Abandoned Fishpond Reversal to Mangrove Forest: Will the Carbon Storage Potential Match the Natural Stand 30 Years after Reforestation?
Abstract
:1. Introduction
2. Materials and Methods
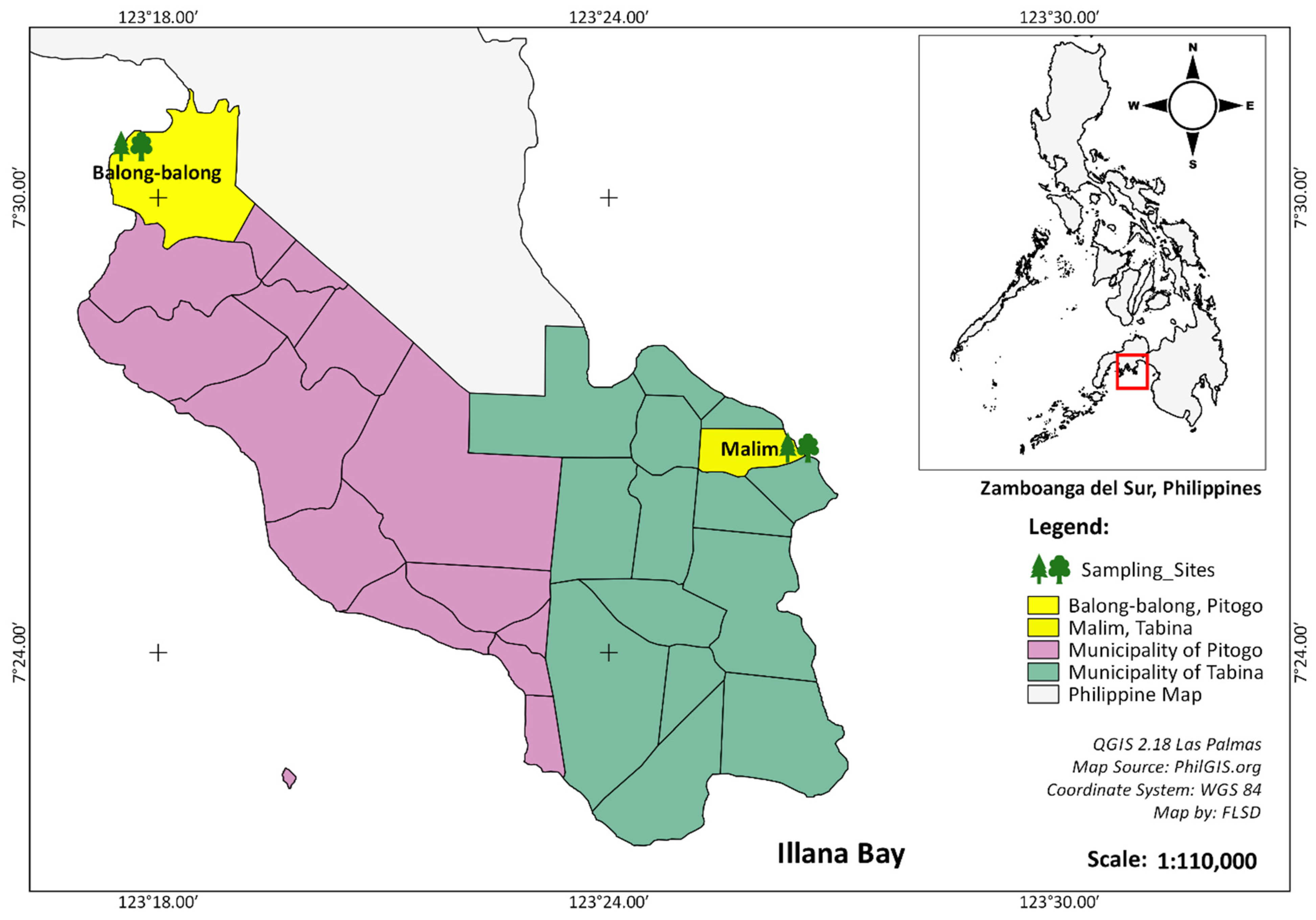
2.1. The Study Site
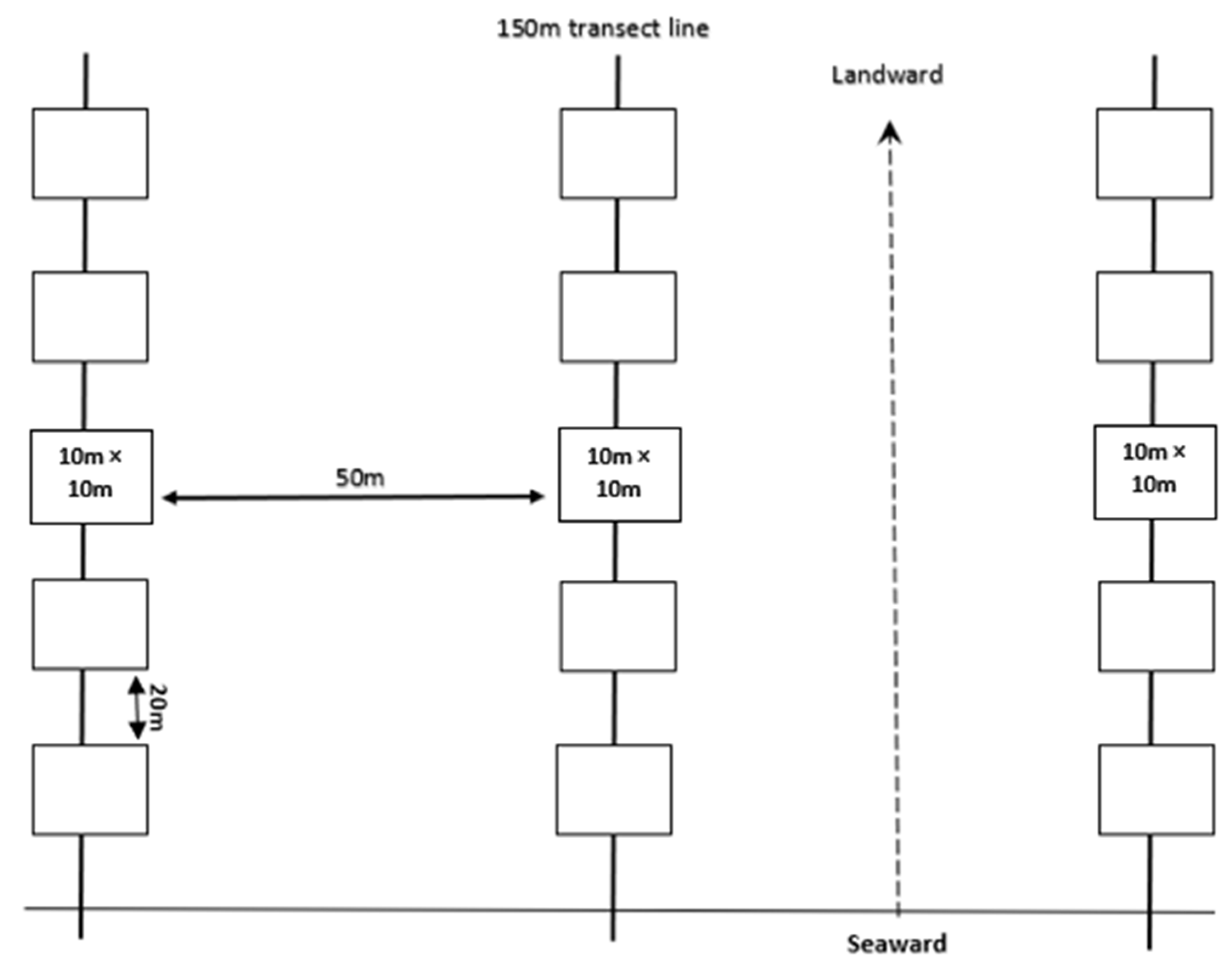
2.2. Sampling Design
2.3. Tree Biomass and Carbon Stock
2.4. Sediment Carbon Stock Determination
2.5. Data Analysis
3. Results
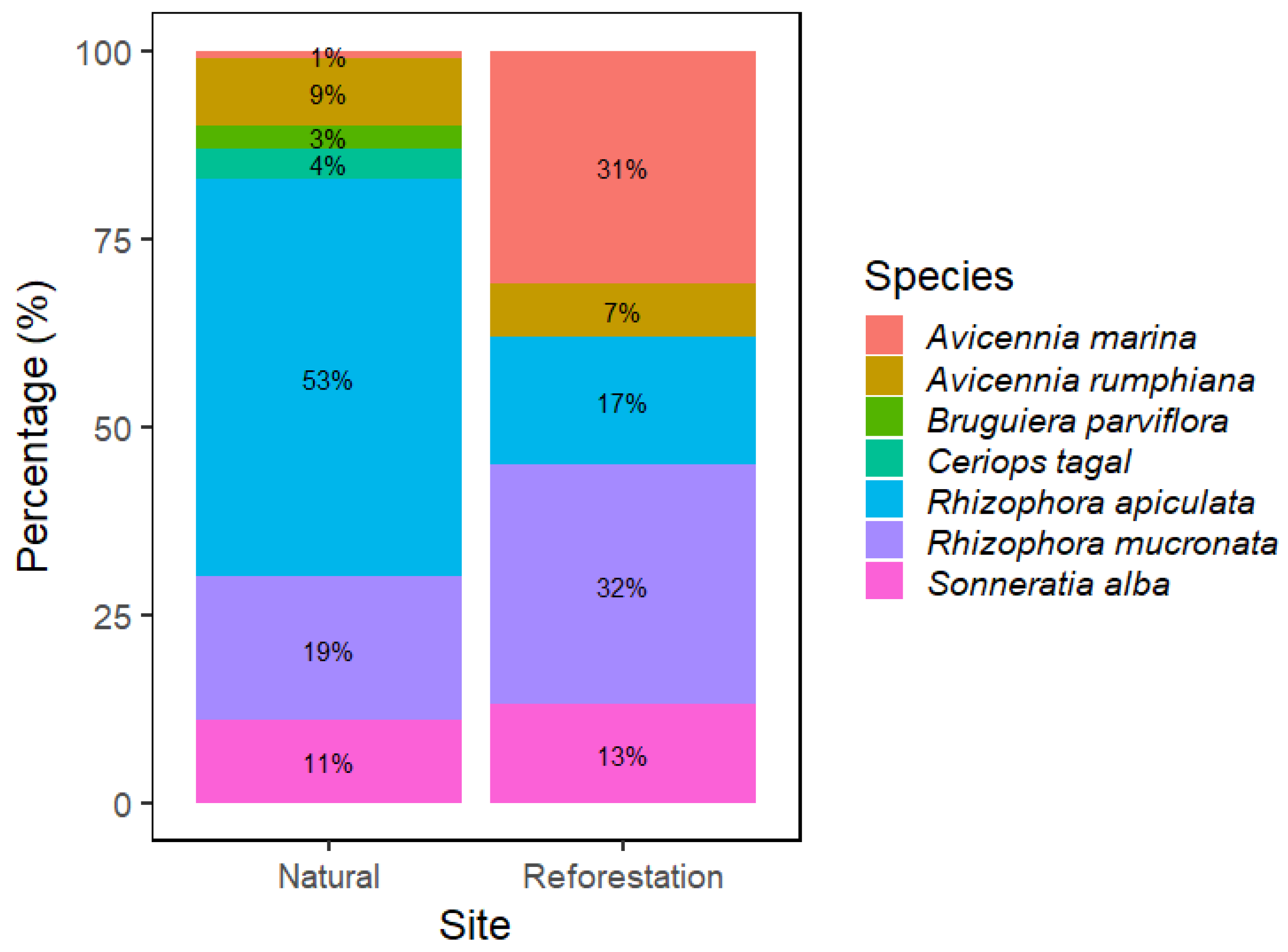
3.1. Major Species Composition
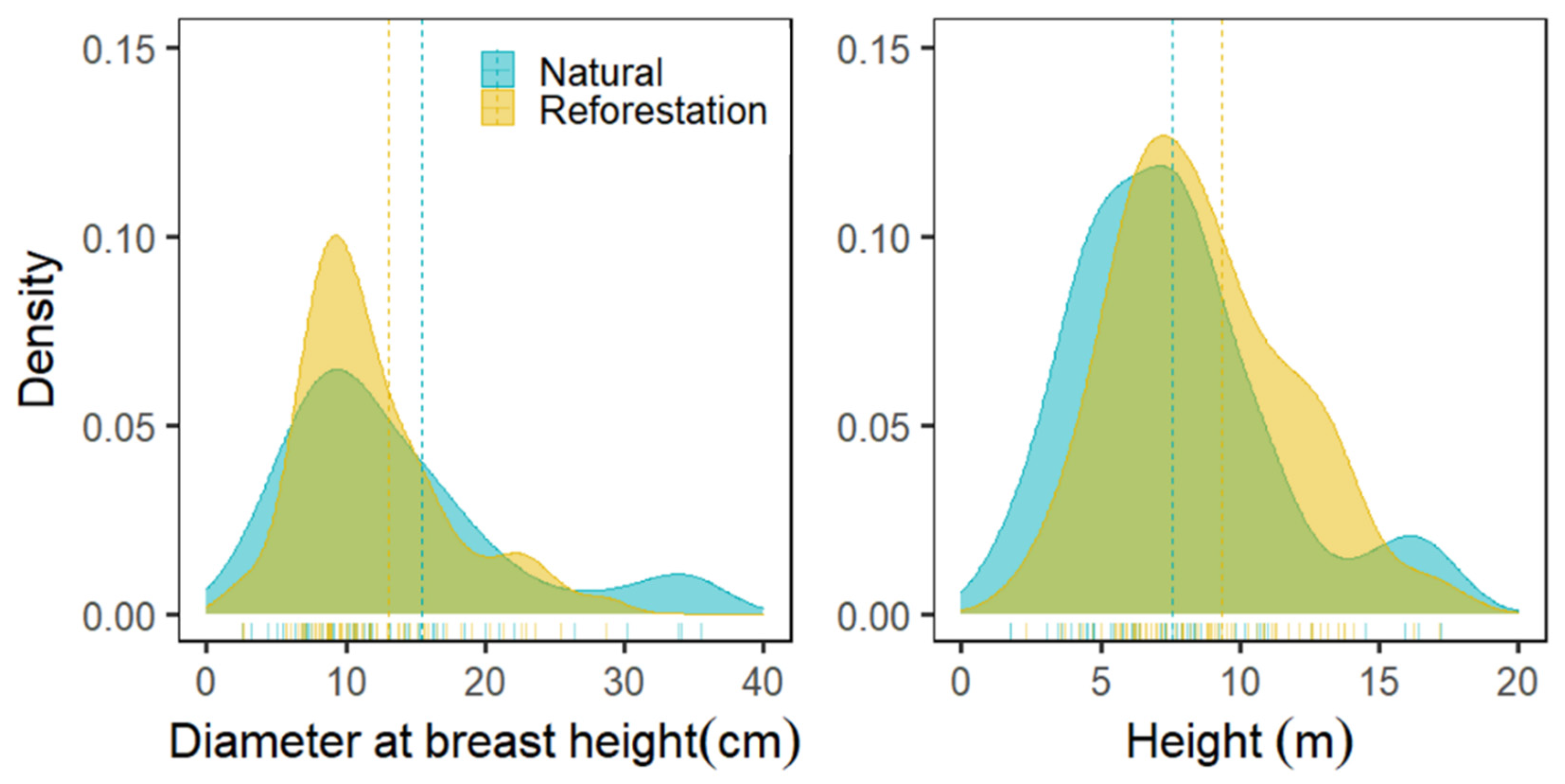
3.2. Diameter and Height Distribution
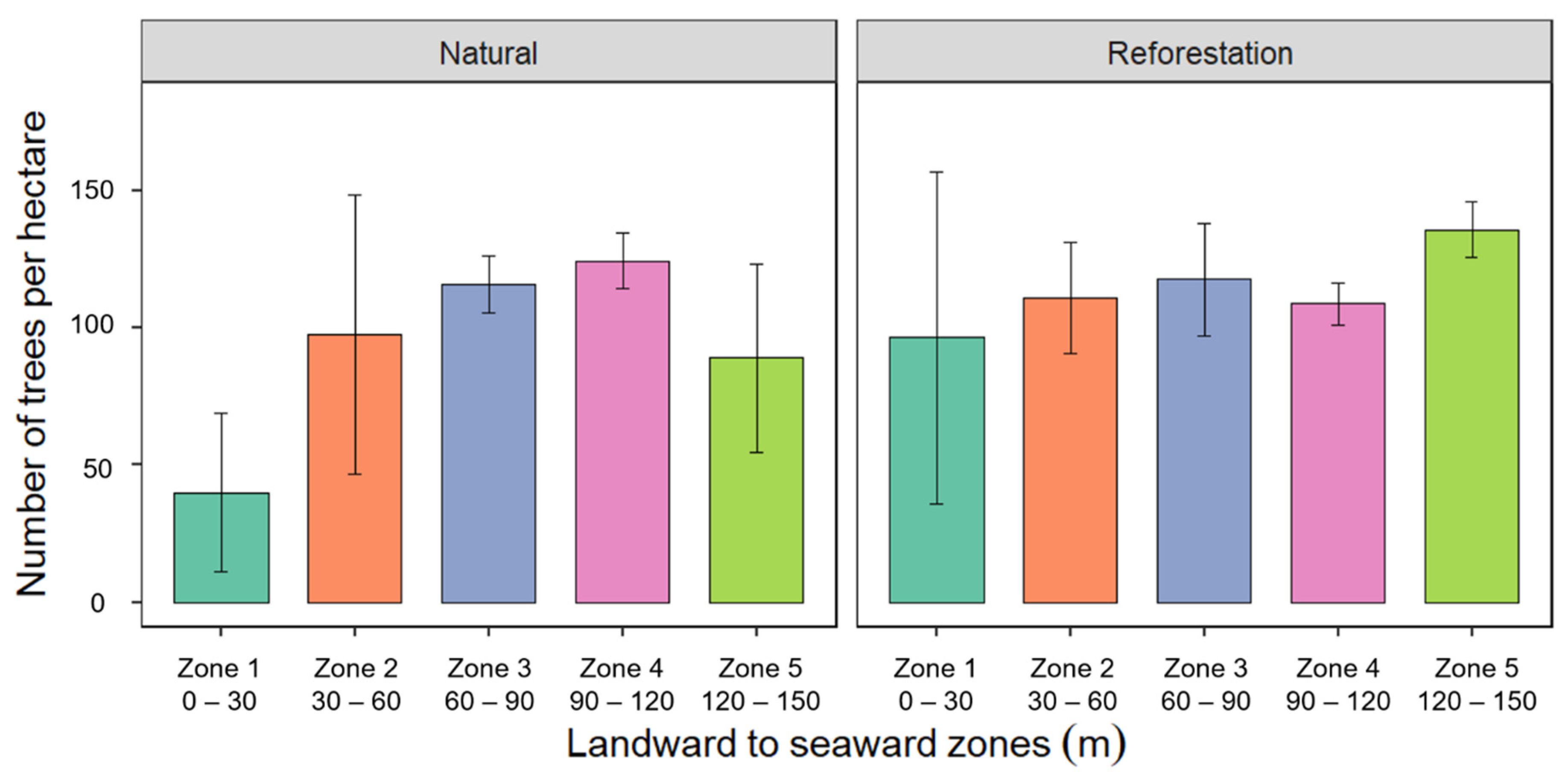
3.3. Tree Density from Landward to Seaward Zones
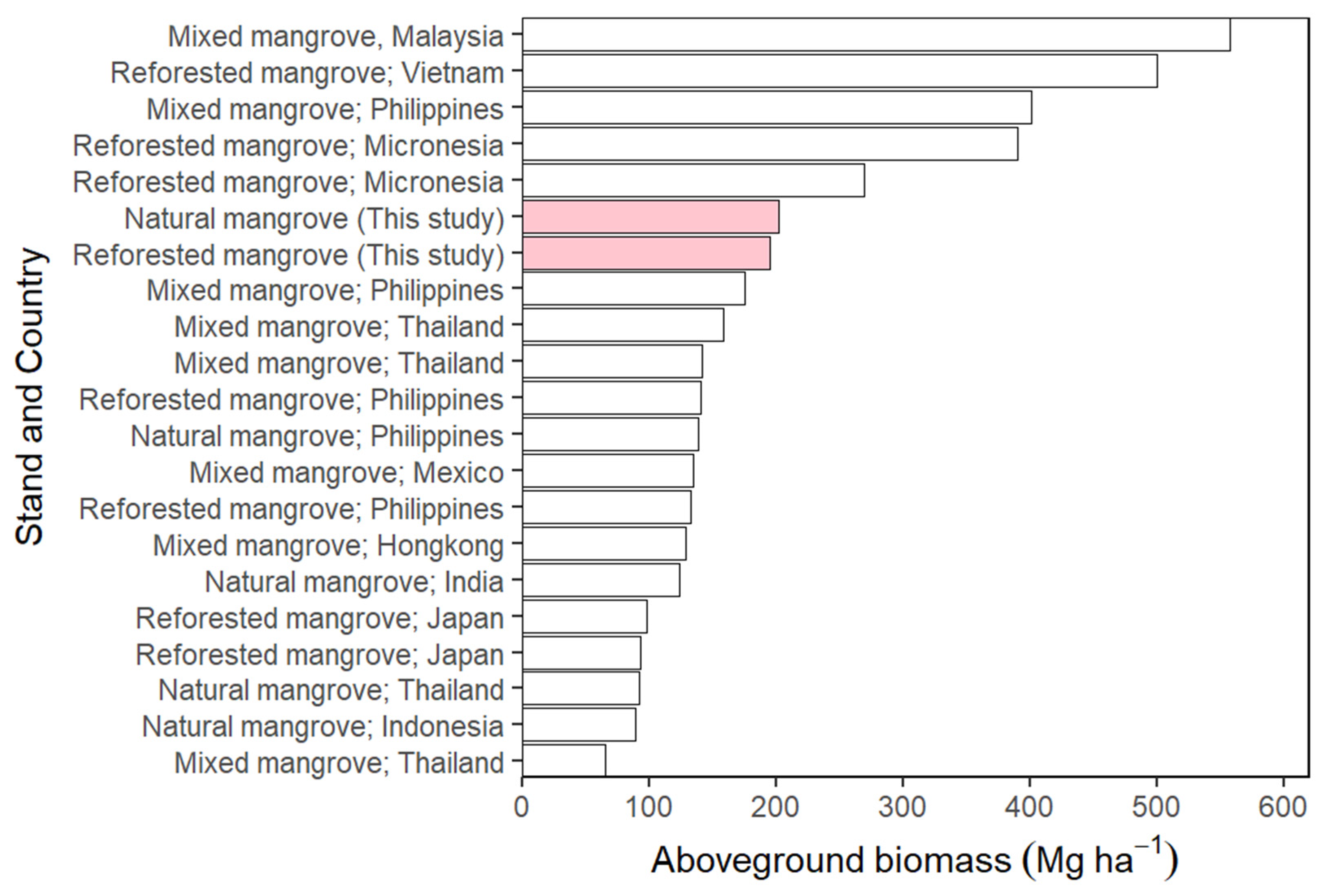
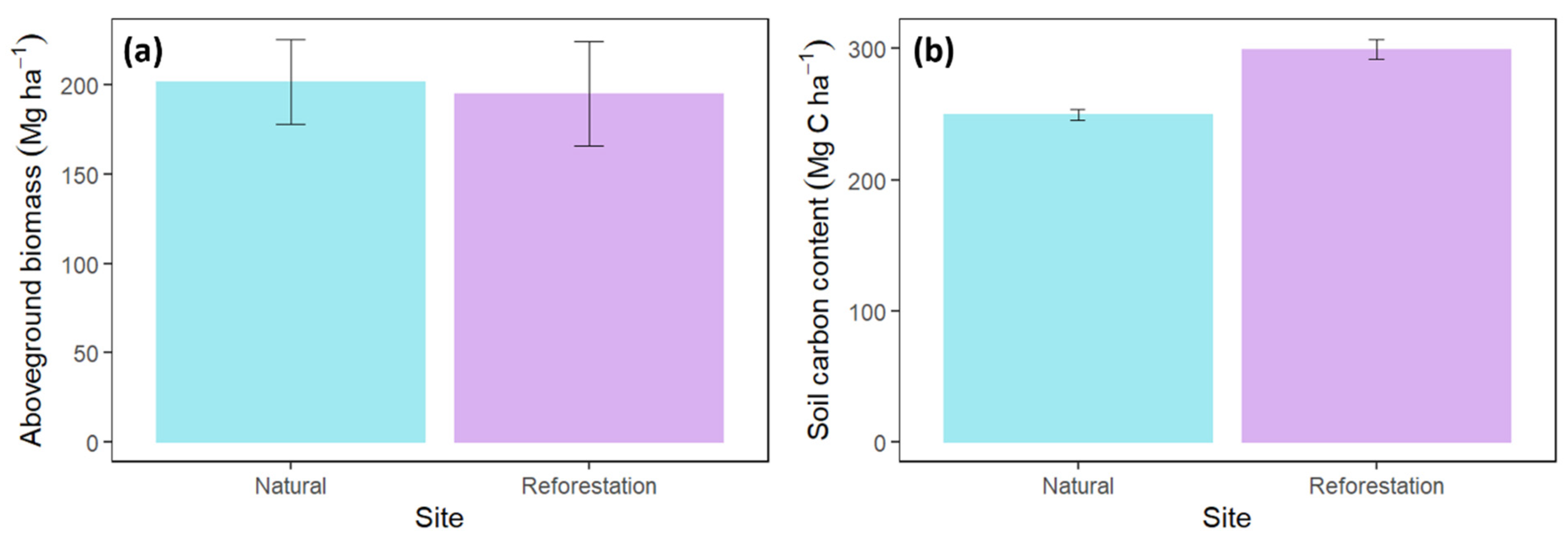
3.4. The Aboveground Biomass
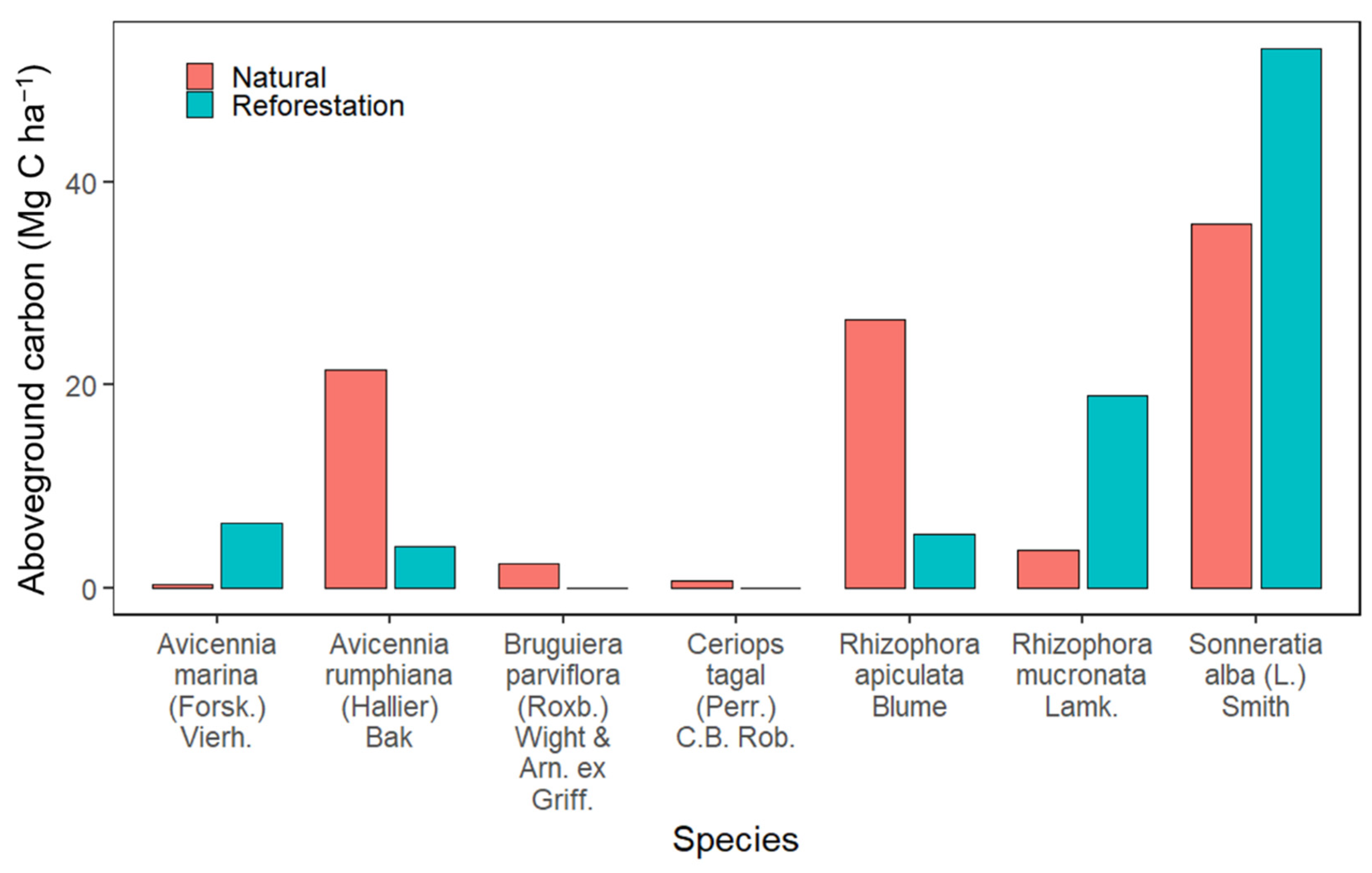
3.5. The Aboveground Carbon Estimates
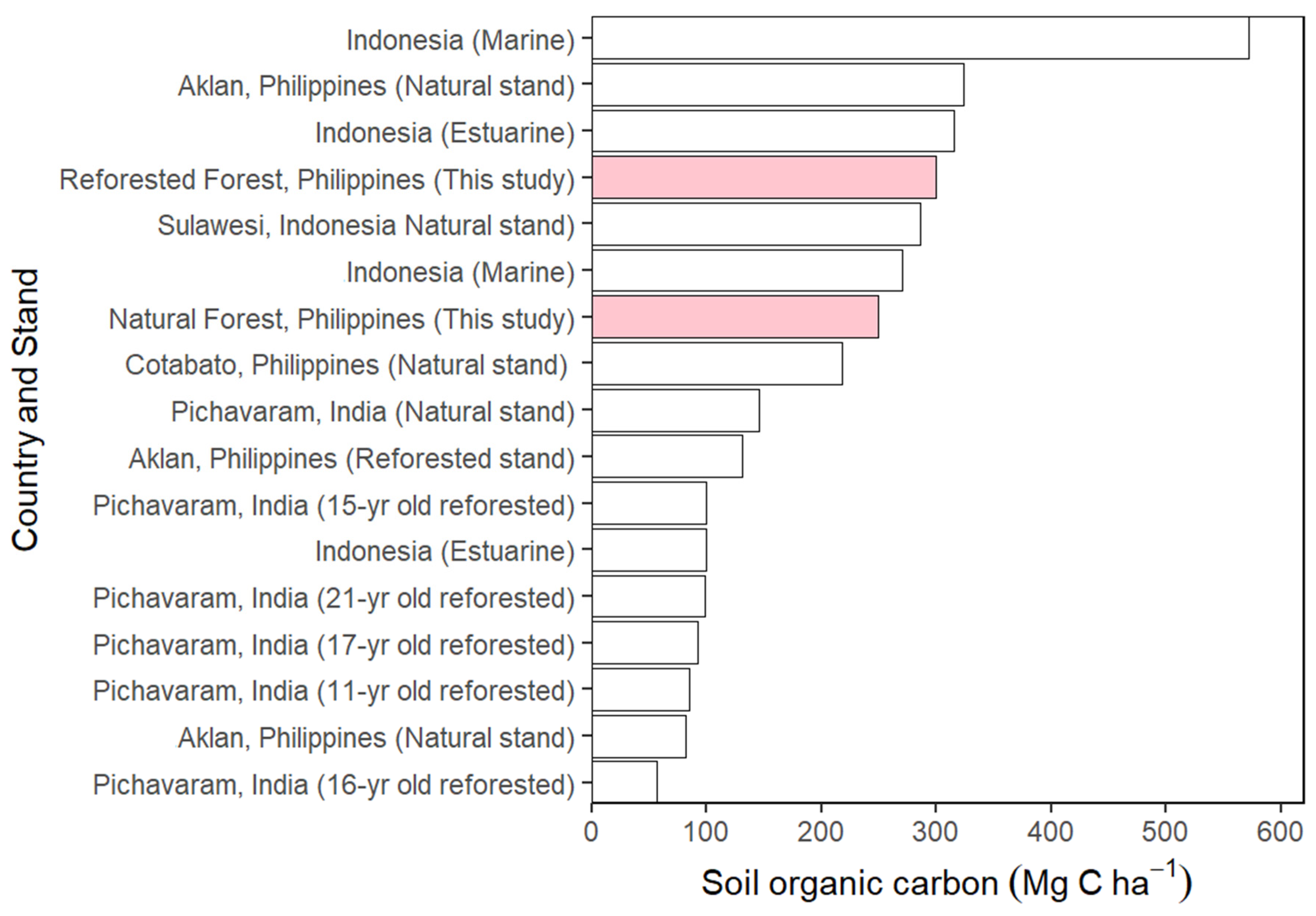
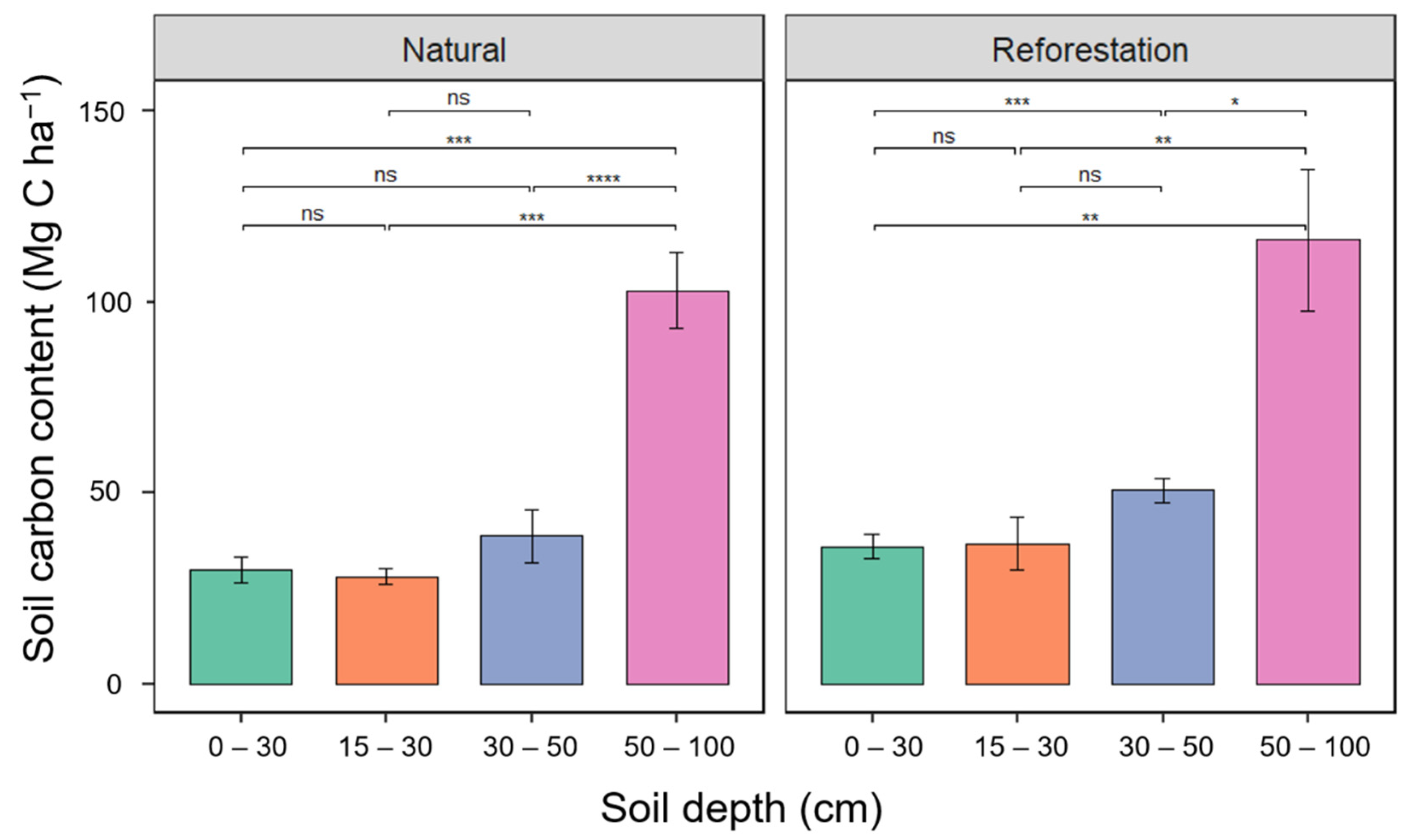
3.6. The Soil Carbon Stock
4. Discussion
4.1. Species Composition
4.2. Diameter and Height Distribution
4.3. Tree Density from Landward to Seaward Zones
4.4. The Aboveground Biomass
4.5. The Aboveground Carbon Stock
4.6. The Soil Carbon Stock
4.7. Implications of Abandoned Fishpond Rehabilitation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Long, J.B.; Giri, C. Mapping the Philippines’ Mangrove Forests Using Landsat Imagery. Sensors 2011, 11, 2972–2981. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.Y.; Primavera, J.H.; Dahdouh-Guebas, F.; Mckee, K.; Bosire, J.O.; Cannicci, S.; Diele, K.; Fromard, F.; Koedam, N.; Marchand, C.; et al. Ecological Role and Services of Tropical Mangrove Ecosystems: A Reassessment. Glob. Ecol. Biogeogr. 2014, 23, 726–743. [Google Scholar] [CrossRef]
- Alongi, D.M. Global Significance of Mangrove Blue Carbon in Climate Change Mitigation. Science 2020, 2, 67. [Google Scholar] [CrossRef]
- Kauffman, J.B.; Adame, M.F.; Arifanti, V.B.; Schile-Beers, L.M.; Bernardino, A.F.; Bhomia, R.K.; Donato, D.C.; Feller, I.C.; Ferreira, T.O.; del Jesus Garcia, M.C.; et al. Total Ecosystem Carbon Stocks of Mangroves across Broad Global Environmental and Physical Gradients. Ecol. Monogr. 2020, 90, e01405. [Google Scholar] [CrossRef] [Green Version]
- Ouyang, X.; Lee, S.Y. Improved Estimates on Global Carbon Stock and Carbon Pools in Tidal Wetlands. Nat. Commun. 2020, 11, 317. [Google Scholar] [CrossRef] [Green Version]
- Duarte, C.M.; Losada, I.J.; Hendriks, I.E.; Mazarrasa, I.; Marbà, N. The Role of Coastal Plant Communities for Climate Change Mitigation and Adaptation. Nat. Clim. Chang. 2013, 3, 961–968. [Google Scholar] [CrossRef] [Green Version]
- Donato, D.C.; Kauffman, J.B.; Murdiyarso, D.; Kurnianto, S.; Stidham, M.; Kanninen, M. Mangroves among the Most Carbon-Rich Forests in the Tropics. Nat. Geosci. 2011, 4, 293–297. [Google Scholar] [CrossRef]
- Gevana, D.T.; Pulhin, F.B.; Pampolina, N.M. Carbon Stock Assessment of a Mangrove Ecosystem in San Juan, Batangas. J. Environ. Sci. Manag. 2008, 11, 15–25. [Google Scholar]
- Primavera, J.H. Philippine Mangroves: Status, Threats and Sustainable Development. In Mangrove Management and Conservation: Present and Future; United Nations University Press: Tokyo, Japan, 2004; pp. 192–207. [Google Scholar]
- Salmo, S.G.; Lovelock, C.E.; Duke, N.C. Assessment of Vegetation and Soil Conditions in Restored Mangroves Interrupted by Severe Tropical Typhoon “Chan-Hom” in the Philippines. Hydrobiologia 2014, 733, 85–102. [Google Scholar] [CrossRef]
- Casey, W.P.; Ewel, K.C. Patterns of Succession in Forested Depressional Wetlands in North Florida, USA. Wetlands 2006, 26, 147–160. [Google Scholar] [CrossRef]
- Cameron, C.; Hutley, L.B.; Friess, D.A.; Brown, B. Community Structure Dynamics and Carbon Stock Change of Rehabilitated Mangrove Forests in Sulawesi, Indonesia. Ecol. Appl. 2019, 29, e01810. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Licuanan, W.Y.; Cabreira, R.W.; Aliño, P.M. The Philippines, 2nd ed.; Elsevier Ltd.: Amsterdam, The Netherlands, 2018; ISBN 9780081008539. [Google Scholar]
- Faridah-Hanum, I.; Latiff, A.; Hakeem, K.R.; Ozturk, M. Mangrove Ecosystems of Asia: Status, Challenges and Management Strategies. In Mangrove Ecosystems of Asia: Status, Challenges and Management Strategies; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2014; pp. 1–471. [Google Scholar] [CrossRef]
- Kathiresan, K.; Bingham, B.L. Biology of Mangroves and Mangrove Ecosystems. Adv. Mar. Biol. 2001, 40, 81–251. [Google Scholar] [CrossRef]
- Primavera, J.H. Development and Conservation of Philippine Mangroves: Institutional Issues. Ecol. Econ. 2000, 35, 91–106. [Google Scholar] [CrossRef]
- Mariano, H.G.; Dagoc, F.L.S.; Espra, A.S.; Amparado, R.F. Mangrove Diversity, Taxonomic of Classification, and and Morphological Characteristics Natural Reforested Mangrove Forests in Selected Municipalities of Zamboanga Del Sur, Mindanao Island. J. Biodivers. Environ. Sci. 2019, 15, 86–99. [Google Scholar]
- Primavera, J.H.; Esteban, J.M.A. A Review of Mangrove Rehabilitation in the Philippines: Successes, Failures and Future Prospects. Wetl. Ecol. Manag. 2008, 16, 345–358. [Google Scholar] [CrossRef]
- Long, J.; Giri, C.; Primavera, J.; Trivedi, M. Damage and Recovery Assessment of the Philippines’ Mangroves Following Super Typhoon Haiyan. Mar. Pollut. Bull. 2016, 109, 734–743. [Google Scholar] [CrossRef]
- Duncan, C.; Primavera, J.H.; Pettorelli, N.; Thompson, J.R.; Loma, R.J.A.; Koldewey, H.J. Rehabilitating Mangrove Ecosystem Services: A Case Study on the Relative Benefits of Abandoned Pond Reversion from Panay Island, Philippines. Mar. Pollut. Bull. 2016, 109, 772–782. [Google Scholar] [CrossRef]
- Forest Management Bureau Philippine Forestry Statistics 2021; Forest Management Bureau, Department of Environment and Natural Resources: Quezon City, Philippines, 2020; p. 2018.
- Ellison, A.M. Mangrove Restoration: Do We Know Enough? Restor. Ecol. 2000, 8, 219–229. [Google Scholar] [CrossRef]
- Lewis, R.R. Ecological Engineering for Successful Management and Restoration of Mangrove Forests. Ecol. Eng. 2005, 24, 403–418. [Google Scholar] [CrossRef]
- Alongi, D. Carbon Cycling and Storage in Mangrove Forests. Annu. Rev. Mar. Sci. 2014, 6, 195–219. [Google Scholar] [CrossRef]
- Kandasamy, K.; Rajendran, N.; Balakrishnan, B.; Thiruganasambandam, R.; Narayanasamy, R. Carbon Sequestration and Storage in Planted Mangrove Stands of Avicennia Marina. Reg. Stud. Mar. Sci. 2021, 43, 101701. [Google Scholar] [CrossRef]
- Climatology and Agrometeorology Division (CAD). Climate Change in the Philippines; Department of Science and Technology—Philippine Atmospheric Geophysical and Astronomical Services Administration: Diliman City, Philippines, 2011. [Google Scholar]
- NAMRIA. Philippines Tide and Current Tables; NAMRIA: Manila, Philippines, 2017.
- Komiyama, A.; Poungparn, S.; Kato, S. Common Allometric Equations for Estimating the Tree Weight of Mangroves. J. Trop. Ecol. 2005, 21, 471–477. [Google Scholar] [CrossRef]
- Fourqurean, J.W.; Johnson, B.; Kauffman, J.B.; Kennedy, H.; Lovelock, C.E.; Megonigal, J.P.; Rahman, A.; Saintilan, N.; Simard, M. Coastal Blue Carbon: Methods for Assessing Carbon Stocks and Emissions Factors in Mangroves, Tidal Salt Marshes, and Seagrasses; Conservation International, International, Intergovernmental Oceanographic Commission of UNESCO, International Union for Conservation of Nature: Arlington, VA, USA, 2014. [Google Scholar]
- Lasco, R.D.; Macdicken, G.; Pulhin, F.; Guillermo, I.Q.; Sales, R.F.; Cruz, R.V.O. Carbon Stocks Assessment of a Selectively Logged Dipterocarp Forest and Wood Processing Mill in the Philippines; Forest Research Institute Malaysia: Kepong, Malaysia, 2006; Volume 18. [Google Scholar]
- Kauffman, J.B.; Sanford, R.L.; Cummings, D.L.; Salcedo, I.H.; Sampaio, E.V.S.B. Biomass and Nutrient Dynamics Associated with Slash Fires in Neotropical Dry Forests. Ecology 1993, 74, 140–151. [Google Scholar] [CrossRef]
- Orella, J.; Africa, D.R.; Bustillo, C.H.; Pascua, N.; Marquez, C.; Adornado, H.; Aguilos, M. Above-and-Belowground Carbon Stocks in Two Contrasting Peatlands in the Philippines. Forests 2022, 13, 303. [Google Scholar] [CrossRef]
- Aguilos, M.; Brown, C.; Minick, K.; Fischer, M.; Ile, O.J.; Hardesty, D.; Kerrigan, M.; Noormets, A.; King, J. Millennial-Scale Carbon Storage in Natural Pine Forests of the North Carolina Lower Coastal Plain: Effects of Artificial Drainage in a Time of Rapid Sea Level Rise. Land 2021, 10, 1294. [Google Scholar] [CrossRef]
- Wickham, H. Ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016; ISBN 978-3-319-24277-4. [Google Scholar]
- Sievert, C. Interactive Web-Based Data Visualization with R, Plotly, and Shiny; Chapman and Hall/CRC: London, UK, 2020; ISBN 9781138331457. [Google Scholar]
- Wickham, H.; Averick, M.; Bryan, J.; Chang, W.; McGowan, L.; François, R.; Grolemund, G.; Hayes, A.; Henry, L.; Hester, J.; et al. Welcome to the Tidyverse. J. Open Source Softw. 2019, 4, 1686. [Google Scholar] [CrossRef]
- Wickham, H. Reshaping Data with the Reshape Package. J. Stat. Softw. 2007, 12, 1–20. [Google Scholar]
- R Core Team R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria. 2021. Available online: https://www.R-project.org/. (accessed on 15 October 2021).
- Tamai, S.; Iampa, P. Establishment and Growth of Mangrove Seedling in Mangrove Forests of Southern Thailand. Ecol. Res. 1988, 3, 227–238. [Google Scholar] [CrossRef]
- Cañizares, L.P.; Seronay, R.A. Diversity and Species Composition of Mangroves in Barangay Imelda, Dinagat Island, Philippines. AACL Bioflux 2016, 9, 518–526. [Google Scholar]
- Alongi, D.M. Patterns of Mangrove Wood and Litter Production Within a Beach Ridge-Fringing Reef Embayment, Northern Great Barrier Reef Coast. Estuaries Coasts 2011, 34, 32–44. [Google Scholar] [CrossRef]
- Patindol, T.; Casas, E. Species Diversity and Composition of Mangroves in Tacloban City, Philippines. Ann. Trop. Res. 2019, 41, 67–75. [Google Scholar] [CrossRef] [Green Version]
- Aguilos, R.; Marquez, C.; Adornado, H.; Aguilos, M. Domesticating Commercially Important Native Tree Species in the Philippines: Early Growth Performance Level. Forests 2020, 11, 885. [Google Scholar] [CrossRef]
- Marquez, C.; Aguilos, R.; Bacsal, R.; Adornado, H.; Aguilos, M. Early Growth of 11 Native and Three Alien Tree Species in Northeastern Mindanao, Philippines. Forests 2021, 12, 909. [Google Scholar] [CrossRef]
- Aguilos, M.; Sun, G.; Noormets, A.; Domec, J.C.; McNulty, S.; Gavazzi, M.; Prajapati, P.; Minick, K.J.; Mitra, B.; King, J. Ecosystem Productivity and Evapotranspiration Are Tightly Coupled in Loblolly pine (Pinus taeda L.) Plantations along the Coastal Plain of the Southeastern U.S. Forests 2021, 12, 1123. [Google Scholar] [CrossRef]
- Aguilos, M.; Stahl, C.; Burban, B.; Hérault, B.; Courtois, E.; Coste, S.; Wagner, F.; Ziegler, C.; Takagi, K.; Bonal, D. Interannual and Seasonal Variations in Ecosystem Transpiration and Water Use Efficiency in a Tropical Rainforest. Forests 2018, 10, 14. [Google Scholar] [CrossRef] [Green Version]
- von Buttlar, J.; Zscheischler, J.; Rammig, A.; Sippel, S.; Reichstein, M.; Knohl, A.; Mahecha, M.D. Impacts of Droughts and Extreme Temperature Events on Gross Primary Production and Ecosystem Respiration: A Systematic Assessment across Ecosystems and Climate Zones. Biogeosciences 2017, 15, 1293–1318. [Google Scholar] [CrossRef] [Green Version]
- Anderegg, W.R.L.; Konings, A.G.; Trugman, A.T.; Yu, K.; Bowling, D.R.; Gabbitas, R.; Karp, D.S.; Pacala, S.; Sperry, J.S.; Sulman, B.N.; et al. Hydraulic Diversity of Forests Regulates Ecosystem Resilience during Drought. Nature 2018, 561, 538–541. [Google Scholar] [CrossRef] [Green Version]
- Desantis, L.R.G.; Bhotika, S.; Williams, K.; Putz, F.E. Sea-Level Rise and Drought Interactions Accelerate Forest Decline on the Gulf Coast of Florida, USA. Glob. Chang. Biol. 2007, 13, 2349–2360. [Google Scholar] [CrossRef]
- Tobias, A.; Umali, A.G.A.; Malabrigo, P.; Galang, M.A. Mangrove Forest Inventory and Estimation of Carbon Storage and Sedimentation in Pagbilao. Wealth Account. Valuat. Ecosyst. Serv. 2017, 1–95. [Google Scholar] [CrossRef]
- Sahu, S.C.; Kumar, M.; Ravindranath, N.H. Carbon Stocks in Natural and Planted Mangrove Forests of Mahanadi Mangrove Wetland, East Coast of India. Curr. Sci. 2016, 110, 2253–2260. [Google Scholar] [CrossRef]
- Aguilos, M.; Sun, G.; Noormets, A.; Domec, J.C.; McNulty, S.; Gavazzi, M.; Minick, K.; Mitra, B.; Prajapati, P.; Yang, Y.; et al. Effects of Land-Use Change and Drought on Decadal Evapotranspiration and Water Balance of Natural and Managed Forested Wetlands along the Southeastern US Lower Coastal Plain. Agric. For. Meteorol. 2021, 303, 108381. [Google Scholar] [CrossRef]
- Aguilos, M.; Mitra, B.; Noormets, A.; Minick, K.; Prajapati, P.; Gavazzi, M.; Sun, G.; McNulty, S.; Li, X.; Domec, J.C.; et al. Long-Term Carbon Flux and Balance in Managed and Natural Coastal Forested Wetlands of the Southeastern USA. Agric. For. Meteorol. 2020, 288, 108022. [Google Scholar] [CrossRef]
- Ile, O.J.; Aguilos, M.; Morkoc, S.; Heitman, J.; King, J.S. Root Biomass Distribution and Soil Physical Properties of Short-Rotation Coppice American Sycamore (Platanus occidentalis L.) Grown at Different Planting Densities. Forests 2021, 12, 1806. [Google Scholar] [CrossRef]
- Gevaña, D.T.; Camacho, L.D.; Camacho, S.C. Stand Density Management and Blue Carbon Stock of Monospecific Mangrove Plantation in Bohol, Philippines. For. Stud. 2017, 66, 75–83. [Google Scholar] [CrossRef] [Green Version]
- Fujimoto, K.; Imaya, A.; Tabuchi, R.; Kuramoto, S.; Utsugi, H.; Murofushi, T. Belowground Carbon Storage of Micronesian Mangrove Forests. Ecol. Res. 1999, 14, 409–413. [Google Scholar] [CrossRef]
- Lasco, R.D.; Pulhin, F.B. Forest Land Use Change in the Philippines and Climate Change Mitigation. Mitig. Adapt. Strateg. Glob. Chang. 2000, 5, 81–97. [Google Scholar] [CrossRef]
- Silver, W.L.; Lugo, A.E.; Keller, M. Soil Oxygen Availability and Biogeochemistry along Rainfall and Topographic Gradients in Upland Wet Tropical Forest Soils. Biogeochemistry 1999, 44, 301–328. [Google Scholar] [CrossRef]
- Samosorn, S.; Sangtiean, T.; Rodtassana, C.; Poungparn, S. Roles of Aboveground Roots Facilitating Sedimentation and Elevation Change in a Mangrove Forest behind Bamboo Seawalls. Songklanakarin J. Sci. Technol. 2018, 40, 1315–1323. [Google Scholar] [CrossRef]
- Poungparn, S.; Komiyama, A.; Intana, V.; Piriyaota, S.; Sangtiean, T.; Tanapermpool, P.; Patanaponpaiboon, P.; Kato, S. A Quantitative Analysis on the Root System of a Mangrove, Xylocarpus Granatum Koenig. Tropics 2002, 12, 35–42. [Google Scholar] [CrossRef]
- Nakasuga, T. Analysis of the Mangrove Stand. Science Bulletin of the College of Agrculture; University of the Ryukus: Okinawa, Japan, 1979; pp. 413–519. [Google Scholar]
- Mall, L.; Singh, V.P.; Garge, A. Study of Biomass, Litter Fall, Litter Decomposition and Soil Respiration Monogeneric and Mixed Mangrove Forests of Andaman Island. Trop. Ecol. 1991, 32, 235–249. [Google Scholar]
- Komiyama, A.; Havanond, S.; Srisawatt, W.; Mochida, Y.; Fujimoto, K.; Ohnishi, T.; Ishihara, S.; Miyagi, T. Top/Root Biomass Ratio of a Secondary Mangrove (Ceriops Tagal (Perr.) C.B. Rob.) Forest. For. Ecol. Manag. 2000, 139, 127–134. [Google Scholar] [CrossRef]
- Camacho, L.D.; Gevaña, D.T.; Carandang, A.P.; Camacho, S.C.; Combalicer, E.A.; Rebugio, L.L.; Youn, Y.C. Tree Biomass and Carbon Stock of a Community-Managed Mangrove Forest in Bohol, Philippines. For. Sci. Technol. 2011, 7, 161–167. [Google Scholar] [CrossRef]
- Suzuki, E.; Tagawa, H. Biomass of a Mangrove Forest and a Sedge Marsh on Ishigaki Island, South Japan. Jpn. J. Ecol. 1983, 33, 231–234. [Google Scholar] [CrossRef]
- Brown, S.; Lugo, A. Tropical Secondary Forests. J. Trop. Ecol. 1990, 6, 1–32. [Google Scholar] [CrossRef]
- Chou, M.-Q.; Lin, W.-J.; Lin, C.-W.; Wu, H.-H.; Lin, H.-J. Allometric Equations May Underestimate the Contribution of Fine Roots to Mangrove Carbon Sequestration. Sci. Total Environ. 2022, 833, 155032. [Google Scholar] [CrossRef] [PubMed]
- Weiss, C.; Weiss, J.; Boy, J.; Iskandar, I.; Mikutta, R.; Guggenberger, G. Soil Organic Carbon Stocks in Estuarine and Marine Mangrove Ecosystems Are Driven by Nutrient Colimitation of P and N. Ecol. Evol. 2016, 6, 5043–5056. [Google Scholar] [CrossRef] [Green Version]
- Rumengan, A.P.; Mantiri, D.M.H.; Rompas, R.; Hutahaean, A.; Kepel, T.L.; Paruntu, C.P.; Kepel, R.C.; Gerung, G.S. Carbon Stock Assessment of Mangrove Ecosystem in Totok Bay, Southeast Minahasa Regency, North Sulawesi, Indonesia. AACL Bioflux 2018, 11, 1280–1288. [Google Scholar]
- Dimalen, F.K.; Rojo, M.J.A. Carbon Stock Assessment of a Mangrove Forest in Cotabato City, Philippines. J. Biodivers. Environ. Sci. 2019, 14, 1–8. [Google Scholar]
- Barrientos, K. Species Diversity and Soil Carbon Sequestration Potential of Mangrove Species at Katunggan It Ibajay (KII) Eco-Park in Aklan, Philippines. Prism 2017, 22, 4. [Google Scholar]
- Gnanamoorthy, P.; Selvam, V.; Ramasubramanian, R.; Chakraborty, S.; Pramit, D.; Karipot, A. Soil Organic Carbon Stock in Natural and Restored Mangrove Forests in Pichavaram South-East Coast of India. Indian J. Geo-Mar. Sci. 2019, 48, 801–808. [Google Scholar]
- Suello, R.H.; Hernandez, S.L.; Bouillon, S.; Belliard, J.-P.; Dominguez-Granda, L.; van de Broek, M.; Rosado Moncayo, A.M.; Ramos Veliz, J.; Ramirez, K.P.; Govers, G.; et al. Mangrove Sediment Organic Carbon Storage and Sources in Relation to Forest Age and Position along a Deltaic Salinity Gradient. Biogeosci. Discuss. 2021, 19, 1571–1585. [Google Scholar] [CrossRef]
- Cierjacks, A.; Kleinschmit, B.; Babinsky, M.; Kleinschroth, F.; Markert, A.; Menzel, M.; Ziechmann, U.; Schiller, T.; Graf, M.; Lang, F. Carbon Stocks of Soil and Vegetation on Danubian Floodplains. J. Plant Nutr. Soil Sci. 2010, 173, 644–653. [Google Scholar] [CrossRef]
- Elsey-Quirk, T.; Seliskar, D.M.; Sommerfield, C.K.; Gallagher, J.L. Salt Marsh Carbon Pool Distribution in a Mid-Atlantic Lagoon, USA: Sea Level Rise Implications. Wetlands 2011, 31, 87–99. [Google Scholar] [CrossRef]
- Ricker, M.C.; Lockaby, B.G. Soil Organic Carbon Stocks in a Large Eutrophic Floodplain Forest of the Southeastern Atlantic Coastal Plain, USA. Wetlands 2015, 35, 291–301. [Google Scholar] [CrossRef]
- Stevenson, N.J. Disused Shrimp Ponds: Options for Redevelopment of Mangrove the Scale of the Problem Environmental Problems Associated with Pond Development; Centre for Tropical Coastal Management Dept. Marine Sciences and Coastal Management, University of Newcastle: Newcastle, UK, 1996; pp. 1–10. [Google Scholar]
- Selvam, V.; Ramasubramanian, R.; Ravichandran, K.K. Genesis and Present Status of Restoration Practices in Saline Blanks in India. In Sharing Lessons on Mangrove Restoration; IUCN: Gland, Switzerland, 2012; ISBN 9782831715582. [Google Scholar]
- Hossain, M.Z.; Lin, K. Diversified Uses of Abandoned Shrimp Ponds—A Case Study in the Upper Gulf of Thailand; Bangkok (Thailand) AIT-ITCZM: Klong Luang, Thailand, 2001; p. 23. [Google Scholar]
- Matsui, N.; Suekuni, J.; Nogami, M.; Havanond, S.; Salikul, P. Mangrove Rehabilitation Dynamics and Soil Organic Carbon Changes as a Result of Full Hydraulic Restoration and Re-Grading of a Previously Intensively Managed Shrimp Pond. Wetl. Ecol. Manag. 2010, 18, 233–242. [Google Scholar] [CrossRef]
- Walton, M.E.M.; Samonte-Tan, G.P.B.; Primavera, J.H.; Edwards-Jones, G.; le Vay, L. Are Mangroves Worth Replanting? The Direct Economic Benefits of a Community-Based Reforestation Project. Environ. Conserv. 2006, 33, 335–343. [Google Scholar] [CrossRef]
- Ile, O.J.; Aguilos, M.; Morkoc, S.; Minick, K.; Domec, J.C.; King, J.S. Productivity of Low-Input Short-Rotation Coppice American Sycamore (Platanus occidentalis L.) Grown at Different Planting Densities as a Bioenergy Feedstock over Two Rotation Cycles. Biomass Bioenergy 2021, 146, 105983. [Google Scholar] [CrossRef]
- Aguilos, M.; Takagi, K.; Liang, N.; Ueyama, M.; Fukuzawa, K.; Nomura, M.; Kishida, O.; Fukazawa, T.; Takahashi, H.; Kotsuka, C.; et al. Dynamics of Ecosystem Carbon Balance Recovering from a Clear-Cutting in a Cool-Temperate Forest. Agric. For. Meteorol. 2014, 197, 26–39. [Google Scholar] [CrossRef] [Green Version]
- Walcker, R.; Gandois, L.; Proisy, C.; Corenblit, D.; Mougin, É.; Laplanche, C.; Ray, R.; Fromard, F. Control of “Blue Carbon” Storage by Mangrove Ageing: Evidence from a 66-Year Chronosequence in French Guiana. Glob. Chang. Biol. 2018, 24, 2325–2338. [Google Scholar] [CrossRef]
- Pham, V.H.; Luu, V.D.; Nguyen, T.T.; Koji, O. Will Restored Mangrove Forests Enhance Sediment Organic Carbon and Ecosystem Carbon Storage? Reg. Stud. Mar. Sci. 2017, 14, 43–52. [Google Scholar] [CrossRef]
- Asadi, M.A.; Guntur, G.; Ricky, A.B.; Novianti, P.; Andik, I. Mangrove Ecosystem C-Stocks of Lamongan, Indonesia and Its Correlation with Forest Age. Res. J. Chem. Environ. 2017, 21, 1–9. [Google Scholar]
- Kamruzzaman, M.; Basak, K.; Paul, S.K.; Ahmed, S.; Osawa, A. Litterfall Production, Decomposition and Nutrient Accumulation in Sundarbans Mangrove Forests, Bangladesh. For. Sci. Technol. 2019, 15, 24–32. [Google Scholar] [CrossRef]
- Navarro-Pedreño, J.; Almendro-Candel, M.B.; Zorpas, A.A. The Increase of Soil Organic Matter Reduces Global Warming, Myth or Reality? Science 2021, 3, 18. [Google Scholar] [CrossRef]
- Bindu, G.; Rajan, P.; Jishnu, E.S.; Ajith Joseph, K. Carbon Stock Assessment of Mangroves Using Remote Sensing and Geographic Information System. Egypt. J. Remote Sens. Space Sci. 2020, 23, 1–9. [Google Scholar] [CrossRef]
- Hastuti, A.W.; Suniada, K.I.; Islamy, F. Carbon Stock Estimation of Mangrove Vegetation Using Remote Sensing in Perancak Estuary, Jembrana District, Bali. Int. J. Remote Sens. Earth Sci. 2018, 14, 137. [Google Scholar] [CrossRef] [Green Version]
- Qiu, P.; Wang, D.; Zou, X.; Yang, X.; Xie, G.; Xu, S.; Zhong, Z. Finer Resolution Estimation and Mapping of Mangrove Biomass Using UAV LiDAR and Worldview-2 Data. Forests 2019, 10, 871. [Google Scholar] [CrossRef] [Green Version]
- Tian, J.; Wang, L.; Li, X.; Yin, D.; Gong, H.; Nie, S.; Shi, C.; Zhong, R.; Liu, X.; Xu, R. Canopy Height Layering Biomass Estimation Model (CHL-BEM) with Full-Waveform LiDAR. Remote Sens. 2019, 11, 1446. [Google Scholar] [CrossRef] [Green Version]
- Wang, D.; Wan, B.; Qiu, P.; Zuo, Z.; Wang, R.; Wu, X. Mapping Height and Aboveground Biomass of Mangrove Forests on Hainan Island Using UAV-LiDAR Sampling. Remote Sens. 2019, 11, 2156. [Google Scholar] [CrossRef] [Green Version]
- Kuenzer, C.; Bluemel, A.; Gebhardt, S.; Quoc, T.V.; Dech, S. Remote Sensing of Mangrove Ecosystems: A Review. Remote Sens. 2011, 3, 878–928. [Google Scholar] [CrossRef] [Green Version]
- Rhyma Purnamasayangsukasih, P.; Norizah, K.; Ismail, A.A.M.; Shamsudin, I. A Review of Uses of Satellite Imagery in Monitoring Mangrove Forests. IOP Conf. Ser. Earth Environ. Sci. 2016, 37, 012034. [Google Scholar] [CrossRef]
- Halder, S.; Samanta, K.; Das, S.; Pathak, D. Monitoring the Inter-Decade Spatial–Temporal Dynamics of the Sundarban Mangrove Forest of India from 1990 to 2019. Reg. Stud. Mar. Sci. 2021, 44, 101718. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mariano, H.; Aguilos, M.; Dagoc, F.L.; Sumalinab, B.; Amparado, R., Jr. Abandoned Fishpond Reversal to Mangrove Forest: Will the Carbon Storage Potential Match the Natural Stand 30 Years after Reforestation? Forests 2022, 13, 847. https://doi.org/10.3390/f13060847
Mariano H, Aguilos M, Dagoc FL, Sumalinab B, Amparado R Jr. Abandoned Fishpond Reversal to Mangrove Forest: Will the Carbon Storage Potential Match the Natural Stand 30 Years after Reforestation? Forests. 2022; 13(6):847. https://doi.org/10.3390/f13060847
Chicago/Turabian StyleMariano, Habagat, Maricar Aguilos, Frandel Louis Dagoc, Bryan Sumalinab, and Ruben Amparado, Jr. 2022. "Abandoned Fishpond Reversal to Mangrove Forest: Will the Carbon Storage Potential Match the Natural Stand 30 Years after Reforestation?" Forests 13, no. 6: 847. https://doi.org/10.3390/f13060847
APA StyleMariano, H., Aguilos, M., Dagoc, F. L., Sumalinab, B., & Amparado, R., Jr. (2022). Abandoned Fishpond Reversal to Mangrove Forest: Will the Carbon Storage Potential Match the Natural Stand 30 Years after Reforestation? Forests, 13(6), 847. https://doi.org/10.3390/f13060847