Response of Spruce Forest Ecosystem CO2 Fluxes to Inter-Annual Climate Anomalies in the Southern Taiga
Abstract
:1. Introduction
2. Materials and Methods
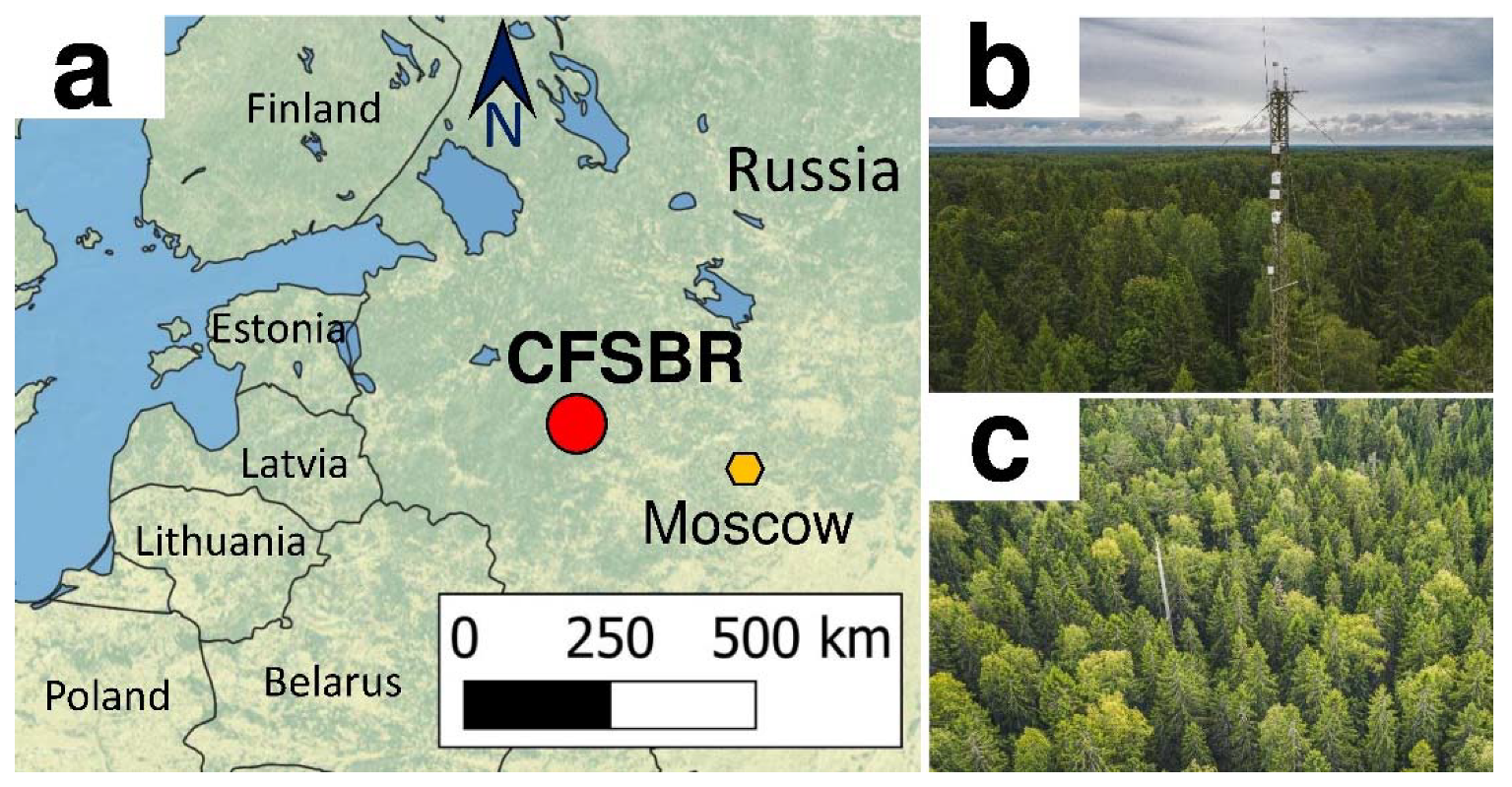
2.1. Study Site
2.2. Flux Measurements
2.3. Data Processing and Statistical Analysis
2.4. Estimation of the Dependence of TER and GPP to Environmental Variables
2.5. Additional Data
3. Results
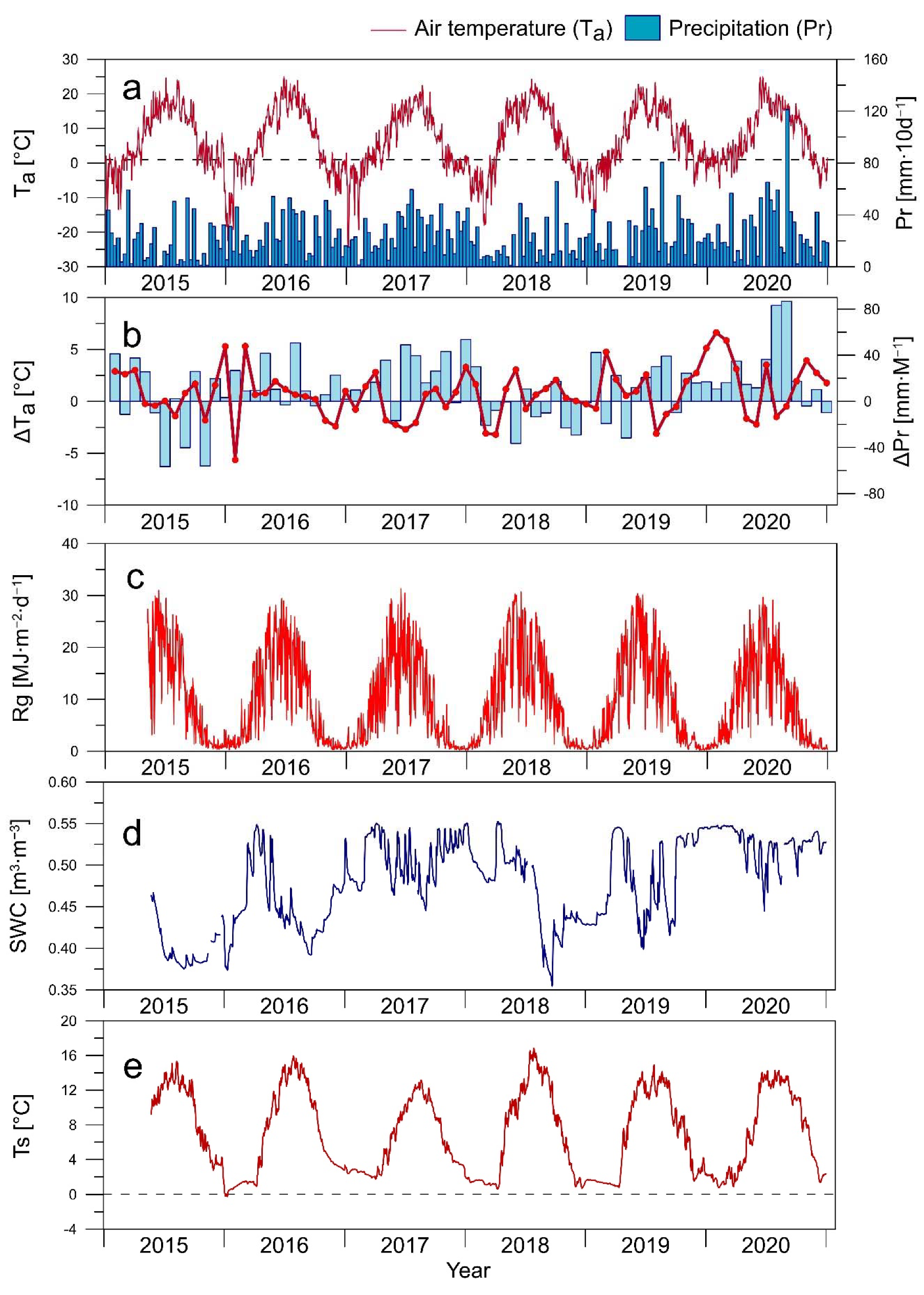
3.1. Environmental Conditions
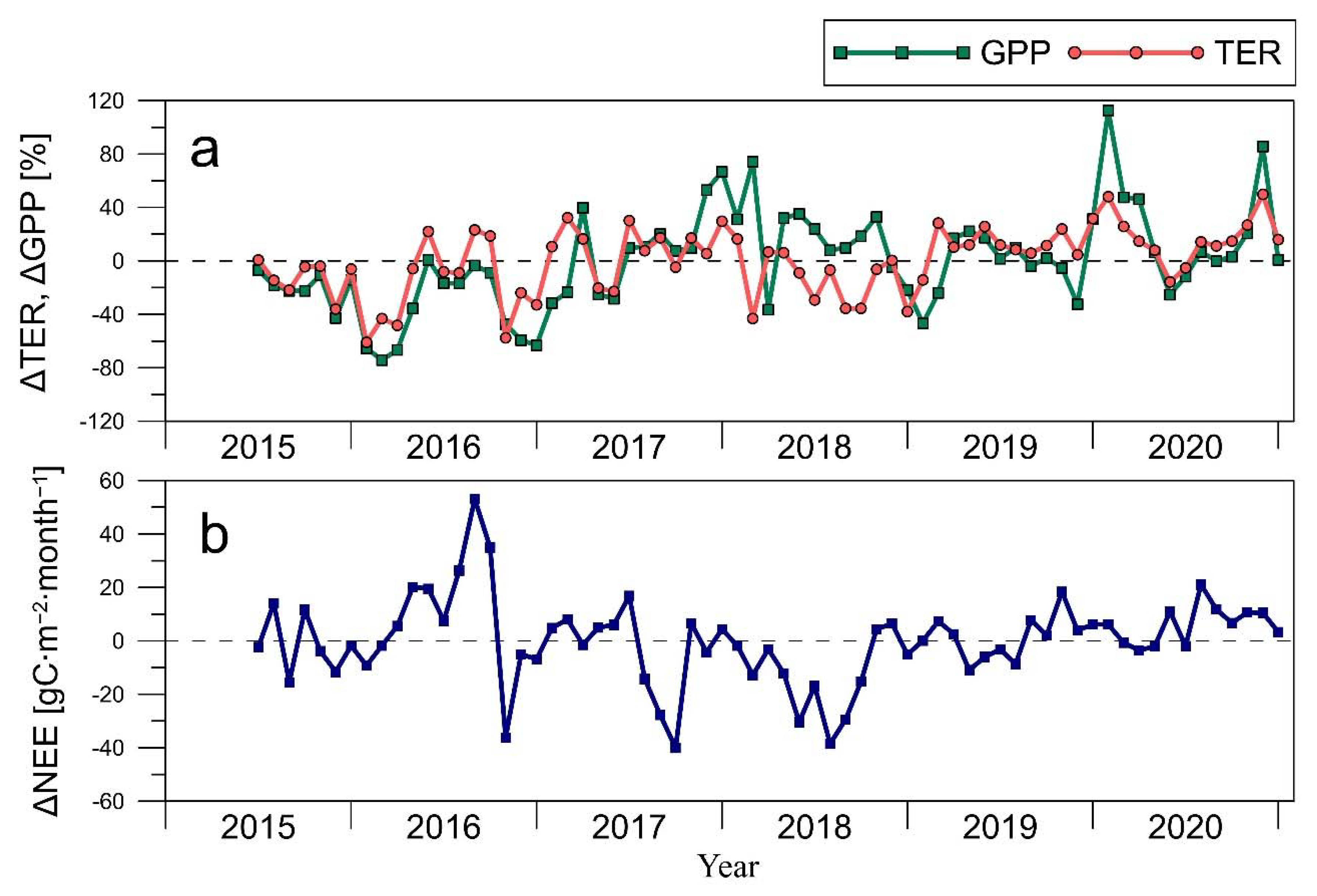
3.2. CO2 Fluxes
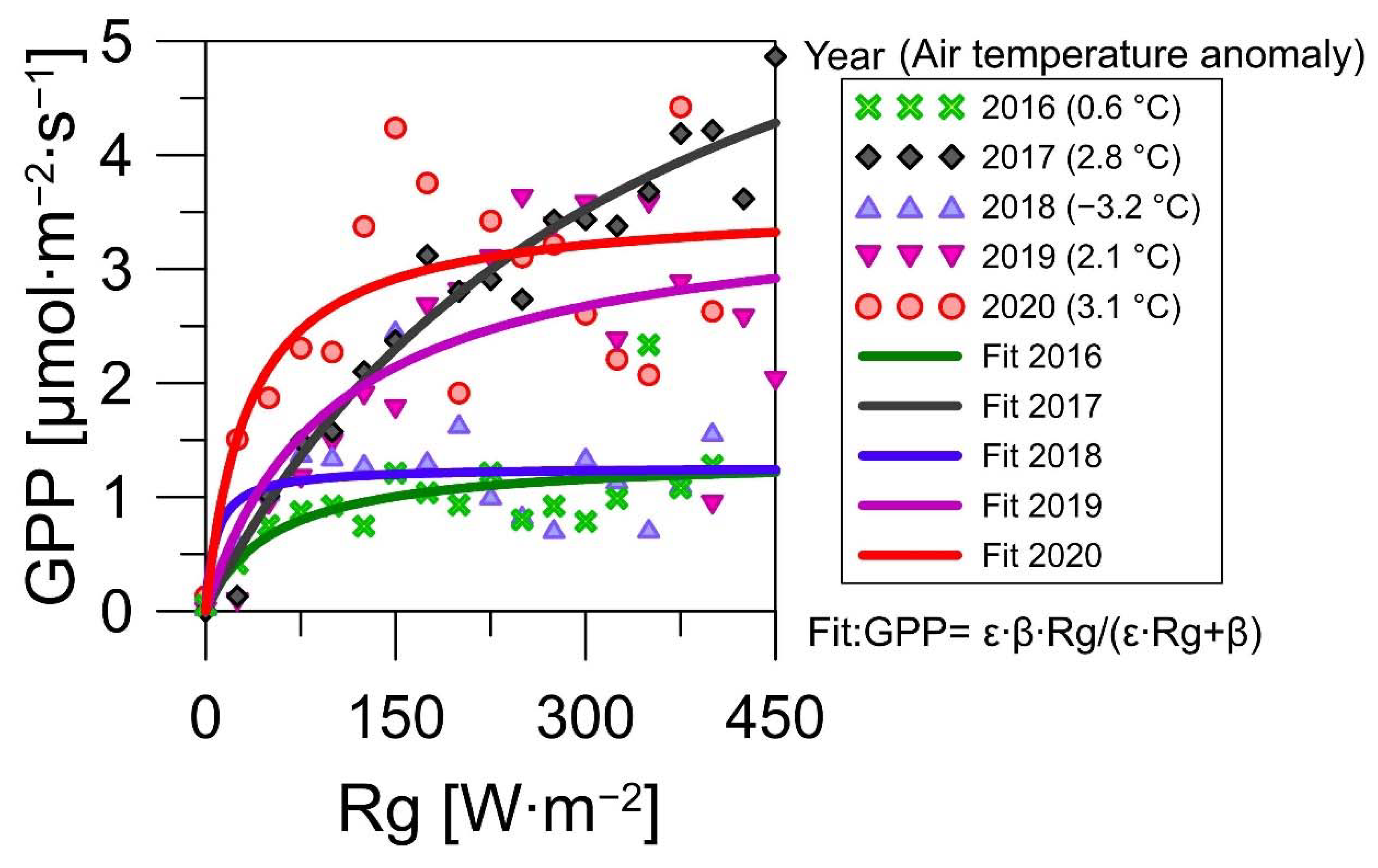
3.3. Environmental Factors of the CO2 Fluxes
4. Discussion
4.1. Ecosystem–Atmosphere CO2 Exchange in the Spruce Forests
4.2. Implications of the Heatwaves for the CO2 Exchange of the Spruce Forests
4.3. Implications of the Local Soil Moisture Regimes for the CO2 Exchange of Spruce Forests
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tagesson, T.; Schurgers, G.; Horion, S.; Ciais, P.; Tian, F.; Brandt, M.; Ahlström, A.; Wigneron, J.-P.; Ardö, J.; Olin, S.; et al. Recent divergence in the contributions of tropical and boreal forests to the terrestrial carbon sink. Nat. Ecol. Evol. 2020, 4, 202–209. [Google Scholar] [CrossRef] [PubMed]
- Chapin, F.S.; Randerson, J.T.; McGuire, A.D.; Foley, J.A.; Field, C.B. Changing feedbacks in the climate–biosphere system. Front. Ecol. Environ. 2008, 6, 313–320. [Google Scholar] [CrossRef]
- Bonan, G.B. Forests and climate change: Forcings, feedbacks, and the climate benefits of forests. Science 2008, 320, 1444–1449. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lal, R.; Smith, P.; Jungkunst, H.F.; Mitsch, W.J.; Lehmann, J.; Nair, P.R.; McBratney, A.B.; Carlos de Moraes Sá, J.; Schneider, J.; Zinn, Y.L.; et al. The carbon sequestration potential of terrestrial ecosystems. J. Soil Water Conserv. 2018, 73, 145A–152A. [Google Scholar] [CrossRef] [Green Version]
- Schewe, J.; Gosling, S.N.; Reyer, C.; Zhao, F.; Ciais, P.; Elliott, J.; Francois, L.; Huber, V.; Lotze, H.K.; Seneviratne, S.I.; et al. State-of-the-art global models underestimate impacts from climate extremes. Nat. Commun. 2019, 10, 1005. [Google Scholar] [CrossRef] [Green Version]
- IPCC. Climate Change 2021. The Physical Science Basis; Cambridge University Press: Cambridge, UK, 2021; in press. [Google Scholar]
- Woodwell, G.M.; Whittaker, R.H. Primary production in terrestrial ecosystems. Am. Zool. 1968, 8, 19–30. [Google Scholar] [CrossRef]
- Chapin, F.S.; Woodwell, G.M.; Randerson, J.T.; Lovett, G.M.; Rastetter, E.B.; Baldocchi, D.D.; Clark, D.A.; Harmon, M.E.; Schimel, D.S.; Valentini, R.; et al. Reconciling carbon-cycle concepts, terminology, and methods. Ecosystems 2006, 9, 1041–1050. [Google Scholar] [CrossRef] [Green Version]
- Coursolle, C.; Margolis, H.A.; Giasson, M.A.; Bernier, P.Y.; Amiro, B.D.; Arain, M.A.; Barr, A.G.; Black, T.A.; Goulden, M.L.; McCaughey, J.H.; et al. Influence of stand age on the magnitude and seasonality of carbon fluxes in Canadian forests. Agric. For. Meteorol. 2012, 165, 136–148. [Google Scholar] [CrossRef] [Green Version]
- Ueyama, M.; Iwata, H.; Harazono, Y. Autumn warming reduces the CO2 sink of a black spruce forest in interior Alaska based on a nine-year eddy covariance measurement. Glob. Chang. Biol. 2014, 20, 1161–1173. [Google Scholar] [CrossRef]
- Lafleur, B.; Fenton, N.J.; Bergeron, Y. Forecasting the development of boreal paludified forests in response to climate change: A case study using Ontario ecosite classification. For. Ecosyst. 2015, 2, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Seidl, R.; Thom, D.; Kautz, M.; Martin-Benito, D.; Peltoniemi, M.; Vacchiano, G.; Wild, J.; Ascoli, D.; Petr, M.; Honkaniemi, J.; et al. Forest disturbances under climate change. Nat. Clim. Chang. 2017, 7, 395–402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, B.; Donnelly, A.; Schwartz, M.D. Evaluating autumn phenology derived from field observations, satellite data, and carbon flux measurements in a northern mixed forest, USA. Int. J. Biometeorol. 2020, 64, 713–727. [Google Scholar] [CrossRef] [PubMed]
- Piao, S.; Friedlingstein, P.; Ciais, P.; Viovy, N.; Demarty, J. Growing season extension and its impact on terrestrial carbon cycle in the Northern Hemisphere over the past 2 decades. Glob. Biogeochem. Cycles 2007, 21, GB3018. [Google Scholar] [CrossRef]
- Thomas, R.T.; Prentice, I.C.; Graven, H.; Ciais, P.; Fisher, J.B.; Hayes, D.J.; Huang, M.; Huntzinger, D.N.; Ito, A.; Jain, A.; et al. Increased light-use efficiency in northern terrestrial ecosystems indicated by CO2 and greening observations. Geophys. Res. Lett. 2016, 43, 11–339. [Google Scholar] [CrossRef]
- Fu, Z.; Dong, J.; Zhou, Y.; Stoy, P.C.; Niu, S. Long term trend and interannual variability of land carbon uptake—The attribution and processes. Environ. Res. Lett. 2017, 12, 014018. [Google Scholar] [CrossRef]
- Piao, S.; Ciais, P.; Friedlingstein, P.; Peylin, P.; Reichstein, M.; Luyssaert, S.; Margolis, H.; Fang, J.; Barr, A.; Chen, A.; et al. Net carbon dioxide losses of northern ecosystems in response to autumn warming. Nature 2008, 451, 49–52. [Google Scholar] [CrossRef]
- Euskirchen, E.S.; Edgar, C.W.; Turetsky, M.R.; Waldrop, M.P.; Harden, J.W. Differential response of carbon fluxes to climate in three peatland ecosystems that vary in the presence and stability of permafrost. J. Geophys. Res.-Biogeosciences 2014, 119, 1576–1595. [Google Scholar] [CrossRef]
- Li, Z.; Xia, J.; Ahlström, A.; Rinke, A.; Koven, C.; Hayes, D.J.; Ji, D.; Zhang, G.; Krinner, G.; Chen, G.; et al. Non-uniform seasonal warming regulates vegetation greening and atmospheric CO2 amplification over northern lands. Environ. Res. Lett. 2018, 13, 124008. [Google Scholar] [CrossRef] [Green Version]
- Parazoo, N.; Arneth, A.; Pugh, T.A.M.; Smith, B.; Steiner, N.; Luus, K.; Commane, R.; Benmergui, J.; Stofferahn, E.; Liu, J.; et al. Spring photosynthetic onset and net CO2 uptake in Alaska triggered by landscape thawing. Glob. Chang. Biol. 2018, 24, 3416–3435. [Google Scholar] [CrossRef]
- Liu, P.; Black, T.A.; Jassal, R.S.; Zha, T.; Nesic, Z.; Barr, A.G.; Helgason, W.D.; Jia, X.; Tian, Y.; Stephens, J.J.; et al. Divergent long-term trends and interannual variation in ecosystem resource use efficiencies of a southern boreal old black spruce forest 1999–2017. Glob. Chang. Biol. 2019, 25, 3056–3069. [Google Scholar] [CrossRef]
- Piao, S.; Liu, Z.; Wang, T.; Peng, S.; Ciais, P.; Huang, M.; Tans, P.P. Weakening temperature control on the interannual variations of spring carbon uptake across northern lands. Nat. Clim. Chang. 2017, 7, 359–363. [Google Scholar] [CrossRef]
- Reichstein, M.; Bahn, M.; Ciais, P.; Frank, D.; Mahecha, M.D.; Seneviratne, S.I.; Wattenbach, M. Climate extremes and the carbon cycle. Nature 2013, 500, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Parida, B.R.; Buermann, W. Increasing summer drying in North American ecosystems in response to longer nonfrozen periods. Geophys. Res. Lett. 2014, 41, 5476–5483. [Google Scholar] [CrossRef]
- Smith, N.E.; Kooijmans, L.M.; Koren, G.; Van Schaik, E.; Van Der Woude, A.M.; Wanders, N.; Ramonet, M.; Xueref-Remy, I.; Siebicke, L.; Manca, G.; et al. Spring enhancement and summer reduction in carbon uptake during the 2018 drought in northwestern Europe. Philos. Trans. R. Soc. B 2020, 375, 20190509. [Google Scholar] [CrossRef]
- Humphrey, V.; Berg, A.; Ciais, P.; Gentine, P.; Jung, M.; Reichstein, M.; Seneviratne, S.I.; Frankenberg, C. Soil moisture–atmosphere feedback dominates land carbon uptake variability. Nature 2021, 592, 65–69. [Google Scholar] [CrossRef]
- Flach, M.; Brenning, A.; Gans, F.; Reichstein, M.; Sippel, S.; Mahecha, M.D. Vegetation modulates the impact of climate extremes on gross primary production. Biogeosciences 2021, 18, 39–53. [Google Scholar] [CrossRef]
- Buermann, W.; Forkel, M.; O’sullivan, M.; Sitch, S.; Friedlingstein, P.; Haverd, V.; Jain, A.K.; Kato, E.; Kautz, M.; Lienert, S.; et al. Widespread seasonal compensation effects of spring warming on northern plant productivity. Nature 2018, 562, 110–114. [Google Scholar] [CrossRef] [Green Version]
- Fischer, E.M.; Seneviratne, S.I.; Lüthi, D.; Schär, C. Contribution of land-atmosphere coupling to recent European summer heat waves. Geophys. Res. Lett. 2007, 34, L06707. [Google Scholar] [CrossRef] [Green Version]
- Bastos, A.; Fu, Z.; Ciais, P.; Friedlingstein, P.; Sitch, S.; Pongratz, J.; Weber, U.; Reichstein, M.; Anthoni, P.; Arneth, A.; et al. Impacts of extreme summers on European ecosystems: A comparative analysis of 2003, 2010 and 2018. Philos. Trans. R. Soc. B 2020, 375, 20190507. [Google Scholar] [CrossRef]
- Seddon, A.; Macias-Fauria, M.; Long, P.; Benz, D.; Willis, K.J. Sensitivity of global terrestrial ecosystems to climate variability. Nature 2016, 531, 229–232. [Google Scholar] [CrossRef] [Green Version]
- Gharun, M.; Hörtnagl, L.; Paul-Limoges, E.; Ghiasi, S.; Feigenwinter, I.; Burri, S.; Buchmann, N. Physiological response of Swiss ecosystems to 2018 drought across plant types and elevation. Philos. Trans. R. Soc. B 2020, 375, 20190521. [Google Scholar] [CrossRef] [PubMed]
- Farquhar, G.D.; von Caemmerer, S.; Berry, J.A. A biochemical model of photosynthetic CO2 assimilation in leaves of C3 species. Planta 1980, 149, 78–90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davidson, E.A.; Janssens, I.A.; Luo, Y. On the variability of respiration in terrestrial ecosystems: Moving beyond Q10. Glob. Chang. Biol. 2006, 12, 154–164. [Google Scholar] [CrossRef]
- Arnone III, J.; Verburg, P.; Johnson, D.; Larsen, J.D.; Jasoni, R.L.; Lucchesi, A.J.; Batts, C.M.; von Nagy, C.; Coulombe, W.G.; Schorran, D.E.; et al. Prolonged suppression of ecosystem carbon dioxide uptake after an anomalously warm year. Nature 2008, 455, 383–386. [Google Scholar] [CrossRef]
- Aubinet, M.; Hurdebise, Q.; Chopin, H.; Debacq, A.; De Ligne, A.; Heinesch, B.; Manise, T.; Vincke, C. Inter-annual variability of Net Ecosystem Productivity for a temperate mixed forest: A predominance of carry-over effects? Agric. For. Meteorol. 2018, 262, 340–353. [Google Scholar] [CrossRef] [Green Version]
- Bond-Lamberty, B.; Peckham, S.D.; Ahl, D.E.; Gower, S.T. Fire as the dominant driver of central Canadian boreal forest carbon balance. Nature 2007, 450, 89–92. [Google Scholar] [CrossRef] [Green Version]
- Kurz, W.A.; Shaw, C.H.; Boisvenue, C.; Stinson, G.; Metsaranta, J.; Leckie, D.; Dyk, A.; Smyth, C.; Neilson, E.T. Carbon in Canada’s boreal forest—A synthesis. Environ. Rev. 2013, 21, 260–292. [Google Scholar] [CrossRef]
- Bradshaw, C.J.; Warkentin, I.G. Global estimates of boreal forest carbon stocks and flux. Glob. Planet Chang. 2015, 128, 24–30. [Google Scholar] [CrossRef]
- Chi, J.; Nilsson, M.B.; Laudon, H.; Lindroth, A.; Wallerman, J.; Fransson, J.E.; Kljun, N.; Lundmark, T.; Ottosson Löfvenius, M.; Peichl, M. The Net Landscape Carbon Balance—Integrating terrestrial and aquatic carbon fluxes in a managed boreal forest landscape in Sweden. Glob. Chang. Biol. 2020, 26, 2353–2367. [Google Scholar] [CrossRef]
- Shvidenko, A.Z.; Schepaschenko, D.G. Carbon budget of Russian forests. Sib. Lesn. Zurnal 2014, 1, 69–92. (In Russian) [Google Scholar]
- Schaphoff, S.; Reyer, C.P.; Schepaschenko, D.; Gerten, D.; Shvidenko, A. Tamm Review: Observed and projected climate change impacts on Russia’s forests and its carbon balance. For. Ecol. Manag. 2016, 361, 432–444. [Google Scholar] [CrossRef] [Green Version]
- Kurbatova, J.; Li, C.; Varlagin, A.; Xiao, X.; Vygodskaya, N. Modeling carbon dynamics in two adjacent spruce forests with different soil conditions in Russia. Biogeosciences 2008, 5, 969–980. [Google Scholar] [CrossRef] [Green Version]
- Zagirova, S.V.; Mikhailov, O.A.; Schneider, J. Carbon dioxide, heat and water vapor exchange in the boreal spruce and peatland ecosystems. Theor. Appl. Ecol. 2019, 3, 12–20. [Google Scholar] [CrossRef]
- Karelin, D.V.; Zamolodchikov, D.G.; Shilkin, A.V.; Popov, S.Y.; Kumanyaev, A.S.; de Gerenyu, V.O.; Tel’nova, N.O.; Gitarskiy, M.L. The effect of tree mortality on CO2 fluxes in an old-growth spruce forest. Eur. J. For. Res. 2020, 140, 287–305. [Google Scholar] [CrossRef]
- Karpov, V.G. Spruce forests of the territory. In Regulation of Factors of Spruce Forest Ecosystems; Karpov, V.G., Ed.; Nauka: Leningrad, Russia, 1983; pp. 7–31. (In Russian) [Google Scholar]
- Vygodskaya, N.N.; Schulze, E.D.; Tchebakova, N.M.; Karpachevskii, L.O.; Kozlov, D.; Sidorov, K.N.; Abrazko, M.A.; Shaposhnikov, E.S.; Solnzeva, O.N.; Minaeva, T.Y.; et al. Climatic control of stand thinning in unmanaged spruce forests of the southern taiga in European Russia. Tellus B 2002, 54, 443–461. [Google Scholar] [CrossRef]
- Peel, M.C.; Finlayson, B.L.; McMahon, T.A. Updated world map of the Köppen-Geiger climate classification. Hydrol. Earth Syst. Sci. 2007, 11, 1633–1644. [Google Scholar] [CrossRef] [Green Version]
- Desherevskaya, O.; Kurbatova, J.; Olchev, A. Climatic conditions of the south part of Valday Hills, Russia, and their projected changes during the 21st century. Open Geogr. J. 2010, 3, 73–79. [Google Scholar] [CrossRef]
- Novenko, E.Y.; Tsyganov, A.N.; Olchev, A.V. Palaeoecological data as a tool to predict possible future vegetation changes in the boreal forest zone of European Russia: A case study from the Central Forest Biosphere Reserve. IOP Conf. Ser. Earth Environ. 2018, 107, 012104. [Google Scholar] [CrossRef] [Green Version]
- Mamkin, V.; Avilov, V.; Ivanov, D.; Varlagin, A.; Kurbatova, J. Interannual variability of the ecosystem CO2 fluxes at paludified spruce forest and ombrotrophic bog in southern taiga. Atmos. Chem. Phys. Discuss. 2021; preprint. [Google Scholar] [CrossRef]
- Mamkin, V.; Kurbatova, J.; Avilov, V.; Ivanov, D.; Kuricheva, O.; Varlagin, A.; Yaseneva, I.; Olchev, A. Energy and CO2 exchange in an undisturbed spruce forest and clear-cut in the Southern Taiga. Agric. For. Meteorol. 2019, 265, 252–268. [Google Scholar] [CrossRef]
- Schulze, E.D.; Vygodskaya, N.N.; Tchebakova, N.M.; Czimczik, C.I.; Kozlov, D.N.; Lloyd, J.; Sidorov, K.N.; Varlagin, A.V.; Wirth, C. The Eurosiberian transect: An introduction to the experimental region. Tellus B 2002, 54, 421–428. [Google Scholar] [CrossRef] [Green Version]
- Kljun, N.; Calanca, P.; Rotach, M.W.; Schmid, H.P. A simple parameterisation for flux footprint predictions. Bound.-Layer Meteorol. 2004, 112, 503–523. [Google Scholar] [CrossRef]
- Mauder, M.; Foken, T. Impact of post-field data processing on eddy covariance flux estimates and energy balance closure. Meteorol. Z. 2006, 15, 597–610. [Google Scholar] [CrossRef]
- Mauder, M.; Cuntz, M.; Drüe, C.; Graf, A.; Rebmann, C.; Schmid, H.P.; Schmidt, M.; Steinbrecher, R. A strategy for quality and uncertainty assessment of long-term eddy-covariance measurements. Agric. For. Meteorol. 2013, 169, 122–135. [Google Scholar] [CrossRef]
- Greco, S.; Baldocchi, D.D. Seasonal variations of CO2 and water vapour exchange rates over a temperate deciduous forest. Glob. Chang. Biol. 1996, 2, 183–197. [Google Scholar] [CrossRef]
- Wutzler, T.; Lucas-Moffat, A.; Migliavacca, M.; Knauer, J.; Sickel, K.; Šigut, L.; Menzer, O.; Reichstein, M. Basic and extensible post-processing of eddy covariance flux data with REddyProc. Biogeosciences 2018, 15, 5015–5030. [Google Scholar] [CrossRef] [Green Version]
- Zięba, A.; Ramza, P. Standard deviation of the mean of autocorrelated observations estimated with the use of the autocorrelation function estimated from the data. Metrol. Meas. Syst. 2011, 18, 529–542. [Google Scholar] [CrossRef] [Green Version]
- Pavelka, M.; Acosta, M.; Marek, M.V.; Kutsch, W.; Janous, D. Dependence of the Q10 values on the depth of the soil temperature measuring point. Plant Soil 2007, 292, 171–179. [Google Scholar] [CrossRef]
- Matthews, B.; Mayer, M.; Katzensteiner, K.; Godbold, D.L.; Schume, H. Turbulent energy and carbon dioxide exchange along an early-successional windthrow chronosequence in the European Alps. Agric. For. Meteorol. 2017, 232, 576–594. [Google Scholar] [CrossRef]
- Lindroth, A.; Holst, J.; Heliasz, M.; Vestin, P.; Lagergren, F.; Biermann, T.; Cai, Z.; Mölder, M. Effects of low thinning on carbon dioxide fluxes in a mixed hemiboreal forest. Agric. For. Meteorol. 2018, 262, 59–70. [Google Scholar] [CrossRef]
- Krasnova, A.; Kukumägi, M.; Mander, Ü.; Torga, R.; Krasnov, D.; Noe, S.M.; Ostonen, I.; Püttsepp, Ü.; Killian, H.; Uri, V.; et al. Carbon exchange in a hemiboreal mixed forest in relation to tree species composition. Agric. For. Meteorol. 2019, 275, 11–23. [Google Scholar] [CrossRef]
- Grünwald, T.; Bernhofer, C. A decade of carbon, water and energy flux measurements of an old spruce forest at the Anchor Station Tharandt. Tellus B 2007, 59, 387–396. [Google Scholar] [CrossRef] [Green Version]
- Jensen, R.; Herbst, M.; Friborg, T. Direct and indirect controls of the interannual variability in atmospheric CO2 exchange of three contrasting ecosystems in Denmark. Agric. For. Meteorol. 2017, 233, 12–31. [Google Scholar] [CrossRef]
- Amiro, B.D.; Barr, A.G.; Barr, J.G.; Black, T.A.; Bracho, R.; Brown, M.; Chen, J.; Clark, K.L.; Davis, K.J.; Desai, A.R.; et al. Ecosystem carbon dioxide fluxes after disturbance in forests of North America. J. Geophys. Res.-Biogeosciences 2010, 115, G4. [Google Scholar] [CrossRef]
- Rödenbeck, C.; Zaehle, S.; Keeling, R.; Heimann, M. The European carbon cycle response to heat and drought as seen from atmospheric CO2 data for 1999–2018. Philos. Trans. R Soc. B 2020, 375, 20190506. [Google Scholar] [CrossRef]
- Krasnova, A.; Mander, Ü.; Noe, S.M.; Uri, V.; Krasnov, D.; Soosaar, K. Hemiboreal forests’ CO2 fluxes response to the European 2018 heatwave. Agric. For. Meteorol. 2022, 323, 109042. [Google Scholar] [CrossRef]
- Kurbatova, J.; Tatarinov, F.; Molchanov, A.; Varlagin, A.; Avilov, V.; Kozlov, D.; Ivanov, D.; Valentini, R. Partitioning of ecosystem respiration in a paludified shallow-peat spruce forest in the southern taiga of European Russia. Environ. Res. Lett. 2013, 8, 045028. [Google Scholar] [CrossRef] [Green Version]
- Tanja, S.; Berninger, F.; Vesala, T.; Markkanen, T.; Hari, P.; Mäkelä, A.; Ilvesniemi, H.; Hänninen, H.; Nikinmaa, E.; Huttula, T.; et al. Air temperature triggers the recovery of evergreen boreal forest photosynthesis in spring. Glob. Chang. Biol. 2003, 9, 1410–1426. [Google Scholar] [CrossRef]
- Kwon, M.J.; Ballantyne, A.; Ciais, P.; Bastos, A.; Chevallier, F.; Liu, Z.; Green, J.K.; Qiu, C.; Kimball, J.S. Siberian 2020 heatwave increased spring CO2 uptake but not annual CO2 uptake. Environ. Res. Lett. 2021, 16, 124030. [Google Scholar] [CrossRef]
- Nemani, R.R.; Keeling, C.K.; Hashimoto, H.; Jolly, W.M.; Piper, S.C.; Tucker, C.J.; Myneni, R.B.; Running, S.W. Climate-driven increases in global terrestrial net primary production from 1982 to 1999. Science 2003, 300, 1560–1563. [Google Scholar] [CrossRef] [Green Version]
- Wolf, S.; Keenan, T.F.; Fisher, J.B.; Baldocchi, D.B.; Desai, A.R.; Richardson, A.D.; Scott, R.L.; Law, B.E.; Litvak, M.E.; Brunsell, N.A.; et al. Warm spring reduced carbon cycle impact of the 2012 US summer drought. Proc. Natl. Acad. Sci. USA 2016, 113, 5880–5885. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beaulne, J.; Garneau, M.; Magnan, G.; Boucher, É. Peat deposits store more carbon than trees in forested peatlands of the boreal biome. Sci. Rep.-UK 2021, 11, 2657. [Google Scholar] [CrossRef] [PubMed]
- Roshydromet. A Report on Climate Features on the Territory of the Russian Federation in 2021; Roshydromet: Moscow, Russia, 2022; p. 104. [Google Scholar]


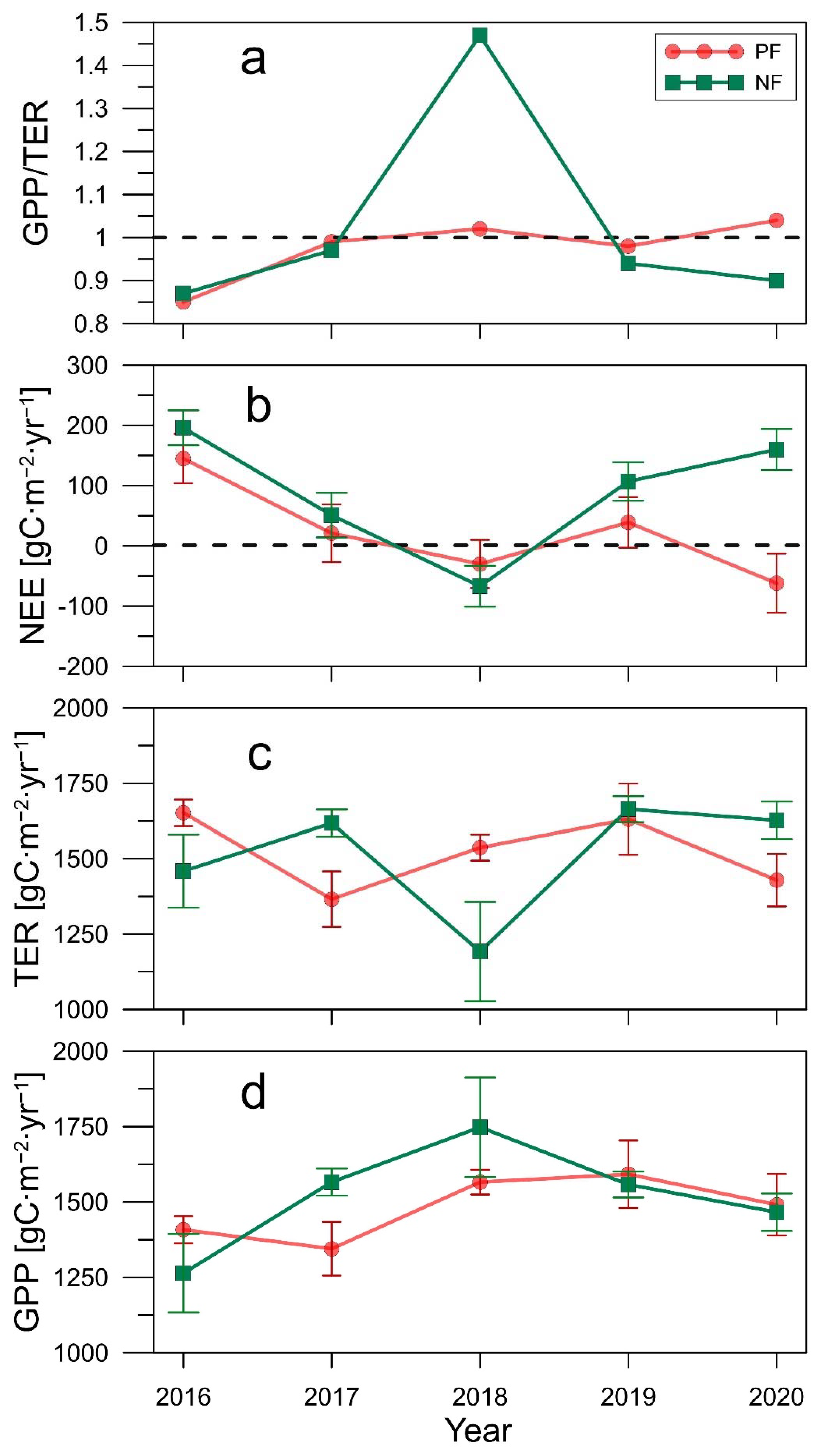
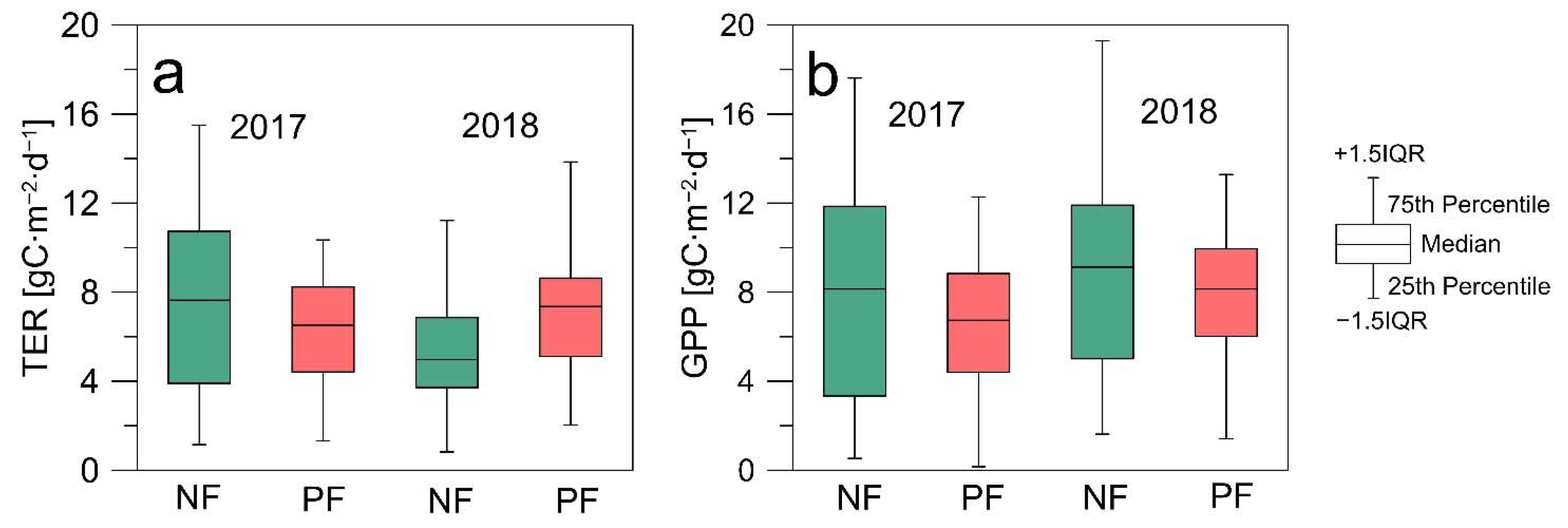
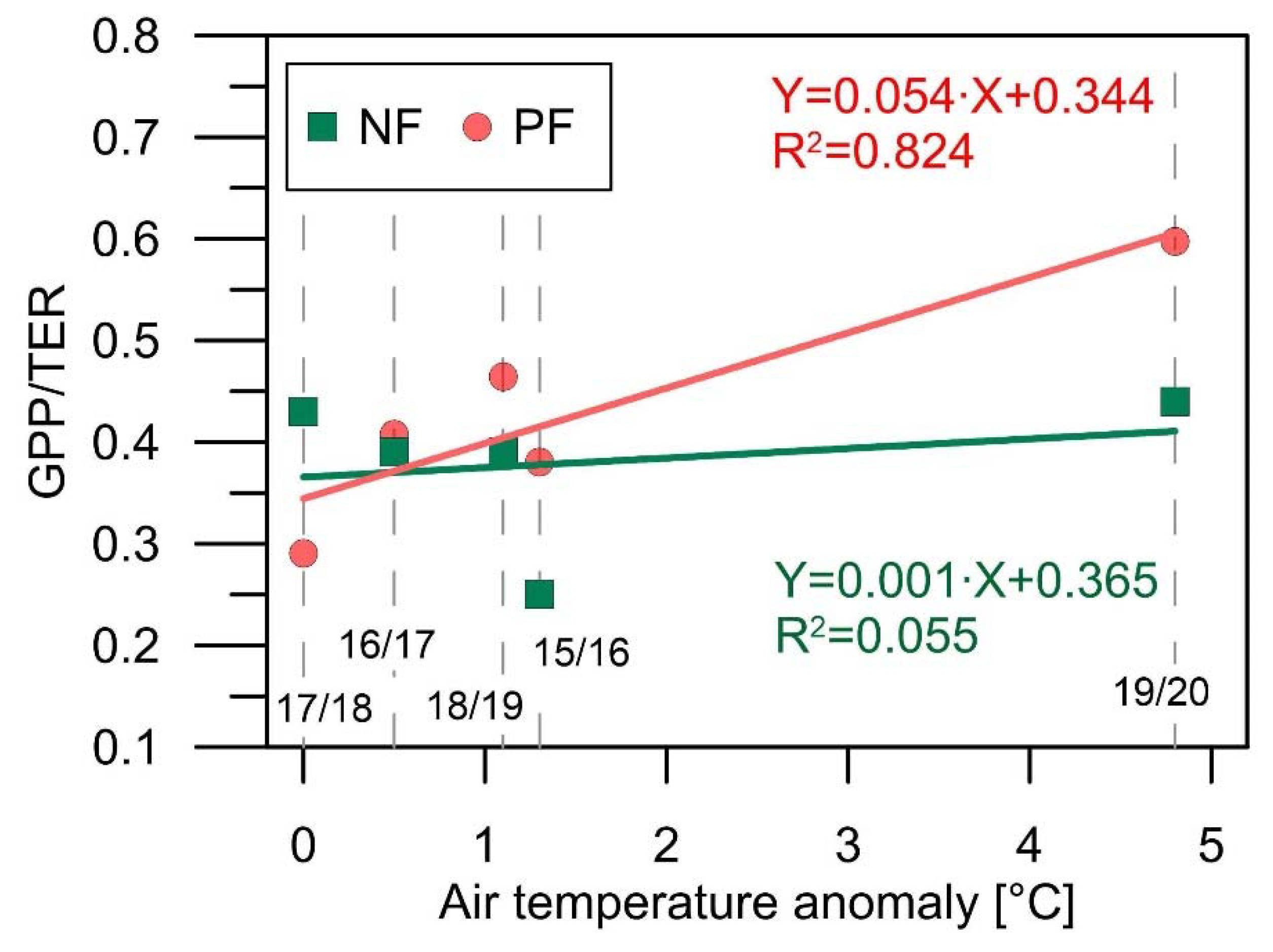
| 2015 | 2016 | 2017 | 2018 | 2019 | 2020 | Long-Term | |
|---|---|---|---|---|---|---|---|
| Ta (°C) | 6.8 | 5.8 | 5.7 | 6.0 | 7.0 | 7.6 | 5.7 ± 0.8 |
| Ta,g.s. (°C) | 13.5 | 14.3 | 12.3 | 14.8 | 13.4 | 13.8 | 13.6 ± 0.8 |
| Start of the g.s. | 09.04 | 07.04 | 28.04 | 13.04 | 16.04 | 22.04 | 12.04 |
| End of the g.s. | 06.10 | 11.10 | 19.10 | 24.10 | 05.10 | 17.10 | 11.10 |
| Pr (mm) | 671 | 864 | 956 | 560 | 848 | 992 | 778 ± 123 |
| Prg.s. (mm) | 300 | 479 | 562 | 343 | 492 | 640 | 445 ± 114 |
| Rg (MJ∙m−2) | 3592 * | 3474 | 3325 | 3733 | 3571 | 3381 | NA |
| Ts (°C) | 9.4 ** | 6.9 | 6.2 | 6.8 | 6.7 | 6.9 | NA |
| SWC (m3∙m−3) | 0.40 ** | 0.45 | 0.51 | 0.47 | 0.48 | 0.53 | NA |
| 2015/2016 | 2016/2017 | 2017/2018 | 2018/2019 | 2019/2020 | Long-Term |
|---|---|---|---|---|---|
| −2.2 | −3.0 | −3.5 | −2.4 | 1.3 | −3.5 ± 1.9 |
| 2016 | 2017 | 2018 | 2019 | 2020 | |
|---|---|---|---|---|---|
| NEE (gC∙m−2) | 196 ± 29 | 51 ± 37 | −67 ± 34 | 107 ± 32 | 160 ± 34 |
| NEEg.s. (gC∙m−2) | 121 ± 55 | −76 ± 76 | −158 ± 64 | −24 ± 65 | 21 ± 71 |
| GPP (gC∙m−2) | 1264 ± 130 | 1566 ± 45 | 1748 ± 165 | 1558 ± 43 | 1466 ± 62 |
| GPPg.s. (gC∙m−2) | 1220 ± 252 | 1458 ± 78 | 1653 ± 281 | 1461 ± 78 | 1336 ± 101 |
| TER (gC∙m−2) | 1459 ± 121 | 1618 ± 45 | 1192 ± 165 | 1664 ± 43 | 1627 ± 62 |
| TERg.s. (gC∙m−2) | 1342 ± 252 | 1382 ± 76 | 1015 ± 301 | 1437 ± 76 | 1358 ± 125 |
| GPP/TER | 0.87 | 0.97 | 1.47 | 0.94 | 0.90 |
| GPP/TER (g.s.) | 0.91 | 1.06 | 1.63 | 1.02 | 0.98 |
| 2015/2016 | 2016/2017 | 2017/2018 | 2018/2019 | 2019/2020 | |
|---|---|---|---|---|---|
| NEE (gC∙m−2) | 64 ± 26 | 82 ± 25 | 65 ± 37 | 94 ± 28 | 95 ± 28 |
| GPP (gC∙m−2) | 22 ± 26 | 52 ± 25 | 62 ± 37 | 52 ± 30 | 74 ± 28 |
| TER (gC∙m−2) | 85 ± 28 | 134 ± 25 | 145 ± 37 | 135 ± 33 | 169 ± 29 |
| GPP/TER | 0.25 | 0.39 | 0.43 | 0.39 | 0.44 |
| α | γ | R2 | Q10 | R10 (µmol∙m−2∙s−1) | |
|---|---|---|---|---|---|
| 2017 (Ts) | 0.136 | 0.638 | 0.480 | 3.90 | 7.37 |
| 2018 (Ts) | 0.086 | 0.530 | 0.458 | 2.36 | 4.02 |
| 2017 (Ta) | 0.072 | 1.071 | 0.489 | 2.05 | 6.00 |
| 2018 (Ta) | 0.046 | 0.853 | 0.261 | 1.58 | 3.72 |
| ε (µmol∙J−1) | β (µmol∙m−2∙s−1) | R2 | |
|---|---|---|---|
| 2016 | 0.026 | 1.361 | 0.689 |
| 2017 | 0.022 | 7.520 | 0.952 |
| 2018 | 0.161 | 1.263 | 0.569 |
| 2019 | 0.036 | 3.561 | 0.600 |
| 2020 | 0.106 | 3.572 | 0.695 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mamkin, V.; Varlagin, A.; Yaseneva, I.; Kurbatova, J. Response of Spruce Forest Ecosystem CO2 Fluxes to Inter-Annual Climate Anomalies in the Southern Taiga. Forests 2022, 13, 1019. https://doi.org/10.3390/f13071019
Mamkin V, Varlagin A, Yaseneva I, Kurbatova J. Response of Spruce Forest Ecosystem CO2 Fluxes to Inter-Annual Climate Anomalies in the Southern Taiga. Forests. 2022; 13(7):1019. https://doi.org/10.3390/f13071019
Chicago/Turabian StyleMamkin, Vadim, Andrej Varlagin, Irina Yaseneva, and Julia Kurbatova. 2022. "Response of Spruce Forest Ecosystem CO2 Fluxes to Inter-Annual Climate Anomalies in the Southern Taiga" Forests 13, no. 7: 1019. https://doi.org/10.3390/f13071019
APA StyleMamkin, V., Varlagin, A., Yaseneva, I., & Kurbatova, J. (2022). Response of Spruce Forest Ecosystem CO2 Fluxes to Inter-Annual Climate Anomalies in the Southern Taiga. Forests, 13(7), 1019. https://doi.org/10.3390/f13071019






