The Analysis Effect of Selected Factors on the Shear Strength of Woodbark at Different Wood Species
Abstract
:1. Introduction
2. Material and Method
3. Results and Discussion
4. Conclusions
- The measured data confirmed a remarkable influence of the direction of the loading force for all tested wood species;
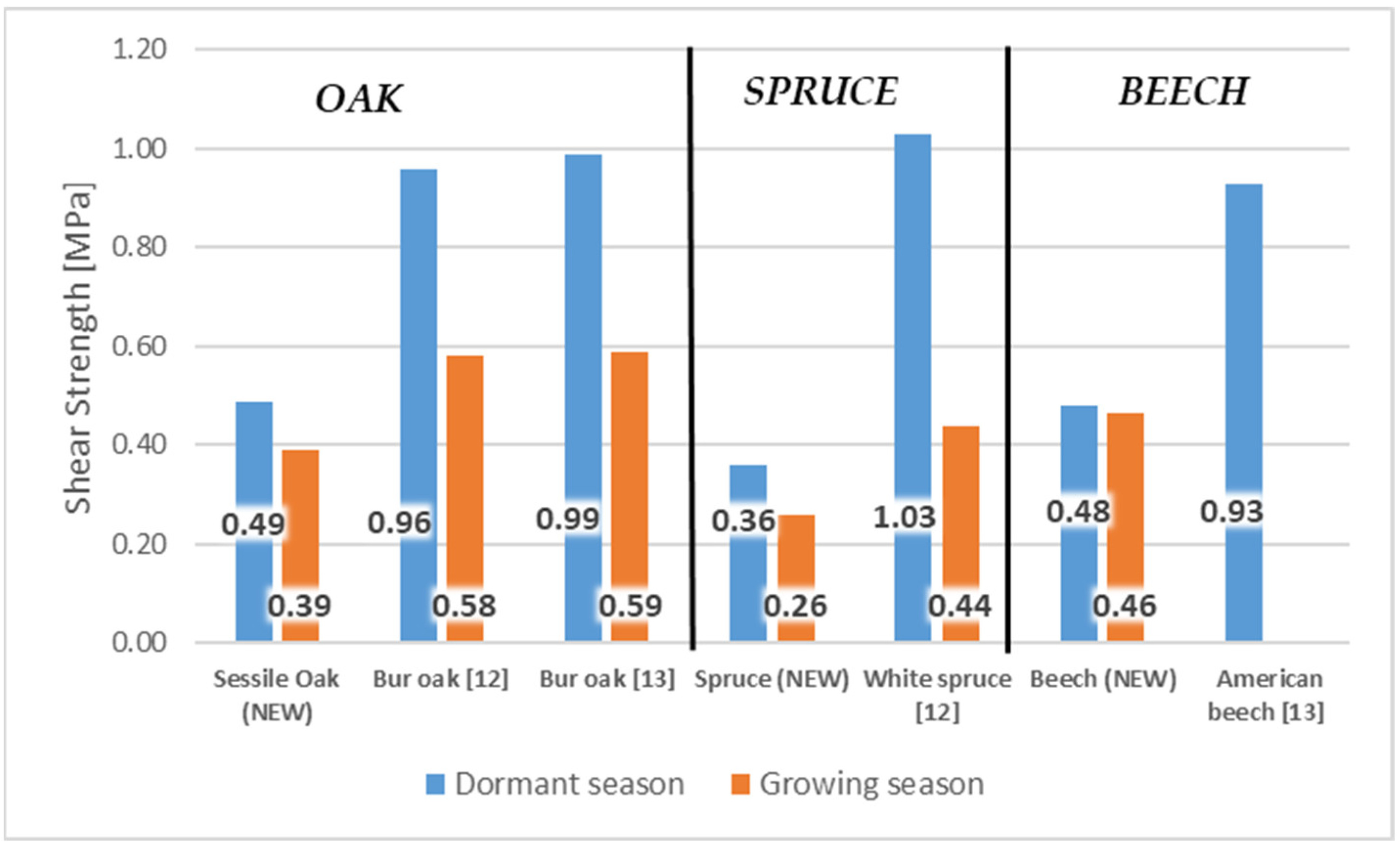
- The highest difference between the tangential and longitudinal direction was measured in sessile oak. The longitudinal direction had average values of shear strength more than 57% higher than the tangential direction during the dormant season; the longitudinal direction in oak had a value of 0.49 MPa, and the tangential direction had a value of 0.21 MPa;
- The results also showed a remarkable effect of the testing season (growing and dormant). The highest values were measured during the dormant season. The rea-son can be found in the structure of cambium, which differs between the dormant and growing season. The differences are mainly in the quality and quantity of the cambial cells;
- The highest difference between the tangential and longitudinal direction was also measured for sessile oak. The longitudinal direction had average values of shear strength almost 49% higher than the tangential direction during the growing season. The value in the longitudinal direction was 0.39 MPa, and in the tangential direction was 0.20 MPa;
- Minor differences in shear strength values were found for the remaining wood species. The smallest difference was found in beech. During the dormant season, the difference was only 8% and during the vegetation season was 13%;
- Based on the analysis of variance (ANOVA), whose value of the significance level was p = 0.0000, a remarkable influence of the wood species on the final value of shear strength was confirmed. This result can be explained mainly by the different structure of the bark between the tested wood species. Mainly, differences in structure can be found in the proportion of mechanical tissues, which oak bark has in the form of phloem fibers and sclereids, or even in several-rows of phloem rays, and vice versa; in the case of beech, these fibers are missing. Unlike oak, beech has a remarkable proportion of sclereids (thick-walled cells) in the structure of the bark. Compared to beech and oak, spruce does not contain any mechanical tissues and only single-row to three-row phloem rays are typical;
- In the final evaluation, we can confirm that values of shear strength are significantly affected by the wood species and period of vegetation, as well as direction of the loading force, but also by other factors. These results can be of great benefit, not only in understanding the protective function of the bark, but also in the debarking process, in which the bark is separated from the wood in the tangential direction.
Author Contributions
Funding
Conflicts of Interest
References
- Morris, H.; Jansen, S. Bark: Its anatomy, function and diversity. Int. Dendrol. Soc. Sect. Trees 2017, 1, 51–61. [Google Scholar]
- Gričar, J. Xylem and phloem formation in sessile oak from slovenia in 2007. Wood Res. 2010, 55, 15–22. [Google Scholar]
- Giannotas, G.; Kamperidou, V.; Barboutis, I. Tree bark utilization in insulating bio-aggregates: A review. Biofuels Bioprod. Bioref. 2021, 15, 1989–1999. [Google Scholar] [CrossRef]
- Trockenbrodt, M. Survey and discussion of the terminology used in bark anatomy. IAWA Bull. 1990, 11, 141–166. [Google Scholar] [CrossRef]
- Bowyer, L.J.; Shmulsky, R.; Haygreen, G.J. Forest Products and Wood Science: An Introduction, 5th ed.; Wiley-Blackwell: Hoboken, NJ, USA, 2007; p. 576. ISBN 0-8138-2654-3. [Google Scholar]
- Kučera, L.; Boshhard, H.H.; Katz, E. Über den Keillwuchs und den welligen Jahrringverlauf in Buche (Fagus sylvatica L.). Holz Als Roh-Und Werkst. 1980, 38, 161–168. [Google Scholar] [CrossRef]
- Požgaj, A.; Chovanec, D.; Kurjatko, S.; Babiak, M. Štruktúra a Vlastnosti Dreva (Structure and Properties Wood), 2nd ed.; Príroda: Bratislava, Slovakia, 1997; p. 485. ISBN 80-07-00960-4. [Google Scholar]
- Panshin, A.J.; Zeeuw, D.C. Textbook of Wood Technology: Structure, Identification, Properties, and Uses of the Commercial Woods of the United States and Canada Subsequent Edition; McGraw-Hill College: Washington, DC, USA, 1980; p. 736. [Google Scholar]
- Chahal, A.; Ciolkosz, D. A review of wood-bark adhesion: Methods and mechanics of debarking for woody biomass. Wood Fiber Sci. 2019, 51, 12. [Google Scholar] [CrossRef]
- Chahal, A.; Ciolkosz, D.; Puri, V.; Liu, J.; Jacobson, M. Factors affecting wood-bark adhesion for debarking of shrub willow. Biosyst. Eng. 2020, 196, 202–209. [Google Scholar] [CrossRef]
- Einspahr, D.W.; Hankey, A.W.; Wink, A.W.; Benson, K.M.; Swanson, W.J. Wood bark adhesion and methods of reducing adhesion in hardwood species. Memb. Group Proj. 1970, 1971, 7–9. [Google Scholar]
- Harder, M.L.; Parham, A.R.; Einspahr, W.D. Bark and wood properties of pulpwood species as related to separation and segregation of chip/bark mixtures. Memb. Inst. Pap. Chem. 1978, 1, 100–112. [Google Scholar]
- Einspahr, D.W.; Van Eperen, R.H.; Harder, M.L. Morphological and bark strength characteristics important to wood/bark adhesion in hardwoods. Wood Sci. Technol. 1984, 16, 339–348. [Google Scholar]
- Gričar, J.; Jagodic, Š.; Prislan, P. Structure and subsequent seasonal changes in the bark of sessile oak (Quercus petraea). Trees 2015, 29, 747–757. [Google Scholar] [CrossRef]
- Gričar, J.; Čufar, K. Seasonal dynamics of phloem and xylem formation in silver fir and Norway spruce as affected by drought, Russ. J. Plant Physiol. 2008, 55, 538–543. [Google Scholar]
- Braun, H.J. Neueste Erkenntnisse über das Rindensterben der Buchen: Grundursache und der Krankheitsablauf, verursacht durch die Buchenwollschildlaus Cryptococcus fagi Bär. Allg. Forst. Jagdztg. 1976, 147, 121–130. [Google Scholar]
- Prislan, P.; Koch, G.; Schmitt, U.; Gričar, J.; Čufar, K. Cellular and topochemical characteristics of secondary changes in bark tissues of beech (Fagus sylvatica L.). Holzforschung 2012, 66, 131–138. [Google Scholar] [CrossRef] [Green Version]
- Čunderlík, I.; Vilkovský, P. Change adhesion wood/bark on the trunk of a beech during dormant and growing period. Acta Fac. Xylologiae 2015, 57, 5–13. [Google Scholar]
- Prislan, P.; Gričar, J.; De Luis, M.; Smith, T.K.; Čufar, K. Phenological variation in xylem and phloem formation in (Fagus sylvatica L.) from two contrasting sites. Aglicultural For. Meteorol. 2013, 180, 142–151. [Google Scholar] [CrossRef]
- Evert, R.F.; Susan, E.E. Esau’s Plant Anatomy Meristems, Cells, and Tissues of the Plant Body: Their Structure, Function, and Development, 3rd ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2006; p. 624. [Google Scholar]
- Chen, H.M.; Han, J.J.; Cui, K.M.; He, X.Q. Modification of cambial cell wall architecture during cambium periodicity in (Populus tomentosa). Carr. Trees 2010, 24, 533–540. [Google Scholar] [CrossRef]
- Gričar, J. Influence of Temperature on Cambial Activity and Cell Differentiation in Quercus Sessiliflora and Acer Pseudoplatanus of Different Ages. Drv. Ind. 2013, 64, 95–105. [Google Scholar] [CrossRef]
- Baduna, S. Prilog proučavanju svojstva kore nekih vrstva drvna. Drevna Ind. 1985, 36, 275–280. [Google Scholar]
- Duchesne, I.; Nylinder, M. Measurement of the bark/wood shear strength: Practical methods to evaluate debarking resistance of Norway spruce and scots pine pulpwood. For. Prod. J. 1996, 46, 1–6. [Google Scholar]
- Ugulino, B.; Caceres, B.C.; Hernández, E.R.; Blais, C. Influence of temperature and moisture content on bark/wood shear strength of black spruce and balsam fir logs. Wood Sci. Technol. 2020, 54, 963–979. [Google Scholar] [CrossRef]
- STN EN 49 0118; Wood. Shear Strength Parallel to the Grain. Slovak Standards Institute: Bratislava, Slovakia, 1979.






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vilkovský, P.; Vilkovská, T.; Klement, I.; Čunderlík, I. The Analysis Effect of Selected Factors on the Shear Strength of Woodbark at Different Wood Species. Forests 2022, 13, 637. https://doi.org/10.3390/f13050637
Vilkovský P, Vilkovská T, Klement I, Čunderlík I. The Analysis Effect of Selected Factors on the Shear Strength of Woodbark at Different Wood Species. Forests. 2022; 13(5):637. https://doi.org/10.3390/f13050637
Chicago/Turabian StyleVilkovský, Peter, Tatiana Vilkovská, Ivan Klement, and Igor Čunderlík. 2022. "The Analysis Effect of Selected Factors on the Shear Strength of Woodbark at Different Wood Species" Forests 13, no. 5: 637. https://doi.org/10.3390/f13050637
APA StyleVilkovský, P., Vilkovská, T., Klement, I., & Čunderlík, I. (2022). The Analysis Effect of Selected Factors on the Shear Strength of Woodbark at Different Wood Species. Forests, 13(5), 637. https://doi.org/10.3390/f13050637





