Abstract
Terpene trilactones (TTLs) are the main secondary metabolites in Ginkgo biloba L. with efficacious pharmacological activity. Jasmonate ZIM-domain (JAZ) protein is a key regulatory factor of the JA signaling pathway, which regulates the biosynthesis of secondary metabolites such as terpenes, alkaloids, and flavonoids. In this study, GbJAZ01~GbJAZ11 were identified from the genome data in G. biloba, which contained TIFY-, Jas-, and weakly conserved NT-domains, and the promoters in most of them contained light, hormone, and stress-responsive elements. Phylogenetic analysis divided all JAZ proteins of Arabidopsis thaliana, Oryza sativa, Picea sitchensis, Taxus chinensis, and G. biloba into nine groups, in which GbJAZs belong to Group VI-IX. GbJAZs have similar functional motifs to A. thaliana and O. sativa, but also contain three specific motifs of gymnosperms, indicating that, although gymnosperms and angiosperms have some conservative structures and functions, their evolutionary processes are independent. Expression pattern analysis showed that the expression levels of GbJAZs were significantly up-regulated by MeJA, but the change pattern and amplitude were different, indicating that the function of GbJAZs in response to a JA signal may be different. After ABA and SA treatment, the expression of GbJAZs was up-regulated or inhibited in varying degrees, and different GbJAZs may be involved in the synergistic or antagonistic effects between JA and other hormone signals. The MeJA significantly increased the content of TTLs in G. biloba leaves, which were significantly positively correlated with the expression levels of GbJAZ01, 02, 07, and 11, and negatively correlated with the expression of GbJAZ04. They may play an important role in JA signaling pathways and the interactions between JA and other hormone signals, and participate in the regulation of the biosynthesis of TTLs. Our results provide a reference for the discovery that GbJAZs are involved in JA signaling pathways, and lay a theoretical foundation for analyzing JA signaling pathways to regulate the synthesis of secondary metabolites.
1. Introduction
Ginkgo biloba L. is a perennial deciduous tree with a long history of cultivation, and is known as a “living fossil”. As a recognized medicinal plant, its extract is effective in the treatment of neurological diseases such as cerebral infarction, Alzheimer’s disease, and ischemic encephalopathy [1]. The active components of G. biloba extract, such as terpenoids, alkaloids, and flavonoids, have the medicinal effects of antioxidation, scavenging free radicals, promoting human blood circulation, regulating vascular relaxation, and reducing blood sugar. Therefore, the pharmacological effects of G. biloba have been widely studied [2].
Jasmonates (Jas) are hormones that play a key role in plant responses to biotic and abiotic stresses, including jasmonic acid (JA), methyl ester derivative methyl jasmonate (MeJA), and amino acid derivatives. In Arabidopsis thaliana, it has been proved that JAs are involved in organ development, cell metabolism and cycle regulation [3,4], and defense against insect pests, bacteria, and abiotic stresses (Wu et al., 2018). As a crucial signal molecule in plant signal networks, JAs play an important role in the development, growth, defense, and regulation of the secondary metabolism in angiosperms and gymnosperms [5].
As the target protein of the SCFCOI1 (skp1-cullin-F-box protein, SCF; coronatine-insensitive 1, COI1) protein complex and the transcriptional suppressor of MYC, Jasmonate ZIM-domain (JAZ) protein is pivotal to JA signal transduction [6]. When JA content in a plant increases due to external stimulation, COI1 binds to JAZ proteins to form a complex receptor of MeJA. At this time, the JAZ protein is degraded by 26S proteasome, releasing the transcriptional activity of transcription factors such as MYC2, thus opening the JA signaling pathway [7]. The JAZ protein contains two main domains, TIFY (N-terminal) and Jas (C-terminal), as well as a weakly conserved NT domain, and does not contain a DNA binding domain, which mainly depends on protein–protein interactions to function [8,9]. The TIFY-domain, also known as the ZIM-domain, is essential for the formation of homologous and heterodimers [10], which regulate the expression level of JAZ gene by participating in the interaction between JAZ protein and its co-inhibitors’ novel interactor of JAZ (NINJA), TOPLESS (TPL) and so on [11]. The Jas-domain, also named the CCT_2 domain, contains a SLx2Fx2KRx2Rx5PY-motif that can interact with COI1 and other transcription factors [12]. The weakly guarded NT-domain can interact with DELLA proteins, a key negative regulator in gibberellin (gibberellins, GA) signaling pathways [13]. The receptors’ non-expressor of pathogenesis-related genes 3 and 4 (NPR3/4) of salicylic acid (salicylic acid, SA) induce the expression and JA biosynthesis of the early responsive genes of the JA signal by promoting the degradation of the JAZ protein [14]. Additionally, the JAZ protein has been reported to have certain functions in the interaction of JA with ethylene (ETH), auxin (IAA), and abscisic acid (ABA) [15]. Yang et al. [16] found that the JAZ protein can regulate plants growth, development, and responses to stress through the co-expression of JA and other related signal molecules in vivo. The JAZs have been identified in many model plants and economic crops; for example, twelve JAZs [5] have been identified in A. thaliana and fifteen JAZs [17] in Oryza sativa, and the regulatory mechanism of JA signaling pathways mediated by them has also been elucidated. JAZs contain a variety of specific motifs, and the JAZ family in angiosperms can be divided into five subgroups according to their specific motifs: the F(A/S)x(A/T)Cx2LS-motif in group I, the SxRx4AIx2I-motif in group III, the LELRLx-motif in group IV, the MERDFLGL- and WxFx2KV-motifs in group V, and a weakly conserved motif in group II [9]. Three newly conserved motifs in the JAZs of the gymnosperms Picea sitchensis and Taxus chinensis were identified, and JAZ containing this motif was significantly different from the JAZs of angiosperms in molecular structure and evolution, and was separated and gathered together in a phylogenetic tree [18]. These motifs are not only the basis of the classification of JAZs, but also the media of JAZ binding according to different transcription factors. Therefore, finding motifs that effectively promote protein binding will help to screen out regulatory factors involved in plant life activities. The study of plant JAZ will be conducive to understanding the origin and evolution of JA signaling systems.
In response to JA signaling induction, transcription factors such as MYC, WRKY, bHLH (basic helix-loop-helix), and ERF (ethylene-responsive factor) are involved in regulating the biosynthesis of secondary metabolites such as flavonoids, terpenoids, and alkaloids in plants [19]. In JA signaling pathways, JAZ protein inhibits the regulatory ability of downstream transcription factors by binding to transcription factors and functional proteins [20]. The identification of JAZ, the key node of JA signaling pathways in G. biloba, contributes to studies of the regulation of JA signaling pathways in G. biloba, which regulate the synthesis of plant secondary metabolites and increase the content of target products. Until now, few JAZ genes have been identified from G. biloba, and whether the GbJAZs have the same structure and function as JAZ in other plants remains to be studied. As they are all gymnosperms, the specificity of family members of GbJAZs is the same as that of P. sitchensis and T. chinensis.
In this study, JAZs of G. biloba were identified based on the genome of G. biloba. The similarities and differences of sequence structure, functional elements, and evolutionary relationships between GbJAZs, GbJAZs and angiosperm JAZs or other gymnosperms JAZs were clarified with bioinformatics analysis, laying a foundation for further functional research. In addition, the research into the changing laws of expression and TTL content under hormone treatment provides a basis for for the study of the involvement of JAZ genes in the metabolic accumulation of terpene lactones induced by hormone signal pathways, and provides a theoretical basis for exploring the synthesis mechanism of TTLs in G. biloba.
2. Materials and Methods
2.1. Identification of GbJAZs
The G. biloba genome data were downloaded from GigaDB (http://gigadb.org/, accessed on 15 January 2021), and the JAZ protein sequences from A. thaliana were derived from Phytozome (https://phytozome.jgi.doe.gov/, accessed on 19 January 2021) [21]. All the sequences from G. biloba were identified based on the Hidden Markov models of the conserved TIFY-domain (PF06200) and Jas-domain (PF09425) belonging to the JAZ family by using HMMER 3.0. At the same time, the homologous genes in the genome of G. biloba were obtained using local BLAST, with the JAZ protein sequences of A. thaliana as seed sequences. Furthermore, the conserved domains of the candidate sequences were looked up using the CD Search in NCBI (https://www.ncbi.nlm.nih.gov/, accessed on 20 January 2021), and the sequences with incompletely conserved domains were deleted to obtain the JAZs in the G. biloba [22].
2.2. Bioinformatics Analysis of GbJAZs
The gff3. file of the G. biloba genome was used to build chromosome localization of GbJAZs with TBtools. The protein physicochemical properties of GbJAZs were analyzed using the ProtParam tool in ExPASy (https://web.expasy.org/, accessed on 3 March 2021). The WoLF PSORT (https://wolfpsort.hgc.jp/, accessed on 3 March 2021) was used to predict the subcellular localization of GbJAZs. The exon–intron structures of GbJAZs were displayed by GSDS 2.0 (http://gsds.cbi.pku.edu.cn/, accessed on 4 March 2021). The conserved motifs of the GbJAZs were searched in MEME (http://meme-suite.org/tools/meme, accessed on 4 March 2021) and analyzed using TBtools. Multiple sequence alignment of the JAZ family genes of G. biloba was carried out with Vector NT1 11.5 [23]. The upstream 2000 bp genomic DNA sequences of the GbJAZs’ start code were downloaded and submitted to PlantCARE (http://bioinformatics.psb.ugent.be/webtools/plantcare/html/, accessed on 6 March 2021) to predict putative cis-elements [24].
To analyze the groups of the putative JAZ family, we downloaded AtJAZ, OcJAZ proteins from Phytozome (https://phytozome.jgi.doe.gov/, accessed on 13 March 2021) and PsJAZ, TcJAZ proteins from NCBI (Supplementary Table S4). A neighbor-joining tree with the whole sequences of these JAZ and GbJAZ proteins was constructed by MEGA 6.0 with bootstrap analysis (1000 replicates) [18]. The protein–protein interaction of JAZ proteins was predicted using STRING (https://string-db.org/, accessed on 1 May 2022) under the following parameters: A. thaliana was selected to perform the comparison analysis, and then the minimum required interaction score was set to middle confidence 0.400 [25].
2.3. Expression Analysis of GbJAZs
Two-year-old seedlings of G. biloba with the same growth potential, which were cultured in the greenhouse of Yangtze University west campus, were used as research materials. MeJA (1 mM), ABA (1 mM), and SA (20 mM) were sprayed on the leaves, respectively, with water as the control, and three biological repetitions were set for each treatment, with five plants for each repetition [26,27]. After 0, 3, 6, 12, 24, and 48 h, the leaves of G. biloba were collected and frozen in liquid nitrogen, and stored at −80 °C for later use. The total RNA was extracted using the MiniBEST Plant RNA Extraction Kit (TaKaRa, Dalian, China), and cDNA was synthesized using HiScript II Q RT SuperMix for qPCR (Vazyme, Nanjing, China). The fluorescent quantitative reaction system was prepared with 2 × ChamQ Universal SYBR Qpcr Master Mix (Vazyme, Nanjing, China). Real-time quantitative PCR according to the method of Xu et al. [28] was performed on Bioer LineGene 9600 Plus fluorescence quantitative PCR amplifier. JAZ protein can regulate its growth, development, and response to stress through the expression of JA and other related signal molecules in vivo. Three biological repeats and three technical repeats were conducted, and the sequence of primers is shown in Table 1. The 2−△△Ct method [29] was used to analyze the expression level of the GbJAZs.

Table 1.
qRT-PCR primer sequence of GbJAZs.
2.4. Determination of TTL Content in G. biloba Leaves
The content of the TTLs in the leaves collected at 0, 3, 6, 12, 24, and 48 h after MeJA treatment was detected through the evaporative light-scattering detector high-performance liquid chromatography (HPLC-ELSD) method [30]. The 5 g leaf powder ground with liquid nitrogen was extracted with 30 mL of ethyl acetate in a 600 W ultrasonic bath for 15 min. The sample was wrapped in a filter paper bag and placed in a Soxhlet extractor, 20 mL ethyl acetate and 2 drops of 2% hydrochloric acid were added, and it was concentrated and refluxed for 1.5 h at 90 °C and evaporated to dryness at 90 °C. The residue was dissolved in 5 mL methanol and then passed through a filter membrane with a pore diameter of 0.45 μm to obtain the sample solution. Ginkgolide A (GA), ginkgolide B (GB), ginkgolide C (GC), ginkgolide J (GJ) and bilobalide (BB) (HPLC ≥ 98%) were used as single and mixed standard samples, and the peak time and standard curve were determined. The mobile phase was composed of methanol-tetrahydrofuran-water (20:8:72) and other conditions of chromatography were set according to the method of Zheng. The injection volume was 10 μL, and three replications were performed. The statistical significance of the TTL content was analyzed with GraphPad Prism 7. All tests were performed in triplicate, and data were represented as means ± SD (n = 3).
3. Results
3.1. Identification and Physicochemical Properties Analysis of GbJAZs
Through the screening of domains, eleven candidate GbJAZs containing both TIFY- and Jas-domains were identified and named GbJAZ01~GbJAZ11 according to their sequences on the chromosome (Supplementary Table S1). The analysis of physical and chemical properties shows that the amino acids of GbJAZs are 157~522, the molecular masses are 17.46~56.38 KDa, the theoretical isoelectric points of GbJAZs are more than 7, which is alkaline, and all are unstable proteins with hydrophilicity between −0.75 and 0.19, except GbJAZ01 (Table 2 and Table S2). The results of the chromosome distribution show that GbJAZs are unevenly distributed on chromosomes 5, 7, 8, 9, 10, and 11. Among them, there are four GbJAZs on chromosome 9, three GbJAZs on chromosome 5, and a GbJAZ on each of the other chromosomes. Furthermore, it appears that there is a clustering of GbJAZ01, GbJAZ02, and GbJAZ03 on chromosome 5 (Figure 1). The results of the subcellular localization prediction show that GbJAZ10 is located in the cell membrane and nucleus, and the other nine GbJAZs are located in the nucleus (Table 2).

Table 2.
Basic information for GbJAZs.
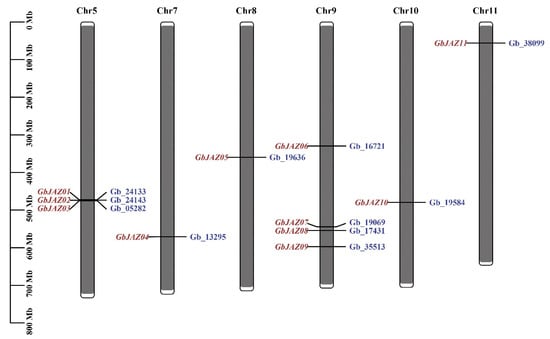
Figure 1.
Chromosome localization of GbJAZs.
3.2. Gene Structure Analysis of GbJAZs
The full-length protein sequences of GbJAZs were predicted using the MEME online website (Figure 2A). The GbJAZ01~GbJAZ11 contained 3~6 conserved motifs. Motif 1, Motif 2, and Motif 3 are all distributed in eleven GbJAZs. Among them, Motif 1 at the N-terminal is a TIFY-domain, Motif 2 at the C-terminal is a Jas-domain, and Motif 3 is a weakly conservative domain. Motif 1, Motif 2, and Motif 3 of the GbJAZs are relatively conservative in evolution. GbJAZ01, GbJAZ02, GbJAZ03, GbJAZ06, and GbJAZ11 all have Motif 5. GbJAZ01, GbJAZ02, GbJAZ04, GbJAZ05, and GbJAZ11 all have a Motif 6 at the N-terminal. GbJAZs in the same subgroup all have the same protein motif and distribution; for example, Motif 9 exists at the C-terminal of GbJAZ04 and GbJAZ11; Motif 4 and Motif 7 are only distributed on GbJAZ05 and GbJAZ10; and GbJAZ07 and GbJAZ08 have Motif 8 (Figure 2A,B). It can be seen that there are differences in the type, quantity, and distribution of the conserved motifs of GbJAZs, indicating that there may be functional differentiation in GbJAZs. The results of intron and exon information analysis of GbJAZs show that there are seven exons in GbJAZ11, three exons in GbJAZ08, six exons in GbJAZ01, GbJAZ02, GbJAZ03, GbJAZ04 and GbJAZ06, five exons in GbJAZ05 and GbJAZ09, and four exons in GbJAZ07 and GbJAZ10 (Figure 2C). Moreover, the corresponding gene structures of the proteins with close evolutionary relationship are also similar. The results of multiple sequence alignment show that there are two obvious conserved regions in GbJAZs, namely the TIFY- and Jas-domains. It was found that the sequences of GbJAZs in the same subgroup are very similar, and with differences in GbJAZs among each of the subgroups (Figure 3).
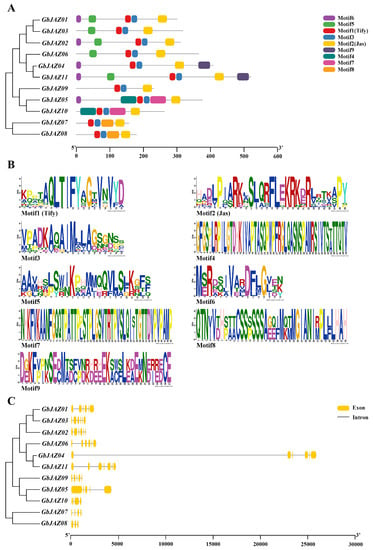
Figure 2.
Genetic structure of GbJAZs. (A) Evolutionary tree and motif distribution map; (B) Motif logo; (C) Exon and intron distribution map.

Figure 3.
Multiple sequence alignment of GbJAZs. The red underlined area is the TIFY domain and the blue underlined area is the Jas domain.
3.3. Cis-Acting Elements Analysis of GbJAZs
The results concerning the cis-acting elements analysis of GbJAZs show that all the promoter regions of GbJAZs contain light responsive elements, mostly including hormone-, stress-, and growth-related cis-acting regulatory elements (Figure 4, Supplementary Table S3). Except for GbJAZ03, all of the gene promoter regions contain MeJA-responsive elements. Both GbJAZ02 and GbJAZ03 contain GA-responsive elements. GbJAZ01, GbJAZ02, GbJAZ03, GbJAZ05, and GbJAZ06 all contain IAA-responsive elements. GbJAZ05, GbJAZ09, GbJAZ07, and GbJAZ10 all contain SA-responsive elements. Except for GbJAZ06, all other genes contain ABA-responsive elements. We also predicted the defense- and stress-responsive component TC-rich repeats in GbJAZ01, GbJAZ02, GbJAZ05, and GbJAZ07, and the low-temperature responsiveness elements LTR and TATA-box in GbJAZ05 and GbJAZ09. Except for GbJAZ04 and GbJAZ11, all of the GbJAZs have anaerobic stress-responsive elements. GbJAZ03 and GbJAZ07 contain drought-stress-responsive MYB-binding sites MBS. GbJAZ01, GbJAZ02, GbJAZ06, and GbJAZ10 all contain light-responsive MYB-binding sites MRE.
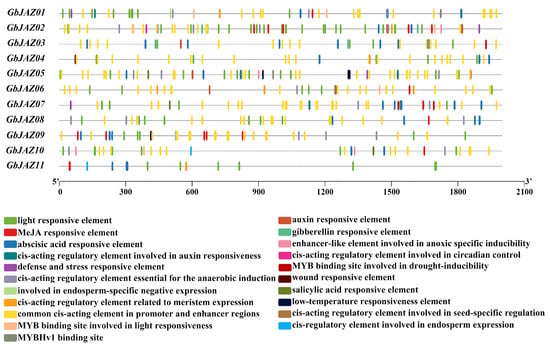
Figure 4.
Distribution of cis-acting elements of GbJAZs.
3.4. Phylogenetic Analysis of GbJAZs
According to the phyla of plants, homology, and the different motifs of genes, A. thaliana (14), O. sativa (15), P. sitchensis (13), T. chinensis (9), and G. biloba JAZ proteins were divided into nine groups in the phylogenetic tree (Figure 5). The JAZ proteins of gymnosperms and angiosperms were divided into two distinct groups, with JAZs of gymnosperms in the group VI-IX, and JAZs of angiosperms in the group I-V. There is a close evolutionary relationship between the JAZ proteins of gymnosperms and angiosperms, and a distant evolutionary relationship between the JAZ proteins of gymnosperms and angiosperms. Thus, the JAZ proteins may have a common ancestor, but with obvious evolution.
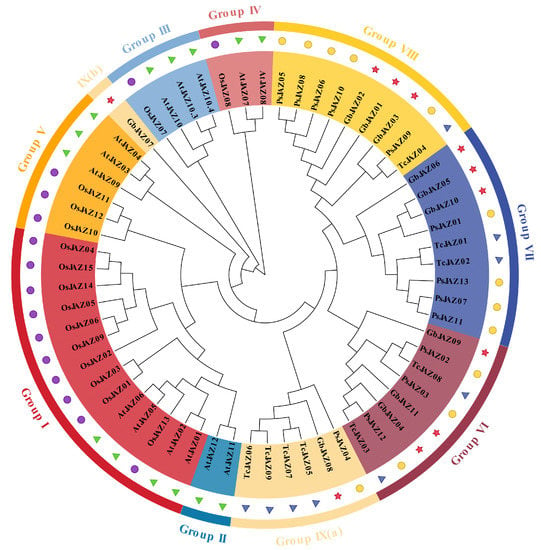
Figure 5.
Phylogenetic tree of the JAZ family in Arabidopsis thaliana (At), Oryza sativa (Os), Picea sitchensis (Ps), Taxus chinensis (Tc) and Ginkgo biloba (Gb) JAZs. Different colored shapes represent different species, Arabidopsis thaliana (green triangle), Oryza sativa (purple circle), Picea sitchensis (yellow circle), Taxus chinensis (blue triangle), Ginkgo biloba (red star).
Three conserved motifs peculiar to gymnosperms were found in GbJAZs, TcJAZs, and PsJAZs of group VI-IX: PxMMx3LS- (Figure 6A), (A/T)(F/M)EEx6G(L/F)(P/G)S- (Figure 6B) and LDLSLx-motifs (Figure 6C), indicating that JAZs have their independent evolution process in gymnosperms and angiosperms. In group VIII, the PxMMx3LS-motifs in GbJAZ01, GbJAZ02, GbJAZ03, PsJAZ05, PsJAZ06, PsJAZ08, PsJAZ09, PsJAZ10, and TcJAZ04 were highly conserved. In group VII, there was little difference in the (A/T)(F/M)EEx6G(L/F)(P/G)S-motifs of GbJAZ05, GbJAZ10, PsJAZ01, PsJAZ07, PsJAZ11, PsJAZ13, TcJAZ01, and TcJAZ02. The LDLSLx-motif of GbJAZ09 in group VI, and of TcJAZ05, TcJAZ06, TcJAZ07, TcJAZ09, and PsJAZ04 in group IX, is a typical ethylene-responsive element binding factor-associated amphiphilic repression-motif (EAR-motif), which is different from the highly conserved LELRLx-EAR-motif in group IV. The JAZs of group VI contain the AVPQARK-motif (Figure 6D) at the front end of the Jas-domain necessary for COI1 binding, which is the same as in OsJAZ10, OsJAZ11, OsJAZ12, and AtJAZ09 of group V. V(E/A)RDF(L/M)GL-motifs (Figure 6E) of the JAZs (N-terminal) in group V were also found in AtJAZ03, AtJAZ04, AtJAZ09, OsJAZ10, OsJAZ11, and OsJAZ12 in group V (Figure 6F). In parallel, the V(E/A)RDF(L/M)GL-motif is highly similar to the ELDFLGL-motif (Figure 6G) of the genes in group III.

Figure 6.
Specific motifs model and sequences alignment. (A) PxMMx3LS-motif; (B) (A/T)(F/M)EE x6G(L/F)(P/G)S-motif; (C) LDLSLx-motif; (D) AVPQARK-motif; (E) V(E/A)RDF(L/M)GL-motif; (F) MERDFLG-motif; (G) ELDFLGL-motif.
3.5. Protein–Protein Interaction Network Prediction of GbJAZs
The interaction of the JAZ protein of A. thaliana corresponding to the homologous JAZ protein of G. biloba was predicted by STRING (Figure 7), as the homologous JAZ proteins of AtJAZ03, GbJAZ04, and GbJAZ11 belonged to group VI and could interact with MYC2, which is a common transcription factor of light, and ABA- and JA-signaling pathways. GbJAZ07 has high homology with AtJAZ09 and may have the function of interacting with MYC3, which is involved in tryptophan-responsive, JA-responsive, and other stress-responsive gene regulation. GbJAZ01, GbJAZ02, and GbJAZ03 may interact with MYC4, which is related to JA gene regulation. All of the above JAZ proteins may have the function of combining with COI1 and recruiting NINJA to be the negative regulator of jasmonate responses. GbJAZ proteins may be related to the negative regulation of genes’ JA responses. It can be concluded that GbJAZ01, GbJAZ02, GbJAZ03, GbJAZ04, GbJAZ07, and GbJAZ11 proteins may interact with some genes and act as negative regulators in JA signaling pathways.
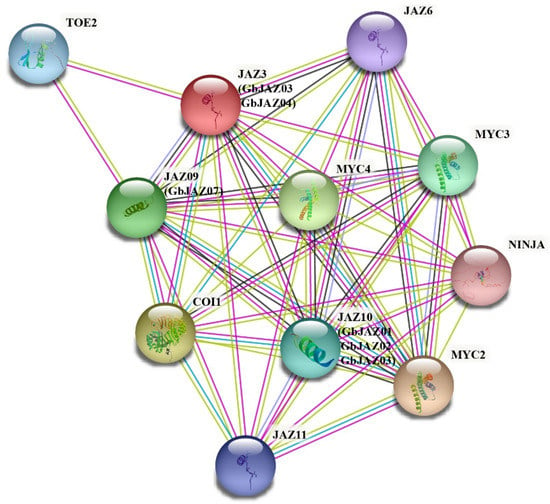
Figure 7.
The functional regulatory network of G. biloba JAZ proteins. The protein–protein interaction of JAZ proteins was predicted using STRING software. The cyan line represents data from curated databases; the purple line experimentally determined data; the green line gene neighborhood; the blue line gene co-occurrence; the yellow line text mining; the black line co-expression; and the gray line protein homology.
3.6. Expression Patterns of GbJAZs
To predict the roles of GbJAZs, we examined their expression profiles in response to MeJA, ABA, and SA (Figure 8, Supplementary Table S5). The GbJAZs responded positively to the induction of MeJA, but the expression levels and time points at the peak were different. The expression levels of GbJAZ01 and GbJAZ08 reached their peaks at 6 h and 3 h, respectively, which was about 80 times higher than that of the control group. Notably, GbJAZ04 showed an upward trend within 48 h of treatment, indicating that each of the GbJAZs may perform different functions in response to JA signals. After the treatment with ABA, the expression levels of the GbJAZs were up-regulated, and then decreased in the following 12~48 h. In particular, GbJAZ06, GbJAZ08, and GbJAZ11 were expressed lower than the control group at 24~48 h. Except for the expression levels of GbJAZ06, GbJAZ10, and GbJAZ11, which decreased after the induction of SA, most GbJAZs increased at first and then decreased, seeming to be lowly expressed. Under the treatment of MeJA, SA and ABA, the expression level of GbJAZ07 increased at first and then decreased, and the expression level at 6~48 h was lower than that of the control group. To sum up, GbJAZs can be induced by MeJA, SA, and ABA hormones, which may regulate related pathways, respond to abiotic stress, or participate in regulating the synthesis of secondary metabolites of G. biloba.
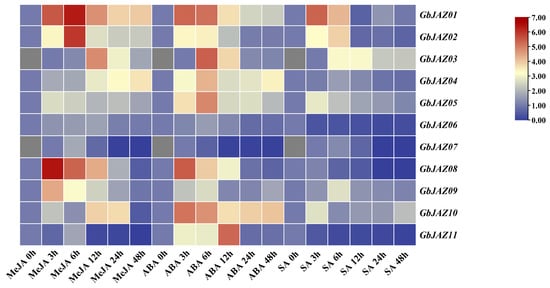
Figure 8.
Heatmap analysis of GbJAZ expression levels under the induction of MeJA, ABA and SA.
3.7. Correlation Analysis between the Expression Levels of GbJAZs and TTL Content in G. biloba
The concentration of TTLs in the samples was calculated according to the standard curve (Supplementary Table S6). The content of TTLs in G. biloba leaves increased at first and then decreased after treatment with exogenous hormone MeJA, and reached the highest value (1.379 mg/g) at 48 h after treatment (Figure 9A). The correlation analysis between the expression of GbJAZs and TTL content after treatment with MeJA showed the expression of GbJAZ01, GbJAZ02, and GbJAZ07 was extremely significantly positively correlated with TTL content, while the expression of GbJAZ04 and GbJAZ11 was significantly negatively correlated with TTL content (Figure 9B, Supplementary Table S7). It is speculated that these five GbJAZs are involved in regulating the content of TTLs in G. biloba leaves.
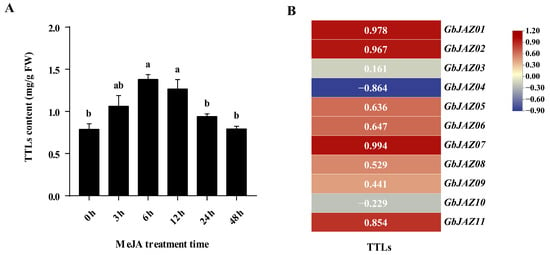
Figure 9.
Correlation analysis. (A) The content of TTLs in the G. biloba leaves treated with MeJA; (B) Correlation analysis between the expression level of GbJAZs and TTL content.
4. Discussion
4.1. Specific Motifs of GbJAZs
The JAZ protein, as a repressor of the JA signaling pathway, contains domains and specific motifs (Supplementary Figure S1) that can bind to different transcription factors, regulate downstream pathways, and perform different functions through interaction [31,32]. The JAZ proteins of group V in the phylogenetic tree all belong to angiosperm genes, which are typically characterized by the presence of MERDFLGL- and WxSx2KV-motifs [9]. The JAZs of gymnosperms in group VI have the V(E/A)RDF(L/M)GL-motif (Figure 6E), which is highly similar to the MERDFLGL-motif (Figure 6F). At the same time, the front end of the Jas-domain in JAZs of group VI is very similar to that of group V, all of which are AVPQARK-motifs (Figure 6D). Meanwhile, the front end of the Jas-domain in AtJAZ09, OsJAZ10, OsJAZ11, and OsJAZ12 can form a ring to bind JA-Ile and COI1 [33]. It is proved that JAZs in group VI may have the function of combining with COI1. V(E/A)RDF(L/M)GL and AVPQARK-motifs are highly conserved in angiosperms and gymnosperms, indicating that JAZs in group VI may play an important role in the JA signaling system. Moreover, the results show that the ELDFLGL-motif at the N-terminal (Figure 6G) of group III is highly similar to the V(E/A)RDF(L/M)GL and MERDFLGL-motifs, so there may be a certain evolutionary relationship among groups VI, V and III.
DELLA and MYC2 in A. thaliana compete to bind JAZ in vivo, and are affected by GA in regulating JA signaling transduction, which is the key to crosstalk between JA and GA signaling systems. At the same time, the binding of the DELLA protein to JAZ is affected by the NT-domain of JAZs [34]. Many distinctions are also found between the structure of the NT-domain in different JAZ genes. The F(A/S)x(A/T)Cx2LS-motif of AtJAZs in group I, the SxRx3SAIx2I-motif of AtJAZs in group III, and the KEEx4SRDSx5Mx2SF-motif of AtJAZ03 in group V, all belong to the NT-domain [35]. In gymnosperm JAZs, the K(D/E)xEx2TKD(S/F) xTDx4DG(S/N)-motif at the back end of the V(E/A)RDF(L/M)GL-motif of GbJAZ04, TcJAZ03, and PsJAZ12 in group VI is similar to the KEEx4SRDSx5Mx2SF-motif of AtJAZ3, and also belongs to the NT-domain. It is suggested that GbJAZ04, TcJAZ3 and PsJAZ12 in group VI may play a crucial part in the crosstalk of pathogen interaction and plant development regulated by GA and JA signals. The JAZs of group VII and group VIII, respectively, contain the (A/T)(F/M)EEx6G(L/F)(P/G)S- and PxMMx3LS-motifs of gymnosperms, and are located at the site of the NT-domain in JAZs. Since these two motifs are not found in the JAZ proteins of angiosperms, and are significantly different from the NT-domain of AtJAZs, the question of whether they are NT-domains or not remains to be further studied.
The EAR motif is a dominant repression motif that suppresses gene expression by binding to a co-repressor directly or by recruiting NINJA [36]. Kagale et al. [37] studied the JAZs of A. thaliana and found that AtJAZ05 in group I contained two EAR-motifs, namely the LxLxL-like EAR-motif at the C-terminal, and the DLNPT-like EAR-motif in the middle. The EAR-motif of AtJAZ05 can directly bind to TPL co-repressors [38]. The LDLSLx-motifs of TcJAZ05, TcJAZ06, TcJAZ07, TcJAZ09, PsJAZ04, and GbJAZ09 also belong to LxLxL-EAR-motifs, and they may have the same function as AtJAZ05.
4.2. Different Expression Patterns of GbJAZs
Some specific genes in plants respond to exogenous hormones, while the degree of response of JAZs are different in each plant [39]. The JAZs in apples (Malus domestica) and grapes (Vitis vinifera) can positively respond to the induction of JA and ABA, but are not sensitive to SA [40,41]. The expression levels of most GbJAZs in the G. biloba leaves treated with ABA and MeJA were significantly up-regulated, which is similar to the response seen in most plants. However, the expression of JAZ genes in A. thaliana, apples, grapes and other plants was higher than that in the early stage after exogenous hormone treatment [42], while the response speeds of GbJAZs were slightly slower, and the expression reached its peak at 6~12 h after treatment. This may be due to the fact that, in the early stage of treatment, a large number of JAZ proteins interact with transcription factors such as MYC, WRKY and ERF, resulting in the specificity of JAZ genes’ time expression.
When the JA signaling pathway is activated by MeJA, JAZ protein is induced to be highly expressed as an inhibitor which inhibits the activity of some transcription factors, such as MYC2, to prevent excessive energy consumption caused by an over-strong response to JA in plants [43]. The results of studies in A. thaliana, cotton (Gossypium hirsutum L.) and other plants also show that JAZ genes are highly expressed in the early stages after hormone treatment and are the negative regulators of JA pathways [44]. After MeJA treatment, the relative expression of GbJAZs also shows an upward trend, indicating that they may also be the negative regulators of JA pathways. After the members of the same family in the same species were treated with exogenous hormones, the expression changes were different, but the expressions of the members of the same subgroup often showed similar changes [45]. However, the expression levels of GbJAZ01~11 were different under the treatment of exogenous hormones, and the GbJAZs with high similarity in the same subgroup also had different expression patterns. A similar situation exists in Populus trichocarpa [46], which may be related to the differentiation of gene transcriptional activity. The relative expression levels of GbJAZ01, GbJAZ02, and GbJAZ03 in group VIII were significantly up-regulated (>5 fold) under the treatment of three exogenous hormones, indicating that members of group VIII may play an important part in the regulation of JA signaling pathways. In addition, with high expression levels under the treatment of three hormones, GbJAZ08, GbJAZ09, and GbJAZ10 may also be regulatory factors in the JA signaling pathway. Due to the possibility of the functional redundancy of genes with low expression but significant changes, it remains to be seen whether other GbJAZs in their subgroup have the same function.
JAZ protein interacts with ABI, the core transcription factor of the ABA signaling pathway, and participates in the interaction between the JA and ABA signaling pathways [14]. NPR3 and NPR4, the receptors of the SA pathway, can bind to JAZ proteins and promote their degradation to initiate JA signaling pathways [15]. The expression levels of GbJAZs increased or decreased to different degrees under the treatment of ABA and SA, indicating that each of the GbJAZs has different functions when participating in the interaction between JA and other hormone signaling pathways, which may be involved in the synergism or antagonism between hormones.
4.3. Identification of Candidate GbJAZs Involved in Regulating the Biosynthesis of TTLs
DELLA represented by RGA has the ability to regulate sesquiterpene synthesis by repressing the activities of MYC2, GL3, EGL3, and GL1 in MBW, together with JAZ, which is determined by the structure of the NT-domain in A. thaliana JAZs [47]. With the high concentration of GA in Artemisia annua, DELLA protein is degraded, and AaJAZ8 can bind to AaMYC2 and inhibit its transcriptional activity, thus regulating the accumulation of sesquiterpenes. The similar NT-domains of GbJAZ04 in group VI, and GbJAZ01, GbJAZ02 and GbJAZ03 in group VIII, may cooperate with DELLA proteins to regulate the synthesis of TTLs. The distribution of genes in the same family is not random in the genome, and the genes adjacent to each other may have functional clustering. Because the transcriptional correlation between genes close to each other is higher, it shows that the functional clustering of genes can affect transcriptional regulation in biosynthesis [48,49]. In this study, GbJAZ01, GbJAZ02, and GbJAZ03 were distributed in chromosome 5 and clustered into a gene cluster; they may have similar levels of transcriptional regulation and jointly regulate the synthesis of secondary metabolites in G. biloba. The JA signaling regulation mode in T. chinensis with TcJAZ03-TcMYC2 as the core, and TcERF2/15 and TASY synthase genes as the target gene, can regulate the synthesis of taxol [19]. As the homologous genes of TcJAZ03, GbJAZ04, and GbJAZ11 in group VI also have the same AVPQARK- and V(E/A)RDF(L/M)GL-motifs as TcJAZ03, they seem to be able to regulate the synthesis of secondary metabolites in G. biloba. In some angiosperms, JAZs containing LxLxL-like EAR-motifs are related to the development of hairy roots. When SmJAZ3 and SmJAZ9 were overexpressed in hairy roots of Salvia miltiorrhiza, the synthesis of tanshinone was inhibited [50]. The combination of AaJAZ8 and AaHD1 in Artemisia annua can change the density of hairy roots, and then change the content of artemisinin [51]. Because of the LDLSLx-motif which belongs to the LxLxL-like EAR-motifs in GbJAZ09, we speculate that GbJAZ09 may also affect the synthesis of secondary metabolites in G. biloba by regulating the growth of hairy roots.
Exogenous hormones are able to induce the biosynthesis of secondary metabolites such as terpenes, alkaloids, and flavonoids in plants. The study of A. thaliana showed that, with an increase in the expression levels of AtJAZ02 and AtJAZ07 under JA and MeJA treatment, the content of phenylpropanoids increased and flavonoids decreased in plants, suggesting that, as key genes, AtJAZ02 and AtJAZ07 can regulate the synthesis of secondary metabolites mediated by Jas [52]. SmJAZ03 in Salvia miltiorrhiza showed significant responses to MeJA, ABA, and SA, respectively, which performed a negative-feedback-regulation function in JA signaling pathways, participated in the interaction between JA and other signaling pathways, and regulated the synthesis of tanshinone [45]. After exogenous MeJA treatment, the content of TTLs in G. biloba leaves increased at first and then decreased to the pre-treatment level. Although the expression of JAZ family members was also significantly up-regulated, the expression levels of GbJAZ01, GbJAZ02, GbJAZ04, GbJAZ07, and GbJAZ11 were extremely significantly or significantly correlated with the content of TTLs, and seemed to be the key genes for the interaction between JA and other hormone signaling pathways that are involved in the composition, content and metabolic pathways of TTL biosynthesis in G. biloba. The expression levels of TcJAZ03 and TcJAZ08 induced by exogenous MeJA were negatively correlated with the content of paclitaxel, which was the negative regulator of paclitaxel synthesis [18]. In addition, as homologous genes of TcJAZ03 and TcJAZ08, GbJAZ04, and GbJAZ11 further confirmed their functions in regulating the synthesis of TTLs, in which GbJAZ04 may be involved in the JA signaling pathway and negatively regulate the synthesis of TTLs. Furthermore, JAZ-MYC coupling was regarded as a protein–protein interface essential for responses to stressors in A. thaliana [53]. Protein–protein interaction network prediction has confirmed some interactions between AtJAZ and MYC, which further indicates that their homologous genes (GbJAZ01, GbJAZ02, GbJAZ03, GbJAZ04, GbJAZ07, and GbJAZ11) may negatively regulate the key transcriptional activator of JA responses. JAZ-MYC coupling provides a way for JAZ to participate in JA signal pathways to regulate the synthesis of secondary metabolites in G. biloba.
5. Conclusions
In this study, eleven JAZ family genes of G. biloba, namely GbJAZ01~11, were identified. All the GbJAZs contain typically conserved TIFY- and Jas-domains, as well as weakly conserved NT-domains and three motifs specific to gymnosperms. The promoter region mostly contains hormone response, stress response, light response, growth and development, and other related cis-acting elements. The JAZs of the gymnosperm G. biloba not only contain some of the same structures and functions as angiosperms, but also have an independent evolutionary process. GbJAZ04 and GbJAZ11, which belong to group VI and have NT-domains with proven functions, may play crucial roles in the JA signaling system. The protein–protein interaction prediction results indicate that GbJAZ proteins may be related to genes’ negative regulation of JA responses. The expression of GbJAZ01, GbJAZ02, GbJAZ04, GbJAZ07, and GbJAZ11 in JAZ family members of G. biloba changed significantly under the treatment of three exogenous hormones, and significantly correlated with the change in TTL content induced by MeJA. They may be the key regulators of the JA pathway, participate in the interactions between JA signaling and other signaling pathways, and regulate the synthesis of TTLs and other secondary metabolites. The results of this study are of great significance for exploring the function of JAZ genes, revealing JA signaling pathways, and enriching the gene resources of G. biloba.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/f13050781/s1, Figure S1: The sequence alignment of specific motifs; Table S1: The sequences of candidate GbJAZs; Table S2: The physicochemical properties of GbJAZs proteins; Table S3: The cis-acting elements of GbJAZs; Table S4: The sequences of JAZs in a phylogenetic tree; Table S5: The expression data statistics of GbJAZs after hormone treatment; Table S6: The data of TTL content determination; Table S7: The data of correlation analysis between the expression levels of GbJAZs and TTL content in G. biloba leaves.
Author Contributions
X.H., X.L. and J.Z. conceived and designed the experimental procedures; X.H. conducted the experiments; X.H. wrote the original manuscript; X.L., J.Z., J.Y., Y.L. and F.X. revised the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by National Natural Science Foundation of China, grant number 31901344.
Data Availability Statement
The data and results are available to every reader upon reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Cui, T. Studies on Regulation of Ginkgo Flavonesand and Ginkgolides Accumulation in Cultured Cells of Ginkgo biloba L. and Their Pharmacology; South China University of Technology: Guangzhou, China, 2002. (In Chinese) [Google Scholar]
- Hu, Z. A research on Extraction and Preparation Technology of Flavonoids from Ginkgo biloba Leaves; Jinan University: Guangzhou, China, 2017. (In Chinese) [Google Scholar]
- Mandaokar, A.; Thines, B.; Shin, B.; Lange, B.; Choi, G.; Koo, Y.; Yoo, Y.; Yang, D.; Choi, G.; Browse, J.; et al. Transcriptional regulators of stamen development in Arabidopsis identified by transcriptional profiling. Plant J. Cell Mol. Biol. 2010, 46, 984–1008. [Google Scholar] [CrossRef] [PubMed]
- Pauwels, L.; Morreel, K.; Witte, E.D.; Lammertyn, F.; Montagu, M.; Boerjan, W.; Inze, D.; Goossens, A. Mapping methyl jasmonate-mediated transcriptional reprogramming of metabolism and cell cycle progression in cultured Arabidopsis cells. Proc. Natl. Acad. Sci. USA 2008, 105, 1380–1385. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pauwels, L.; Goossens, A. The JAZ proteins: A crucial interface in the jasmonate signaling cascade. Plant Cell 2011, 23, 3089–3100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wager, A.; Browse, J. Social network: JAZ protein interactions expand our knowledge of Jasmonate signaling. Front. Plant Sci. 2012, 3, 41. [Google Scholar] [CrossRef] [Green Version]
- Staswick, P.E. JAZing up jasmonate signaling. Trends Plant Sci. 2008, 13, 66–71. [Google Scholar] [CrossRef]
- Vanholme, B.; Grunewald, W.; Bateman, A.; Kohchi, T.; Gheysenet, G. The Tify family previously known as ZIM. Trends Plant Sci. 2007, 12, 239–244. [Google Scholar] [CrossRef]
- Bai, Y.; Meng, Y.; Huang, D.; Qi, Y.; Chen, M. Origin and evolutionary analysis of the plant-specific TIFY transcription factor family. Genomics 2011, 98, 128–136. [Google Scholar] [CrossRef] [Green Version]
- Chung, H.S.; Howe, G.A. A critical role for the TIFY motif in repression of jasmonate signalng by a stabilized splice variant of the JASMONATE ZIM-domain protein JAZ10 in Arabidopsis. Plant Cell 2009, 21, 131–145. [Google Scholar] [CrossRef] [Green Version]
- Han, B.; Liu, J.; Zhao, X.; Tang, Z. Bioinformatics analysis of JAZ gene family in maize. J. Shanxi Agric. Sci. 2020, 48, 1552–1556. (In Chinese) [Google Scholar]
- Sheard, L.B.; Tan, X.; Mao, H.B.; Withers, J.; Nissan, G.B.; Hinds, T.R.; Kobayashi, Y.; Hsu, F.F.; Sharon, M.; Browse, J.; et al. Jasmonate perception by inositol-phosphate-potentiated COI1–JAZ co-receptor. Nature 2010, 468, 400–405. [Google Scholar] [CrossRef]
- Kazan, K.; Manners, J.M. JAZ repressors and the orchestration of phytohormone crosstalk. Trends Plant Sci. 2012, 17, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Sonbol, F.M.; Huot, B.; Gu, Y.; Withers, J.; Mwimba, M.; Jian, Y.; Sheng, Y.; Dong, X. Salicylic acid receptors activate jasmonic acid signalling through a non-canonical pathway to promote effector-triggered immunity. Nat. Commun. 2016, 7, 13099. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ju, L.; Jing, Y.; Shi, P.; Liu, J. JAZ proteins modulate seed germination through interaction with ABI5 in bread wheat and Arabidopsis. New Phytol. 2019, 223, 246–260. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Liu, Z.; Qu, S.; Yu, L.; Zhan, Y. Effects of transient infection of FmJAZ1 gene on JA pathway related gene expression in Fraxinus mandshurica. Guihaia 2021, 41, 662–670. (In Chinese) [Google Scholar]
- Taniguchi, S.; Hosokawa, S.Y.; Tamaoki, D.; Yamada, S.; Akimitsu, K.; Gomi, K. Jasmonate induction of the monoterpene linalool confers resistance to rice bacterial blight and its biosynthesis is regulated by JAZ proteinin rice. Plant Cell Environ. 2014, 37, 451–461. [Google Scholar] [CrossRef]
- Zhang, M.; Chen, Y.; Nie, L.; Jin, X.; Fu, C.; Yu, L. Molecular, structural, and phylogenetic analyses of Taxus chinensis JAZs. Gene 2017, 620, 66–74. [Google Scholar] [CrossRef]
- Zhang, M. The JA Regulation Mechanism on Taxol Biosynthetic in Taxus chinensis; Huazhong University of Science and Technology: Wuhan, China, 2016. (In Chinese) [Google Scholar]
- Dong, Y.; Zhang, W.; Ling, Z. Advances in transcription factors regulating plant terpenoids biosynthesis. Bull. Bot. 2020, 55, 340–350. (In Chinese) [Google Scholar]
- Guan, R.; Zhao, Y.; Zhang, H.; Fan, G.; Liu, X.; Zhou, W.; Shi, C.; Wang, J.; Liu, W.; Liang, X.; et al. Draft genome of the living fossil Ginkgo biloba. Gigascience 2016, 5, 49. [Google Scholar] [CrossRef] [Green Version]
- Huang, J.; Deng, S.; Shen, J. Identification and response to phytophthora sojae infections of JAZ gene familyin Soybean. Soybean Sci. 2019, 38, 868–878. (In Chinese) [Google Scholar]
- Chen, S.; Liu, X.; Liao, Y.; Zhang, W.; Xu, F. Genome-wide identification of WRKY family genes and analysis of their expression in response to abiotic stress in Ginkgo biloba L. Not. Bot. Horti Agrobot. Cluj Napoca 2019, 47, 1100–1115. [Google Scholar] [CrossRef] [Green Version]
- Ye, Y.; Xin, H.; Gu, X. Genome-wide identification and functional analysis of the Basic Helix-Loop-Helix (bHLH) transcription family reveals candidate PtFBH genes involved in the flowering process of Populus trichocarpa. Forests 2021, 12, 1439. [Google Scholar] [CrossRef]
- Zhou, X.; Liao, Y.; Kim, S.; Chen, Z.; Xu, F. Genome-wide identifcation and characterization of bHLH family genes from Ginkgo bilob. Sci. Rep. 2020, 10, 13723. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Xu, F.; Song, Q.; Ye, J.; Liao, Y.; Xu, F. Isolation, characterization and functional analysis of a novel 3-hydroxy-3-methylglutaryl-coenzyme A synthase gene (GbHMGS2) from Ginkgo biloba. Acta Physiol. Plant. 2018, 40, 72. [Google Scholar] [CrossRef]
- Ye, J.; Mao, D.; Cheng, S.; Zhang, X.; Xu, F. Comparative transcriptome analysis reveals the potential stimulatory mechanism of terpene trilactone biosynthesis by exogenous salicylic acid in Ginkgo biloba. Ind. Crops Prod. 2020, 145, 112104. [Google Scholar] [CrossRef]
- Xu, F.; Ning, Y.; Zhang, W.; Liao, Y.; Li, L.; Cheng, H.; Cheng, S. An R2R3-MYB transcription factor as a negative regulator of the flavonoid biosynthesis pathway in Ginkgo biloba. Funct. Integr. Genom. 2014, 14, 177–189. [Google Scholar] [CrossRef] [PubMed]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative C(T) method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef]
- Zheng, J.; Zhang, X.; Fu, M.; Zeng, H.; Ye, J.; Zhang, W.; Liao, Y.; Xu, F. Effects of different stress treatments on the total terpene trilactone content and expression levels of key genes in Ginkgo biloba leaves. Plant Mol. Biol. Rep. 2020, 38, 521–530. [Google Scholar] [CrossRef]
- Chini, A.; Fonseca, S.; Chico, J.M.; Calvo, P.F.; Solano, R. The ZIM domain mediates homo- and heteromeric interactions between Arabidopsis JAZ proteins. Plant J. Cell Mol. Biol. 2009, 59, 77–87. [Google Scholar] [CrossRef]
- Melotto, M.; Mecey, C.; Niu, Y.; Chung, H.S.; Katsir, L.; Yao, J.; Zeng, W.; Thines, B.; Staswick, P.; Browse, J.; et al. A critical role of two positively charged amino acids in the Jas motif of Arabidopsis JAZ proteins in mediating coronatine and jasmonoyl isoleucine-dependent interactions with the COI1 F-box protein. Plant J. 2008, 55, 979–988. [Google Scholar] [CrossRef] [Green Version]
- Thines, B.; Katsir, L.; Melotto, M.; Niu, Y.; Mandaokar, A.; Liu, G.; Nomura, K.; He, S.Y.; Howe, G.A.; Browse, J.; et al. JAZ repressor proteins are targets of the SCF(COI1) complex during jasmonate signalling. Nature 2007, 448, 661–665. [Google Scholar] [CrossRef]
- Hou, X.; Li, Y.; Cheng, C.; Xia, K. DELLAs modulate jasmonate signaling via competitive binding to JAZs. Dev. Cell 2010, 19, 884–894. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.; Ma, B.; Qi, X.; Guo, Q.; Wang, X.; Zeng, Q.; He, N. Identification and characterization of genes involved in the jasmonate biosynthetic and signaling pathways in mulberry (Morus notabilis). J. Integr. Plant Biol. 2014, 56, 663–672. [Google Scholar] [CrossRef] [PubMed]
- Kazan, K. Negative regulation of defence and stress genes by EAR-motif-containing repressors. Trends Plant Sci. 2006, 11, 109–112. [Google Scholar] [CrossRef]
- Kagale, S.; Rozwadowski, L.K. Genome-wide analysis of ethylene-responsive element binding factor-associated amphiphilic repression motif-containing transcriptional regulators in Arabidopsis. Plant Physiol. 2010, 152, 1109–1134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hiratsu, K.; Matsui, K.; Koyama, T.; Takagi, M.O. Dominant repression of target genes by chimeric repressors that include the EAR motif, a repression domain, in Arabidopsis. Plant J. Cell Mol. Biol. 2003, 34, 733–739. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhang, Z.; Wu, Z. Progress of the structural and functional analysis of plant transcription factor TIFY protein family. Biotechnol. Bull. 2020, 36, 121–128. (In Chinese) [Google Scholar]
- Li, X.; Yin, X.; Wang, H.; Guo, C.; Gao, H.; Zheng, Y.; Fan, C.; Wang, X. Genome-wide identification and analysis of the apple (Malus × domestica Borkh.) TIFY gene family. Tree Genet. Genomes 2015, 11, 1–13. [Google Scholar] [CrossRef]
- Zhang, Y.; Gao, M.; Singer, S.D.; Fei, Z.; Hua, W.; Wang, X.; Yang, H. Genome-wide identification and analysis of the TIFY gene family in grape. PLoS ONE 2012, 7, e44465. [Google Scholar] [CrossRef] [Green Version]
- Liu, S.; Zhang, P.; Cheng, C.; Xia, G. The moss jasmonate ZIM-domain protein PnJAZ1 confers salinity tolerance via crosstalk with the abscisic acid signalling pathway. Plant Sci. 2019, 280, 1–11. [Google Scholar] [CrossRef]
- Wen, J.; Li, Y.; Qi, T.; Hua, G.; Song, S. The C-terminal domains of Arabidopsis GL3/EGL3/TT8 interact with JAZ proteins and mediate dimeric interactions. Plant Signal. Behav. 2018, 13, e1422460. [Google Scholar] [CrossRef] [Green Version]
- Cai, X.; Zhen, J.; Jiang, Z. Cloning and expression analysis of cold response gene GhJAZ1 from Gossypium hirsutum L. Acta Agric. Boreali Sin. 2018, 33, 7–13. (In Chinese) [Google Scholar]
- Pei, T. The Function of SmJAZ in Regulating the Biosynthesis of Salvianolic Acids and Tanshinones in Salvia miltiorrhiza; Northwest A&F University: Xianyang, China, 2019. (In Chinese) [Google Scholar]
- Xue, L.; Liu, X.; Luo, Y. Expansion and expression analysis of PtMAP65 gene family in Populus trichocarpa. For. Res. 2021, 34, 92–101. (In Chinese) [Google Scholar]
- Hong, G.; Xue, X.; Mao, Y.; Wang, L.; Chen, X. Arabidopsis MYC2 interacts with DELLA proteins in regulating sesquiterpene synthase gene expression. Plant Cell 2012, 24, 2635–2648. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hagee, D.; Hardan, A.; Botero, J.; Arnone, J.T. Genomic clustering within functionally related gene families in Ascomycota fungi. Comput. Struct. Biotechnol. J. 2020, 18, 3267–3277. [Google Scholar] [CrossRef]
- Arnone, T. Genomic considerations for the modification of Saccharomyces cerevisiae for biofuel and metabolite biosynthesis. Microorganisms 2020, 8, 321. [Google Scholar] [CrossRef] [Green Version]
- Shi, M.; Zhou, W.; Zhang, J.; Huang, S.; Wang, H.; Kai, G. Methyl jasmonate induction of tanshinone biosynthesis in Salvia miltiorrhiza hairy roots is mediated by JASMONATE ZIM-DOMAIN repressor proteins. Sci. Rep. 2016, 6, 20919. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, T.; Chen, M.; Shen, Q.; Li, L.; Fu, X.; Pan, Q.; Tang, Y.; Shi, P.; Lv, Z.; Jiang, W.; et al. HOMEODOMAIN PROTEIN 1 is required for jasmonate-mediated glandular trichome initiation in Artemisia annua. New Phytol. 2016, 213, 1145–1155. [Google Scholar] [CrossRef] [PubMed]
- Yan, H. Role of JAZ2 and JAZ7 in Regulating Jasmonic Acid-Mediated Transcriptional and Metabolic Reprogramming; Zhejiang University: Hangzhou, China, 2014. (In Chinese) [Google Scholar]
- Chuquimarca, S.; Ayala-Ruano, S.; Goossens, J.; Pauwels, L.; Goossens, A.; Leon-Reyes, A.; Méndez, M.Á. The molecular basis of JAZ-MYC coupling, a protein-protein interface essential for plant response to stressors. Front. Plant Sci. 2020, 11, 1139. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).