UAVs Technology as a Complementary Tool in Post-Fire Vegetation Recovery Surveys in Mediterranean Fire-Prone Forests
Abstract
:1. Introduction
2. Material and Methods
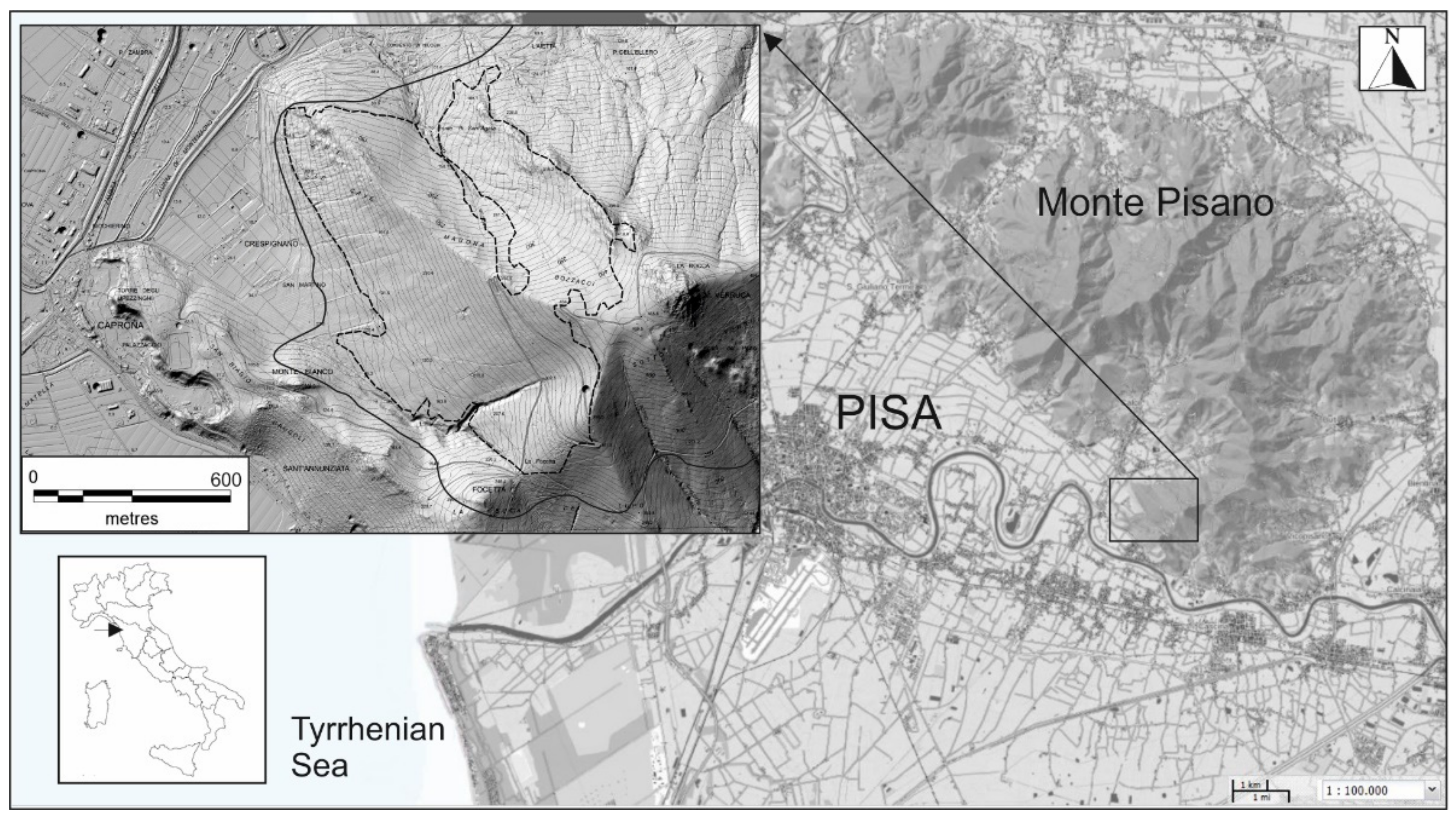
2.1. Study Area
- areas crossed twice by the fire;
- homogeneous vegetation existing before the two fires (maritime pine forests);
- homogeneous sites for morphology, exposure, and substrate;
- different vegetation regrowth after the first fire.
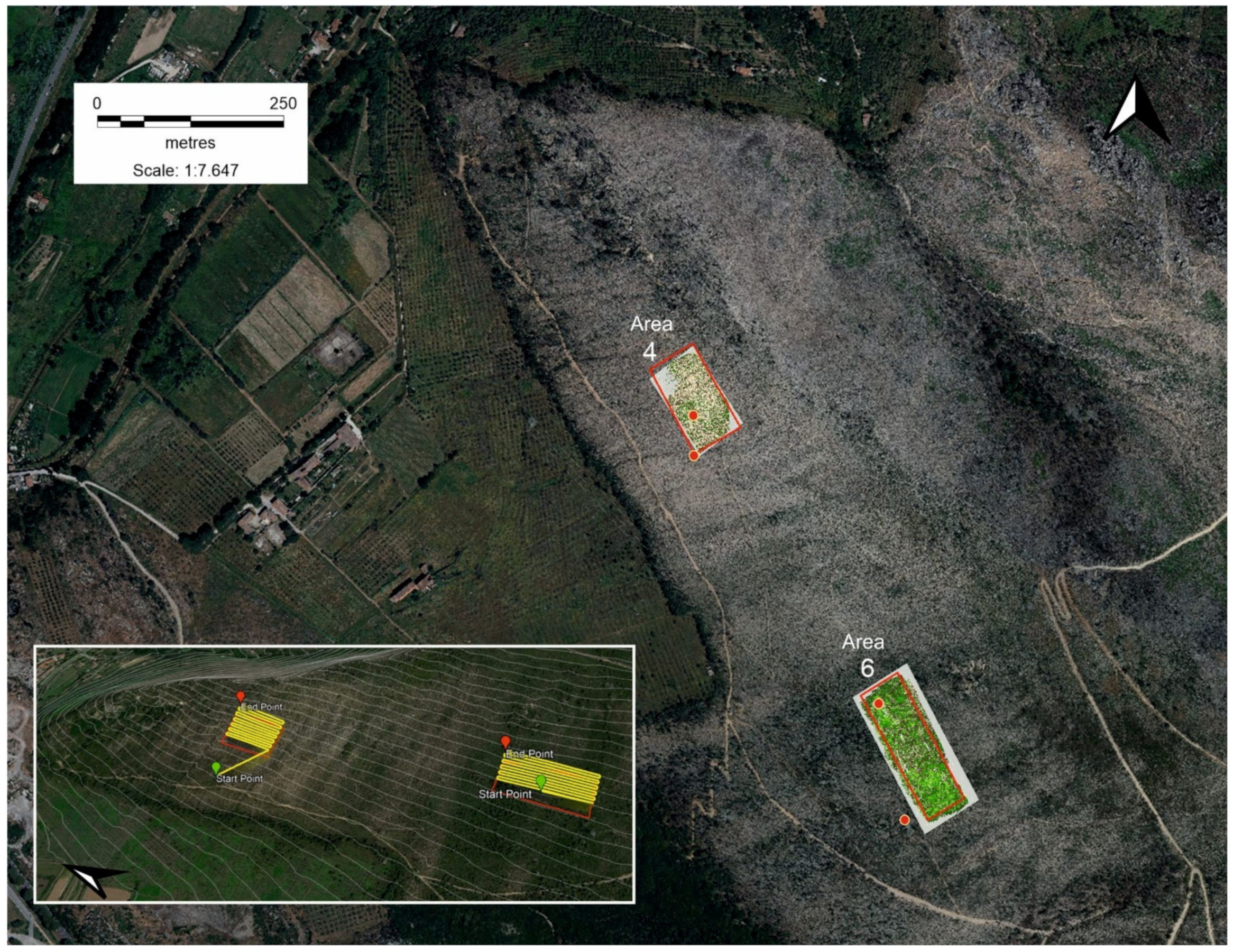
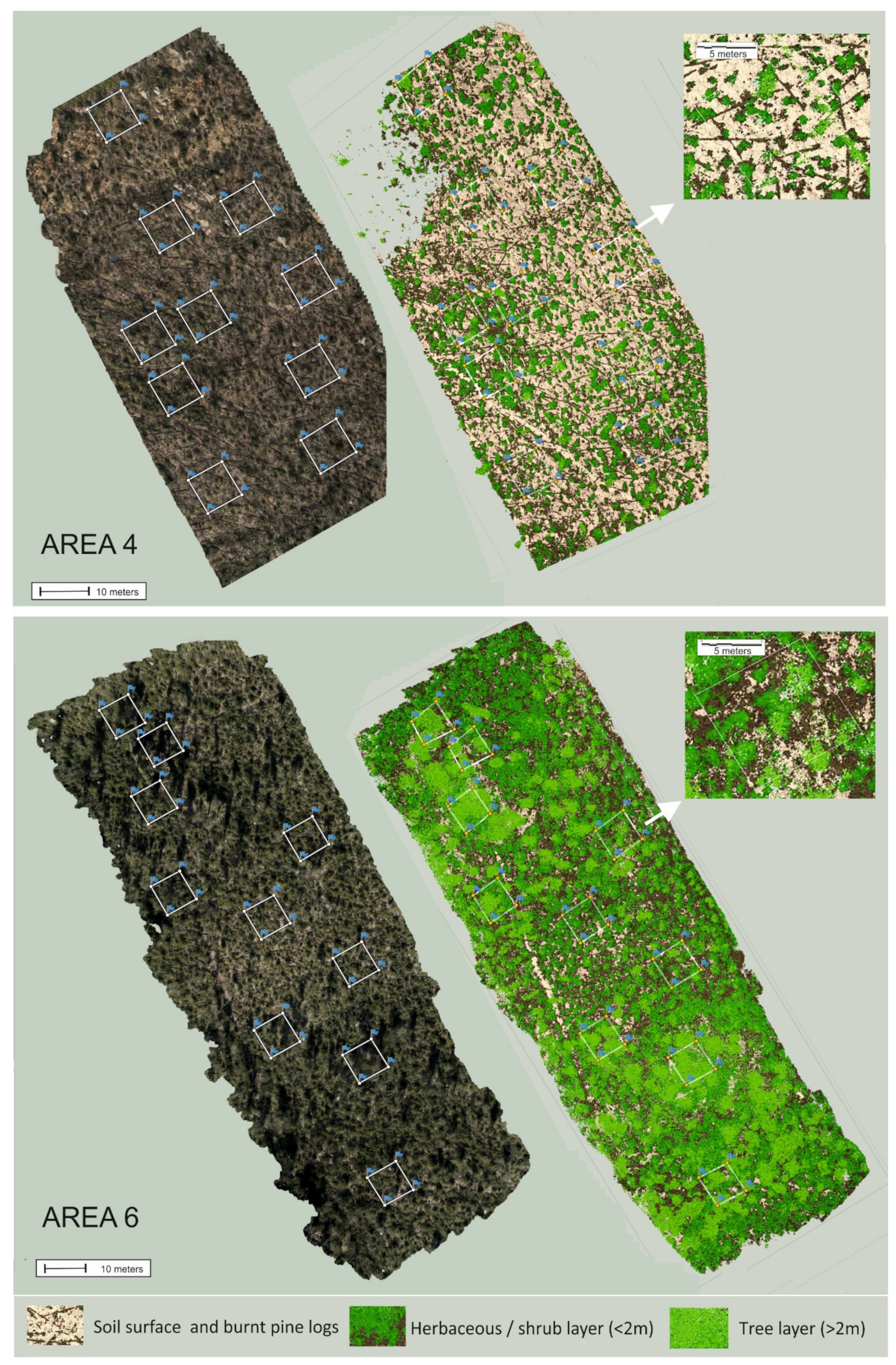
2.2. Remote Sensing and Ground Surveys
2.3. Data Analysis
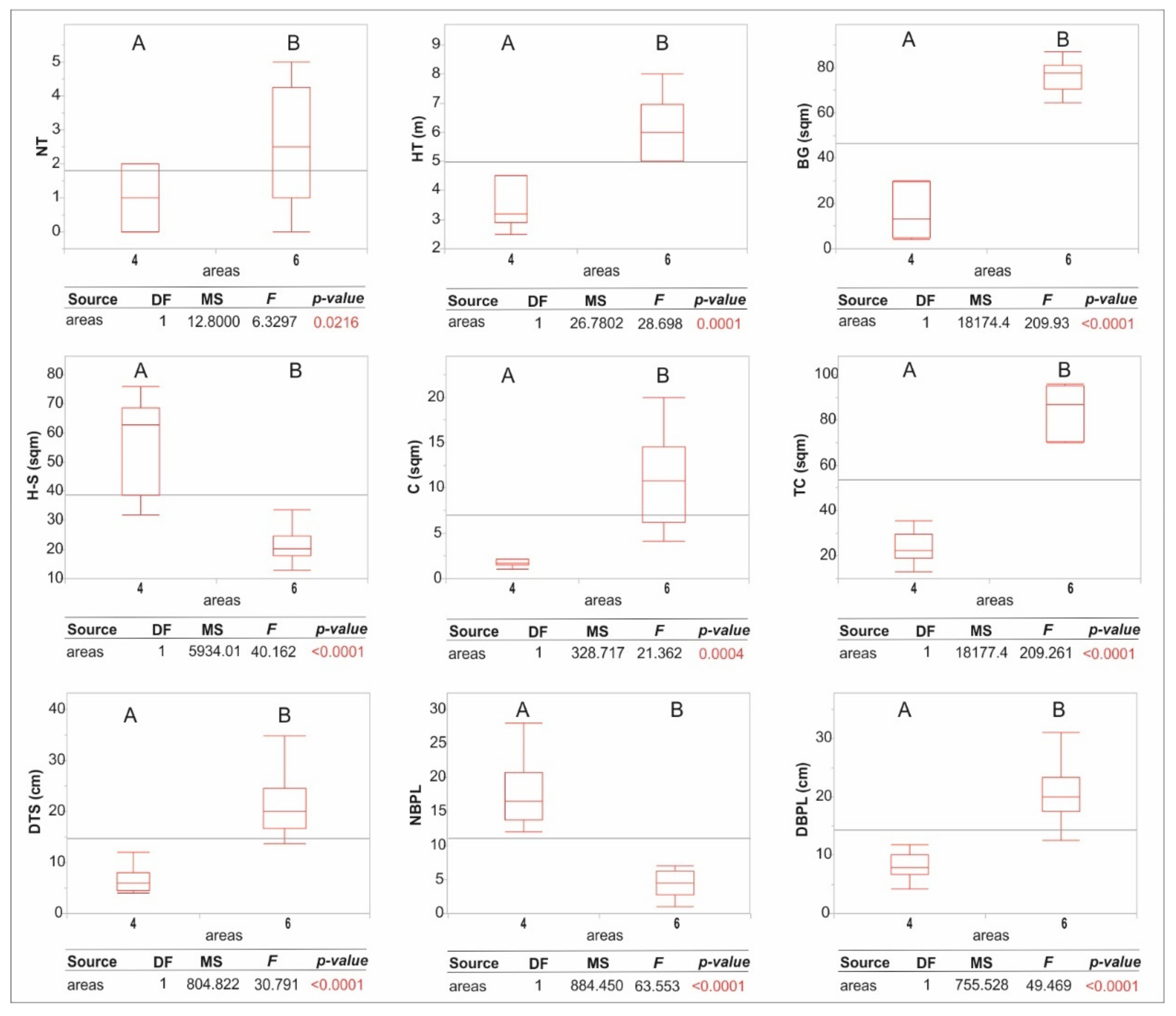
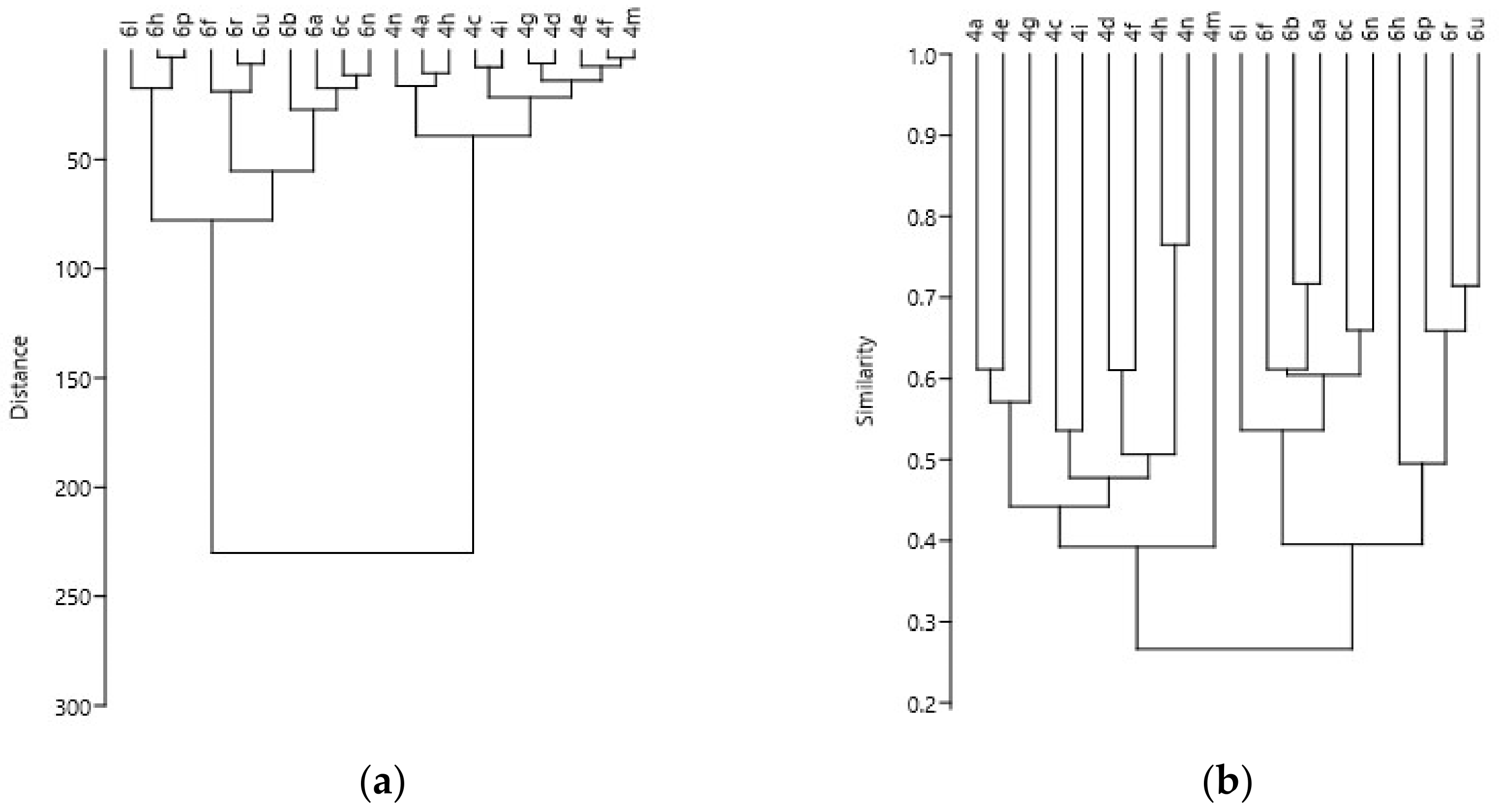
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- He, T.; Lamont, B.B.; Pausas, J.G. Fire as a key driver of Earth’s biodiversity. Biol. Rev. 2019, 94, 1983–2010. [Google Scholar] [CrossRef] [PubMed]
- Scott, A.C. The pre-Quaternary history of fire. Palaeogeogr. Palaeoclim. Palaeoecol. 2000, 164, 281–329. [Google Scholar] [CrossRef]
- Trabaud, L. Man and Fire: Impacts on mediterranean vegetation. In Ecosystems of the World 11: Mediterranean Type Shrublands; Di Castri, F., Goodall, D.W., Specht, R.L., Eds.; Elsevier: Amsterdam, The Netherlands, 1981; pp. 523–537. [Google Scholar]
- Naveh, Z. Fire in the Mediterranean—A landscape ecological perspective. In Fire in Ecosystems Dynamics; Goldammer, J.G., Jenkins, M.J., Eds.; SPB Academic Publishing: The Hague, The Netherlands, 1989; pp. 1–20. [Google Scholar]
- Bond, W.J.; Keeley, J.E. Fire as a global ‘herbivore’: The ecology and evolution of flammable ecosystems. Trends Ecol. Evol. 2005, 20, 387–394. [Google Scholar] [CrossRef]
- Rundel, P.; Arroyo, T.K.; Cowling, R.M.; Keeley, J.E.; Lamont, B.B.; Pausas, J.G.; Vargas, P. Fire and plant diversification in mediterranean-climate regions. Front. Plant Sci. 2018, 9, 851. [Google Scholar] [CrossRef] [PubMed]
- Blasi, C.; Bovio, G.; Corona, P.; Marchetti, M.; Maturani, A. Quadro dei contenuti e dei riferimenti concettuali. In Incendi e Complessità Ecosistemica. Dalla Pianificazione Forestale al Recupero Ambientale; Palombi Editori: Roma, Italy, 2004; pp. 12–15. [Google Scholar]
- Bowman, D.M.J.; Balch, J.; Artaxo, P.; Bond, W.J.; Cochrane, M.A.; D’Antonio, C.M.; DeFries, R.; Johnston, F.H.; Keeley, J.E.; Krawchuk, M.A.; et al. The human dimension of fire regimes on Earth. J. Biogeogr. 2011, 38, 2223–2236. [Google Scholar] [CrossRef] [Green Version]
- Molina, J.R.; Rodríguez y Silva, R.; Herrera, M.A. Economic vulnerability of fire-prone landscapes in protected natural areas: Application in a Mediterranean Natural Park. Eur. J. For. Res. 2017, 136, 609–624. [Google Scholar] [CrossRef]
- Turco, M.; Palazzi, E.; von Hardenberg, J.; Provenzale, A. Observed climate change hotspots. Geophys. Res. Lett. 2015, 42, 3521–3528. [Google Scholar] [CrossRef]
- San-Miguel-Ayanz, J.; Durrant, T.; Boca, R.; Maianti, P.; Libertà, G.; Artes Vivancos, T.; Jacome Felix Oom, D.; Branco, A.; De Rigo, D.; Ferrari, D.; et al. Forest Fires in Europe, Middle East and North Africa 2019.’ EUR 30402 EN. 2020. Available online: https://data.europa.eu/doi/10.2760/893 (accessed on 24 October 2011).
- De Rigo, D.; Libertà, G.; Houston Durrant, T.; Artés Vivancos, T.; San-Miguel-Ayanz, J. Forest Fire Danger Extremes in Europe under Climate Change: Variability and Uncertainty; Publication Office of the European Union: Luxembourg, 2017; Available online: https://data.europa.eu/doi/10.2760/13180 (accessed on 24 October 2020).
- Turco, M.; Rosa-Cánovas, J.J.; Bedia, J.; Jerez, S.; Pedro Montávez, J.; Llasat, M.C.; Provenzale, A. Exacerbated fires in Mediterranean Europe due to anthropogenic warming projected with non-stationary climate-fire models. Nat. Commun. 2018, 9, 3821. [Google Scholar] [CrossRef]
- Trabaud, L. Dynamics after fire of sclerophyllous plant communities in the Mediterranean basin. Ecol. Medit. 1987, 13, 25–34. [Google Scholar] [CrossRef]
- Reilly, M.J.; Wimberly, M.C.; Newell, C.L. Wildfire effects on β-diversity and species turnover in a forested landscape. J. Veg. Sci. 2006, 17, 447–454. [Google Scholar] [CrossRef]
- Capitanio, R.; Carcaillet, C. Post-fire Mediterranean vegetation dynamics and diversity: A discussion of succession models. For. Ecol. Manag. 2008, 255, 431–439. [Google Scholar] [CrossRef]
- Maia, P.; Pausas, J.G.; Vasques, A.; Keizer, J.J. Fire severity as a key factor in post-fire regeneration of Pinus pinaster (Ait.) in Central Portugal. Ann. For. Sci. 2012, 69, 489–498. [Google Scholar] [CrossRef] [Green Version]
- Pausas, J.G.; Keeley, J.E. Evolutionary ecology of resprouting and seeding in fire-prone ecosystems. New Phytol. 2014, 204, 55–65. [Google Scholar] [CrossRef] [PubMed]
- Sunar, F.; Özkan, C. Forest fire analysis with remote sensing data. Int. J. Remote Sens. 2001, 22, 2265–2277. [Google Scholar] [CrossRef]
- Esquin, S.; Navarro, R.; Fernández, P. Fire severity assessment by using NBR (Normalized Burn Ratio) and NDVI (Normalized Difference Vegetation Index) derived from LANDSAT TM/ETM images. Int. J. Remote Sens. 2008, 29, 1053–1073. [Google Scholar] [CrossRef]
- Talucci, A.C.; Forbath, E.; Kropp, H.; Alexander, H.D.; DeMarco, J.; Paulson, A.K.; Zimov, N.S.; Zimov, S.; Loranty, M.M. Evaluating Post-Fire Vegetation Recovery in Cajander Larch Forests in Northeastern Siberia Using UAV Derived Vegetation Indices. Remote Sens. 2020, 12, 2970. [Google Scholar] [CrossRef]
- Husson, E.; Hagner, O.; Ecke, F. Unmanned aircraft systems help to map aquatic vegetation. App. Veg. Sci. 2014, 17, 567–577. [Google Scholar] [CrossRef]
- Baena, S.; Moat, J.; Whaley, O.; Boyd, D.S. Identifying species from the air: UAVs and the very high resolution challenge for plant conservation. PLoS ONE 2017, 12, e0188714. [Google Scholar] [CrossRef] [Green Version]
- Bertacchi, A.; Giannini, V.; Di Franco, C.; Silvestri, N. Using unmanned aerial vehicles for vegetation mapping and identification of botanical species in wetlands. Landsc. Ecol. Eng. 2019, 15, 231–240. [Google Scholar] [CrossRef]
- Larrinaga, A.R.; Brotons, L. Greenness Indices from a Low-Cost UAV Imagery as Tools for Monitoring Post-Fire Forest Recovery. Drones 2019, 3, 6. [Google Scholar] [CrossRef] [Green Version]
- Dainelli, R.; Toscano, P.; Di Gennaro, S.F.; Matese, A. Recent Advances in Unmanned Aerial Vehicle Forest Remote Sensing—A Systematic Review. Part I: A General Framework. Forests 2021, 12, 327. [Google Scholar] [CrossRef]
- De Castro, A.I.; Shi, Y.; Maja, J.M.; Peña, J.M. UAVs for Vegetation Monitoring: Overview and Recent Scientific Contributions. Remote Sens. 2021, 13, 2139. [Google Scholar] [CrossRef]
- Rapetti, F.; Vittorini, S. I caratteri del clima. In La Pianura di Pisa e i Rilievi Contermini; Società Geografica Italiana: Roma, Italy, 1994; pp. 103–132. [Google Scholar]
- Pesaresi, S.; Biondi, E.; Casavecchia, S. Bioclimates of Italy. J. Maps 2017, 13, 955–960. [Google Scholar] [CrossRef]
- Bertacchi, A.; Sani, A.; Tomei, P.E. La Vegetazione del Monte Pisano; Felici Editore: Pisa, Italy, 2004. [Google Scholar]
- Bonari, G.; Fernández-González, F.; Çoban, S.; Monteiro-Henriques, T.; Bergmeier, E.; Didukh, Y.P.; Xystrakis, F.; Angiolini, C.; Chytrý, K.; Acosta, A.T.R.; et al. Classification of the Mediterranean lowland to submontane pine forest vegetation. App. Veg. Sci. 2021, 24, e12544. [Google Scholar] [CrossRef]
- Bertacchi, A.; Borgia, A. Paesaggio forestale e incendi in aree forestali del Monte Pisano: Il caso di studio della valle di Crespignano (Pi)—Toscana Nord-Occidentale. Atti Soc. Tosc. Sci. Nat. Mem. Ser. B 2020, 127, 5–20. [Google Scholar] [CrossRef]
- Braun–Blanquet, J. Plant Sociology; Koeltz Scientific Books: Koenigstein, Germany, 1983. [Google Scholar]
- Van der Maarel, E. Transformation of cover-abundance values in phytosociology and its effects on community similarity. Vegetatio 1979, 39, 97–114. [Google Scholar]
- Bartolucci, F.; Peruzzi, L.; Galasso, G.; Albano, A.; Alessandrini, A.N.M.G.; Ardenghi, N.M.G.; Astuti, G.; Bacchetta, G.; Ballelli, S.; Banfi, E.; et al. An updated checklist of the vascular flora native to Italy. Plant Biosyst. 2018, 152, 179–303. [Google Scholar] [CrossRef]
- Sothe, C.; Dalponte, M.; Almeida, C.M.d.; Schimalski, M.B.; Lima, C.L.; Liesenberg, V.; Miyoshi, G.T.; Tommaselli, A.M.G. Tree Species Classification in a Highly Diverse Subtropical Forest Integrating UAV-Based Photogrammetric Point Cloud and Hyperspectral Data. Remote Sens. 2019, 11, 1338. [Google Scholar] [CrossRef] [Green Version]
- Targioni Tozzetti, G. Relazioni D’alcuni Viaggi Fatti in Diverse Parti della Toscana per Osservare le Produzioni Naturali, e gli Antichi Monumenti di Essa; Edizione Seconda con Copiose Giunte; Stamperia Granducale: Firenze, Italy, 1768; Volume 1, p. 480. [Google Scholar]
- Buccianti, M. Il pino marittimo in Toscana. Ann. Dell’accademia Ital. Sci. For. 1974, 23, 219–260. [Google Scholar]
- AIB. Piani Specifici di Prevenzione, Regione Toscana. Available online: https://www.regione.toscana.it/piani-specifici-di-prevenzione (accessed on 24 June 2011).
- Alvarez, R.; Valbuena, L.; Calvo, L. Effect of high temperatures on seed germination and seedling survival in three pine species (Pinus pinaster, P. sylvestris and P. nigra). Int. J. Wildland Fire 2007, 16, 63–70. [Google Scholar] [CrossRef]
- Calvo, L.; Santalla, S.; Valbuena, L.; Marcos, E.; Tárrega, R.; Luis-Calabuig, E. Post-fire natural regeneration of a Pinus pinaster forest in NW Spain. Plant Ecol. 2008, 197, 81–90. [Google Scholar] [CrossRef]
- Luis-Calabuig, E.; Torres, O.; Valbuena, L.; Calvo, L.; Marcos, E. Impact of large fires on a community of Pinus pinaster. In Fire and Biological Processes; Trabaud, L., Prodon, R., Eds.; Backhuys Publishers: Leiden, The Netherlands, 2002; pp. 1–12. [Google Scholar]
- Fernandes, P.M.; Rigolot, E. The fire ecology and management of maritime pine (Pinus pinaster Ait.). For. Ecol. Manag. 2007, 241, 1–13. [Google Scholar] [CrossRef]
- Vega, J.A.; Fernández, C.; Pérez-Gorostiaga, P.; Fonturbel, T. The influence of fire severity, serotiny, and post-fire management on Pinus pinaster Ait. recruitment in three burnt areas in Galicia (NW Spain). For. Ecol. Manag. 2008, 256, 1596–1603. [Google Scholar] [CrossRef]
- Maugé, J.P. Le pin Maritime, Premier Résineux de France; Centre de Productivité & d’Action Forestière d’Aquitaine: Bordeaux, France; Institut pour le Développement Forestier: Paris, France, 1987; p. 192. [Google Scholar]
- Bernetti, G. Selvicoltura Speciale; UTET: Torino, Italy, 1995; p. 415. [Google Scholar]
- Pausas, J.G. Resprouting of Quercus suber in NE Spain after fire. J. Veg. Sci. 1997, 8, 703–706. [Google Scholar] [CrossRef] [Green Version]
- Silva, J.S.; Catry, F. Forest fires in cork oak (Quercus suber L.) stands in Portugal. Int. J. Environ. Stud. 2006, 63, 235–257. [Google Scholar] [CrossRef]
- Pons, J.; Pausas, J.G. Oak regeneration in heterogeneous landscapes: The case of fragmented Quercus suber forests in the eastern Iberian Peninsula. For. Ecol. Manag. 2006, 2311, 196–204. [Google Scholar] [CrossRef]
- Acacio, V.; Holmgren, M.; Jansen, P.A.; Schrotter, O. Multiple recruitment limitation causes arrested succession in Mediterranean cork oak systems. Ecosystems 2007, 107, 1220–1230. [Google Scholar] [CrossRef] [Green Version]
- Calvo, L.; Santalla, S.; Marcos, E.; Valbuena, L.; Tarrega, R.; Luis, E. Regeneration after wildfire in communities dominated by Pinus pinaster an obligate seeder and in others dominated by Quercus pyrenaica a typical resprouter. For. Ecol. Manag. 2003, 1841, 209–223. [Google Scholar] [CrossRef]
- Hill, D.; Fasham, M.; Tucker, G.; Shrewry, M.; Shaw, P. Handbook of Biodiversity Methods: Survey, Evaluation and Monitoring; Cambridge University Press: Cambridge, UK, 2005. [Google Scholar] [CrossRef]
- Santana, V.M.; Alday, J.G.; Baeza, M.J. Effects of fire regime shift in Mediterranean Basin ecosystems: Changes in soil seed bank composition among functional types. Plant Ecol. 2014, 215, 555–566. [Google Scholar] [CrossRef]
- Baudena, M.; Santana, V.M.; Baeza, M.J.; Bautista, S.; Eppinga, M.B.; Hemerik, L.; Garcia Mayor, A.; Rodriguez, F.; Valdecantos, A.; Vallejo, V.R.; et al. Increased aridity drives post-fire recovery of Mediterranean forests towards open shrublands. New Phytol. 2020, 2254, 1500–1515. [Google Scholar] [CrossRef] [Green Version]
- Vasques, A.; Baudena, M.; Vallejo, V.R.; Kéfi, S.; Bautista, S.; Santana, V.M.; Baeza, M.J.; Maia, P.; Keizer, J.J.; Rietkerk, M. Post-fire Regeneration Traits of Understorey Shrub Species Modulate Successional Responses to High Severity Fire in Mediterranean Pine Forests. Ecosystems 2022, 1–15. [Google Scholar] [CrossRef]

| NT | HT (m) | BG (sqm) | H-S (sqm) | C (sqm) | TC (sqm) | DTT (cm) | NBPL | DBPL (cm) | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Area 4 | a | 2 | 2.9 | 70.6 | 22.7 | 3.3 | 29.4 | 3.95 | 13 | 7.6 |
| c | 0 | 0 | 80.9 | 19.1 | 0 | 19.1 | 0 | 16 | 7.2 | |
| d | 1 | 3 | 81.1 | 17.2 | 1.7 | 18.9 | 12 | 16 | 10.3 | |
| e | 1 | 3.2 | 80.3 | 18.2 | 1.5 | 19.7 | 4.5 | 23 | 4.2 | |
| f | 2 | 4.5 | 77.4 | 20.9 | 1.05 | 22.5 | 6 | 18 | 6.4 | |
| g | 1 | 2.5 | 77.5 | 21 | 1.5 | 22.5 | 8 | 12 | 11.7 | |
| h | 1 | 4 | 64.6 | 33.6 | 1.8 | 35.4 | 7 | 17 | 6.7 | |
| i | 0 | 0 | 87 | 13 | 0 | 13 | 0 | 14 | 9.5 | |
| m | 2 | 4.5 | 76.2 | 19.4 | 2.2 | 23.8 | 5.2 | 20 | 10 | |
| n | 0 | 0 | 70.2 | 29.8 | 0 | 29.8 | 0 | 28 | 8 | |
| 1.4 ± 0.2 | 3.5 ± 0.3 | 76.6 ± 2 | 21.5 ± 1.9 | 1.8 ± 0.3 | 22.8 ± 2 | 6.6 ± 1 | 17.7 ± 1.5 | 8.1 ± 0.7 | ||
| Area 6 | a | 4 | 7.4 | 13.9 | 36.8 | 49.3 | 86.1 | 19.9 | 1 | 31 |
| b | 4 | 6.2 | 26.5 | 31.8 | 41.7 | 73.5 | 24.3 | 5 | 25 | |
| c | 5 | 6.5 | 6.9 | 39.3 | 53.8 | 93.1 | 24.6 | 2 | 19.5 | |
| f | 1 | 8 | 4.2 | 75.8 | 22 | 95.8 | 34.9 | 4 | 20.3 | |
| h | 1 | 5 | 29.4 | 68.1 | 10 | 70.6 | 16.6 | 7 | 22.7 | |
| l | 0 | 0 | 29.9 | 70.1 | 0 | 70.1 | 0 | 6 | 12.5 | |
| n | 5 | 5 | 12.6 | 44.8 | 42.6 | 87.4 | 13.6 | 7 | 16.1 | |
| p | 1 | 6 | 30.1 | 66.1 | 5 | 69.9 | 18 | 4 | 21 | |
| r | 3 | 5 | 5.3 | 66.9 | 27.8 | 94.7 | 16.6 | 3 | 18.5 | |
| u | 2 | 6 | 4.1 | 59.7 | 46.2 | 95.9 | 20 | 5 | 18 | |
| 2.8 ± 0.5 | 6.1 ± 0.3 | 16.3 ± 3.6 | 55.9 ± 5 | 33.1 ± 5.8 | 83.7 ± 3.6 (*) | 20.9 ± 2 | 4.4 ± 0.6 | 20.4 ± 1.5 |
| DTS_UAV (cm) | DTS_GS (cm) | DBPL_UAV (cm) | DPBL_GS (cm) | ||
|---|---|---|---|---|---|
| Area 4 | a | 3.95 | 4.2 | 7.6 | 8.2 |
| c | 0 | 0 | 7.2 | 7.8 | |
| d | 12 | 13.6 | 10.3 | 11.5 | |
| e | 4.5 | 5.2 | 4.2 | 4.7 | |
| f | 6 | 7.2 | 6.4 | 6.6 | |
| g | 8 | 7.5 | 11.7 | 12.2 | |
| h | 7 | 6.2 | 6.7 | 7.3 | |
| i | 0 | 0 | 9.5 | 9.3 | |
| m | 5.2 | 6.5 | 10 | 10.5 | |
| n | 0 | 0 | 8 | 8.2 | |
| 6.6 ± 1 | 7.2. ± 1 | 8.1 ± 0.7 | 8.6 ± 0.7 | ||
| Area 6 | a | 19.9 | 21.9 | 31 | 31.5 |
| b | 24.3 | 25.5 | 25 | 25.6 | |
| c | 24.6 | 23.1 | 19.5 | 18.7 | |
| f | 34.9 | 36.5 | 20.3 | 21 | |
| h | 16.6 | 17.7 | 22.7 | 23.5 | |
| l | 0 | 0 | 12.5 | 13.1 | |
| n | 13.6 | 12.8 | 16.1 | 17.3 | |
| p | 18 | 17.5 | 21 | 22.1 | |
| r | 16.6 | 17.8 | 18.5 | 19.2 | |
| u | 20 | 21.8 | 18 | 18.7 | |
| 20.9 ± 2 | 21.6 ± 2.2 | 20.4 ± 1.5 | 21.1 ± 1.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bertacchi, A. UAVs Technology as a Complementary Tool in Post-Fire Vegetation Recovery Surveys in Mediterranean Fire-Prone Forests. Forests 2022, 13, 1009. https://doi.org/10.3390/f13071009
Bertacchi A. UAVs Technology as a Complementary Tool in Post-Fire Vegetation Recovery Surveys in Mediterranean Fire-Prone Forests. Forests. 2022; 13(7):1009. https://doi.org/10.3390/f13071009
Chicago/Turabian StyleBertacchi, Andrea. 2022. "UAVs Technology as a Complementary Tool in Post-Fire Vegetation Recovery Surveys in Mediterranean Fire-Prone Forests" Forests 13, no. 7: 1009. https://doi.org/10.3390/f13071009
APA StyleBertacchi, A. (2022). UAVs Technology as a Complementary Tool in Post-Fire Vegetation Recovery Surveys in Mediterranean Fire-Prone Forests. Forests, 13(7), 1009. https://doi.org/10.3390/f13071009






