Abstract
Lycium ruthenicum Murr. is one of the most important shrubs grown in northwest China. Healthy buds and leaves of L. ruthenicum Murr. were selected for the present study. Flower development was divided into six stages, namely, flower bud pre-differentiation (I), late flower differentiation (II), squaring stage (III), dew crown period (IV), open stage (V) and senescent stage (VI). Endogenous hormone content and specific value, soluble sugar, sucrose, starch, and soluble protein were measured, and ABA/IAA, ABA/GA3, ZR/IAA, ZR/GA3, and C/N were calculated in buds and leaves at stage VI. The results showed that ABA, GA3, and ZR content of buds significantly increased from flower bud pre-differentiation to late flower differentiation stage. However, ABA, GA3, and ZR content of leaves had the opposite change trend. From open stage to senescent stage, IAA, ABA, and GA3 content of buds and leaves significantly increased in L. ruthenicum Murr. However, ZR content of buds and leaves significantly decreased from open stage to senescent stage. ABA/IAA, ABA/GA3, ZR/IAA, and ZR/GA3 values of buds significantly increased from lower bud pre-differentiation to late flower differentiation stage. However, ABA/IAA, ABA/GA3, ZR/IAA, and ZR/GA3 values of leaves significantly decreased from lower bud pre-differentiation to late flower differentiation stage. ABA/IAA and ABA/GA3 of buds significantly increased from open stage to senescent stage, but ZR/IAA and ZR/GA3 of buds significantly decreased from open to senescent. At this stage, ABA/IAA, ABA/GA3, ZR/IAA, and ZR/GA3 significantly decreased in L. ruthenicum Murr. The higher soluble sugar and sucrose content in the buds and leaves were beneficial to the flower bud differentiation of L. ruthenicum. The increasing of soluble sugar improved the energy basis to florescence and senescent. The carbohydrates metabolism enhanced from open stage to senescent stage and nitrogen metabolism reduced from open stage to senescent stage of L. ruthenicum.
1. Introduction
Lycium ruthenicum Murr. is a perennial deciduous shrub of the Solanaceae family. The plant is distributed mainly across northwest China. Its fruit is rich in anthocyanins, polysaccharides, and proteins, has positive effects on eyesight as an anti-hypertensive, and lowers cholesterol [1,2]. With the increase in interest to L. ruthenicum, natural vegetation was seriously damaged. The population of L. ruthenicum has dropped in recent years. Thus, research on a key link plant breeding system is important to L. ruthenicum survival.
Flower development is the key link in plant breeding system and a highly complex process that is characterized by two distinct physiological phases: (a) bud initiation and (b) floral bud development [3]. Flowering has a correlation with the endogenous hormone levels. The ABA was controversial during the floral transitions, as both positive and negative effects of ABA have been reported [4]. IAA was also controversial [5]. Carbohydrate and nitrogen content had the important role of flower development, which were the basis of flower development. Florescence is an important component of plant breeding systems [6]. The flowering phase of L. ruthenicum takes 5–9 months to complete. Its flowers are bisexual and have purple and pink petals and clearly visible veins. Sexual reproduction is an important period in L. ruthenicum growth and development and has a crucial influence on L. ruthenicum fruit yield. Florescence is controlled by complex physiological processes [7] and involves internal and external factors. External factors include light, temperature, water, and nutrients, while internal factors include hormones, carbohydrates, and nutrient substances [8,9].
Researchers have investigated the relationship between flower development and hormones, and the physiology and biochemistry in different fruit types. However, similar research papers did not include L. ruthenicum.
Therefore, the aims of this study were to clarify the changes in endogenous hormones and metabolites in the leaves and buds of L. ruthenicum and assess the effects of these concentrations on flower development. We will reveal the regulatory mechanism of flower development in L. ruthenicum and provide the theoretical basis on L. ruthenicum flowering phase management.
2. Materials and Methods
2.1. Plant Material
The floral buds of L. ruthenicum grown in the agronomy practice base of Hexi University under natural conditions, an annual mean temperature of 6 °C, annual mean evaporation of 2291 mm, and mean rainfall of 113–120 mm, were selected for the present study.
Flower development and florescence were observed, and divided into six stages. These stages were designated as flower bud pre-differentiation (I), late flower differentiation (II), squaring stage (III), dew crown period (IV), open stage (V), and senescent stage (VI), the buds and leaves of L. ruthenicum were taken in April to June 2021 (Figure 1). Leaves were taken from buds at every stage. Hormones, soluble sugar, sucrose, starch, and soluble protein levels were measured in leaf and bud samples during flowering development.
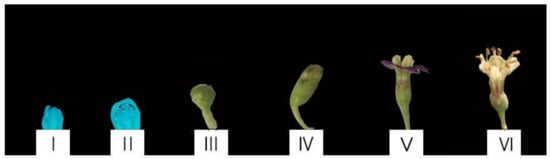
Figure 1.
Flower development stages in Lycium ruthenicum Murr.
2.2. Hormone Analysis
Buds and leaves from the different developmental stages were immediately frozen in liquid nitrogen and stored at –80 °C for subsequent analyses. Endogenous hormones, including indole acetic acid (IAA), indolebutyric acid (IBA) abscisic acid (ABA), gibberellic acid (GA3), and zeatin riboside (ZR), were measured by using high efficiency liquid chromatography (HPLC). Leaf and bud samples (0.4 g) were put in 10 mL centrifuge tube, and added to cold 80% methanol containing 0.5% methanoic acid at 4 °C in 2 mL. Ultrasonic extraction was performed by 30 min and let stand for the night. The extracts were centrifuged at 12,000× g for 15 min. The residue added to a 2 mL precool extracting solution, and an ultrasonic extraction for 30 min was done. The methanol phase was reduced to an aqueous phase under reduced pressure on a rotary evaporator at 38 °C. After that, was incubated at −20 °C refrigerator freeze for 30 min, and then were centrifuged at 12,000× g for 10 min. the supernatants were reserved and pigment and lipid were discarded. The purification pipe underwent severe concussion for 30 s, and then was centrifuged at 4000× g for 5 min. The supernatants were concentrated to near dry by pressure blowing concentrator, and then added to 1.0 mL acetonitrile solution. Ultrasonic extraction was performed by 30 min, and filtered by 0.22 μm Millipore filter. The filter liquor was detected by HPLC as described previously [10]. Every sample repeat measured three times, and calculated the average value.
2.3. Determination of Soluble Total Sugar and Sucrose
The 0.5 g buds or leaves were powdered, and then 5 mL of 80% ethanol was added. The mixture was incubated in boiling water for 30 min. Homogenates were centrifuged at 6000× g for 5 min. Residues were re-suspended in 3 mL of 80% ethanol and centrifuged at 6000× g for 5 min. The supernatants of the two obtained centrifugates were mixed and then added with 0.1 g of activated charcoal. The mixture was filtered, and the filtrate was used in the determination of soluble total sugar and sucrose. Total sugar and sucrose concentrations were evaluated at 630 nm via the spectrophotometer according to the anthrone method [11]. Soluble sugar was measured at 640 nm using methanol as blank. The concentration of soluble sugar was calculated using glucose solution as standard [12].
2.4. Starch Content
Approximately 0.5 g of the buds or leaves were powdered and then mixed with 6 mL of 80% ethanol. The mixture was incubated in boiling water for 30 min. The homogenates were centrifuged at 3000× g for 5 min. The supernatants were discarded, and 10 mL of 3 mol/L HCl was added to the sediment in boiling water for 45 min. Then, 10 mL 3 mol/L NaOH was added to the sediment. Around 2 mL of the sample solution was diluted to 10 mL, and warm 6 mL of 0.4% anthrone was added in a boiling water bath. Starch content was measured at 640 nm via the spectrophotometer according to the anthrone method [13].
2.5. Soluble Proteins
Soluble proteins were estimated by the method of Bradford [14]. A mixture of 0.3 g of buds and leaves was homogenized in 20 mL of 20% trichloroacetic acid (TCA), and then centrifuged at 800 rpm for 15 min. Supernatant was discarded. Pellets were mixed with 5 mL of 0.1 N NaOH to solubilize proteins, and solution was centrifuged again at 800 rpm for 15 min. The supernatant was mixed with 10 mL of 0.1 N NaOH and used for estimation of protein content. Absorbance was evaluated at 595 nm.
2.6. Statistical Analysis
All data were subjected to one-way analyses of variance (ANOVA). The data analyses were performed with SPSS18.0 statistical software package for Windows. LSD multiple comparison tests were used to separate significant differences among all of the treatments at 0.05 level. SE was showed in figures and tables. C/N value was calculated from soluble total sugar and soluble protein.
3. Results
3.1. Endogenous Hormones Content
Buds and leaves showed different behaviors with respect to endogenous hormone changes at different developmental stages of L. ruthenicum. IAA levels in leaves and ABA, GA3, and ZR levels in buds significantly increased from stage I to II (p < 0.05; Figure 2B,C and Figure 3A,C). From stage II to III, IAA levels of buds and ZR level of leaves increased 19.38- and 0.52-fold, respectively (Figure 2A and Figure 3D). From stage III to IV, GA3 levels of buds and leaves increased 44.50% and 17.15%, respectively (Figure 3A,B). ABA, ZR levels in buds and leaves and GA3 levels in leaves significantly increased from stage IV to V (p < 0.05; Figure 2C,D and Figure 3B–D). From stage V to VI, the IAA, ABA, and GA3 levels of buds and leaves significantly increased, while the ZR levels of buds and leaves significantly decreased at this stage (Figure 2 and Figure 3).
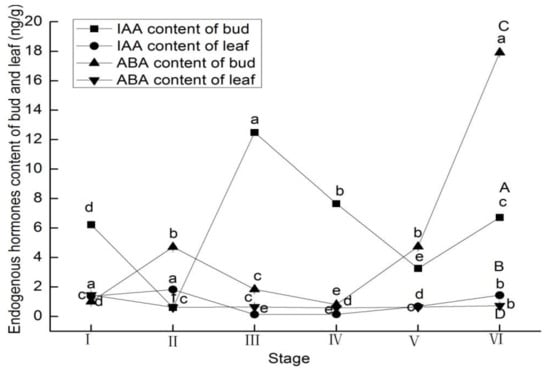
Figure 2.
Endogenous hormones contents of indole acetic acid (IAA) in buds (A) and in leaves (B), and abscisic acid (ABA) in buds (C) and in leaves (D) of Lycium ruthenicum Murr. at different flower development stages. Vertical bars represent standard error (SE) of means (n = 3). Mean values significantly different at p ≤ 0.05 are indicated by different letters.
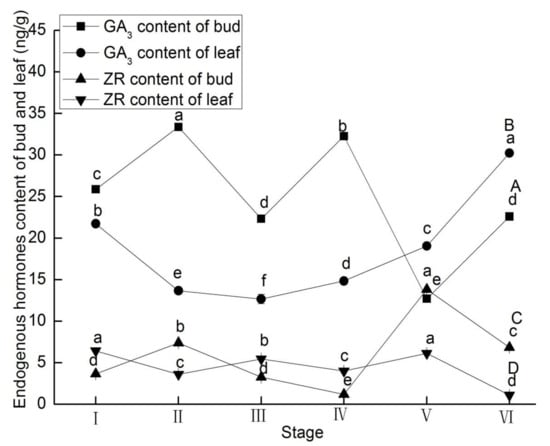
Figure 3.
Endogenous hormones contents of gibberellic acid in buds (A) and in leaves (B), and zeatin riboside in buds (C) and in leaves (D) of Lycium ruthenicum Murr. at different flower development stages. Vertical bars represent standard error (SE) of means (n = 3). Mean values significantly different at p ≤ 0.05 are indicated by different letters.
3.2. Endogenous Hormones Specific Value
In the buds, the values of ABA/IAA, ABA/GA3, and ZR/IAA, ZR/GA3 had a similar change tendency from stage I to IV, and significantly increased from stage I to II then decreased from stage II to IV (Figure 4). From stage V to VI, the values of ABA/IAA and ABA/GA3 significantly increased, while the values of ZR/IAA and ZR/GA3 significantly decreased at this stage.
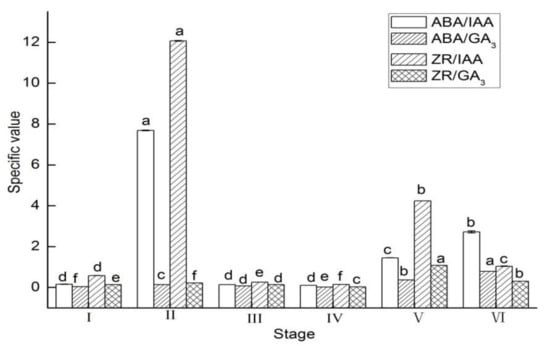
Figure 4.
Specific value changes of ABA/IAA and ABA/GA3 and ZR/IAA and ZR/GA3 in buds of Lycium ruthenicum Murr. at different flower development stages. Vertical bars represent standard error (SE) of means. Mean values significantly different at p ≤ 0.05 are indicated by different letters.
In the leaves, the values of ABA/IAA, ABA/GA3, ZR/IAA, and ZR/GA3 significantly decreased from stage I to II, and then increased from stage II to III (Figure 5). However, the values of ABA/IAA, ABA/GA3, ZR/IAA, and ZR/GA3 significantly decreased from stage III to VI.
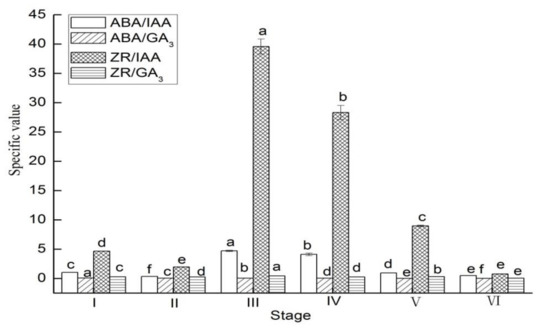
Figure 5.
The specific value of ABA/IAA and ABA/GA3 and ZR/IAA and ZR/GA3 in leaves of Lycium ruthenicum Murr. at different flower development stages. Vertical bars represent standard error (SE) of means. Mean values significantly different at p ≤ 0.05 are indicated by different letters.
3.3. Carbohydrates
Soluble sugar in the buds significantly increased from stage I to III by 8.51%, and increased 4.15-fold from stage IV to VI (p < 0.05; Table 1). However, soluble sugar in the leaves significantly decreased 85.89% from stage I to stage V, and significantly increased 9.27-fold from stage V to VI. Sucrose in the buds significantly increased and then decreased from stage I to VI (p < 0.05). The maximum sucrose value in the leaves appeared at stage III and was lowest at stage II. Starch content of bud and leaves were significantly variable during every stage (p < 0.05).

Table 1.
Carbohydrates content in buds and leaves of Lycium ruthenicum Murr. at different flower development stages.
3.4. Soluble Protein
Soluble protein contents in the buds and leaves had a similar change tendency. Soluble protein contents in the buds increased 39.28-fold from stage I to III, and decreased by 98.38% from stage III to VI (Figure 6). Soluble protein contents in the leaves increased 48.66-fold from stage Ito III, and decreased by 96.56-fold from stage III to VI.
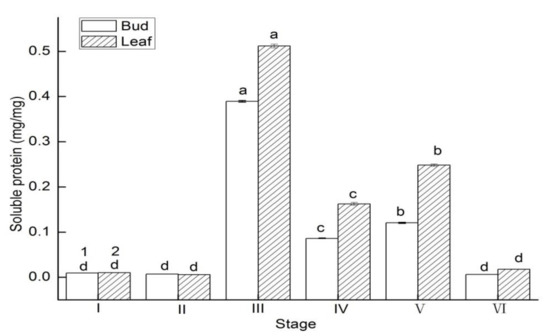
Figure 6.
Soluble protein changes of in buds and leaves of Lycium ruthenicum Murr. at different flower development stages. Vertical bars represent standard error (SE) of means. Mean values significantly different at p ≤ 0.05 are indicated by different letters.
3.5. C/N Value
C/N values in buds and leaves were significantly variable during flower development (p < 0.05).
C/N values decreased by 26% and increased 20.75% from stage I to II and between stages V to VI, respectively, in buds (Figure 7). C/N values increased by 39% and 143.34% from stage I to II and between stages V to VI, respectively, in leaves.
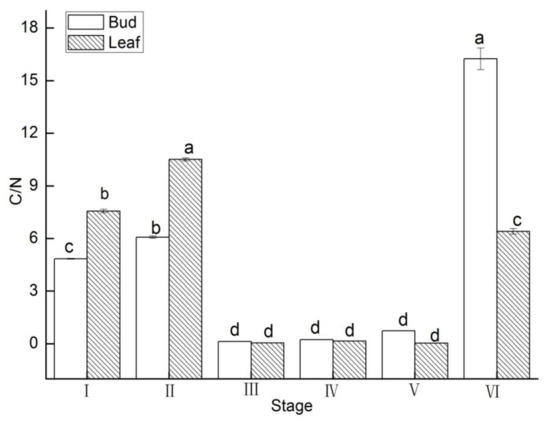
Figure 7.
C/N value changes of in buds and leaves of Lycium ruthenicum Murr. at different flower development stages. Vertical bars represent standard error (SE) of means. Mean values significantly different at p ≤ 0.05 are indicated by different letters.
4. Discussion
Flower bud differentiation is a complex process, and is affected by multiple factors. Endogenous hormone content and their balance are important effects for flower bud differentiation [15,16,17]. The relation of balance is mutual restraint and promotion, and control the metabolism nutrient substance to adjust and control flower bud differentiation. IAA is the main factor responsible for inflorescence stalk elongation [18]. IAA was also controversial, as both positive and negative effects of IAA have been reported [19]. Our study showed that IAA content of buds significantly decreased from flower bud pre-differentiation to late flower differentiation. The results suggest that floral induction need lower IAA levels in L. ruthenicum. Similar results have been reported for Chrysanthemum [20]. However, IAA content of leaves significantly increased at this stage. The results showed a relation of IAA transportation from morphological upper buds to morphological lower leaves at this stage. Similar results have been reported for Chrysanthemum [20]. At the squaring stage, the IAA content of buds significantly increased compare with flower bud differentiation stage. The results indicated that squaring need higher IAA levels in L. ruthenicum. Similar results have been reported in apricot [21]. ABA is essential in flowering and plays an important role in flower development [22]. The ABA content of buds significantly increased from flower bud pre-differentiation to late flower differentiation. The results suggest that ABA could promote flower differentiation of L. ruthenicum. ABA content of buds and leaves maintained the higher level from open stage to senescent stage in L. ruthenicum. It indicated that ABA can promote the flower opening and senescence in L. ruthenicum. Some research showed that GA3 had an inhibitory effect to florescence [23,24,25,26,27]. However, other research found that lower concentration GA3 can promote floral development in terminal buds of apple trees [8]. Our research showed that GA3 content of buds significantly increased from flower bud pre-differentiation to late flower differentiation. The results suggest that the higher GA3 could promote the flower differentiation of L. ruthenicum, similar to olive [7]. The ZR content in buds significantly increased from flower bud pre-differentiation to late flower differentiation. The results showed that ZR could promote flower bud differentiation of L. ruthenicum, similar to potatoes [28]. At the open stage, the ZR content reached the maximum in buds and leaves of L. ruthenicum. Flower formation is the result of all kinds of hormone interaction. Our results showed that for all hormone levels analyzed, their proportion changed at different development stages of L. ruthenicum.
ABA/IAA in the buds gradually increased before the open stage. The increase indicated that ABA/IAA in buds benefits flower bud differentiation and flower development in L. ruthenicum. Increased ABA/GA3 values in the buds and leaves were in accordance with the flower development in L. ruthenicum. Research in Jujube [25] and Lycoris radiata [29] obtained similar results. ABA/GA3, ABA/IAA, and ZR/IAA in the buds were higher than in the leaves. This finding indicated that hormone levels and proportions in the buds had an important effect on L. ruthenicum flower development. Nutrient substance accumulation is the basis of the flower bud differentiation. Carbohydrates have an important contribution to the flower bud differentiation. Priestly [30] suggested that a high carbohydrate level alone does not promote flowering. In our research, the higher soluble sugar and sucrose content in the buds and leaves were in accordance with the flower bud differentiation of L. ruthenicum. Similar results have been reported in olive [7] and Olea europaea var [31]. Florescence and senescent process of plant need to consume abundant respiratory substrate, and need enough energy to push florescence and senescent. Our research showed that the increasing of soluble sugar improved the energy basis to florescence and senescent. Nitrogen is the main nutrient element of plant growth. The soluble protein levels of buds and leaves had the same variation tendency in L. ruthenicum. The soluble protein levels were unaltered from flower bud pre-differentiation to late flower differentiation. At the squaring stage, soluble protein levels of buds and leaves reached maximum in L. ruthenicum. At the open and senescent stage, soluble protein levels of buds and leaves significantly decreased in L. ruthenicum. Research on Nicotiana plumbaginifolia Viv [9] obtained similar results. The decrease was associated with improved proteolytic cleavage or decreased protein biosynthesis and was regarded a prerequisite to the open stage in various flowers [32,33]. The C/N ratios reflect the basic state of carbon and nitrogen metabolism. The C/N ratios of buds and leaves were higher at the flower bud differentiation stage of L. ruthenicum, because carbohydrate can improve vascular sap concentration and provided important nutrient substances to flower bud differentiation stage. The C/N ratios in the buds and leaves significantly increased from the open stage to senescent stage. This result showed that the carbohydrate metabolism enhanced from the open stage to senescent stage and that the nitrogen metabolism reduced from the open stage to senescent stage of L. ruthenicum.
5. Conclusions
Our current study demonstrated that a higher ABA, GA3, and ZR content and ABA/IAA, ABA/GA3, ZR/IAA, and ZR/GA3 values in buds could contribute to the promotion of the flower bud differentiation of L. ruthenicum. Higher ABA/IAA, ABA/GA3, ZR/IAA, and ZR/GA3 values in leaves were correlated to flower development in L. ruthenicum. Higher IAA, ABA, and GA3 values in buds and leaves correlated with the senescent of flowers in L. ruthenicum. Higher soluble sugar and sucrose content in the buds and leaves was beneficial to the flower bud differentiation of L. ruthenicum. The increase of soluble sugar improved the energy basis to florescence and senescent.
Author Contributions
Y.G. designed experiments, performed data analysis, and wrote the paper; L.A. performed data analysis and field detection; H.Y. and M.Y. performed data analysis and revised the paper. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by programs of the National Natural Science Foundation (Grant No. 32060334; Grant No.31660193).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Zheng, J.; Ding, C.-X.; Wang, L.-S.; Li, G.-L.; Shi, J.-Y.; Li, H.; Wang, H.-L.; Suo, Y.-R. Anthocyanins composition and antioxidant activity of wild Lycium ruthenicum Murr. from Qinghai-Tibet Plateau. Food Chem. 2011, 126, 859–865. [Google Scholar] [CrossRef]
- Wang, J.-H.; Chen, W. Responses of seed germination and seedling growth of Lycium ruthenicum to salt stress. Chin. J. Ecol. 2012, 31, 804–810. [Google Scholar]
- Mert, C.; Barut, E.; İpek, A. Variation in flower bud differentiation and progression of floral organs with respect to crop load in olive. Not. Bot. Horti Agrobot. Clujnapoca 2013, 41, 79–85. [Google Scholar] [CrossRef][Green Version]
- Riboni, M.; Robustelli, T.-A.; Galblati, M.; Tonelli, C.; Cont, L. ABA-dependent control of gigantea signalling enables drought escape via up-regulation of flowering locust in Arabidopsis thaliana. J. Exp. Bot. 2016, 67, 6309–6322. [Google Scholar] [CrossRef]
- Cao, S.-Y.; Zhang, Q.-M.; Wu, S. Advances in research on the mechanism of flower-bud differentiation of fruit trees. J. Fruit Sci. 2003, 20, 345–350. [Google Scholar]
- Wyatt, R. Pollinator plant interactions and the evolution of breeding system. In Pollination Biology; Real, L., Ed.; Academic Press: New York, NY, USA, 1983. [Google Scholar]
- Ulger, S.; Sonmez, S.; Karkacier, M.; Ertoy, N.; Akdesir, O.; Aksu, M. Determination of endogenous hormones, sugars and mineral nutrition levels during the induction, initiation and differentiation stage and their effects on flower formation in olive. Plant Growth Regul. 2004, 42, 89–95. [Google Scholar] [CrossRef]
- Zhang, Y.-J.; Li, W.-Q.; Liu, X.-H.; Lu, Y.-M. Changes in the morphology of the apical meristem and the levels of endogenous hormones during flower bud development of different Lily (Lilium) cultivars. Philipp. Agric. Sci. 2013, 96, 296–300. [Google Scholar]
- Shaziya, N.; Inayatullah, T.; Syed, S.-A.; Riyaz, A.D. Physiological and biochemical aspects of flower development and senescence in Nicotiana plumbaginifolia Viv. Folia Hort. 2017, 29, 25–31. [Google Scholar]
- Abreu, M.-E.; Munne, B.-S. Salicylic acid deficiency in NahG transgenic lines and sid2 mutants increases seed yield in the annual plant Arabidopsis thaliana. J. Exp. Bot. 2009, 60, 1261–1271. [Google Scholar] [CrossRef]
- Nazari, M.; Zarinkamar, F.; Soltani, B.-m. Physiological, biochemical and molecular responses of Mentha aquatica L. to manganese. Plant Physiol. Biochem. 2017, 120, 202–212. [Google Scholar] [CrossRef]
- Robyt, J.-F.; White, B.-J. Protein Purification: Principles and Practices, Biochemical Techniques, Theory and Practice; Cole Publ.: Belmont, CA, USA, 1987; pp. 40–72. [Google Scholar]
- Yan, F. Study on Morphological Differentiation of Flower Bud of Hippeastrum; Northwest Agriculture and Forestry University: Xianyang, China, 2009. [Google Scholar]
- Bradford, M.-M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein dye binding. Ann. Biochem. 1976, 72, 248–253. [Google Scholar] [CrossRef]
- Anton, J.-M.; Will, G.; Gerard, W.-M.; George, J.-W. In vitro flower bud formation in tobacco: Interaction of hormones. Plant Physiol. 1991, 97, 402–408. [Google Scholar]
- Davis, S.-J. Integrating hormones into the floral-transition pathway of Arabidopsis thaliana. Plant Cell Environ. 2009, 32, 1201–1210. [Google Scholar] [CrossRef]
- Shalit, A.; Rozman, A.; Alvarez, J.-P.; Bowman, J.-L.; Eshed, Y.; Lifschitz, E. The flowering hormone florigen functions as a general systemic regulator of growth and termination. Proc. Natl. Acad. Sci. USA 2009, 106, 8392–8397. [Google Scholar] [CrossRef]
- Banasik, L.; Saniewski, M. The effect of different auxins on tulip stem elongation. Acta Hortic. 1985, 167, 193–204. [Google Scholar] [CrossRef]
- Wan, C.-Y.; Mi, L.; Chen, B.-Y.; Li, J.-F.; Huo, H.-Z.; Xu, J.-T.; Chen, X.-P. Effects of nitrogen during nursery stage on flower bud differentiation and early harvest after transplanting in strawberry. Braz. J. Bot. 2018, 41, 1–10. [Google Scholar] [CrossRef]
- Feng, F.; Yang, J.-S. Relationship between floral bud differentiation and endogenous hormones in autumn-cutting Chrysanthemum morifolium Jinba. Sci. Agric. Sin. 2011, 44, 552–561. [Google Scholar]
- Hao, Y. The Study of the Endogenesis Hormone and Dynamic Growth of Apricot Varieties during Florescence and Fruit Period; North West Agriculture and Forestry University: Xianyang, China, 2009. [Google Scholar]
- Luckwill, L.-C. Proceedings of the XLX International Horticultural Congress; International Society for Horticultural Science: Vienna, Austria, 1974; Volume 11, pp. 169–177. [Google Scholar]
- Wu, Z.-X.; Zhou, Z.D.; Tao, Z.-L.; Wang, L.-X. Change of endogenous hormones in Feizixiao and Edan litchi during flower bud differentiation. Chin. J. Trop. Crops 2005, 26, 42–45. [Google Scholar]
- Laia, A.; Sergi, M.-B. Hormonal changes during flower development in floral tissues of Lilium. Planta 2012, 236, 343–356. [Google Scholar]
- Niu, H.-L.; Zhang, H.-W.; Bian, Y.; Li, X.-G. Flower Formation and Endogenous Hormones Dynamic in Chinese Jujube. Acta Hortic. Sin. 2015, 42, 655–664. [Google Scholar]
- Wang, Y.H.; Fan, C.-H.; Shen, X.; Qu, G.-M.; Shi, J.D. Changes in endogenous hormones during the flower bud differentiation of sweet cherry. Acta Agric. Boreali-Occident. Sin. 2002, 1, 64–67. [Google Scholar]
- Gao, X.-J.; Wu, X.-E.; Wang, S.-Y.; Chen, C.-L.; Cheng, J.-H.; Dong, G.-P.; Peng, L. Change in endogenous hormone contents of mango during floral differentiation after heading-back. Fujian J. Agri. Sci. 2009, 24, 227–230. [Google Scholar]
- Ai, X.-M.; He, R.-Y.; Hu, Y.-F. Flower bud differentiation and their relationships with cntent changes of endogenous hormones in potatoes. Acta Bot. Boreali-Occident. Sin. 2018, 38, 87–94. [Google Scholar]
- Wang, L.; Tang, G.-G.; Liu, T. Variation of endogenous hormone and nucleic acid content during flower bud differentiation in Lycoris radiata. J. Nanjing For. Univ. 2008, 32, 67–70. [Google Scholar]
- Priestly, G.-A. The annual turnover resources in young olive trees. J. Hortic. Sci. 1977, 52, 105–112. [Google Scholar] [CrossRef]
- Sarmiento, R.; Valpuestra, V.; Catalina, L.; Gonzoles, G.-F. Variation of the contents of starch and soluble carbohydrate of leaves and buds of plants of Olea europaea var Manzanillo in relation to their vegetative or reproductive process. Aneles Edafol. Agrobiol. 1976, 35, 683–695. [Google Scholar]
- Macnish, A.-J.; Jiang, C.-Z.; Negre, Z.-F.; Reid, M.-S. Physiological and molecular changes during opening and senescence of Nicotiana mutabilis flowers. Plant Sci. 2010, 179, 267–272. [Google Scholar] [CrossRef]
- Doorn, W.-G.; Woltering, E.-J. Physiology and molecular biology of petal senescence. J. Exp. Bot. 2008, 59, 453–480. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).