Abstract
Reforestation through assisted natural regeneration usually accumulates more biomass carbon than through tree planting, but its effects on soil respiration (Rs) and its components, autotrophic respiration (Ra) and heterotrophic respiration (Rh), are poorly understood despite the importance in forest carbon cycling. In this study, we clear-cut part of a 35-year-old secondary Castanopsis carelesii (C. carelesii) forest and reforested the logged land with C. carelesii via two approaches—active tree planting and assisted natural regeneration—and measured Rs, Ra, and Rh as well as soil temperature and moisture in these forests. In the first two years following reforestation, Rs, Ra and Rh rates were mostly reduced in the two young forests compared to the secondary forest, likely due to reduced photosynthate production and thus carbon substrate input associated with the clear-cut. However, the Rh:Rs ratio was significantly greater in the young plantation than in the other two forests in the first two years, suggesting a greater loss of soil organic carbon from the young plantation. In the third year, the mean Rs, Rh, and Ra rates of the young forest established via assisted natural regeneration were similar to those of the secondary forest, but significantly greater than those of the young plantation. The rates of Rs, Rh, and Ra mostly increased exponentially with increasing soil temperature in all forests, but mostly lack significant relationships with soil moisture. These findings indicate that, compared with reforestation via tree plantation, assisted natural regeneration not only reduced the loss of soil organic carbon via soil respiration, but also had a more rapid recovery of soil respiration to the level of the secondary forest. Our study highlights that, in addition to temperature, carbon substrate availability is also important in regulating soil respiration following reforestation.
1. Introduction
To limit the future global temperature rise to <1.5 °C, approximately 730 billion tons of CO2 (or 199 billion tons of carbon, Pg C) need to be removed from the atmosphere by the end of this century [1,2]. Some believe that plantation forests can be effective in mitigating increases in atmospheric CO2 concentration [3,4,5]. However, certain forest management practices, such as slash burning and site preparation, often increase soil carbon emissions [6,7,8] and reduce the carbon sequestration potential of plantation forests. A recent synthesis reported that, compared with plantation forests, naturally regenerated forests are on average 40 times more effective in biomass carbon sequestration [9].
Soil respiration (Rs) is an important component of the forest carbon cycle, accounting for 30–80% of total forest respiration [10]. Thus, changes in Rs induced by forest management practices can have a significant impact on forest carbon cycling [11,12,13]. For example, slash burning, site preparation, and weeding can enhance soil respiration through increasing soil temperature, reducing soil moisture, destabilizing soil aggregates, and enhancing nutrient availability for soil microorganisms [8,14,15,16,17,18,19].
In contrast to plantation forests, assisted natural regeneration (ANR) involves minimum human intervention and typically commences with harvest residues retained on the forest floor [20,21]. The most fundamental practices of ANR are protecting and facilitating the growth of parent trees present in the area, rather than establishing an entirely new population of trees [20,22]. Retaining parent trees and harvest residues provides a rich carbon source for soil respiration [23]. However, little is known on how differences in carbon substrate inputs between plantation forests and ANR forests affect soil respiration.
Soil respiration consists of heterotrophic microbial respiration (Rh) and autotrophic root respiration (Ra). While photosynthates transported into fine roots are the main carbon source of Ra, soil organic carbon (SOC) is another major carbon source of Rh. Thus, separating Ra and Rh can help to elucidate the relative contributions of photosynthates and microbial decomposition of SOC to carbon efflux, which is of great importance. For example, the effects of the widely reported warming-induced enhancement of Rs [24,25] would have major effects on soil carbon stock if it is mainly due to the mineralization of SOC via Rh. On the other hand, the effects on soil carbon stock could be minimal if much of the enhanced Rs are due to elevated Rh stimulated by increases in belowground photosynthate allocation associated with warming-enhanced plant growth.
Differences in management practices between ANR and tree planting lead to their differences in Rs and its components [26,27,28,29]. For example, in the early stages of afforestation via tree planting, high temperatures due to low plant cover and high nutrient availability due to slash burning may increase SOC mineralization [30,31,32,33,34]. In contrast, retaining plant residues, parent trees, and understory plants (because of no slash burning) in ANR would maintain substantial Ra in the initial stages. With continuing stand development, Ra can be expected to increase through time in both ANR and plantation forests as root biomass increases, whereas the gradual depletion of nutrients left from slash burning would decrease their effects on enhancing Rh in plantation forests [23,35,36]. The high demand for nutrients during the rapid stand development stage in both ANR and plantation forests may also stimulate the decomposition of SOC [37,38,39,40]. Currently, how changes in biotic and abiotic conditions associated with different reforestation approaches affect Rs and its components over time are not well understood despite their importance on carbon cycling.
Because of the high carbon sink, biodiversity, and ecosystem service potential of forests established via ANR, this regeneration strategy has been practiced for nearly 60 years in several Asian countries, such as the Philippines, Thailand, and China [20,41,42]. However, we are unaware of studies examining the effects of ANR on soil respiration. In this study, we investigated Rs and its components and soil microclimate for three years in a secondary Castanopsis forest (Castanopsis carelesii (Hemsl.) Hayata), along with young C. carlesii forests established via ANR and active tree planting. We carried out the study for three years because the young forests are during the fast-growing stage, during which the quantity of fine roots, where autotrophic respiration take place, could vary considerably. In addition, the input of carbon substrate through aboveground and belowground litter as well as recent photosynthates (e.g., via root exudation) that are important for soil microbial respiration can also change considerably. Based on this experimental design, we aimed to test the following hypotheses. Compared with the young forest established through ANR, Rs and Rh were greater in the young C. carlesii plantation (H1) due to the higher temperature and nutrient availability favorable for soil respiration. Moreover, we hypothesized that the differences would vary through time (H2) due to the differences in the temporal patterns of soil biotic (e.g., plant residue availability and root biomass) and abiotic (e.g., soil temperature and moisture) conditions among the forests. Specifically, we expected that Rs and its components recovered more rapidly in the young forest established via ANR than the forest plantation due to its greater vegetation cover and more rapid vegetation growth in the first few years [23].
2. Materials and Methods
2.1. Study Site
The study was conducted at the National Field Research Station on Forest Ecosystems located in Chenda Town, Sanming City, Fujian Province, China (26°19′ N, 117°36′ E). The area is characterized by low elevation mountains and hills with an average slope of approximately 30°. The soils in this area are sandy and iron-rich Ferric Acrisol derived from black mica granite (Food and Agriculture Organization of the United Nations, 2017). The characteristic vegetation of the region is subtropical evergreen broad-leaved forest. The tree layer mostly dominated by C. carlesii, Elaeocarpus decipiens Hemsl., and Schima superba Gardn. et Champ., with a stand density of 3788 trees ha−1. The shrub layer was dominated by Itea chinensis Hook. et Arn, and Symplocos sumuntia Buch.-Ham. ex D. Don, and the herb layer was dominated by Gahnia tristis Nees and Dicranopteris dichotoma (Thunb.) Bernh. The average annual temperature is 20.1 °C (10 °C in January and 30 °C in July) between 2011 and 2015 and the main growing season extends from April to October. Mean annual precipitation is approximately 1550 mm, with 80% falling between March and August.
2.2. Experimental Design
The selected experimental site was a natural secondary forest (hereafter NSF) converted from a natural forest dominated by C. carlesii in 1976. In November 2011, an area of 1.1 ha on the southeast slope of the 35-year NSF was harvested to establish nine 20 m × 5 m experimental plots. The nine plots were randomly and evenly assigned to three treatments, C. Carlesii established through ANR, C. carlesii plantation, and Chinese fir (Cunninghamia lanceolate (Lamb.) Hook) plantation, using a randomized block design. Three replicates of the same treatment were randomly established in the upper, intermediate, and lower slopes. Three additional plots of the same size were also established on the three slope locations in the unlogged part of the NSF adjacent to the logged site. To remove differences resulting from different tree species, we focused the young C. carlesii ANR forest (hereafter YNR) and the young C. carlesii plantation (hereafter YCP) in this study, with the uncut NSF served as the control.
For the YNR, 4500–6000 evenly distributed seedlings of C. carlesii per hectare were retained when the NSF was harvested. Following traditional stem-only harvesting, the bole wood was removed, whereas branches, twigs, and leaves were retained and evenly spread over the logged land. The seedlings were carefully conserved during the first three years following logging and thereafter left to undergo secondary succession. By 2015, the tree layer mostly consisted of C. carlesii (55%) and Litsea cubeba (Lour.) Pers. (10%), the shrub layer was dominated by Callicarpa macrophylla Vahl and Ardisia punctata Lindl., and the herb layer was dominated by Gahnia tristis Nees and Hedyotis caudatifolia Merr. et Metcalf.
For the Castanopsis plantation, residues (branches, twigs, and leaves) were evenly spread over the logged area following the logging and subsequently burnt in March 2012. Then, C. carlesii seedlings were planted at a density of 2400 seedlings per hectare mimicking the commercial management practice of the region. During the first three to five years (prior to canopy closure), weeding was conducted twice annually, one in June–July and the other in November–December. By 2015, the tree layer mostly consisted of C. carlesii (82%), the shrub layer mainly consisted of Rhus chinensis Mill., Litsea mollis Hemsl., and Maesa japonica (Thunb.) Moritzi. ex Zoll, and the herb layer mainly consisted of G. tristis Nees and Carex cruciata Walhlenb.
In March 2012, seven 1 m × 1 m subplots were established in each plot for measuring soil respiration (Rs) and heterotrophic respiration (Rh). A polyvinyl chloride (PVC) collar (20.4 cm inner diameter and 7.5 cm height) was inserted 5 cm into the soil in four of the seven subplots (1 m × 1 m) for Rs measurements. Heterotrophic respiration was measured in the remaining three subplots using the trenching method. The trenches were lined with Nylon mesh sheets (100 mesh, Sefar, Heiden, Switzerland), which allowed lateral water movement but inhibited root ingrowth, and then refilled carefully with the excavated soil. In April 2012, PVC collars (20 cm in diameter) were inserted 5 cm into the soil for Rh measurements. Surface vegetation was removed from the sampling sites during the study period.
2.3. Measurement of Soil Respiration, Temperature, and Moisture
From 15 April 2012 to 15 April 2015, Rs and Rh were measured using an automated soil CO2 flux system (LiCOR Inc., Lincoln, NE, USA) equipped with a 20 cm measurement chamber (Model 8100-103). Rs and Rh were measured biweekly from 09:00 to 12:00 and Ra was calculated as the difference between Rs and Rh. Soil temperature was measured at a depth of 5 cm using a hand-held probe (Model SK-250WP; Sato Keiryoki Mfg.) and soil moisture (0–12 cm) was measured close to each PVC collar, using a time domain reflectometer (Model TDR300, Spectrum Technologies Inc., Plainfield, IL, USA) at the same time of Rs and Rh measurements.
2.4. Data Analysis
Our experimental design allowed us to examine the effects of management practices (i.e., forest type), sampling time and their interaction on Rs, Rh, Ra, and soil microclimate parameters using a two-way analysis of variance followed by least significant difference (LSD) tests. The differences of Rs, Rh, and Ra and Rh to Rs ratio among the forests were analyzed using one-way analysis of variance followed by LSD tests. Because Rs, Rh, Ra, and soil microclimate were expected to vary through time, the measurements were divided by year for the above tests. To minimize the transient effects caused by the decomposition of pre-existing dead roots, the Rh and Ra data of the first six months after trenching were not used for the above analyses. The relationships between soil microclimate and soil respiration and its components were examined by regression analysis for each forest type. The significance level of all analyses was set at p < 0.05.
The sensitivity of Rs, Rh, and Ra to temperature was characterized by Q10 values, using the following equations [43,44].
where R is soil respiration (total and its components, in μmol CO2 m−2 s−1), T (°C) is soil temperature at 5 cm depth, and α and β are fitted parameters. The differences of Q10 among the forests were analyzed using a one-way analysis of variance followed by LSD tests. PASW Statistics 18 (IBM SPSS software) was used for statistical analysis.
R = αexpβT
Q10 = exp10β
3. Results
3.1. Rainfall, Soil Moisture, and Temperature
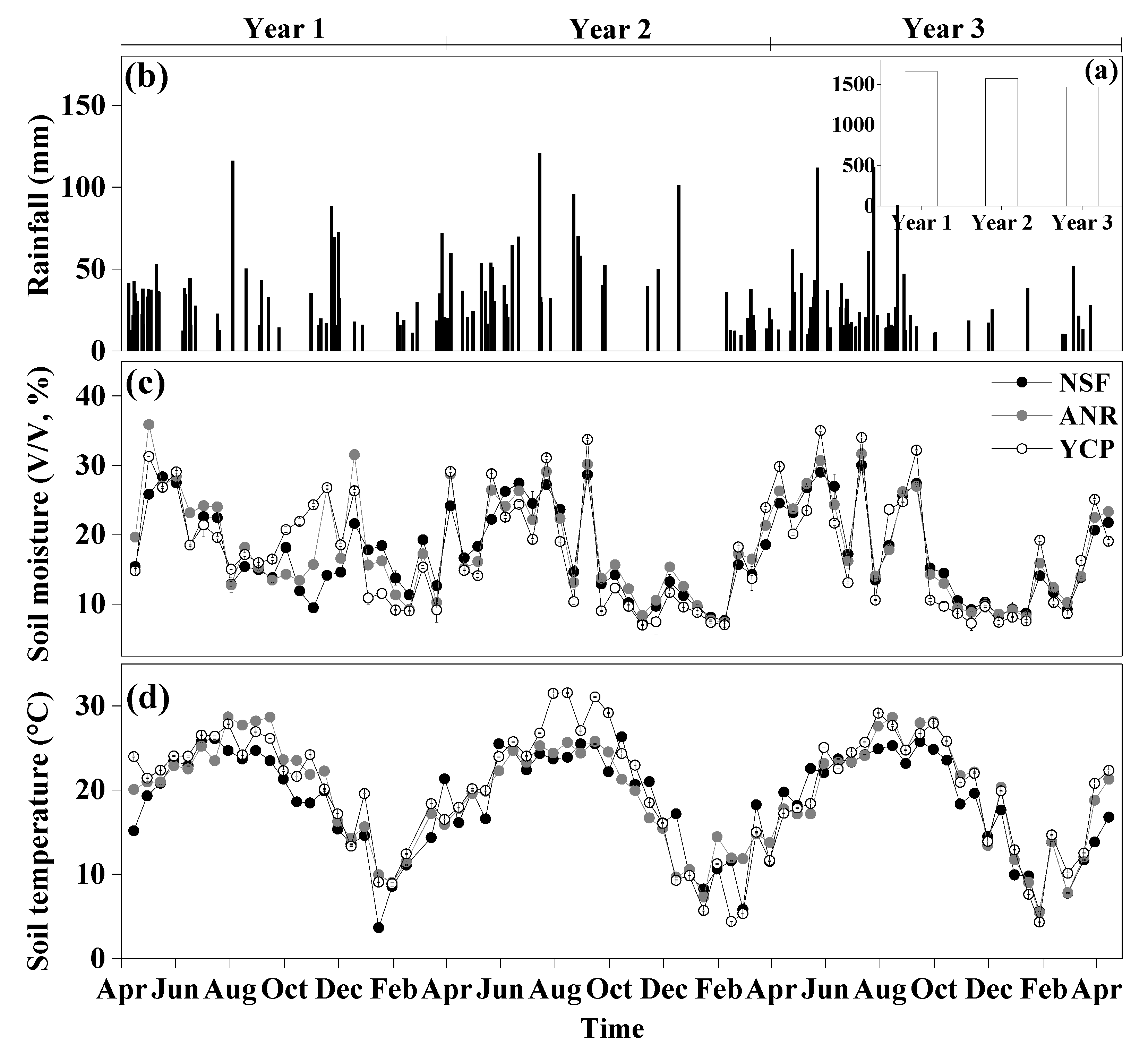
Rainfall, soil temperature, and moisture all showed significant differences among the forests for each of the three years, except that soil temperature was not significantly different among the forests in the second year (Table 1). Annual rainfall was approximately 1500 mm during the study period, mostly in March–August (Figure 1). The temporal pattern of soil moisture largely followed the rainfall pattern (Figure 1). Soil temperature also showed clear seasonal variation, near 30 °C in June–August but less than 10°C in December–February (Figure 1d).

Table 1.
Results of the two-way ANOVA showing the effects of forest type, sampling time and their interaction on total soil respiration (Rs), soil heterotrophic respiration (Rh), soil autotrophic respiration (Ra), soil temperature, and soil moisture.
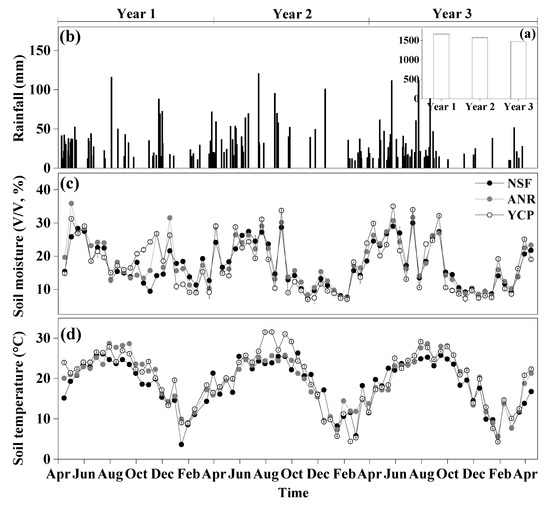
Figure 1.
Variation in rainfall, soil temperature, and soil moisture at a depth of 5 cm from 15 April 2012 to 15 April 2015, in a natural secondary forest (NSF), a young forest established through assisted natural regeneration (YNR), and a young Castanopsis carlesii plantation (YCP) (mean ± standard deviation, n = 3).
In the first year following the reforestation, the average soil temperature of the YNR (20.3 °C) and YCP (20.6 °C) was significantly higher than that of the NSF (18.6 °C). In the second year, the soil temperature was not significantly different among the forests (18.6–19.2 °C) and, in the third year, the soil temperatures of the YNR (19.7 °C) and YCP (20.1 °C) were again significantly higher than that of the NSF (18.2 °C).
Soil moisture was higher in the YNR (19.8%) than the YCP (19.1%), which in turn was higher than the NSF (17.7%) in the first year. In the second year, soil moisture was not significantly different between the NSF (16.9%) and YCP (16.4%), but both were lower than that of the YNR (17.5%). In the third year, it was significantly higher in the YNR (17.5%) than the YCP (16.9%), but neither was significantly different from that in the NSF (17.3%).
3.2. Total, Heterotrophic, and Autotrophic Soil Respiration
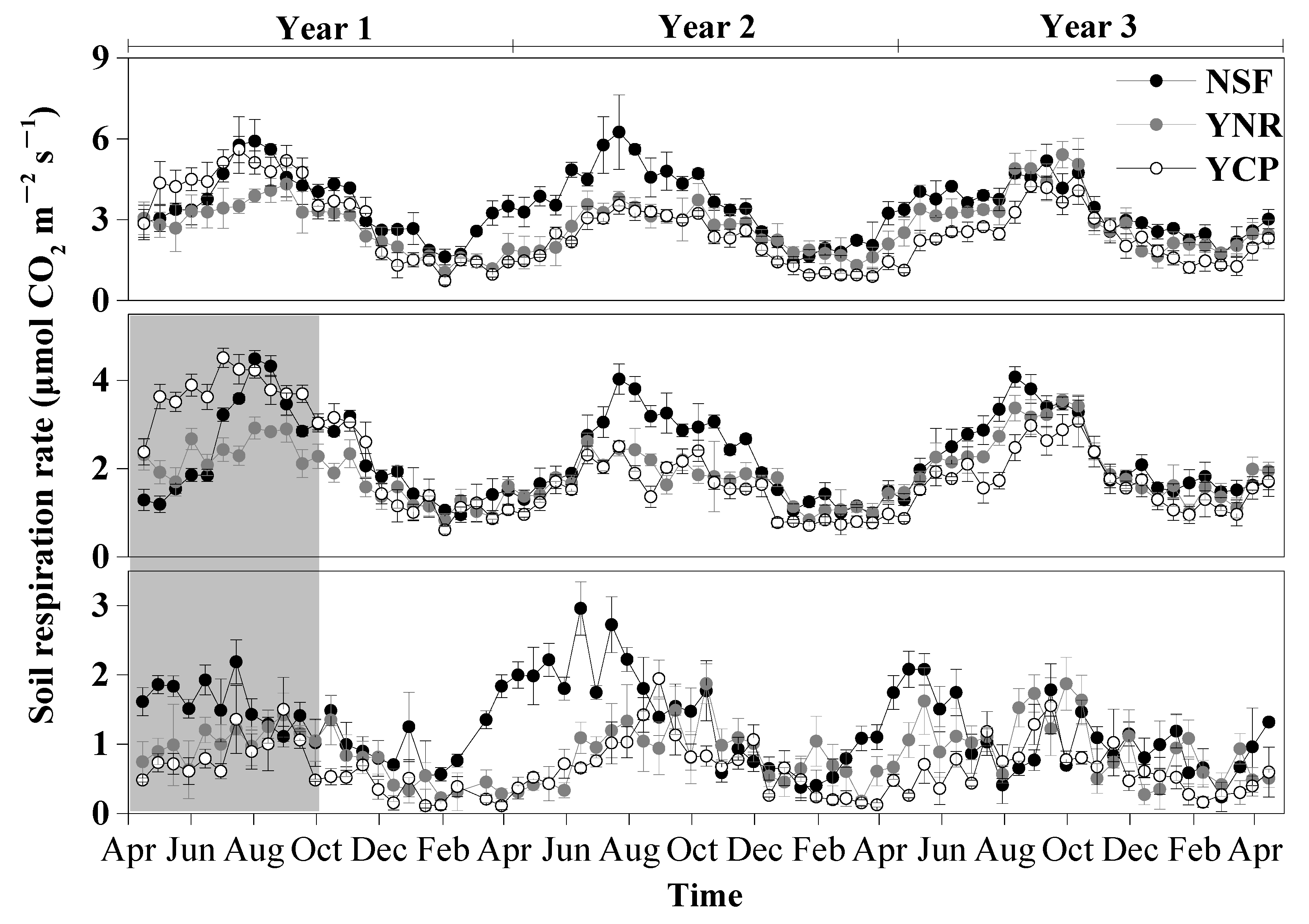
The rates of Rs and Rh exhibited strong seasonality, peaking in July–September and being the lowest in January–March (Figure 2). The seasonality of Ra was weaker than that of Rs and Rh (Figure 2).
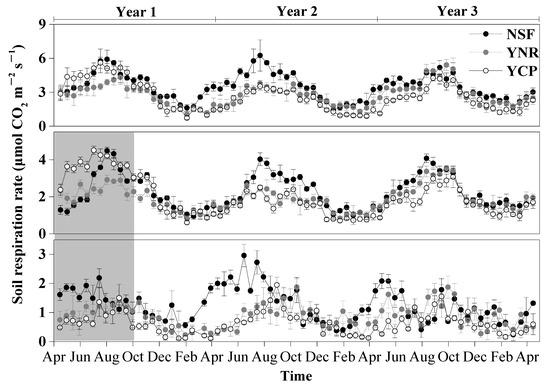
Figure 2.
Temporal variation of mean (±standard deviation) soil respiration (Rs), heterotrophic respiration (Rh), and autotrophic respiration (Ra) rates in the first three years following a reforestation experiment from 15 April 2012 to 15 April 2015 in a mature natural secondary forest (NSF), a young forest established through assisted natural regeneration (YNR), and a young Castanopsis carlesii plantation (YCP). Heterotrophic respiration rates measured in the first six month were shaded in gray and not used in the comparisons among the forests to account for transient effects caused by the decomposition of dead roots.
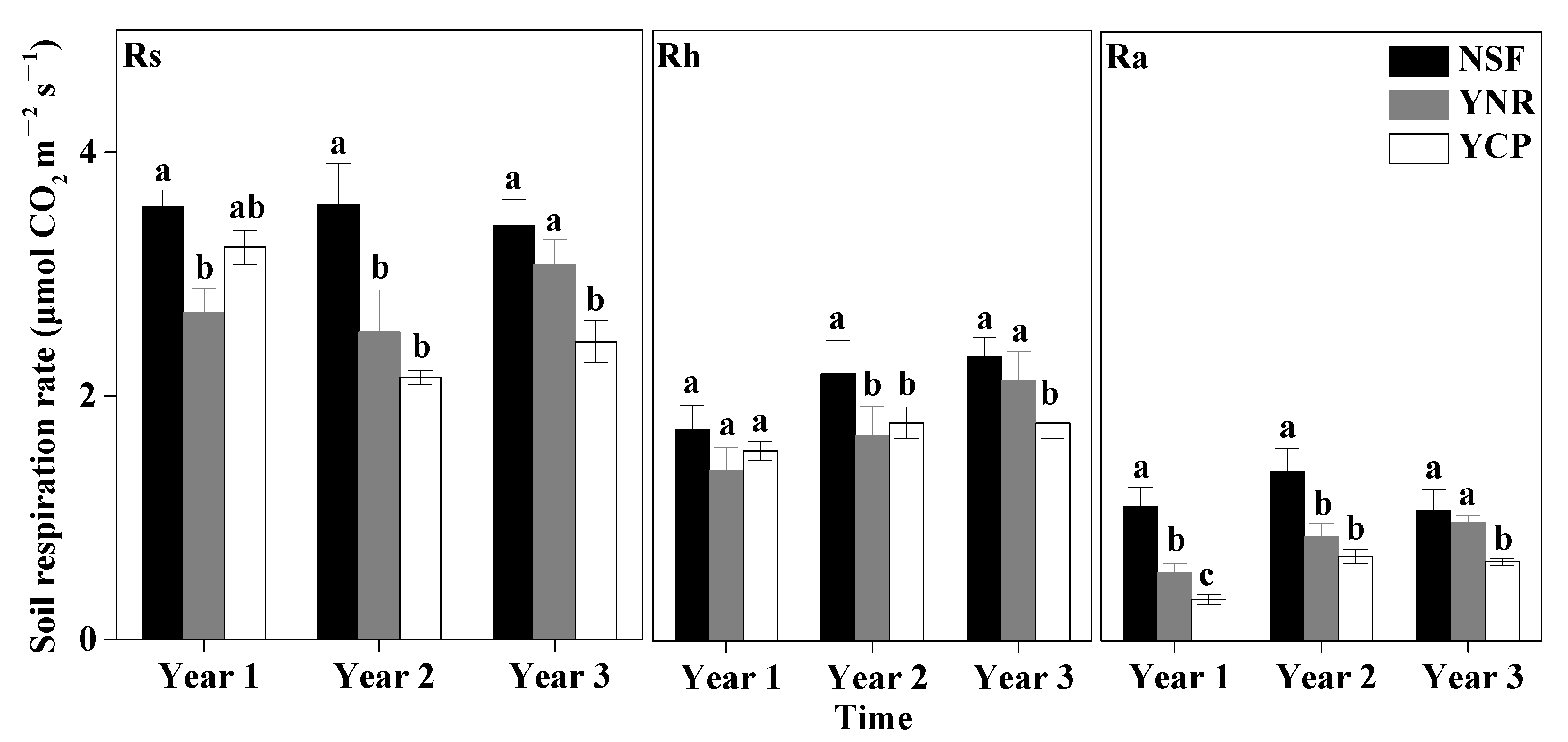
The effects of forest type and sampling time as well as their interaction on Rs, Rh, and Ra were all significant for each sampling year (Table 1). The differences of Rs, Rh, and Ra among the forests also varied among different sampling years. In the first year, the mean Ra rate was significantly higher in the NSF than in the YCP and the YNR. The mean Rs of the first year was significantly higher in the NSF than in the YNR, but not the YCP (p = 0.126). In contrast, the Rh rate was not significantly different among the forests (p = 0.064) (Figure 3).
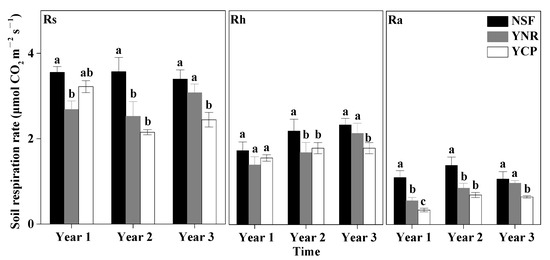
Figure 3.
Mean (±standard deviation) soil respiration (Rs), heterotrophic respiration (Rh), and autotrophic respiration (Ra) rates in the first three years following a reforestation experiment in a mature natural secondary forest (NSF), a young forest established through assisted natural regeneration (YNR), and a young Castanopsis carlesii plantation (YCP). For the first year, only data of the second half of the year were used to account for transient effects caused by the decomposition of dead roots. Different letters indicate significant different between forests at p < 0.05. For a given period of a give respiration rate (Rs, Rh, or Ra), forests sharing no common letters are significantly different at p < 0.05.
In the second year, none of the mean rates of Rs, Rh, and Ra was significantly different between the YCP and the YNR, but the rates of the two forests were significantly lower than those in the NSF, with the largest difference in March–September for Ra, in March–August for Rh, and in June–October for Ra (Figure 2 and Figure 3).
In the third year, none of the mean rates of Rs, Rh, and Ra of the YNR were significantly different from those of the NSF, but the rates of the two forests were significantly higher than those of the YCP by 26% (Rs), 19% (Rh), and 50% (Ra), with the largest difference in March–October for Rs, March–August for Ra, and June–October for Rh (Figure 2 and Figure 3).
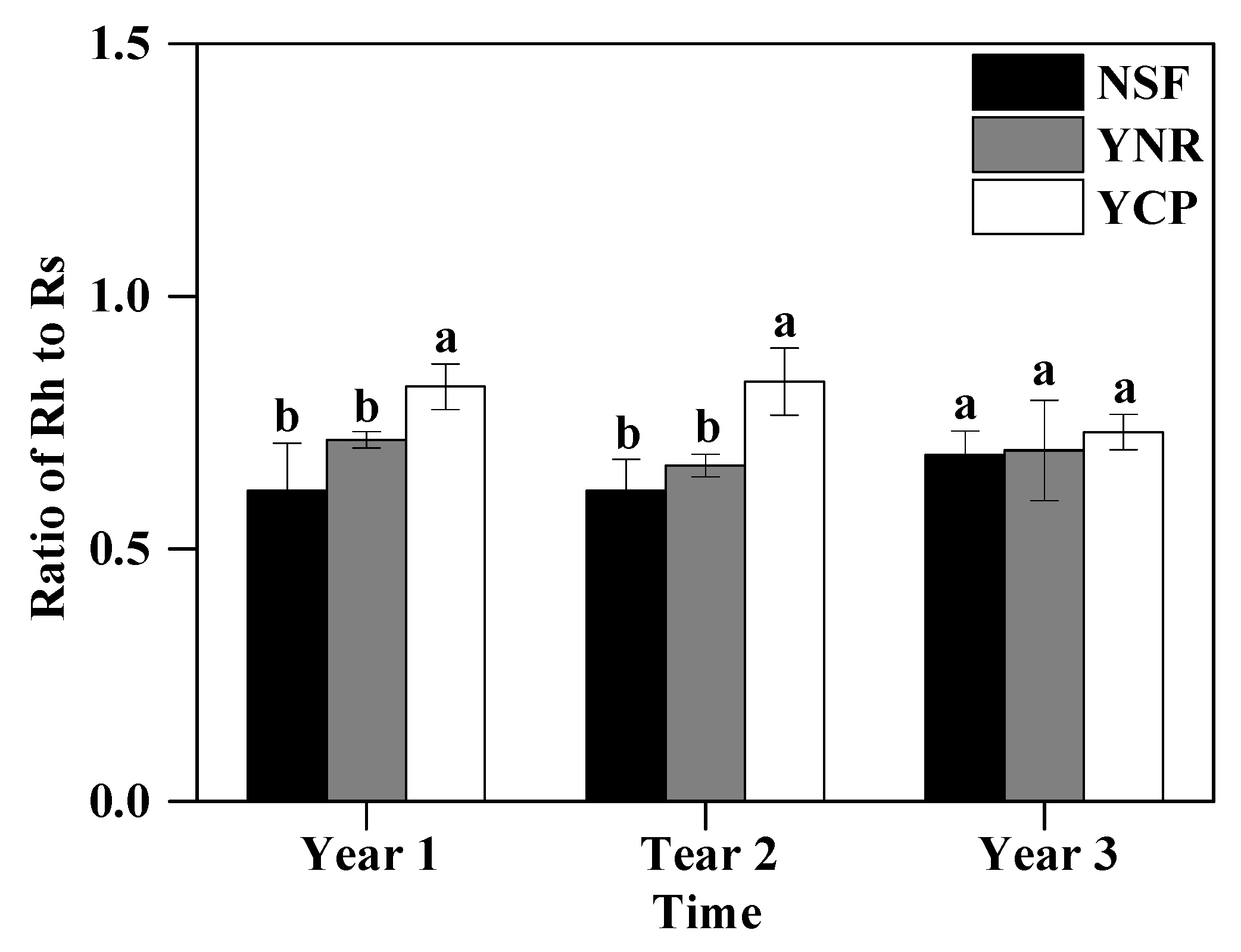
In the first and second years, the Rh-to-Rs ratio was significantly greater in the YCP (first year 0.82 and second year 0.83) than in the YNR (0.70 and 0.67) and the NSF (0.63 and 0.61), respectively, whereas no significant differences were detected between the YNR and the NSF (first year p = 0.133, second year p = 0.246) (Figure 4). The Rh-to-Rs ratio (0.61–0.73) was no longer significantly different among the forests in the third year (p = 0.708) (Figure 4).
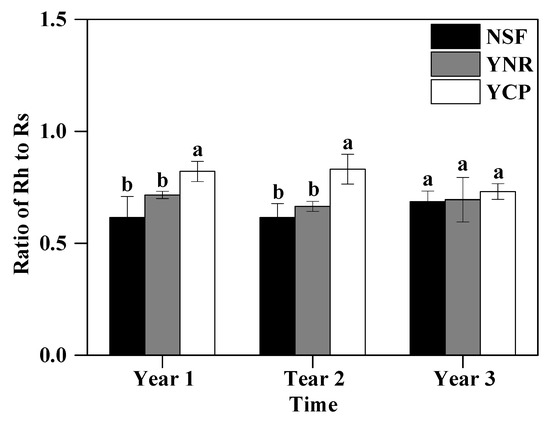
Figure 4.
Mean (± standard deviation) ratio of heterotrophic respiration (Rh) to total respiration (Rs) in the first three years following a reforestation experiment in a mature, natural secondary forest (NSF), a young forest established through assisted natural regeneration (YNR), and a young Castanopsis carlesii plantation (YCP). For the first year, only data for the second half of the year were used to account for transient effects caused by the decomposition of dead roots. Different letters indicate significant different between forests at p < 0.05. For a given period, forests sharing no common letters are significantly different at p < 0.05.
3.3. Relationships between Soil Temperature, Moisture, and Rs and Its Components
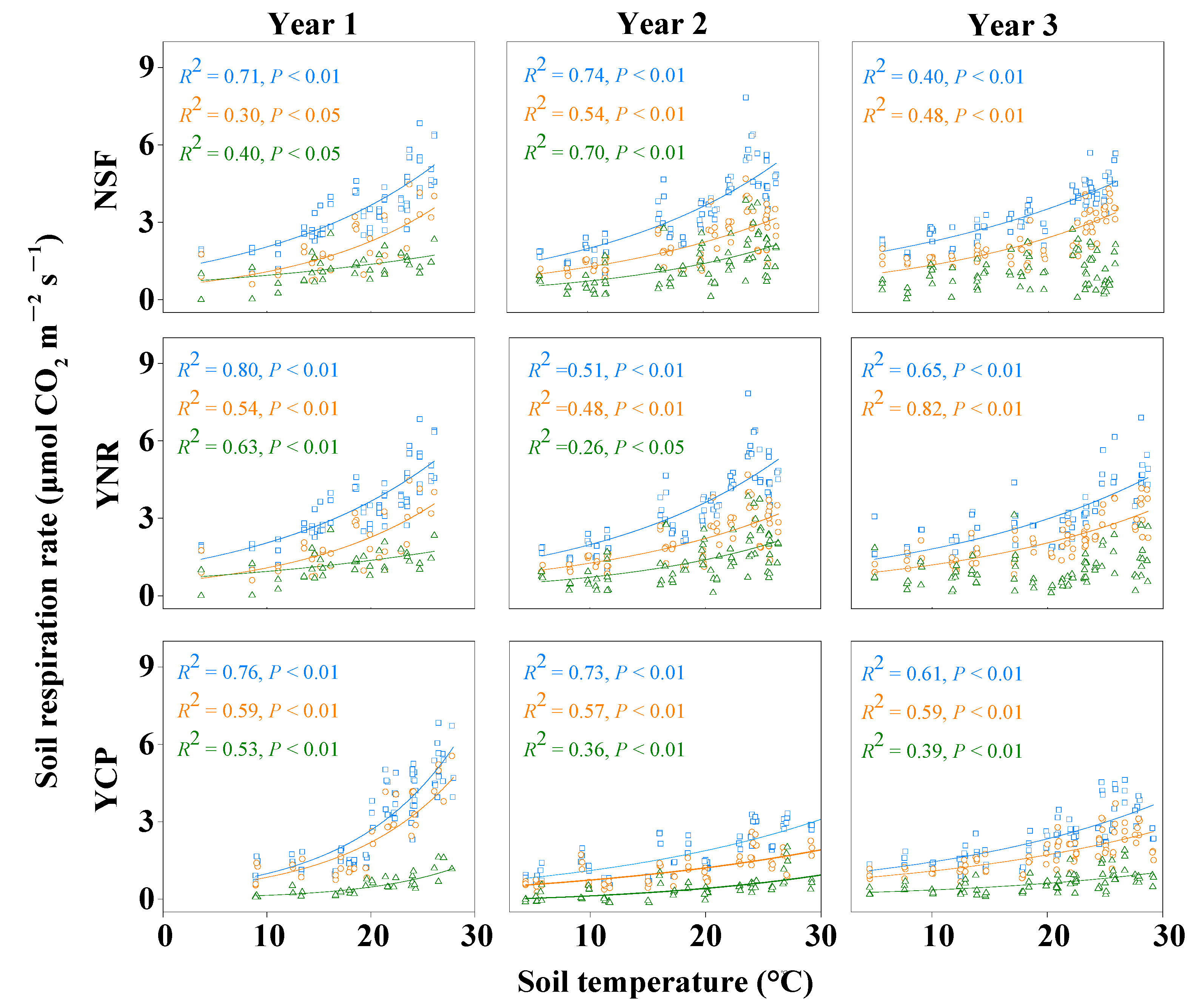
The rates of Rs, Rh, and Ra increased exponentially with increasing soil temperatures in all forests, except that Ra was not significantly related to soil temperature in the third year (Figure 5). Soil temperature explained 40–80% of the variation of Rs, 30–82% of Rh, and 26% to 70% of Ra across the three forest types over the three years (Figure 5). In the first year, the Q10 values of Rs, Rh, and Ra of the YCP were significantly higher than those of the NSF and YNR, and, in the second year, they were significantly higher in the NSF than the YCP and the YNR (p < 0.05); whereas, in the third year, Q10 values were similar among the forests: all approximately 1.6 (Table 2). Soil moisture was a poor predictor of Rs and its components, with most of the relationships not being significant (Table 3).
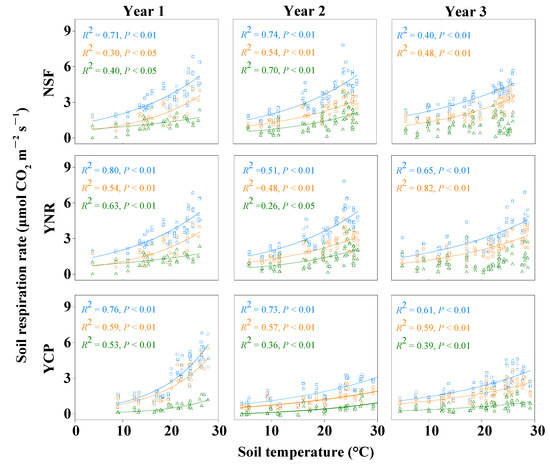
Figure 5.
The relationship between soil respiration (Rs, open squares and blue lines), heterotrophic respiration (Rh, open circles and red lines), and autotrophic respiration (Ra, open triangles and green lines) rates and soil temperature during the first three years of sampling time following a reforestation experiment. The fitted curves in the graph all reach the significant level (p < 0.05). NSF: natural secondary forest, YNR: young forest established via assisted natural regeneration, YCP: young Castanopsis carlesii plantation.

Table 2.
The temperature sensitivity (Q10) of total soil respiration (Rs), soil heterotrophic respiration (Rh), and soil autotrophic respiration (Ra).

Table 3.
The relationship between total soil respiration (Rs), soil heterotrophic respiration (Rh), soil autotrophic respiration (Ra), and soil moisture.
4. Discussion
4.1. Temporal Patterns of Soil Respiration and Its Components among Different Forests
The significant interactive effect of sampling time and forest type on Rs, Rh, and Ra indicate that different reforestation methods resulted in different temporal variations of total soil respiration and its components (Table 1) supporting our two hypotheses. The higher Rs in the secondary forest compared to the young forests in the first year was due to its higher Ra rate, because the Rh rate was not significantly different among the three forests (Figure 2 and Figure 3). The lower Ra in the young forests compared to the NSF could be largely explained by the great loss of roots, and therefore root respiration associated with logging, because root respiration is positively related to root biomass [45]. For example, a study of a Pinus banksiana Lamb stand reported that CO2 emissions reduced more than 50% in the first growing season following the logging, with the decreased root biomass as a major cause of the reduction [46]. Although we did not have the root biomass data for 2012, the coverage of trees could be used as a surrogate of root biomass. Tree cover was 75% in the YNR and only 17% in the YCP compared to 100% (closed canopy) of the NSF in 2012 [47]. The much greater tree cover in the YNR than the YCP can be used to explain the greater Ra in the YNR than the YCP.
The similar soil temperature and the lack of significant differences in Rs and Rh between YNR and YCP in the first year (Figure 1d and Figure 3) were contradictory to our H1. Possibly, the considerable loss of roots associated with clear-cutting led to the similarly lower soil respiration of the two young forests relative to the NSF at the very early stage of reforestation. In contrast to the reduced Ra, the young forests were able to maintain Rh similar to that of the secondary forest (Figure 3), despite their reduced production of litterfall and root exudates, as can be inferred from their lower tree cover described above, indicating the different responses of Ra and Rh to different reforestation approaches. Many studies have reported increases in soil temperature, moisture, and C-substrate efficacy following slash burning, all of which contribute to enhance Rh [30,31,32,33,34,48,49]. Thus, if substrate availability was similar among the forests, we would expect that, compared to the NSF, the higher temperature of the young forests would result in a higher Rh in the two young forests, not the observed similar Rh among the forests. In other words, it is possible that the positive effect of temperature on Rh in the young forests was largely offset by the lower carbon substrate input leading to the lack of significant differences of Rh between the young forests and the NSF.
However, the Rh became significantly lower in the young forests than the NSF in the second year (Figure 3). The similar temperatures, but lower Rs, Rh, and Ra, in the young forests than the NSF during the second year suggest that factors other than temperature were key to the variation rate of respiration among the forests. Soil respiration constrained by substrate availability in plantation forests relative to natural forests has been reported for subtropical forests in China [50]. In 2015, SOC was 2.56 kg ha−1 in the YNR, but only 1.99 kg ha−1 in the YCP [47]. Possibly, the limited new litter input from the young trees could not make up the gradual depletion of the pre-existing C substrate in the young forests so that Rh was limited by substrate availability. Similar to the first year, the lower production of photosynthates and smaller root biomass of the young forests likely contributed to their lower Ra than that of the NSF. The differences in soil respiration between YNR and YCP were most evident in the third year. During this period, the Rs, Rh, and Ra of the YNR were similar to those of the NSF, but the rates remained significantly lower in the YCP (Figure 3), confirming our expectation that predicted a more rapid recovery of Rs and its components in the YNR than the YCP. Several factors contributed to the more rapid recovery of soil respiration of the YNR than the YCP. Plant growth, including aboveground biomass and fine root growth, were more rapid in the YNR than the YCP. In 2013, fine root biomass was 4.2 t ha−1 in the ANR compared to 2.5 t ha−1 in the YCP [51], and by 2015 it was 53.6 t ha−1 in YNR compared to only 11.5 t ha−1 in YCP [23]. The more rapid plant growth of the ANR than the YCP was also evident from the near-closed canopy (95% tree cover) in the ANR compared to 67% tree cover in the YCP by 2015 [47]. More rapid tree growth means more photosynthate and fine root production, and therefore more below-ground carbon allocation to enhance Rh. Greater root biomass also contributed to greater Ra. The more rapid recovery of Rh and Ra together led to the more rapid recovery of Rs in the YNR than the YCP, and highlighted the role of productivity in regulating soil respiration as previously reported by several CO2-enrichment experiments [52,53,54,55,56]. In addition, a global synthesis of Rs partitioning studies has suggested that less productive ecosystems are associated with smaller proportions of Ra and greater proportions of Rh [57].
Dissecting Rs into autotrophic and heterotrophic components helps to reveal important insights into soil carbon cycling. While root respiration relies on recently produced photosynthates and those stored in plant biomass as the carbon source, microbial respiration also uses SOC as the carbon source. Therefore, soil presenting a rise in the Rh-to-Rs ratio could be prone to carbon loss. In our study, the significantly greater Rh-to-Rs ratio in the YCP compared to in the NSF and YNR in the first two years (Figure 4) indicates that, despite the similarly lower total soil respiration of the YCP and YNR relative to the NSF, proportionally more SOC was lost from the YCP than the YNR [57,58]. The greater SOC loss from the YCP than the YNR was supported by the greater SOC in the YNR than the YCP described above. Although photosynthate put into the soil was reduced following logging in both the YCP and the YNR, the reduction was greater in the YCP due to the consumption of plant residues by slash-burning and weeding that were only practiced in the YCP [8]. In addition to the direct reduction in photosynthate input, slash-burning and other site preparation practices (e.g., hole-digging) have been shown to disrupt the stability of soil aggregates, which may mobilize aggregate protected C, thereby making it more vulnerable to microbial decomposition [16,17]. However, with the rapid plant growth, inputs of photosynthates increased so that the Rh-to-Rs ratio was no longer significantly different among the forests in the third year (Figure 4).
4.2. The Responses of Q10 to the Forest Regeneration Approach
The temperature sensitivity of soil respiration (Q10) is important in predicting the response of soil respiration to warming [44,59]. Interestingly, Q10 values were significantly greater in the YCP than in the NSF and YNR in the first year, but they were significantly greater in the NSF than the YNR and YCP in the second year and became similar among the forests in the third year (Table 2). In the very early stage (the first year) following reforestation, the increases in temperature and nutrients associated with slash-burning likely led to higher microbial activity and root respiration in the YCP (than the NSF and YNR) when substrate and nutrient availability was not limited [16,60,61]. Although temperature also increased in the YNR, there was no increased nutrient availability from burning ash (as burning was not prescribed in YNR) so that microbial activity was not significantly enhanced relative to the NSF. However, with the gradual depletion of pre-existing carbon substrate, the temperature sensitivity of soil respiration was constrained by substrate availability in the young forests leading to their lower Q10 values than that in the NSF in the second year (Table 2). The substrate constraint in the young forests in the second year was supported by their lower Q10 values (YNR: 1.42, YCP: 1.50) relative to the values in the first year (YNR: 1.60, YCP: 2.10). With the rapid plant growth, substrate was no longer limited and temperature differences diminished. As a result, both the constrain by substrate availability and enhancement by elevated temperatures damped, leading to the similar Q10 (1.58–1.62) of soil respiration among the forests in the third year (Table 2), and the values were close to the global mean of 1.6 [62].
4.3. General Discussion
A variety of reforestation approaches involve different management practices that could have very different impacts on important ecological processes, including soil respiration. Our results clearly illustrate that reforestation via tree planting together with slash-burning and site preparation, among others, results in different temporal patterns of Rs and its component from the patterns associated with reforestation via assisted natural regeneration. Through maintaining plant residues that are important substrates for Rs, mainly microbial respiration, the YNR was able to maintain Rh similar to that of the NSF. More importantly, by the third year, Rs, Rh, and Ra of the YNR were similar to those of the NSF, while they remained significantly lower in the YCP. We attributed the more rapid recovery of Rs and its components of the YNR to its more rapid vegetation growth and thus greater litter input and root biomass, although we are lacking data on the litter input and root biomass of plants other than trees. Adding to the previously documented advantages [9,41,42], our study provided empirical evidence showing that assisted natural regeneration can restore soil respiration to the levels seen in mature natural forests in a relatively short period of time.
5. Limitations and Conclusions
Although we have clearly shown the differences in soil respiration and its components between reforestation via tree planting and assisted natural regeneration, the exact sources of the differences cannot be fully revealed from our measurements. For example, in addition to tree roots, the roots of shrubs and herbaceous plants also contribute to autotrophic respiration. Although we had information concerning the tree root biomass, density, and coverage of the forests, we did not have quantitative information for the coverage or root biomass of shrubs and herbaceous plants. For soil microbial respiration, part or even most of the differences between the young Chinese fir plantation and the young forest established through assisted natural regeneration could be due to residue burning that consumed SOC and damaged soil microorganisms that was practiced only in the plantation. However, the tree densities in our study basically followed the common practices of forest plantations and assisted natural regeneration of the region. Similarly, the differences in shrub and herbaceous plant coverage and composition reflected the differences that are to be observed between different reforestation practices and residue burning as part of a conventional practice of forest plantations of the region. Thus, it is fair to attribute the differences of soil respiration and its components between the forests to the different reforestation practices, and forest plantation versus assisted natural regeneration. However, to attain a more comprehensive and perhaps mechanistic understanding of the differences of soil respiration and its components between the forests, the coverage and root biomass of plants other than trees, and litter input and important soil properties (e.g., soil pH, texture, clay content, bulk density, soil porosity, microbial biomass) should be measured in future studies as much as possible.
Our study illustrates that the response of soil respiration and its components (autotrophic respiration and heterotrophic respiration) of reforestation varied through time and between different reforestation approaches. In the first year following the reforestation, the young Castanopsis forests were able to maintain the heterotrophic respiration rate similar to that of the 35-year NSF, possibly because carbon substrate was not limited. However, with the gradual depletion of pre-existing carbon substrate and the low input of new photosynthates, soil respiration, and its components, was significantly lower in the young forests than the secondary forest in the second year. The Castnopsis plantation had a significantly higher ratio of Rh to Rs than that of the young Catanopsis forest established through assisted nature regeneration in the first two years of reforestation, indicating the greater mineralization of SOC in the Catanopsis plantation during this period. In the third year, soil respiration, and its components of the forest established through assisted nature regeneration was not different from those of the secondary forests, but both were greater than those of the Castanopsis plantation. We also found that Q10 of soil respiration was not only influenced by temperature, but also by carbon substrate availability. Thus, the effect of warming on soil respiration and, therefore, the soil carbon pool could be confounded by its effect on substrate availability. Collectively, our study indicates that soil respiration and its components of the young forest established via assisted regeneration recovered to the levels similar to those of the secondary forest in the third year, whereas the young plantation had not. Consequently, our study illustrates that the adoption of assisted natural regeneration could make a valuable contribution to forest carbon sequestration.
Author Contributions
C.L., X.L., Z.Y. and Y.Y. designed the experiments; D.X., C.X., X.L. and S.C. performed the experiments; Z.W., Z.Y. and T.L. analyzed the data, wrote and revised the paper. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Nature Science Foundation of China (NO. 32192433, 32101495 and 31930071).
Informed Consent Statement
Written informed consent has been obtained from the patient(s) to publish this paper.
Data Availability Statement
Data used in this study are available upon reasonable request from the corresponding author.
Acknowledgments
We thank everyone who contributed to this article.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Lewis, S.L. The Paris agreement has solved a troubling problem. Nature 2016, 532, 283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- IPCC. Summary for policymarkers. In An IPCC Special Report on the Impacts of Global Warming of 1.5 °C above Pre-Industrial Levels and Related Global Greenhouse Gas Emission Pathways, in the Context of Strengthening the Global Response to the Threat of Climate Change, Sustainable Development, and Efforts to Eradicate Poverty; Allen, M., Babiker, M., Chen, Y., Coninck, H.D., Connors, S., Diemen, R.V., Dube, O.P., Ebi, K.L., Engelbrecht, F., Ferrat, M., et al., Eds.; Global warming of 1.5 °C; International Panel for Climate Change: Geneva, Switzerland, 2018; p. 26. [Google Scholar]
- Guedes, B.S.; Olsson, B.A.; Egnell, G.; Sitoe, A.A.; Karltun, E. Plantations of Pinus and Eucalyptus replacing degraded mountain miombo woodlands in Mozambique significantly increase carbon sequestration. Glob. Ecol. Conserv. 2018, 14, e00401. [Google Scholar] [CrossRef]
- Shao, P.; Liang, C.; Lynch, L.; Xie, H.T.; Bao, X.L. Reforestation accelerates soil organic carbon accumulation: Evidence from microbial biomarkers. Soil Biol. Biochem. 2019, 131, 182–190. [Google Scholar] [CrossRef]
- Wang, X.G.; Zhou, M.H.; Li, T.; Ke, Y.; Zhu, B. Land use change effects on ecosystem carbon budget in the Sichuan Basin of Southwest China: Conversion of cropland to forest ecosystem. Sci. Total Environ. 2017, 609, 556–562. [Google Scholar] [CrossRef] [PubMed]
- Giasson, M.A.; Coursolle, C.; Margolis, H.A. Ecosystem-level CO2 fluxes from a boreal cutover in eastern Canada before and after scarification. Agric. For. Meteorol. 2006, 140, 23–40. [Google Scholar] [CrossRef]
- La Scala, N.; Lopes, A.; Spokas, K.; Bolonhezi, D.; Archer, D.W.; Reicosky, D.C. Short-term temporal changes of soil carbon losses after tillage described by a first-order decay model. Soil Tillage Res. 2008, 99, 108–118. [Google Scholar]
- Yang, Z.J.; Chen, G.S.; Liu, X.F.; Xiong, D.C.; Xu, C.; Mary, A.A.; Rebecca, L.M.; Si, S.H.; Yang, Y.S. Loss of soil organic carbon following natural forest conversion to Chinese fir plantation. Forest Ecol. Manag. 2019, 449, 117476. [Google Scholar] [CrossRef]
- Lewis, S.L.; Wheeler, C.E.; Mitchard, E.T.; Koch, A. Regenerate natural forests to store carbon. Nature 2019, 568, 25–28. [Google Scholar] [CrossRef]
- Davidson, E.A.; Richardson, A.D.; Savage, K.E.; Hollinger, D.Y. A distinct seasonal pattern of the ratio of soil respiration to total ecosystem respiration in a spruce-dominated forest. Glob. Chang. Biol. 2006, 12, 230–239. [Google Scholar] [CrossRef]
- IPCC. Summary for policymarkers. Climate Change: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Stocker, T.F., Qin, D., Plattner, G.K., Tignor, M., Allen, S.K., Boschung, J., Nauels, A., Xia, Y., Bex, V., Midgley, P.M., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2013; pp. 1–29. [Google Scholar]
- Jandl, R.; Lindner, M.; Vesterdal, L.; Bauwens, B.; Baritz, R.; Hagedorn, F.; Johnson, D.W.; Minkkinen, K.; Byrne, K.A. How strongly can forest management influence soil carbon sequestration? Geoderma 2007, 137, 253–268. [Google Scholar] [CrossRef]
- Zhou, L.; Zhou, X.; Shao, J.; Nie, Y.; He, Y.; Jiang, L.; Wu, Z.T.; Hosseini Bai, S. Interactive effects of global change factors on soil respiration and its components: A meta-analysis. Glob. Chang. Biol. 2016, 22, 3157–3169. [Google Scholar] [CrossRef] [PubMed]
- Bergner, B.; Johnstone, J.; Treseder, K.K. Experimental warming and burn severity alter soil CO2 flux and soil functional groups in a recently burned boreal forest. Glob. Chang. Biol. 2010, 10, 1996–2004. [Google Scholar] [CrossRef] [Green Version]
- Bernoux, M.; Cerri, C.C.; Cerri, C.E.P.; Neto, M.S.; Metay, A.; Perrin, A.S.; Scopel, E.; Razafimbelo, T.; Blavet, D.; Piccolo, M.C.; et al. Cropping systems, carbon sequestration and erosion in Brazil: A review. Agron. Sustain. Dev. 2006, 26, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Mastrolonardo, G.; Rumpel, C.; Forte, C.; Doerr, S.H.; Certini, G. Abundance and composition of free and aggregate-occluded carbohydrates and lignin in two forest soils as affected by wildfires of different severity. Geoderma 2015, 245–246, 40–51. [Google Scholar] [CrossRef] [Green Version]
- Mataix Solera, J.; Cerdà, A.; Arcenegui, V.; Jordán, A.; Zavala, L.M. Fire effects on soil aggregation: A review. Earth Sci. Rev. 2011, 109, 44–60. [Google Scholar] [CrossRef]
- McLaughlin, J.W.; Gale, M.R.; Jurgensen, M.F.; Trettin, C.C. Soil organic matter and nitrogen cycling in response to harvesting, mechanical site preparation, and fertilization in a wetland with a mineral substrate. Forest Ecol. Manag. 2000, 129, 7–23. [Google Scholar] [CrossRef]
- Post, W.M.; Kwon, K.C. Soil carbon sequestration and land-use change: Processes and potential. Glob. Chang. Biol. 2010, 6, 317–327. [Google Scholar] [CrossRef] [Green Version]
- Dugan, P.C.; Durst, P.B.; Ganz, D.J.; Mckenzie, P.J. Advancing Assisted Natural Regeneration (ANR) in Asia and the Pacific; FAO Regional Office for Asia and the Pacific: Bangkok, Thailand, 2003. [Google Scholar]
- Li, Y.H. Methods of the natural regeneration of broad-leaved forests. J. Fujian Coll. For. 1985, 5, 21–26. (In Chinese) [Google Scholar]
- Shoo, L.P.; Catterall, C.P. Stimulating natural regeneration of tropical forest on degraded land: Approaches, outcomes, and information gaps. Restor. Ecol. 2013, 21, 670–677. [Google Scholar] [CrossRef]
- Yang, Y.S.; Wang, L.X.; Yang, Z.J.; Xu, C.; Xie, J.S.; Chen, G.S.; Lin, C.F.; Guo, J.F.; Liu, X.F.; Xiong, D.C.; et al. Large ecosystem service benefits of assisted natural regeneration. J. Geophys. Res.-Biogeo. 2018, 123, 676–687. [Google Scholar] [CrossRef]
- Hicks Pries, C.E.; Castanha, C.; Porras, R.C.; Torn, M.S. The whole-soil carbon flux in response to warming. Science 2017, 355, 1420–1423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, N.; Quesada, B.; Xia, L.; Butterbach-Bahl, K.; Goodale, C.L.; Kiese, R. Effects of climate warming on carbon fluxes in grasslands-A global meta-analysis. Glob. Chang. Biol. 2019, 25, 1839–1851. [Google Scholar] [CrossRef] [PubMed]
- Carvalhais, N.; Forkel, M.; Khomik, M.; Bellarby, J.; Jung, M.; Migliavacca, M.; Mu, M.Q.; Saatchi, S.; Santoro, M.; Thurner, M.; et al. Global covariation of carbon turnover times with climate in terrestrial ecosystems. Nature 2014, 514, 213–217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hardiman, B.S.; Gough, C.M.; Halperin, A.; Hofmeister, K.L.; Nave, L.E.; Bohrer, G.; Curtis, P.S. Maintaining high rates of carbon storage in old forests: A mechanism linking canopy structure to forest function. Forest Ecol. Manag. 2013, 298, 111–119. [Google Scholar] [CrossRef]
- Laganiere, J.; Angers, D.A.; Pare, D. Carbon accumulation in agricultural soils after afforestation: A meta-analysis. Glob. Chang. Biol. 2010, 16, 439–453. [Google Scholar] [CrossRef]
- Schlesinger, W.H.; Andrews, J.A. Soil respiration and the global carbon cycle. Biogeochemistry 2000, 48, 7–20. [Google Scholar] [CrossRef]
- Gordon, A.M.; Schlentner, R.E.; Cleve, K.V. Seasonal patterns of soil respiration and CO2 evolution following harvesting in the white spruce forests of interior Alaska. Can. J. For. Res. 1987, 17, 304–310. [Google Scholar] [CrossRef]
- Gupta, S.D.; DeLuca, T.H. Short-term changes in belowground C, N stocks in recently clear felled Sitka spruce plantations on podzolic soils of North Wales. Forest Ecol. Manag. 2012, 281, 48–58. [Google Scholar] [CrossRef]
- Hendrickson, O.Q.; Chatarpaul, L.; Burgess, D. Nutrient cycling following whole-tree and conventional harvest in northern mixed forest. Can. J. For. Res. 1989, 19, 725–735. [Google Scholar] [CrossRef]
- Londo, A.J.; Messina, M.G.; Schoenholtz, S.H. Forest harvesting effects on soil temperature, moisture, and respiration in a bottomland hardwood forest. Soil Sci. Soc. Am. J. 1999, 63, 637–644. [Google Scholar] [CrossRef]
- Lytle, D.E.; Cronan, C.S. Comparative soil CO2 evolution, litter decay, and root dynamics in clearcut and uncut spruce-fir forest. Forest Ecol. Manag. 1998, 103, 121–128. [Google Scholar] [CrossRef]
- Don, A.; Schumacher, J.; Freibauer, A. Impact of tropical land-use change on soil organic carbon stocks-a meta-analysis. Glob. Chang. Biol. 2011, 17, 1658–1670. [Google Scholar] [CrossRef] [Green Version]
- Van Noordwijk, M.; Cerri, C.; Woomer, P.L.; Nugroho, K.; Bernoux, M. Soil carbon dynamics in the humid tropical forest zone. Geoderma 1997, 79, 187–225. [Google Scholar] [CrossRef]
- Högberg, P.; Högberg, M.N.; Göttlicher, S.G.; Betson, N.R.; Keel, S.G.; Metcalfe, D.B.; Campbell, C.; Schindlbacher, A.; Hurry, V.; Lundmark, T.; et al. High temporal resolution tracing of photosynthate carbon from the tree canopy to forest soil microorganisms. New Phytol. 2007, 177, 220–228. [Google Scholar] [CrossRef]
- Liu, X.F.; Lin, T.C.; Yang, Z.J.; Vadeboncoeur, M.A.; Lin, C.F.; Xiong, D.C.; Lin, W.S.; Chen, G.S.; Xie, J.S.; Li, Y.Q.; et al. Increased litter in subtropical forests boosts soil respiration in natural forests but not plantations of Castanopsis carlesii. Plant Soil 2017, 418, 141–151. [Google Scholar] [CrossRef]
- McGuire, K.L.; Treseder, K.K. Microbial communities and their relevance for ecosystem models: Decomposition as a case study. Soil Biol. Biochem. 2010, 42, 529–535. [Google Scholar] [CrossRef] [Green Version]
- Tang, J.; Baldocchi, D.D.; Xu, L. Tree photosynthesis modulates soil respiration on a diurnal time scale. Glob. Chang. Biol. 2005, 11, 1298–1304. [Google Scholar] [CrossRef]
- Shono, K.; Cadaweng, E.A.; Durst, P.B. Application of assisted natural regeneration to restore degraded tropical forestlands. Restor. Ecol. 2007, 15, 620–626. [Google Scholar] [CrossRef]
- Yao, G.; Wang, H. Silviculture technique of natural second stand in Heilongjiang Province. J. Northeast. For. Univ. 1988, 16, 86–91. (In Chinese) [Google Scholar]
- Lloyd, J.; Taylor, J.A. On the temperature dependence of soil respiration. Funct. Ecol. 1994, 8, 315–323. [Google Scholar] [CrossRef]
- Li, Q.; Song, X.; Chang, S.X.; Peng, C.; Xiao, W.; Zhang, J.; Wang, W. Nitrogen depositions increase soil respiration and decrease temperature sensitivity in a Moso bamboo forest. Agric. For. Meteorol. 2019, 268, 48–54. [Google Scholar] [CrossRef]
- Striegl, R.G.; Wickland, K.P. Effects of a clear-cut harvest on soil respiration in a jack pine-lichen woodland. Can. J. For. Res. 1998, 28, 534–539. [Google Scholar] [CrossRef]
- Makita, N.; Pumpanen, J.; Köster, K.; Berninger, F. Changes in very fine root respiration and morphology with time since last fire in a boreal forest. Plant Soil 2016, 402, 303–316. [Google Scholar] [CrossRef]
- Xu, C.; Yang, Z.J.; Qian, W.; Chen, S.D.; Liu, X.F.; Lin, W.S.; Xiong, D.C.; Jiang, M.H.; Chang, C.T.; Huang, J.C.; et al. Runoff and soil erosion responses to rainfall and vegetation cover under various afforestation management regimes in subtropical montane forest. Land Degrad. Dev. 2019, 30, 1711–1724. [Google Scholar] [CrossRef]
- Guo, J.F.; Yang, Y.S.; Chen, G.S.; Xie, J.S.; Gao, R. Effects of clear-cutting and slash burning on soil respiration in Chinese fir and evergreen broadleaved forests in mid-subtropical China. Plant Soil 2010, 333, 249–261. [Google Scholar] [CrossRef]
- Hu, T.; Zhao, B.; Li, F. Effects of fire on soil respiration and its components in a Dahurian larch (Larix gmelinii) forest in northeast China: Implications for forest ecosystem carbon cycling. Geoderma 2021, 402, 115273. [Google Scholar] [CrossRef]
- Sheng, H.; Yang, Y.S.; Yang, Z.J.; Chen, G.S.; Xie, J.S.; Guo, J.F.; Zou, S.Q. The dynamic response of soil respiration to land-use changes in subtropical China. Glob. Chang. Biol. 2010, 16, 1107–1121. [Google Scholar] [CrossRef]
- Hu, S.C.; Xiong, D.C.; Huang, J.X.; Deng, F.; Chen, Y.Y.; Liu, X.F.; Chen, G.S. Fine root production in initial stage of Castanopsis carlesii under different regeneration modes in sanming, fujian province, China. Chin. J. Appl. Ecol. 2015, 25, 3259–3267. (In Chinese) [Google Scholar]
- Dias, A.; Ruijven, J.V.; Berendse, F. Plant species richness regulates soil respiration through changes in productivity. Oecologia 2010, 163, 805–813. [Google Scholar] [CrossRef] [Green Version]
- Drake, J.E.; Macdonald, C.A.; Tjoelker, M.G.; Crous, K.Y.; Gimeno, T.E. Short-term carbon cycling responses of a mature eucalypt woodland to gradual stepwise enrichment of atmospheric CO2 concentration. Glob. Chang. Biol. 2016, 22, 380–390. [Google Scholar] [CrossRef]
- Giardina, C.P.; Litton, C.M.; Crow, S.E. Warming-related increases in soil CO2 efflux are explained by increased below-ground carbon flux. Nat. Clim. Chang. 2014, 4, 822–827. [Google Scholar] [CrossRef] [Green Version]
- Macdonald, C.A.; Anderson, I.C.; Khachane, A.; Singh, B.P.; Barton, C.V.; Duursma, R.A.; Ellsworth, A.S.; Singh, B.K. Plant productivity is a key driver of soil respiration response to climate change in a nutrient-limited soil. Basic Appl. Ecol. 2021, 50, 155–168. [Google Scholar] [CrossRef]
- Schlesinger, W.H.; Lichter, J. Limited carbon storage in soil and litter of experimental forest plots under increased atmospheric CO2. Nature 2001, 411, 466–469. [Google Scholar] [CrossRef] [PubMed]
- Bond-Lamberty, B.; Wang, C.; Gower, S.T. A global relationship between the heterotrophic and autotrophic components of soil respiration? Glob. Chang. Biol. 2004, 10, 1756–1766. [Google Scholar] [CrossRef]
- Cahoon, S.; Sullivan, P.F.; Gamm, C.; Welker, J.M.; Eissenstat, D.; Post, E. Limited variation in proportional contributions of auto- and heterotrophic soil respiration, despite large differences in vegetation structure and function in the low arctic. Biogeochemistry 2016, 127, 339–351. [Google Scholar] [CrossRef]
- Xu, X.; Yang, B.; Wang, H.; Cao, Y.; Li, K.; Gao, S. Temperature sensitivity of soil heterotrophic respiration is altered by carbon substrate along the development of Quercus Mongolica forest in northeast China. Appl. Soil Ecol. 2019, 133, 52–61. [Google Scholar] [CrossRef]
- Barreiro, A.; Martin, A.; Carballas, T.; Diazravina, M. Long-term response of soil microbial communities to fire and fire-fighting chemicals. Biol. Fertil. Soils 2016, 52, 963–975. [Google Scholar] [CrossRef]
- Stirling, E.; Macdonald, L.M.; Smernik, R.J.; Cavagnaro, T.R. Post fire litters are richer in water soluble carbon and lead to increased microbial activity. Appl. Soil Ecol. 2019, 136, 101–105. [Google Scholar] [CrossRef]
- Mahecha, M.D.; Reichstein, M.; Carvalhais, N.; Lasslop, G.; Lange, H.; Seneviratne, S.I.; Richardson, A.D. Global convergence in the temperature sensitivity of respiration at ecosystem level. Science 2010, 329, 838–840. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).