Abstract
Soil enzymes are involved in the process of mineralization of soil organic matters. The close-to-nature transformation (CNT) of plantations changes the soil enzyme activities by changing the composition of stand vegetation, which in turn affects the change process of soil organic carbon. We therefore selected two typical coniferous plantations in southwest China, Pinus massoniana and Cunninghamia lanceolate, to explore the effects of CNT on soil enzyme activities and soil organic carbon, and the relationship between them is explored through comparative study. Compared with control stands (CCK and PCK), CNT enhanced soil organic carbon; the content of water-soluble organic carbon in the 0–10 cm soil layer of the transformed C. lanceolata plantations (CCN) is 81.29% higher than those in the control stands (p < 0.05); the contents of particulate organic carbon and water-soluble organic carbon in the 10–30 cm soil layer are 95.42% and 48.68% higher than those in the control stands (p < 0.05), respectively; after the CNT, the protease, urease, and acid phosphatase in C. lanceolata plantations were higher than control stands, while protease and catalase in P. massoniana plantations were higher than control stands. Correlation analysis showed that catalase and protease were more closely related to organic carbon components than other organic enzymes; redundancy analysis (RDA) results show that pH and total nitrogen are key factors that cause changes in carbon fractions after the CNT. In general, CNT enhanced soil organic carbon in coniferous plantations, which was more conducive to soil organic carbon accumulation but had a negative effect on soil organic carbon stability to a certain extent. Therefore, the effect of tree species configuration on soil carbon stability components should be considered in the forest management practice.
1. Introduction
The increase in forest carbon sink is an important way to achieve “peak emissions and carbon neutrality”. The forest soil is the main organic carbon pool in the terrestrial carbon cycle. The changes in its carbon storage play an important role in regulating the global carbon balance and mitigating the greenhouse effect [1]. The area of plantations in China ranks first in the world, with an area of about 79.5428 million hm2, which has a huge carbon sink capability [2]. It is considered to be the possible mechanism and the most potential choice for mitigating global change. It plays an increasingly significant role in mitigating climate change and promoting the realization of “peak emissions and carbon neutrality” [3]. Therefore, how to increase the storage and stability of soil carbon in plantations has become a hot topic and an urgent problem to be solved for scientists at home and abroad.
Soil organic carbon (SOC) is an important part of forest soil carbon pool and an important indicator of forest soil fertility [4]. However, due to the high background value of soil organic carbon, the response to changes in the short-term management model shows a certain lag, which cannot accurately reflect the change in soil quality and transformation rate in a short time [5]. It is found in the study that the active organic carbon of soil is more sensitive to forest management measures than total organic carbon, and can be taken as an indicator of early changes in organic carbon, which are usually represented by water-soluble organic carbon, readily oxidation organic carbon, particulate organic carbon, etc. The inactive organic carbon represents the accumulation and sequestration of soil organic carbon, such as recalcitrant organic carbon and recombinant organic carbon [6,7]. Therefore, the soil carbon pool fractions are used to quantitatively analyze the changes in soil organic carbon in south subtropical plantations, which is more conducive to studying the changes in soil organic carbon pool in this region.
“Close-to-nature transformation (CNT)” is a forest management model that is inclusive of forestry production and ecological protection, which originated in Germany. It is a feasible way to achieve multi-objective management of plantations [8]. The long-term practice has proved that this model can achieve different harvest objectives under the premise of stable development of forest ecosystems so as to maximize the comprehensive benefits of forests [9]. Compared with the monoculture plantations, the CNT has changed the vegetation community, tree species composition, quantity, and quality of litterfall, which affects the soil microhabitat, resulting in changes in soil nutrients, stand productivity, and species diversity [10,11]. The reduction in soil carbon input and the increase in carbon emissions in the early stage of CNT led to the loss of soil organic carbon and certain nutrient elements [12]. However, CNT was able to improve the species diversity of plantations, increase the carbon storage, improve the physical and chemical properties of soil, and reduce the greenhouse gas emissions [13], indicating that the impact of CNT on soil carbon was more complicated.
In addition, soil enzyme activity greatly affected the decomposition, transformation, and storage of soil organic carbon in the process of soil carbon cycling [14]. Among them, urease and acid phosphatase indirectly affect the soil carbon cycle by affecting soil nitrogen and phosphorus content [15], and catalase is also related to the conversion rate of soil organic matter [16]. On these grounds, the level of enzyme activities could characterize the demand for soil microbial nutrients and their relationship with the nutrient supply level. Although the relationship between soil enzyme activities and soil organic carbon components has been widely concerned, what are the effects of close-to-nature transformation on soil organic carbon components and enzyme activities? Is it beneficial to improve soil carbon sequestration and stability of stand? And what is the relationship between soil organic carbon fractions and enzyme activities and soil environmental factors? There is still no in-depth discussion and understanding of these issues.
Therefore, in order to elucidate the change characteristics of soil organic carbon components in P. massoniana and C. lanceolata plantations after CNT, the soil organic carbon components and enzyme activities of P. massoniana and C. lanceolata close-to-nature forests (PCN and CCN) and their control plantations (PCK and CCK) were compared in this study. We hypothesized that: (1) Compared with the control forests, CNT can promote soil organic carbon and stability; (2) The change of soil organic carbon was regulated by enzyme activity. The purpose of this study is to provide a scientific basis and decision-making reference for improving the understanding of the carbon cycle mechanism of plantations and the scientific management of the plantation ecosystem in the process of realizing dual carbon goals under the background of global change.
2. Materials and Methods
2.1. Site Description
The study site was conducted at the Guangxi Youyiguan Forest Ecosystem Research Station, the Experimental Center of Tropical Forestry, Chinese Academy of Forestry (22°10′ N, 106°50′ E), Pingxiang city, Guangxi Zhuang Autonomous Region in southern China. The region belongs to a subtropical monsoon climate, with an annual rainfall of 1200–1500 mm and an annual temperature of 20.5–21.7 °C. The soil is classified as an Oxisol in the USDA taxonomy [17]. The subtropical evergreen broad-leaved forest is the main vegetation type in this region.
2.2. Experimental Design
The experiment was conducted in the plantations of P. massoniana and C. lanceolata with an initial planting density of 2500 plants ha−1 in 1993. In 2004, the thinning was carried out, and the retention density was 1200 plants ha−1. In 2007, half of the plantations were transformed into mixed coniferous and broad-leaved uneven-aged plantations by thinning to 450 plants ha−1. In early 2008, two-year-old seedlings of Castanopsis fissa and Erythrophleum fordii were interplanted in the transformed plantations of P. massoniana and C. lanceolata, and the replanting density of C. fissa and E. fordii was both 375 plants ha−1. The non-transformed plantations of P. massoniana and C. lanceolata, which have the same plant density as the transformed plantations considered as the controls. There were four plantation types, including non-transformed C. lanceolata pure plantation (CCK), transformed C. lanceolata plantation (CCN), non-transformed P. massoniana pure plantation (PCK), and transformed P. massoniana plantation (PCN). Each plantation type was replicated four times, with an area of 20 m × 20 m for each plot. The stand characteristics are shown in Table 1.

Table 1.
The stand characteristics in four plantations measure in August 2018.
2.3. Samplings and Measurements
Soil samples were collected in August 2018 to determine soil chemical properties. In total, 144 soil sampling points were randomly selected within the 16 sampling plots in each of the 4 stands. The fresh and partially decomposed litter on the surface were removed, and then soil samples were collected at three depth, 0–10, 10–30, and 30–50 cm. A total of 27 soil cores were collected using an 8.7 cm diameter stainless steel core from each plot and bulked to one composite sample per depth. Each soil sample was divided into two parts: one part (0–50 cm) was naturally air-dried to determine the soil’s organic carbon components and chemical properties of the soil, and the other part (0–10 cm) was stored in a refrigerator at 4 °C for determining the soil enzyme activities, ammonium nitrogen, and nitrate nitrogen.
The content of soil organic carbon (SOC) was measured by K2Cr2O7 outside heating method [18]; soil pH was measured in deionized water using a glass electrode. Total phosphorus (TP) was measured by sodium hydroxide alkali fusion-molybdenum antimony colorimetry, and available phosphorus (AP) was measured by the ammonium fluoride-hydrochloric acid extraction method [19]. Total nitrogen (TN), ammonium nitrogen (NH4+-N), and nitrate nitrogen (NO3−-N) of soil were measured by the Kjeldahl method and spectrophotometry, respectively, and alkali-hydrolyzable nitrogen (AN) was measured by alkaline hydrolysis diffusion method [18]. Total potassium (TK) was measured by sodium hydroxide alkali fusion method and flame photometer, and available potassium (AK) was measured by ammonium acetate extraction method and flame photometer [20].
Particle organic carbon (POC) was measured with [(NaPO3)6] as the dispersant for separation, which was dispersed by hand shaking (15 min) and oscillator (90 r min−1, 18 h) respectively, and dried by a 53 μm sieve (60 °C) and then analyzed with an elemental analyzer [21]. Readily oxidation organic carbon (ROOC) was measured by the KMnO4 oxidation method [22]. Water-soluble organic carbon (WSOC) was extracted by KCL and then measured with a TOC instrument [17], and recalcitrant organic carbon (ROC) was digested with hydrochloric acid [23].
Catalase (CAT) activities were measured by ultraviolet spectrophotometry [24], protease (PRO) activities were measured by casein colorimetry [25], urease (URE) activities were measured by sodium phenol colorimetry [26], and acid phosphatase (ACP) activities were measured by sodium diphenyl phosphate colorimetry [27].
2.4. Statistical Analyses
The analysis of environmental factors affecting SOC and its fractions was conducted by using the “vegan”, “agricolae”, and “plyr” packets. The analysis process is completed on R 4.1.0 [28]. The differences in chemical properties of different stands were tested by the use of one-way analysis of variance (one-way ANOVA) and least significant difference (LSD). The correlation between soil enzyme activities, organic carbon fractions, and physical and chemical properties was tested by Pearson correlation analysis. Redundancy analysis (RDA) with Canoco 4.5 software (Microcomputer Power, Inc., Ithaca, NY, USA) was carried out for soil chemical properties, enzyme activities, soil organic carbon, and its fractions. All the statistics in this paper conform to normal distribution and meet the variance homogeneity test. The statistical significance test was set as p < 0.05.
3. Results
3.1. Soil Chemical Properties
Soil organic carbon, total nitrogen, alkali-hydrolyzable nitrogen, total phosphorus, and available phosphorus of all stands are decreasing with the increase in soil layers, and the vertical distribution is obvious. The contents of soil organic carbon, total nitrogen, alkali-hydrolyzable nitrogen, total phosphorus, nitrate nitrogen, and ammonium nitrogen at different soil levels of CNT were greater than those of the control forests in the C. lanceolata plantations. The contents of soil organic carbon, total phosphorus, nitrate nitrogen, and ammonium nitrogen of transformed P. massoniana plantations were higher than those of the control plantations. However, no significant difference was detected in the contents of all nutrients of P. massoniana and C. lanceolata between the close to natural and control forests (Table 2).

Table 2.
Effects of close-to-nature transformation on soil chemical properties of Pinus—Cuninghamia plantations. Mean ± SE.
3.2. Soil Organic Carbon Fractions
The four kinds of organic carbon content in the same stand had obvious vertical distribution characteristics. Except for readily oxidation organic carbon, they all decrease significantly with the increase in soil depth. Four organic carbons in transformed plantations are higher than those in the control plantations (except for 10–30 cm, readily oxidation organic carbon) in C. lanceolata plantations. In the 0–10 cm soil layer, the content of water-soluble organic carbon in the transformed plantations is 81.29%, significantly higher than those in the control stands, and in the 10–30 cm soil layer, the contents of particulate organic carbon and water-soluble organic carbon in the transformed plantations are 95.42% and 48.68% significantly higher than those in the control stands, respectively (p < 0.05). However, there is no obvious change law for the contents of four organic carbons in P. massoniana plantations, nor a significant difference between the reference plantations and the transformed plantations. In general, regardless of whether it is transformed or not, the four soil organic carbon fractions of P. massoniana plantations are higher than those of C. lanceolata plantations, and some are significantly higher than those of C. lanceolata plantations (Figure 1).
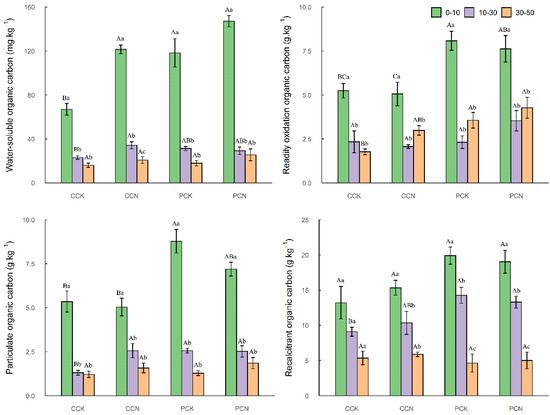
Figure 1.
Characteristics of soil organic carbon fractions after close-to-nature transformation. CCK, CCN, PCK, and PCN represent the control and close-to-nature transformation of C. lanceolata plantation and the control and close-to-nature transformation of P. massoniana plantation, respectively. Different small letters meant significant differences among different soil layers in the same stands, and different capital letters meant significant differences among different stands in the same soil layers at p < 0.05.
3.3. Soil Enzyme Activities
The activities of protease, urease, and acid phosphatase in transformation C. lanceolata plantation are 5.62%, 18.75%, and 28.40% higher than in control stands, and in P. massoniana plantations after transformation, the activities of protease and catalase are 7.37% and 6.00% higher than control stands, respectively, while the activities of urease and acid phosphatase are reduced (Figure 2). Except for protease, the changes in the activities of other enzymes in C. lanceolata and P. massoniana plantations are exactly opposite, but the activities of four enzymes in the 0–10 cm soil layer of C. lanceolata and P. massoniana plantations (p > 0.05) are not significantly affected by the CNT.
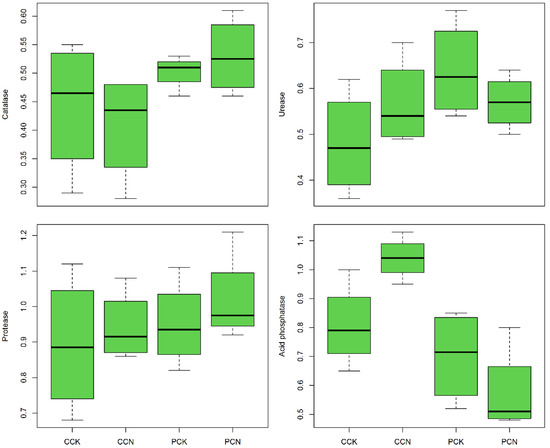
Figure 2.
Characteristics of soil enzyme activities after close-to-nature transformation (0–10 cm). CCK, CCN, PCK, and PCN represent the control and close-to-nature transformation of C. lanceolata plantation and the control and close-to-nature transformation of P. massoniana plantation, respectively.
3.4. Correlation Analysis of Organic Carbon Fractions with Enzyme Activities and Chemical Factors
There was no correlation between catalase and water-soluble organic carbon, but a positive correlation between catalase and other organic carbon components and a significant positive correlation between protease and recalcitrant organic carbon. Soil pH, total potassium, and available potassium are significantly negatively correlated with organic carbon fractions. Total nitrogen is significantly positively correlated with organic carbon fractions, alkali-hydrolyzable nitrogen is significantly positively correlated with recalcitrant organic carbon, and available phosphorus is significantly positively correlated with water-soluble organic carbon, readily oxidation organic carbon, and particulate organic carbon. Acid phosphatase, total phosphorus, ammonium nitrogen, and nitrate nitrogen are not significantly correlated with organic carbon fractions (Table 3).

Table 3.
Correlation coefficients of soil enzyme activity, organic carbon fractions, and chemical factors (0–10 cm).
RDA was used to determine the relationship among the organic carbon fractions, soil chemical properties, and enzyme activities (Figure 3). The result showed that the variations in the cumulative interpretation of the first and second axes were 76.3%. The first axis indicates the variables in 64.9%, and the second axis explains the variables in 11.4%. Permutation tests showed that the variations observed in overall organic carbon fractions were best explained by the vectors of soil chemical properties (pH, total nitrogen, total potassium, available potassium, and available phosphorus) (p < 0.05). The change in the content of organic carbon fractions was negatively correlated with pH, total potassium, and available potassium and was positively correlated with the total nitrogen and available phosphorus.
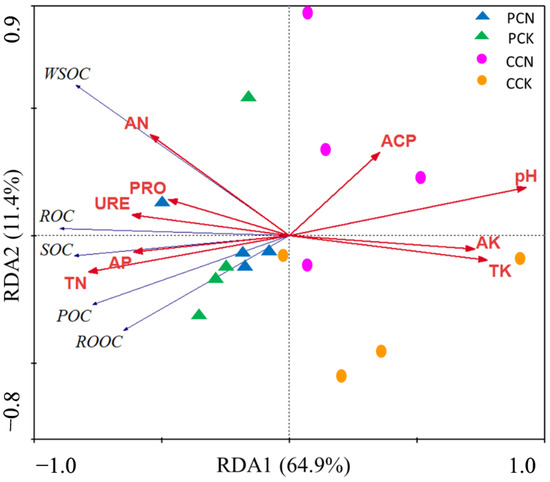
Figure 3.
Redundancy analysis (RDA) of soil chemical properties and enzyme activities on soil organic carbon and its organic carbon fractions. Organic carbon fractions (SOC: Total organic carbon; WSOC: Water-soluble organic carbon; ROOC: Readily oxidation organic carbon; ROC: Recalcitrant organic carbon; POC: Particulate organic carbon) are the response variables, and soil chemical properties and enzyme activities (PRO: Protease; URE: Urease; ACP: Acid phosphatase; TN: Total nitrogen; TN: Total nitrogen; AN: Alkali-hydrolyzable nitrogen; AP: Available phosphorus; TK: Total potassium; AK: Available potassium) are explanatory variables. CCK, CCN, PCK, and PCN represent the control and close-to-nature transformation of C. lanceolata plantation and the control and close-to-nature transformation of P. massoniana plantation, respectively.
4. Discussion
In this study, the content of soil organic carbon in C. lanceolata and P. massoniana plantations after close-to-nature transformation was higher than that of their control plantations in 2018 (10 years after the CNT). At the same time, after the transformation, the difference in available phosphorus content in the C. lanceolata plantations was reduced, while the available phosphorus content in deep soil was increased (Table 2), and other nutrient contents increased to varying degrees. Previous studies in 2010 (2 years after the CNT) showed that the content of available phosphorus in the transformed C. lanceolata plantations was significantly lower than that in the reference plantations, and the content of soil organic carbon in the transformed P. massoniana plantations was significantly lower than that in the reference plantations [12]. It can be found that the loss of soil organic carbon and some nutrient elements caused by the early close-to-nature transformation has been improved, which is conducive to the sequestration of soil carbon and the accumulation of nutrients in the plantations. Different study durations will also lead to different study results. The CNT time is not long enough so that the difference among stands with different treatments is not significantly different, and the transformation effect is not obvious. However, with the passage of time, the productivity of stand after CNT will gradually increase, which will gradually increase the input of soil organic matter and reduce the decomposition rate of organic matter [29]. Therefore, the long-term effects of close-to-nature transformation on the content of organic carbon at different stages need to be continuously studied.
The readily oxidation organic carbon content of soil in C. lanceolata and P. massoniana plantations decreased first and then increased with the soil depth. This may be because the readily oxidation organic carbon is more easily oxidized and decomposed in the soil, and the decomposition products move from the topsoil layer to the lower layer with water, which are sequestrated by the microorganisms in the lower layer and then become a potential carbon source for its growth [30], thereby increasing the accumulation of readily oxidation organic carbon in the deep layer. In contrast, no significant difference was observed in readily oxidation organic carbon tween the transformed C. lanceolata and P. massoniana plantations and their control plantations. On the one hand, it may be the result of mutual transformation between readily oxidation organic carbon and other forms of organic carbon in the sample plot, which maintains the stable fluctuation of the readily oxidation organic carbon content in soil [31]. On the other hand, the readily oxidized organic carbon was not sensitive to the change in tree species composition. However, the more detailed reasons need to be further analyzed.
Particulate organic carbon in soil can quickly and effectively reflect the changes in overall soil quality so that it can be used as a sensitive indicator of changes in soil organic carbon pool and can also indicate the evolution of soil fertility [32]. The content of particulate organic carbon in the close-to-nature transformation plantation at 10–30 cm soil layer was significantly higher than that in the control plantation. The results showed that the CNT was not only beneficial to the accumulation of soil organic carbon but also could effectively improve soil quality. However, there was no significant difference in particulate organic carbon content between topsoil and deep soil. The reason may be that the topsoil layer has a large amount of litterfall and incomplete decomposition, and the failure to remove the semi-decomposed litterfall during sample collection and subsequent treatment, resulting in little difference in the particulate organic carbon content of the topsoil in the sample plot [31]. Moreover, the deep soil has a weaker agglomeration capacity and fewer root exudates [31]. Therefore, the content of particulate organic carbon between the transformed plantations and the reference plantations is not significantly different.
Recalcitrant organic carbon is an organic carbon fraction that has existed in the soil for a long time and is difficult to be decomposed and utilized by microorganisms, and it is also a reserve carbon source for microbial decomposition [33]. In this study, it is found that recalcitrant organic carbon and organic carbon have the highest correlation coefficient (0.97), which is consistent with previous study results [34]. Recalcitrant organic carbon is closely related to the accumulation of organic carbon. Alkali-hydrolyzable nitrogen, available phosphorus, total potassium, and available potassium showed consistent changes with recalcitrant organic carbon in P. massoniana plantations after CNT, suggesting that soil available nutrients may be correlated with soil organic carbon components. The chemical properties of soil (pH, total nitrogen, total potassium, available potassium) in Table 3 are significantly correlated with soil organic carbon and organic carbon fractions. Therefore, whether the change of soil chemical properties is an important factor causing the change of soil carbon components in stands with different treatments is worth further study. At the same time, the decrease in recalcitrant organic carbon also indicates that the CNT has an impact on the stability of soil carbon, which intends to promote the conversion of recalcitrant organic carbon to active fractions [35]. The correlation analysis in this study also shows that recalcitrant organic carbon is significantly positively correlated with particulate organic carbon and readily oxidation organic carbon (Table 3).
The results revealed that soil pH and total nitrogen were the main environmental factors that drive the variation of organic carbon and its fractions (Figure 3). This is consistent with the results of many previous studies that soil pH can change the organic carbon by affecting microorganisms [36]. The study by Liao Dan et al. [37] also showed that the contents of activated carbon, chronic carbon, and recalcitrant organic carbon were highly significantly positively correlated with TN. With the higher N content, the soil carbon pooling can be significantly increased, the carbon:nitrogen ratio can be reduced, and the microbial activity can be improved. With the increase in soil nitrogen, the decomposition of organic carbon can be accelerated as well [38]. In this study, the total nitrogen content in the soil of transformed C. lanceolata plantations was higher than that of the reference plantations, while the P. massoniana forest plantations showed differently, which may also be one of the reasons for the different response of C. lanceolata and P. massoniana plantations to organic carbon and its fractions. Generally, after the CNT, the nutrient content of forest soil will be gradually accumulated, and the soil organic matter with high nitrogen content was easily decomposed, transported, and transformed by microorganisms within a certain range [39], and then affects the process of soil organic carbon sequestration.
Organic carbon is taken as the carrier of enzymes. With the high-level organic carbon content, the production of extracellular enzymes can be induced, and the synthesis of enzymes and the growth and reproduction of microorganisms can be promoted [40]. The results of this study showed that the activities of protease, urease, and acid phosphatase in C. lanceolata plantations after transformation are 5.62%, 18.75%, and 28.40% higher than control stands, and the activities of protease and catalase activities in P. massoniana plantations are 7.37% and 6.00% higher than control plantations. Through the CNT, the content of readily decomposable organic carbon in the stands was increased, thus promoting the utilization of readily decomposable carbon by microorganisms. This can be explained by the fact that the contents of water-soluble organic carbon and particulate organic carbon in Figure 2 were significantly higher than that of the reference plantations. It is found in the study that the impact of soil organic carbon on enzyme activity was closely related to the type of organic carbon [41]. This study showed that catalase was significantly correlated with organic carbon fractions (except water-soluble organic carbon), and protease was significantly correlated with recalcitrant organic carbon, indicating that organic carbon was sensitive to changes in the activities of these two enzymes, and the strength of enzyme activities also affected its mineralization rate. Different from the above two enzymes, acid phosphatase and urease were not significantly correlated with organic carbon and its fractions, indicating that their impact on soil organic carbon fractions may depend more on microbial species, biomass, and activities. Moreover, the sampling was conducted in the rainy season, and the soil moisture of the four stands was similar so that acid phosphatase was more vulnerable to soil moisture than organic carbon [42]. However, due to the coupling relationship between various nutrient elements in the soil, it was uncertain whether the higher enzyme activities led to the increase in soil organic carbon content or whether the larger soil organic carbon pool provided sufficient substrate for enzyme activities, thus promoting the improvement of enzyme activity [43]. Most scholars believed that soil enzyme activities were simultaneously regulated by key physical and chemical properties of soil, soil microbial biomass, and soil organic matter content [44]. It is found in the study of Sinsabaugh et al. [45] that higher nitrogen availability would promote the degradation of unstable organic carbon. In addition, seasonal changes and microflora changes affected soil carbon pool storage and enzyme activities. Due to sampling only in the growing season, this study lacks relevant data to study seasonal changes and microbial community structure. This may also be an important reason why there is no significant difference in enzyme activities between the transformed plantations and the reference plantations in this study. Therefore, in order to better explore the relationship between soil organic carbon fractions and enzyme activities, the seasonal dynamics and microorganisms need to be further studied in the future.
5. Conclusions
Close-to-nature transformation can improve the soil organic carbon and enzyme activity in coniferous plantation, and the change components mainly are active carbon, which is closely related to soil enzyme activities and soil chemical properties, among them, soil pH, total nitrogen and catalase were the main factors affecting organic carbon components. The results showed that close-to-nature transformation will promote soil organic carbon accumulation by changing the composition of tree species but had a negative effect on soil organic carbon stability to a certain extent. Therefore, the effect of tree species configuration on soil carbon stability components should be considered in the practice of forest management.
Author Contributions
Conceptualization, A.M. and J.Z. (Ji Zeng); methodology, A.M.; software, W.S.; validation, W.S. and A.M.; formal analysis, A.M. and J.Z. (Ji Zeng); investigation, H.L., H.M., J.M., K.Y., Z.L. (Zhongguo Li) and J.Z. (Jihui Zhang); resources, Z.L. (Zhaoying Li); data curation, W.S. and Y.T.; writing—original draft preparation, W.S.; writing—review and editing, W.S.; visualization, W.S.; supervision, J.W.; project administration, J.Z. (Ji Zeng); funding acquisition, A.M. and J.Z. (Ji Zeng). All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (32071764), National Natural Science Foundation of Guangxi Province (2020GXNSFAA297208), Fundamental Research Funds of the Chinese Academy of Forestry (CAFYBB2021ZW001-2) and the National Natural Science Foundation of China (31971655).
Acknowledgments
We gratefully acknowledge the guidance of Junkun Lu from Research Institute of Tropical Forestry, China Academy of Forestry, Hui Wang from Institute of Forest Ecology, Environment and Nature Conservation, Chinese Academy of Forestry, and You Yeming, Xiaoguo Zhou from Guangxi University in manuscript writing and revision; as well as Hai Chen and Maofeng Zhu for assistance in field work and laboratory analysis.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Matos, E.S.; Freese, D.; Ślązak, A.; Bachmann, U.; Veste, M.; Hüttl, R. Organic-carbon and nitrogen stocks and organic-carbon fractions in soil under mixed pine and oak forest stands of different ages in NE Germany. J. Plant Nutr. Soil Sci. 2010, 173, 654–661. [Google Scholar] [CrossRef]
- Xiao, N.; Mo, X.Q.; Tan, X.M.; Su, X.Y.; Yan, J.L.; Gao, G.N.; Zhang, W.; Hang, X.M.; You, Y.M. Effects of muti-layer and mixed-age forest management of Pinus massoniana plantations on soil carbon components and transformation. Guangxi Zhiwu 2022, 42, 595–607. [Google Scholar] [CrossRef]
- Liu, S.R.; Yang, Y.J.; Wang, H. Development strategy and management countermeasures of planted forests in China: Transforming from timber-centered single objective management towards multi-purpose management for enhancing quality and benefits of ecosystem services. Acta Ecol. Sin. 2018, 38, 1–10. [Google Scholar]
- Bai, Y.X.; Sheng, M.Y.; Hu, Q.J.; Zhao, C.; Wu, J.; Zhang, M.S. Effects of land use change on soil organic carbon and its components in karst rocky desertification of southwest China. J. Appl. Ecol. 2020, 31, 10. [Google Scholar]
- Davidson, E.A.; Janssens, I.A. Temperature sensitivity of soil carbon decomposition and feedbacks to climate change. Nature 2006, 440, 165–173. [Google Scholar] [CrossRef]
- Pang, D.B.; Cui, M.; Liu, Y.G.; Wang, G.Z.; Cao, J.H.; Wang, X.R.; Zhou, J.X. Responses of soil labile organic carbon fractions and stocks to different vegetation restoration strategies in degraded karst ecosystems of southwest China. Ecol. Eng. 2019, 138, 391–402. [Google Scholar] [CrossRef]
- Yang, X.; Wang, D.; Lan, Y.; Meng, J.; Jiang, L.L.; Sun, Q.; Cao, D.Y.; Sun, Y.Y.; Chen, W.F. Labile organic carbon fractions and carbon pool management index in a 3-year field study with biochar amendment. J. Soils Sediments 2018, 18, 1569–1578. [Google Scholar] [CrossRef]
- Zeng, W.S. Close-to-Nature forest management: A practical approach to improve the forest quality of China. Forest Res. Manag. 2009, 2, 6–11. [Google Scholar]
- Moradi, M.; Mohadjer, M.R.M.; Sefidi, K.; Zobiri, M.; Omidi, A. Over-mature beech trees (Fagus orientalis Lipsky) and close-to-nature forestry in northern Iran. J. For. Res. 2012, 23, 6. [Google Scholar] [CrossRef]
- Jiang, J.; Liu, X.Z.; Jia, H.Y.; Ming, A.G.; Chen, B.B.; Lu, Y.C. Effects of stand density on understory species diversity and soil physicochemical properties after close-to-nature transformation management of Chinese fir plantation. J. Beijing For. Univ. 2019, 41, 174–181. [Google Scholar]
- Feng, C.; Ma, Y.H.; Jin, X.; Wang, Z.; Ma, Y.; Fu, S.L.; Chen, H.Y.H. Soil enzyme activities increase following restoration of degraded subtropical forests. Geoderma 2019, 351, 180–187. [Google Scholar] [CrossRef]
- He, Y.J.; Liang, X.Y.; Qin, L.; Li, Z.Y.; Shao, M.X.; Tan, L. Community characteristics and soil properties of coniferous plantation forest monocultures in the early stages after close-to-nature transformation management in southern subtropical China. Acta Ecol. Sin. 2013, 33, 2484–2495. [Google Scholar]
- Lai, J.M.; Li, K.Z.; Huang, C.D.; Zhan, J.; Yang, W.Q. Effect of improvement measures on soil labile organic carbon of low-efficiency Pinus massoniana forest. For. Res. 2013, 26, 167–173. [Google Scholar]
- Lu, G.; Huang, H.X.; Zhou, X.L.; Cao, X.P.; Zhao, A. Characteristics of soil organic carbon and changes of enzyme activities in burned area of spruce-fir forests in diebu forest region. Acta Agrestia Sin. 2022, 30, 943–949. [Google Scholar]
- Sinsabaugh, R.L.; Phenol, O. Peroxidase, and organic matter dynamics of soil. Soil Biol. Biochem. 2010, 42, 391–404. [Google Scholar] [CrossRef]
- Tai, T.Y. Studies on Soil Microbiological Characteristics of Da Xinganling Mountains Burned areas in Inner Mongolia. Master’s Thesis, Inner Mongolia Normal University, Hohhot, China, 2015. [Google Scholar]
- Wang, H.; He, Z.L.; Lu, Z.M.; Zhou, J.Z.; Nostrand, J.V.; Xu, X.H.; Zhang, Z.J. Genetic linkage of soil carbon pools and microbial functions in subtropical freshwater wetlands in response to experimental warming. Appl. Environ. Microb. 2012, 78, 7652–7661. [Google Scholar] [CrossRef] [Green Version]
- Sparks, D.L.; Page, A.; Helmke, P.; Loeppert, R.; Soltanpour, P.; Tabatabai, M.; Johnston, C.; Sumner, M. Methods of Soil Analysis. Part 3—Chemical Methods; Soil Science Society of America Inc.: Madison, WI, USA, 1996. [Google Scholar]
- Bao, S. Soil Agricultural Chemistry Analysis, 3rd ed.; China Agriculture Press: Beijing, China, 2000. [Google Scholar]
- Liu, G.; Jiang, N.; Zhang, L. Soil Physical and Chemical Analysis and Description of Soil Profiles; Standard Press of China: Beijing, China, 1996. [Google Scholar]
- Garten, C.T., Jr.; Post, W.M., III; Cooper, P. Forest soil carbon inventories and dynamics along an elevation gradient in the southern Appalachian Mountains. Biogeochemistry 1999, 45, 115–145. [Google Scholar] [CrossRef]
- Blair, G.; Lefroy, R.; Lisle, L. Soil carbon fractions based on their degree of oxidation, and the development of a carbon management index for agricultural systems. Aust. J. Agric. Res. 1995, 46, 393–406. [Google Scholar] [CrossRef]
- Xiang, H.M.; Wen, D.Z.; Zhang, L.L.; Li, J. Altitudinal changes in active and recalcitrant soil carbon pools of forests in the Dinghu Mountains. Acta Ecol. Sin. 2015, 35, 6089–6099. [Google Scholar]
- Yang, L.F.; Zeng, Q.; Hai-Bo, L.I.; Yan, J.J. Measurement of catalase activity in soil by ultraviolet spectrophotometry. Chin. J. Soil Sci. 2011, 42, 207–210. [Google Scholar]
- Zheng, H.Y.; Zhang, D.S. Determination and properties of soil protease activity. Chin. J. Soil Sci. 1981, 3, 32–34. [Google Scholar] [CrossRef]
- Kandeler, E.; Gerber, H. Short-term assay of soil urease activity using colorimetric determination of ammonium. Biol. Fert. Soils 1988, 6, 68–72. [Google Scholar] [CrossRef]
- Tabatabai, M.A.; Bremner, J.M. Use of p-nitrophenyl phosphate for assay of soil phosphatase activity. Soil Biol. Biochem. 1969, 1, 301–307. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022; Available online: https://www.R-project.org/ (accessed on 10 June 2021).
- Jandl, R.; Lindner, M.; Vesterdal, L.; Auwens, B.B.; Baritz, R.; Hagedorn, F.; Johnson, D.W.; Minkkinen, K.; Byrne, K.A. How strongly can forest management influence soil carbon sequestration? Geoderma 2007, 137, 253–268. [Google Scholar] [CrossRef]
- Vieira, F.; Bayer, C.; Zanatta, J.A.; Dieckow, J.; Mielniczuk, J.; He, Z.L. Carbon management index based on physical fractionation of soil organic matter in an Acrisol under long-term no-till cropping systems. Soil Tillage Res. 2007, 96, 195–204. [Google Scholar] [CrossRef]
- Zhang, X.; Han, S.J.; Wang, S.Q.; Gu, Y.; Chen, Z.J. Change of soil organic carbon fractions at different successional stages of betula platyphylla forest in changbai mountains. Acta Ecol. Sin. 2016, 35, 282–289. [Google Scholar]
- Yang, Y.S.; Liu, Y.L.; Chen, G.S.; Li, L.; Xie, J.S.; Lin, P. Content and distribution of unprotected soil organic carbon in natural and monoculture plantation forests of Castanopsis kawakamii in subtropical China. Acta Ecol. Sin. 2004, 24, 1–8. [Google Scholar]
- Zhang, L.; Zhang, D.L.; Mao, Z.J. Charicateristic of Soil oranic carbon and its components in different successional series of broadleaved Korean Pine Forest in Xiaoxing’an Mountains. Sci. Silvae Sin. 2017, 53, 11–17. [Google Scholar]
- Xi, D.; Yu, Z.P.; Xiong, Y.; Liu, X.Y.; Liu, J. Altitudinal changes of soil organic carbon fractions of evergreen broadleaved forests in Guanshan Mountain Jiangxi, China. Chin. J. Appl. Ecol. 2020, 31, 3349–3356. [Google Scholar]
- Xi, D.; Weng, H.D.; Hu, Y.L.; Wu, J.P. Effects of canopy nitrogen addition and understory removal on soil organic carbon fractions in a Chinese fir plantation. Acta Ecol. Sin. 2021, 41, 8525–8534. [Google Scholar]
- Gao, Y.; Ma, H.L.; Gao, R.; Yin, Y.F.; Zhang, W.; Zhu, X.M.; Yang, Y.S. Effects of simulated nitrogen deposition on phenolics and soluble sugar in forest soils. Soils 2014, 46, 41–46. [Google Scholar]
- Liao, D.; Yu, D.S.; Zhao, Y.C.; Wang, N.; Zhang, H.D.; Pan, J.J.; Shi, X.Z. Composition of organic carbon in paddy soil in typical area of Chengdu and its influencing factors. Acta Pedol. Sin. 2015, 52, 517–527. [Google Scholar]
- Xiao, S.S. Carbon Sequestration and Response of Soil Organic Carbon Pool to Exogenous Nitrogen Input in Temperate Semi-Arid Grassland Ecosystem. Ph.D. Thesis, Graduate School of Chinese Academy of Sciences, Beijing, China, 2010. [Google Scholar]
- Huang, X.M.; Liu, S.R.; You, Y.M.; Wen, Y.G.; Hui, W.; Wang, J.X. Microbial community and associated enzymes activity influence soil carbon chemical composition in Eucalyptus urophylla plantation with mixing N2-fixing species in subtropical China. Plant Soil 2016, 414, 199–212. [Google Scholar] [CrossRef]
- Liu, X.D.; Chen, L.; Yang, X.G.; Zhang, Y.F.; Zhao, W.; Li, X.B. Characteristics of soil labile organic carbon fractions and their relationship with soil enzyme activities in four typical communities in desert steppe. Acta Bot. Boreali-Occident. Sin. 2016, 36, 1882–1890. [Google Scholar]
- Zhang, S.Y.; Yuan, H.H.; Lu, H.; Jiang, C.F.; Xiang, S.M. The soil enzyme activities of different land use types and the relationships between the soil enzyme activities and physical-chemical properties or microorganism in Mountainous Area of Northwest Yunnan Province. Subtrop. Soil Water Conserv. 2010, 22, 13–16. [Google Scholar]
- Marhan, S.; Kandeler, E.; Scheu, S. Phospholipid fatty acid profiles and xylanase activity in particle size fractions of forest soil and casts of Lumbricus terrestris L. (Oligochaeta, Lumbricidae). Appl. Soil Ecol. 2007, 35, 412–422. [Google Scholar] [CrossRef]
- Cenini, V.L.; Fornara, D.A.; Mcmullan, G.; Ternan, N.; Carolan, R.; Crawley, M.J.; Clément, J.C.; Lavorel, S. Linkages between extracellular enzyme activities and the carbon and nitrogen content of grassland soils. Soil Biol. Biochem. 2016, 96, 198–206. [Google Scholar] [CrossRef] [Green Version]
- Tursová, M.; Baldrian, P. Effects of soil properties and management on the activity of soil organic matter transforming enzymes and the quantification of soil-bound and free activity. Plant Soil 2013, 38, 99–110. [Google Scholar] [CrossRef]
- Sinsabaugh, R.L.; Carreiro, M.M.; Repert, D.A. Allocation of extracellular enzymatic activity in relation to litter composition, N deposition, and mass loss. Biogeochemistry 2002, 60, 1–24. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).