Y.J.; Lim, H.I. The role of aging and wind in inducing death and/or growth reduction in Korean Fir (Abies koreanaWilson) on Mt. Halla, Korea. Atmosphere 2021, 12, 1135. [Google Scholar] [CrossRef]
- Song, K.M.; Kang, Y.J.; Hyeon, H.J. Vegetation structure at the slope direction and 5characteristic of seedlings of Abies koreana in Hallasan Mountain. J. Environ. Sci. Int. 2014, 23, 39–46. [Google Scholar] [CrossRef] [Green Version]
- Doležal, J.; Matsuki, S.; Hara, T. Effects of dwarf-bamboo understory on tree seedling emergence and survival in a mixed-oak forest in northern Japan: A multi-site experimental study. Community Ecol. 2009, 10, 225–235. [Google Scholar] [CrossRef]
- Yoshida, T.; Noguchi, M. Vulnerability to strong winds for major tree species in a northern Japanese mixed forests: Analyses of historical data. Ecol. Res. 2009, 24, 909–919. [Google Scholar] [CrossRef]
- Zolbrod, A.N.; Peterson, D.L. Response of high-elevation forests in the Olympic mountains to climatic change. Can. J. For. Res. 1999, 29, 1966–1978. [Google Scholar] [CrossRef]
- Evangelista, A.; Frate, L.; Carranza, M.L.; Attorre, F.; Pelino, G.; Stanisci, A. Changes in composition, ecology and structure of high-mountain vegetation: A re-visitation study over 42 years. AoB Plants 2016, 8, plw004. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koo, K.A.; Park, W.K.; Kong, W.S. Dendrochronological analysis of Abies koreana W. at Mt. Halla, Korea: Effects of climate change on the growth. Korean J. Ecol. 2001, 24, 281–288. [Google Scholar]
- Koo, K.A.; Kong, W.S.; Park, S.U.; Lee, J.H.; Kim, J.; Jung, H. Sensitivity of Korean fir (Abies koreana Wils.), a threatened climate relict species, to increasing temperature at an island subalpine area. Ecol. Model. 2017, 353, 5–16. [Google Scholar] [CrossRef]
- Yun, J.H.; Nakao, K.; Tsuyama, I.; Matsui, T.; Park, C.H.; Lee, B.Y.; Tanaka, N. Vulnerability of subalpine fir species to climate change: Using species distribution modelling to assess the future efficiency of current protected areas in the Korean Peninsula. Ecol. Res. 2018, 33, 341–350. [Google Scholar] [CrossRef]
- Altman, J.; Treydte, K.; Pejcha, V.; Cerny, T.; Petrik, P.; Srutek, M.; Song, J.S.; Trouet, V.; Dolezal, J. Tree growth response to recent warming of two endemic species in Northeast Asia. Clim. Chang. 2020, 162, 1345–1364. [Google Scholar] [CrossRef]
- Jonášová, M.; Vávrová, E.; Cudlín, P. Western Carpathian mountain spruce forest after a windthrow: Natural regeneration in cleared and uncleared areas. For. Ecol. Manag. 2010, 259, 1127–1134. [Google Scholar] [CrossRef]
- Webb, S.L. Contrasting windstorm consequences in two forests, Itasca State Park, Minnesota. Ecology 1989, 70, 1167–1180. [Google Scholar] [CrossRef]
- Abe, M.; Izaki, J.; Miguchi, H.; Masaki, T.; Makita, A.; Nakashizuka, T. The effect of Sasa and canopy gap formation on tree regeneration in an old beech forest. J. Veg. Sci. 2002, 13, 565–574. [Google Scholar] [CrossRef]
- Cho, S.; Lee, K.; Choung, Y. Distribution, abundance, and effect on plant species diversity of Sasa borealis in Korean forests. J. Ecol. Environ. 2018, 42, 9. [Google Scholar] [CrossRef] [Green Version]
- Jeon, D.U.; Kim, D.H. Application of an augmented predator-prey model to the population dynamics of roe deer in Jeju. Korean Syst. Dyn. Rev. 2011, 12, 95–126, (In Korean with English Summary). [Google Scholar]
- Grisez, T.J. Slash helps protect seedlings from deer bowsing. J. For. 1960, 58, 385–387. [Google Scholar]
- Bowers, M.A. Influence of herbivorous mammals on an old-field plant community: Years 1–4 after disturbance. Oikos 1993, 67, 129–141. [Google Scholar] [CrossRef]
- Lim, C.H.; An, J.H.; Jung, S.H.; Lee, C.S. Allogenic succession of Korean fir (Abies koreana Wils.) forests in different climate conditions. Ecol. Res. 2018, 33, 327–340. [Google Scholar] [CrossRef]
- Royo, A.A.; Carson, W.P. On the formation of dense understory layers in forests worldwide: Consequences and implications for forest dynamics, biodiversity, and succession. Can. J. For. Res. 2006, 36, 1345–1362. [Google Scholar] [CrossRef]
- George, L.O.; Bazzaz, F. The fern understory as an ecological filter: Emergence and establishment of canopy-tree seedlings. Ecology 1999, 80, 833–845. [Google Scholar] [CrossRef]
- Altman, J.; Doležal, J.; Černý, T.; Song, J.S. Forest response to increasing typhoon activity on the Korean peninsula: Evidence from oak tree-rings. Glob. Chang. Biol. 2013, 19, 498–504. [Google Scholar] [CrossRef] [PubMed]
- Akaji, Y.; Fujiyoshi, K.; Wu, C.; Hattori, I.; Hirobe, M.; Sakamoto, K. Survival and recruitment of Sasa kurilensis culms in response to local light conditions in a cool temperate forest. J. For. Res. 2019, 24, 365–370. [Google Scholar] [CrossRef]
- Tomimatsu, H.; Yamagishi, H.; Tanaka, I.; Sato, M.; Kondo, R.; Konno, Y. Consequences of forest fragmentation in an understory plant community: Extensive range expansion of native dwarf bamboo. Plant Species Biol. 2011, 26, 3–12. [Google Scholar] [CrossRef]
- Kudo, G.; Amagai, Y.; Hoshino, B.; Kaneko, M. Invasion of dwarf bamboo into alpine snow-meadow in northern Japan: Pattern of expansion and impact on species diversity. Ecol. Evol. 2011, 1, 85–96. [Google Scholar] [CrossRef]
- Kim, H.C. The growth characteristics of Sasa quelpaertensis Nakai by an elevation in Mt. Halla. Res. Rep. Mt. Halla Res. Inst. Mt. Halla 2002, 2, 63–71, (In Korean with English Summary). [Google Scholar]
- Tsuyama, I.; Horikawa, M.; Nakao, K.; Matsui, T.; Kominami, Y.; Tanaka, N. Factors determining the distribution of a keystone understory taxon, dwarf bamboo of the section Crassinodi, on a national scale: Application to impact assessment of climate change in Japan. J. For. Res. 2012, 17, 137–148. [Google Scholar] [CrossRef]
- Suh, M.S.; Oh, S.G.; Lee, Y.S.; Ahn, J.B.; Cha, D.H.; Lee, D.K.; Hong, S.Y.; Min, S.K.; Park, S.C.; Kang, H.S. Projections of high resolution climate changes for South Korea using Multiple-Regional Climate Models based on four RCP scenarios. Part 2: Precipitation. Asia-Pac. J. Atmos. Sci. 2016, 52, 171–189. [Google Scholar] [CrossRef]
- Scatena, F.N.; Moya, S.; Estrada, C.; Chinea, J.D. The first five years in the reorganization of aboveground biomass and nutrient use following hurricane Hugo in the Bisley Experimental Watersheds, Luquillo Experimental Forest, Puerto Rico. Biotropica 1996, 28, 424–440. [Google Scholar] [CrossRef]
- Silver, W.L.; Scatena, F.N.; Johnson, A.H.; Siccama, T.G.; Watt, F. At what temoral scales does disturbance affect belowground nutrient pools? Biotropica 1996, 28, 441–457. [Google Scholar] [CrossRef]
- Swanson, F.J.; Clayton, J.L.; Megahan, W.F.; Bush, G. Erosional processes and long-term site productivity. In Maintaining the Long-Term Productivity of Pacific Northwest Forest Ecosystems; Perry, D.A., Meurisse, R., Thomas, B., Miller, R., Boyle, J., Means, J., Perry, C.R., Powers, R.F., Eds.; Timber Press: Portland, OR, USA, 1989; pp. 67–82. [Google Scholar]
- Blanco, H.; Lal, R. Principles of Soil Conservation and Management; Springer Science+Business Media B.V.: Heidelberg, Germany, 2008. [Google Scholar]
- Zimmerman, J.K.; Aide, T.M.; Herrera, L.J.; Rosario, M.A.; Serrano, M.I. Effects of land management and a recent hurricane on forest structure and composition in the Luquillo Experimental Forest, Puerto Rico. For. Ecol. Manag. 1995, 77, 65–76. [Google Scholar] [CrossRef]
- Dolezal, J.; Fibich, P.; Altman, J.; Leps, J.; Uemura, S.; Takahashi, K.; Hara, T. Determinants of ecosystem stability in a diverse temperate forest. Oikos 2020, 129, 1692–1703. [Google Scholar] [CrossRef]
- Past Typhoon. Typhoon Service of Korean Meteorological Administration. Available online: https://www.weather.go.kr/w/typhoon/typ-history.do (accessed on 15 April 2022).
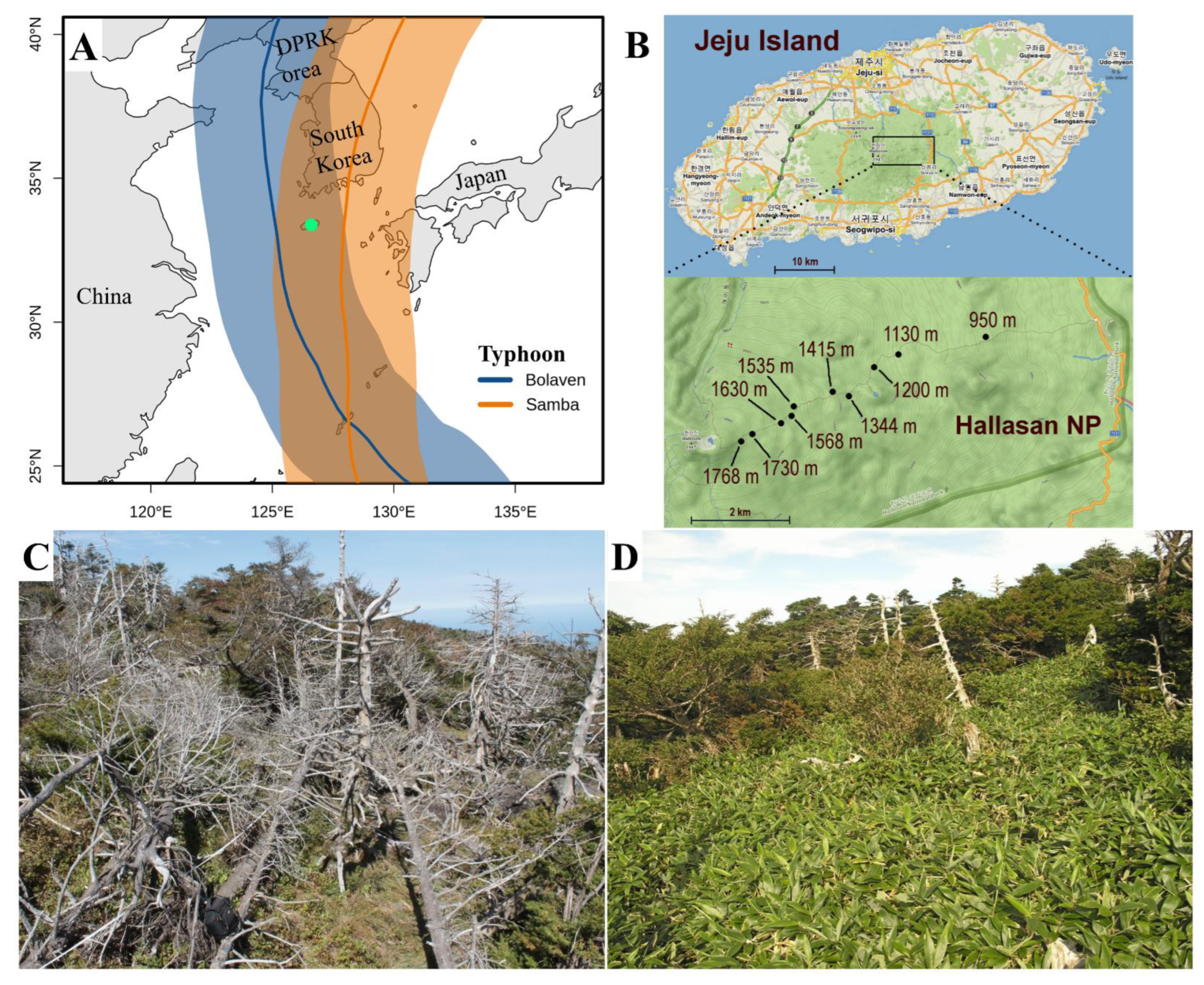
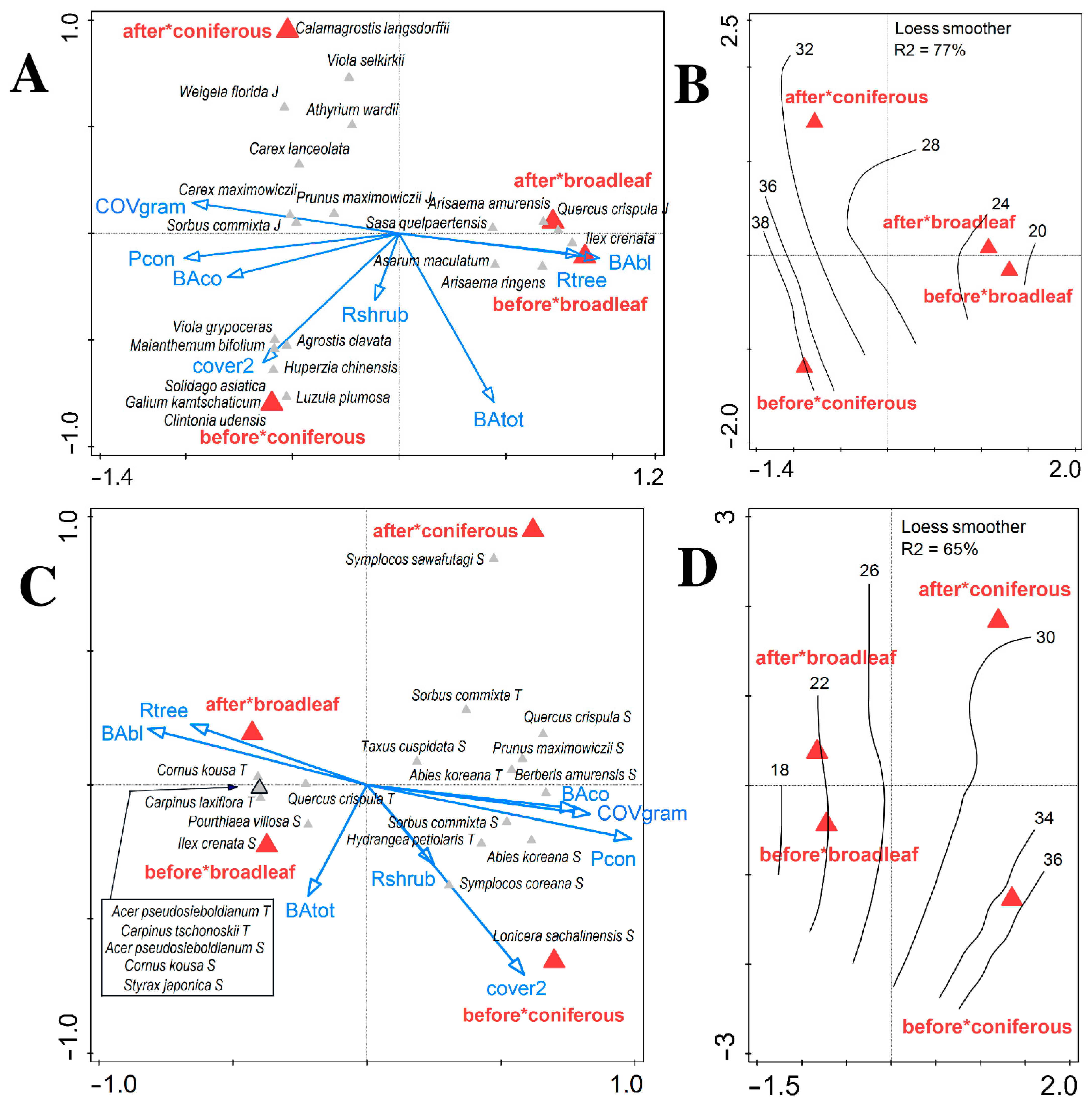
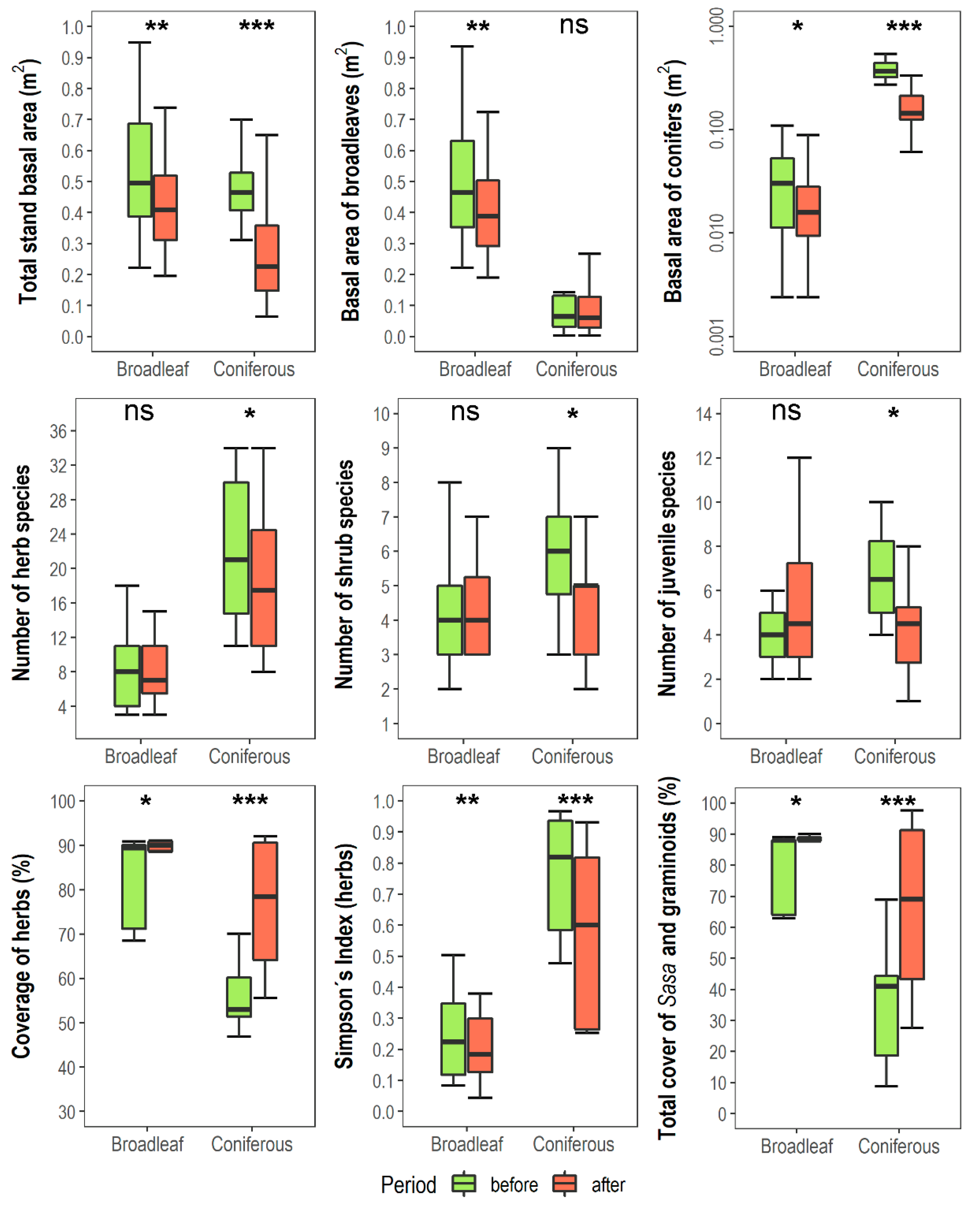
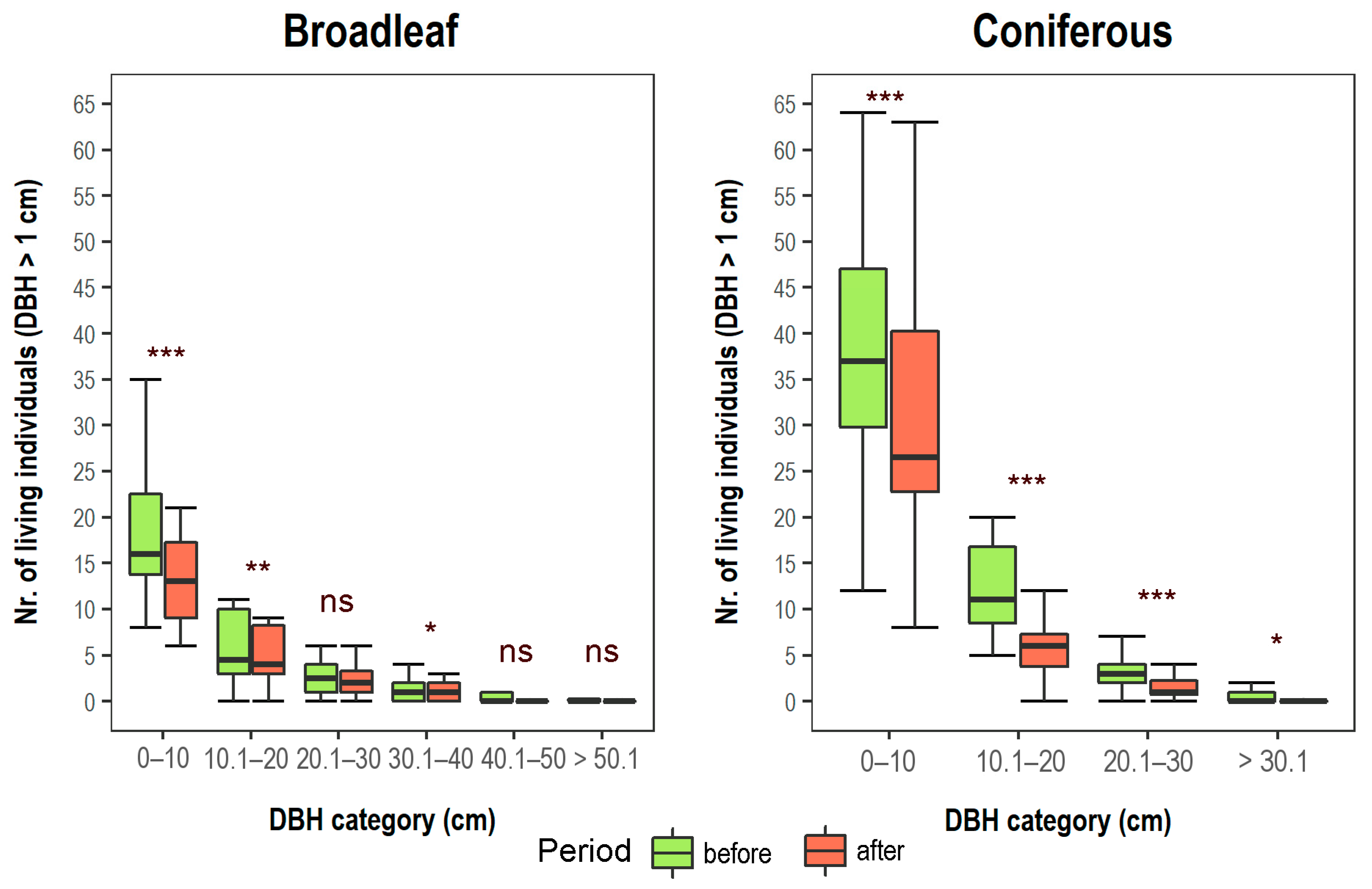

| Forest Type | Altitude (m a.s.l.) | Aspect (°) | Slope (°) |
|---|---|---|---|
| Broadleaf | 949–950 | 38 | 12 |
| 1130 | 160–165 | 4 | |
| 1199–1200 | 130 | 6–10 | |
| 1344–1345 | 80 | 12–15 | |
| 1413–1415 | 75–80 | 20–23 | |
| Coniferous | 1534–1535 | 85 | 7–8 |
| 1567–1569 | 58–60 | 8–15 | |
| 1629–1631 | 55 | 10 | |
| 1730–1731 | 100–110 | 10–12 | |
| 1766–1770 | 110–120 | 23–25 |
| Deciduous | Coniferous | Repeated-Measures LMM | ||||||
|---|---|---|---|---|---|---|---|---|
| Abbreviation | Before | After | Before | After | FT | TD | FT × TD | |
| Diversity and abundance variables | ||||||||
| Number of tree species | Rtree | 4.9 | 4.95 | 2 | 2 | 34.1 *** | ns | ns |
| Number of shrub species | Rshrub | 4.3 | 4.35 | 5.69 | 4.37 | ns | 4.1 * | 4.8 * |
| Number of herbaceous species | Rherb | 8.05 | 8.25 | 22.3 | 18.8 | 39.4 *** | 6.8 * | 8.5 ** |
| Number of species of woody juveniles | Rjuv | 4.3 | 5.1 | 6.8 | 4.18 | ns | ns | 10.4 * |
| Simpson index for herbaceous species | 1/Dherb | 0.24 | 0.2 | 0.76 | 0.57 | 60.1 *** | 55.7 *** | 21.4 *** |
| Total cover of graminoids a (%) | COVgram | 0.25 | 0.85 | 17 | 25 | 18.1 *** | 9.6 ** | 6.9 * |
| Total cover of Sasa quelp. (%) | COVSasa | 79.3 | 84.9 | 18.1 | 40.9 | 41.9 *** | 20.7 *** | 6.1 * |
| Canopy structural variables | ||||||||
| Total cover of the tree layer (%) | cover3 | 82.45 | 74.4 | 34.25 | 27.5 | 41.9 *** | 8.2 ** | ns |
| Total cover of the shrub layer (%) | cover2 | 17.5 | 16.3 | 54.3 | 23.6 | 24.1 *** | 29.1 *** | 25.6 *** |
| Total stand BA b (m2·ha−1) | BAtot | 53 | 43 | 48 | 26 | 5.0 * | 62.1 *** | 8.1 ** |
| BA of broadleaved species (m2·ha−1) | BAbl | 5 | 41 | 12 | 9 | 61.4 *** | 9.6 ** | 7.7 ** |
| BA of conifers (m2·ha−1) | BAco | 3 | 2 | 37 | 16 | 236.1 *** | 66.8 ** | 55.2 ** |
| BA of Abies koreana (m2·ha−1) | BAfir | 1 | 0 | 32 | 11 | 137.5 *** | 63.6 *** | 56.2 *** |
| Fraction of BA of conifers (%) | Pcon | 5.8 | 4.5 | 80.6 | 66.7 | 243.2 *** | 25.4 *** | 14.1 *** |
| Soil variables | ||||||||
| pH (H2O) | pHact | 4.4 | 4.3 | 4.5 | 4.3 | ns | 6.6 * | ns |
| Potential soil acidity c | ΔpH | 0.77 | 0.67 | 0.78 | 0.66 | ns | 8.1 ** | ns |
| Total carbon (% of dry weight) | C | 20.7 | 16.6 | 23.7 | 20.5 | 10.5 ** | 27.8 *** | ns |
| Total nitrogen (% of dry weight) | N | 1.38 | 1.15 | 1.53 | 1.41 | 12.9 *** | 13.6 *** | ns |
| Carbon/nitrogen ratio | C/N | 14.9 | 14.5 | 15.4 | 14.4 | ns | 13.9 *** | ns |
| Available P (ppm d) | P | 11.9 | 6.5 | 5.8 | 10.7 | ns | ns | 9.6 ** |
| Mg ions (ppm) | Mg | 217.6 | 126.6 | 137.1 | 129.7 | 4.9 * | 27.7 *** | 18.7 *** |
| Ca ions (ppm) | Ca | 835.3 | 160.1 | 410.1 | 228.8 | 3.8 * | 35.4 *** | 11.8 ** |
| CCA Analyses | Exp. Variables | Covariables | Permutation | Exp. Variation | F All | P All |
|---|---|---|---|---|---|---|
| Herbs and woody juveniles in the herb layer | ||||||
| CCA1a | TD × FT | - | Freely, freely | 18.5 | 6.4 | 0.001 |
| pCCA2a | FT | TD | Freely, no | 12.9 | 11.3 | 0.001 |
| pCCA3a | TD | FT | No, freely | 4.0 | 3.9 | 0.04 |
| pCCA4a | TD × FT | TD, PI | Freely, TS | 6.3 | 3.3 | 0.001 |
| Woody species in the shrub and the tree layers | ||||||
| CCA1b | TD × FT | - | Freely, freely | 16.7 | 5.8 | 0.001 |
| pCCA2b | FT | TD | Freely, no | 16.3 | 14.7 | 0.001 |
| pCCA3b | TD | FT | No, freely | 0.6 | 1.4 | 0.75 |
| pCCA4b | TD × FT | TD, PI | Freely, TS | 6.7 | 3.5 | 0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Černý, T.; Doležal, J.; Petřík, P.; Šrůtek, M.; Song, J.-S.; Altman, J. Tropical Cyclone Disturbances Induce Contrasting Impacts on Forest Structure, Plant Composition, and Soil Properties in Temperate Broadleaf and Coniferous Forests. Forests 2022, 13, 1033. https://doi.org/10.3390/f13071033
Černý T, Doležal J, Petřík P, Šrůtek M, Song J-S, Altman J. Tropical Cyclone Disturbances Induce Contrasting Impacts on Forest Structure, Plant Composition, and Soil Properties in Temperate Broadleaf and Coniferous Forests. Forests. 2022; 13(7):1033. https://doi.org/10.3390/f13071033
Chicago/Turabian StyleČerný, Tomáš, Jiří Doležal, Petr Petřík, Miroslav Šrůtek, Jong-Suk Song, and Jan Altman. 2022. "Tropical Cyclone Disturbances Induce Contrasting Impacts on Forest Structure, Plant Composition, and Soil Properties in Temperate Broadleaf and Coniferous Forests" Forests 13, no. 7: 1033. https://doi.org/10.3390/f13071033
APA StyleČerný, T., Doležal, J., Petřík, P., Šrůtek, M., Song, J.-S., & Altman, J. (2022). Tropical Cyclone Disturbances Induce Contrasting Impacts on Forest Structure, Plant Composition, and Soil Properties in Temperate Broadleaf and Coniferous Forests. Forests, 13(7), 1033. https://doi.org/10.3390/f13071033







