Pollen Viability of Fraxinus excelsior in Storage Experiments and Investigations on the Potential Effect of Long-Range Transport
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Material
2.2. Viability Tests
2.2.1. TTC Test
2.2.2. Alexander’s Stain
2.2.3. Acetocarmine
2.2.4. Pollen Germination
2.3. Storage Experiments
2.4. Experiments on Pollen Viability after Potential Atmospheric Transport
2.4.1. Climate Chamber
2.4.2. Volumetric Pollen Trap
3. Results
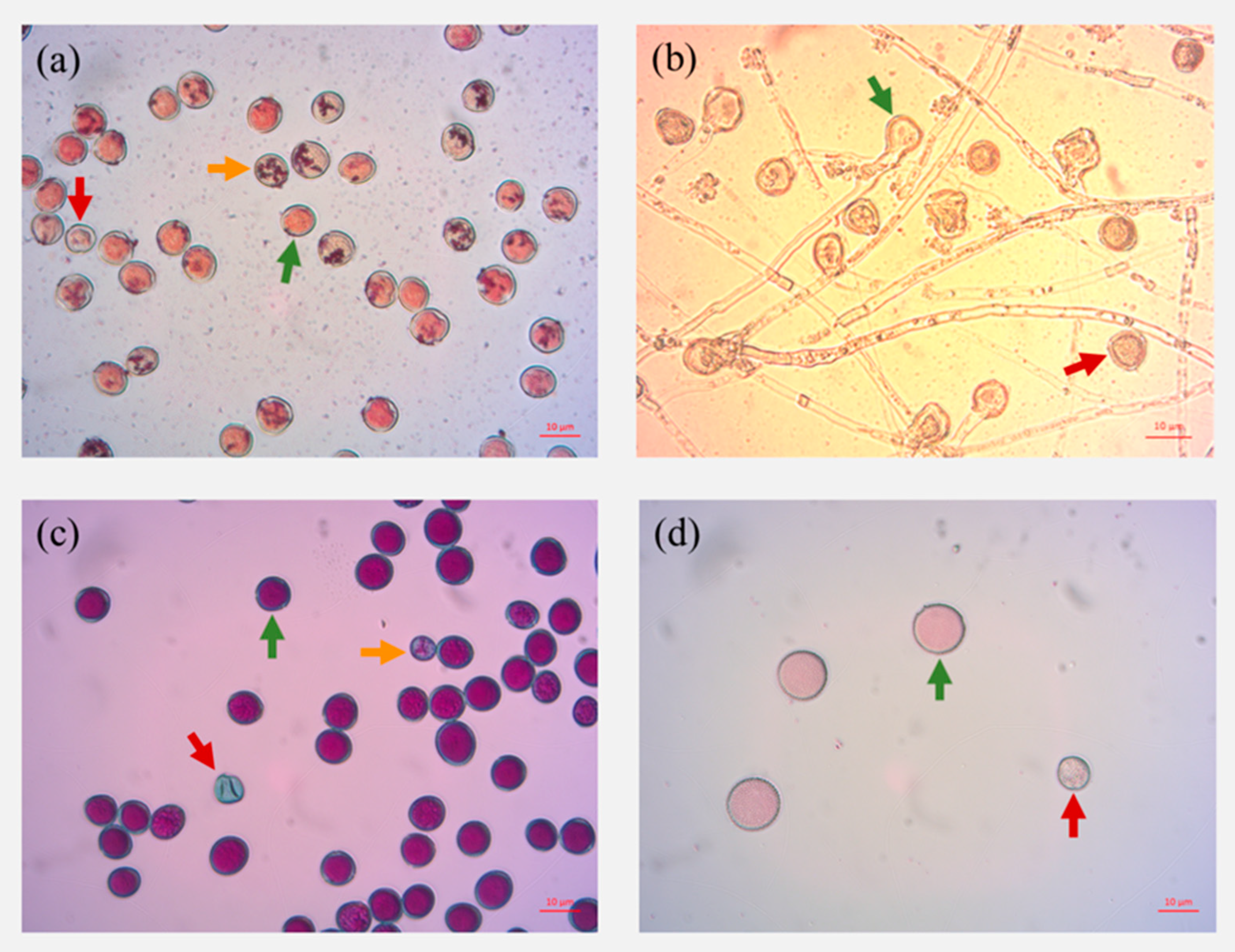
3.1. Viability Tests
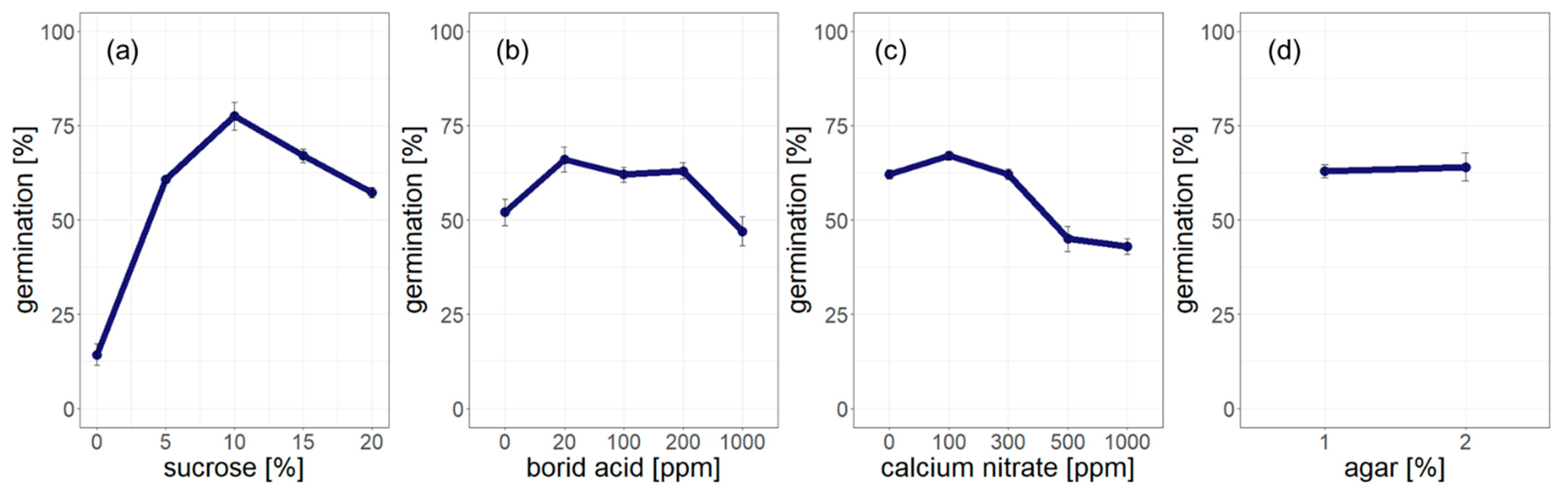
3.2. Optimization of Pollen Germination
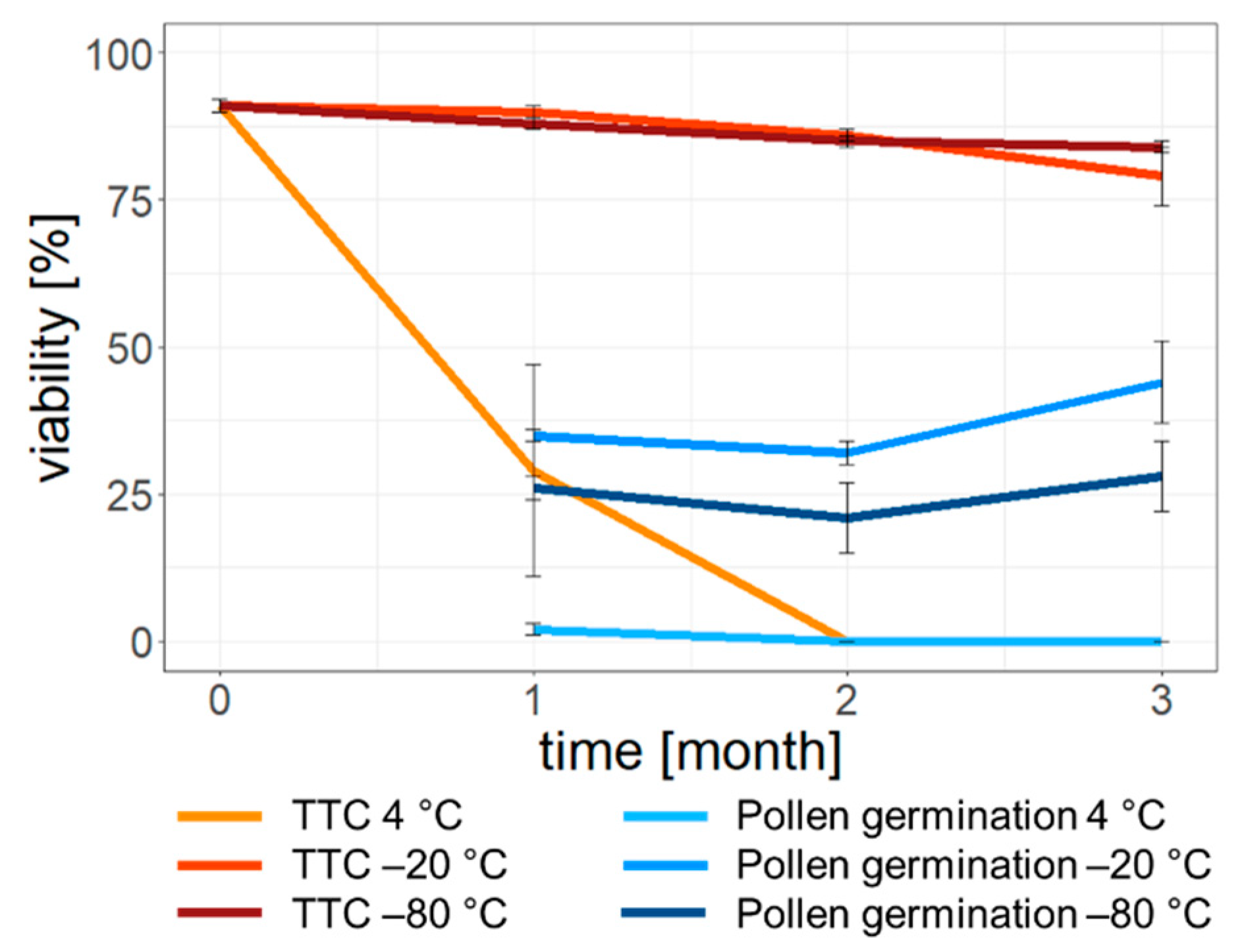
3.3. Storage Experiments
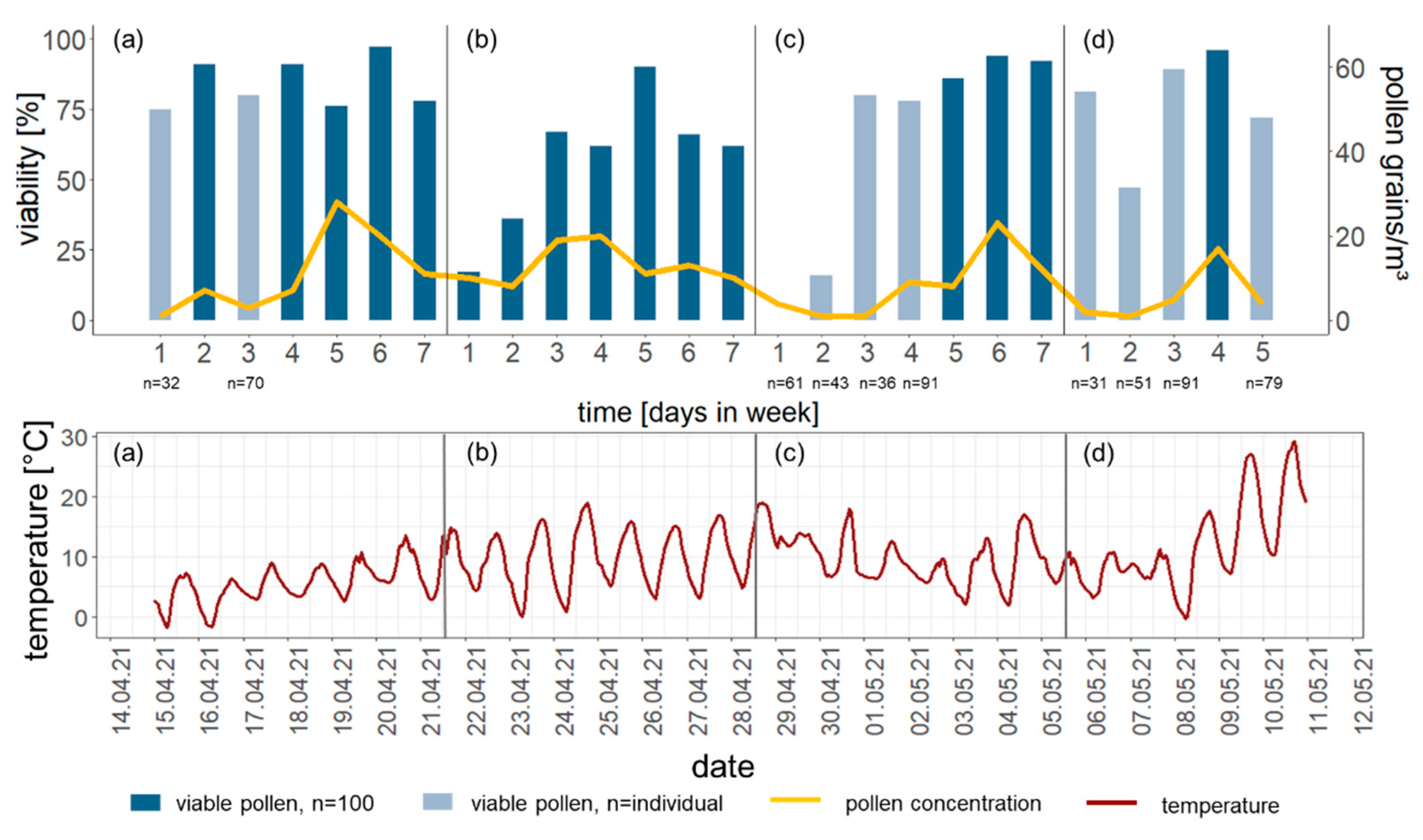
3.4. Atmospheric Transport
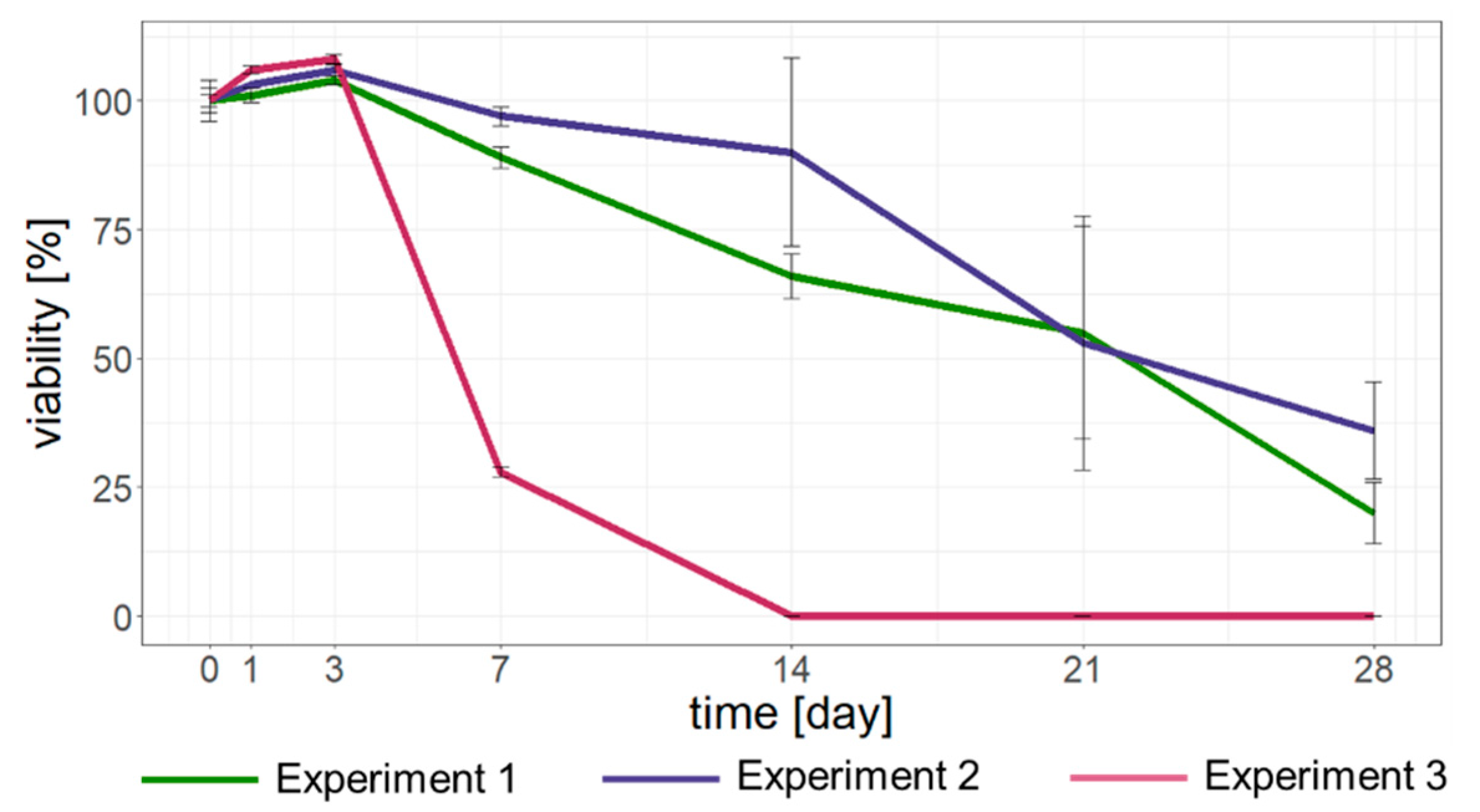
3.4.1. Simulations in Climate Chamber
3.4.2. Samples from Atmosphere
4. Discussion
4.1. Suitability of Viability Tests for Ash Pollen
4.2. Effects of Storage Conditions on Ash Pollen
4.3. Potential Effects of Long-Range Transport of Ash Pollen
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Scherrer, D.; Bader, M.K.-F.; Körner, C. Drought-sensitivity ranking of deciduous tree species based on thermal imaging of forest canopies. Agric. For. Meteorol. 2011, 151, 1632–1640. [Google Scholar] [CrossRef]
- Kölling, C.; Zimmermann, L. Die Anfälligkeit der Wälder Deutschlands gegenüber dem Klimawandel. Gefahrenst. Reinh. Luft 2007, 67, 259–268. [Google Scholar]
- Offenberger, M. Aktuelles zur Entwicklung des Eschentriebsterbens. Anl. Nat. 2017, 39, 22–27. [Google Scholar]
- Stener, L.-G. Clonal differences in susceptibility to the dieback of Fraxinus excelsior in southern Sweden. Scand. J. For. Res. 2013, 28, 205–216. [Google Scholar] [CrossRef]
- McKinney, L.V.; Nielsen, L.R.; Hansen, J.K.; Kjær, E.D. Presence of natural genetic resistance in Fraxinus excelsior (Oleraceae) to Chalara fraxinea (Ascomycota): An emerging infectious disease. Heredity 2011, 106, 788–797. [Google Scholar] [CrossRef]
- Bakys, R.; Vasaitis, R.; Barklund, P.; Ihrmark, K.; Stenlid, J. Investigations concerning the role of Chalara fraxinea in declining Fraxinus excelsior. Plant Pathol. 2009, 58, 284–292. [Google Scholar] [CrossRef]
- Kirisits, T. Ash dieback caused by Hymenoscyphus pseudoalbidus in a seed plantation of Fraxinus excelsior in Austria. J. Agric. Ext. Rural Dev. 2012, 4, 184–191. [Google Scholar] [CrossRef]
- McKinney, L.V.; Thomsen, I.M.; Kjaer, E.D.; Nielsen, L.R. Genetic resistance to Hymenoscyphus pseudoalbidus limits fungal growth and symptom occurrence in Fraxinus excelsior. For. Pathol. 2012, 42, 69–74. [Google Scholar] [CrossRef]
- Pliura, A.; Lygis, V.; Suchockas, V.; Bartkevicius, E. Performance of twenty four european Fraxinus excelsior populations in three lithuanian progeny trials with a special emphasis on resistance to Chalara fraxinea. Balt For. 2011, 17, 17–34. [Google Scholar]
- Enderle, R.; Nakou, A.; Thomas, K.; Metzler, B. Susceptibility of autochthonous German Fraxinus excelsior clones to Hymenoscyphus pseudoalbidus is genetically determined. Ann. For. Sci. 2015, 72, 183–193. [Google Scholar] [CrossRef]
- Enderle, R. An overview of ash (Fraxinus spp.) and the ash dieback disease in Europe. CAB Rev. 2019, 14, 1–12. [Google Scholar] [CrossRef]
- Wohlmuth, A.; Essl, F.; Heinze, B. Genetic analysis of inherited reduced susceptibility of Fraxinus excelsior L. seedlings in Austria to ash dieback. For. Int. J. For. Res. 2018, 91, 514–525. [Google Scholar] [CrossRef]
- McKinney, L.V.; Nielsen, L.R.; Collinge, D.B.; Thomsen, I.M.; Hansen, J.K.; Kjaer, E.D. The ash dieback crisis: Genetic variation in resistance can prove a long-term solution. Plant Pathol. 2014, 63, 485–499. [Google Scholar] [CrossRef]
- Kjær, E.D.; McKinney, L.V.; Nielsen, L.R.; Hansen, L.N.; Hansen, J.K. Adaptive potential of ash (Fraxinus excelsior) populations against the novel emerging pathogen Hymenoscyphus pseudoalbidus. Evol. Appl. 2012, 5, 219–228. [Google Scholar] [CrossRef] [PubMed]
- Enderle, R.; Fussi, B.; Lenz, H.D.; Langer, G.; Nagel, R.; Metzler, B. Ash dieback in Germany: Research on disease development, resistance and management options. In Dieback of European Ash (Fraxinus spp.): Consequences and Guidelines for Sustainable Management; Vasaitis, R., Enderle, R., Eds.; European Cooperation in Science & Technology (COST): Uppsala, Sweden, 2017; ISBN 978-91-576-8696-1. [Google Scholar]
- Coker, T.L.R.; Rozsypálek, J.; Edwards, A.; Harwood, T.P.; Butfoy, L.; Buggs, R.J.A. Estimating mortality rates of European ash (Fraxinus excelsior) under the ash dieback (Hymenoscyphus fraxineus) epidemic. Plants People Planet 2019, 1, 48–58. [Google Scholar] [CrossRef]
- Semizer-Cuming, D.; Kjær, E.D.; Finkeldey, R. Gene flow of common ash (Fraxinus excelsior L.) in a fragmented landscape. PLoS ONE 2017, 12, e0186757. [Google Scholar] [CrossRef]
- Bacles, C.F.E.; Ennos, R.A. Paternity analysis of pollen-mediated gene flow for Fraxinus excelsior L. in a chronically fragmented landscape. Heredity 2008, 101, 368–380. [Google Scholar] [CrossRef]
- Bacles, C.F.E.; Burczyk, J.; Lowe, A.J.; Ennos, R.A. Historical and contemporary mating patterns in remnant populations of the forests tree Fraxinus excelsior L. Evolution 2005, 59, 979. [Google Scholar] [CrossRef]
- Šikoparija, B.; Smith, M.; Skjøth, C.A.; Radisić, P.; Milkovska, S.; Simić, S.; Brandt, J. The Pannonian plain as a source of Ambrosia pollen in the Balkans. Int. J. Biometeorol. 2009, 53, 263–272. [Google Scholar] [CrossRef]
- Hernandez-Ceballos, M.A.; Soares, J.; García-Mozo, H.; Sofiev, M.; Bolivar, J.P.; Galán, C. Analysis of atmospheric dispersion of olive pollen in southern Spain using SILAM and HYSPLIT models. Aerobiologia 2014, 30, 239–255. [Google Scholar] [CrossRef]
- De Weger, L.A.; Pashley, C.H.; Šikoparija, B.; Skjøth, C.A.; Kasprzyk, I.; Grewling, Ł.; Thibaudon, M.; Magyar, D.; Smith, M. The long distance transport of airborne Ambrosia pollen to the UK and the Netherlands from Central and south Europe. Int. J. Biometeorol. 2016, 60, 1829–1839. [Google Scholar] [CrossRef] [PubMed]
- Myszkowska, D.; Piotrowicz, K.; Ziemianin, M.; Bastl, M.; Berger, U.; Dahl, Å.; Dąbrowska-Zapart, K.; Górecki, A.; Lafférsová, J.; Majkowska-Wojciechowska, B.; et al. Unusually high birch (Betula spp.) pollen concentrations in Poland in 2016 related to long-range transport (LRT) and the regional pollen occurrence. Aerobiologia 2021, 37, 543–559. [Google Scholar] [CrossRef]
- Šikoparija, B.; Skjøth, C.A.; Alm Kübler, K.; Dahl, A.; Sommer, J.; Grewling, Ł.; Radišić, P.; Smith, M. A mechanism for long distance transport of Ambrosia pollen from the Pannonian Plain. Agric. For. Meteorol. 2013, 180, 112–117. [Google Scholar] [CrossRef]
- Kearns, C.A.; Inouye, D.W. Techniques for Pollination Biologists; University Press of Colorado: Niwot, CO, USA, 1993; ISBN 9780870812811. [Google Scholar]
- Shivanna, K.R.; Rangaswamy, N.S. Pollen Biology: A Laboratory Manual; Springer: Berlin/Heidelberg, Germany, 1992; ISBN 3-540-55170-0. [Google Scholar]
- Capitani, L.C.; Rovedder, A.P.M.; Da Silva, J.C.C., Jr.; Peccatti, A. Pollen Viability and Autogamy Fitness in Bauhinia forficata Link (Fabaceae). Floresta Ambient. 2018, 25, 1–8. [Google Scholar] [CrossRef]
- Sulusoglu, M.; Cavusoglu, A. In vitro pollen viability and pollen germination in cherry laurel (Prunus laurocerasus L.). Sci. World J. 2014, 2014, 657123. [Google Scholar] [CrossRef]
- Gaaliche, B.; Majdoub, A.; Trad, M.; Mars, M. Assessment of pollen viability, germination, and tube growth in eight tunisian caprifig (Ficus carica L.) cultivars. ISRN Agron. 2013, 2013, 1–4. [Google Scholar] [CrossRef]
- Abdelgadir, H.A.; Johnson, S.D.; van Staden, J. Pollen viability, pollen germination and pollen tube growth in the biofuel seed crop Jatropha curcas (Euphorbiaceae). S. Afr. J. Bot. 2012, 79, 132–139. [Google Scholar] [CrossRef]
- Kelen, M.; Demirtas, I. Pollen viability, germination capability and pollen production level of some grape varieties (Vitis vinifera L.). Acta Physiol. Plant 2003, 25, 229–233. [Google Scholar] [CrossRef]
- Luo, S.; Zhang, K.; Zhong, W.-P.; Chen, P.; Fan, X.-M.; Yuan, D.-Y. Optimization of in vitro pollen germination and pollen viability tests for Castanea mollissima and Castanea henryi. Sci. Hortic. 2020, 271, 109481. [Google Scholar] [CrossRef]
- Du, X.; Zhu, X.; Yang, Y.; Zhao, F.; Liu, H. Pollen ultra-morphology and pollen viability test of Lilium Oriental hybrids. Int. J. Agric. Biol. 2018, 20, 1903–1907. [Google Scholar] [CrossRef]
- Castiñeiras, P.; Vázquez-Ruiz, R.A.; Fernández-González, M.; Rodríguez-Rajo, F.J.; Aira, M.J. Production and viability of Fraxinus pollen and its relationship with aerobiological data in the northwestern Iberian Peninsula. Aerobiologia 2019, 35, 227–241. [Google Scholar] [CrossRef]
- Alexander, M.P. Differential staining of aborted and nonaborted pollen. Stain Technol. 1969, 44, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Peterson, R.; Slovin, J.P.; Chen, C. A simplified method for differential staining of aborted and non-aborted pollen grains. Int. J. Plant Biol. 2010, 1, 13. [Google Scholar] [CrossRef]
- Brewbaker, J.; Kwack, B. The essential role of calcium ion in pollen germination and pollen tube growth. Am. J. Bot. 1963, 50, 859–865. [Google Scholar] [CrossRef]
- Rodriguez-Riano, T.; Dafni, A. A new procedure to asses pollen viability. Sex. Plant Reprod. 2000, 12, 241–244. [Google Scholar] [CrossRef]
- Masson-Delmotte, V.; Zhai, P.; Pirani, A.; Connors, S.L.; Péan, C.; Chen, Y.; Goldfarb, L.; Gomis, M.I.; Matthews, J.B.R.; Berger, S.; et al. (Eds.) Summary for Policymakers. In Climate Change 2021: The Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, MA, USA, 2021. [Google Scholar]
- Hirst, J.M. An automatic volumetric spore trap. Ann. Appl. Biol. 1952, 39, 257–265. [Google Scholar] [CrossRef]
- Galán, C.; Smith, M.; Thibaudon, M.; Frenguelli, G.; Oteros, J.; Gehrig, R.; Berger, U.; Clot, B.; Brandao, R. Pollen monitoring: Minimum requirements and reproducibility of analysis. Aerobiologia 2014, 30, 385–395. [Google Scholar] [CrossRef]
- De Souza, M.M.; Pereira, T.N.; Viana, A.P.; Pereira, M.G.; Bernacci, L.C.; Sudré, C.P.; Silva, L.d.C. Meiotic irregularities and pollen viability in Passiflora edmundoi Sacco (Passifloraceae). Caryologia 2003, 56, 161–169. [Google Scholar] [CrossRef]
- Frescura, V.; Laughinghouse, H.; Cantodorow, T.; Tedesco, S. Pollen viability of Polygala paniculata L. (Polygalaceae) using different staining methods. Biocell 2012, 36, 143–145. [Google Scholar] [CrossRef]
- Coelho, A.; Morais, K.; Laughinghouse, H.; Giacomini, S.; Tedesco, S. Pollen grain viability in accessions of Crotalaria juncea L. (Fabaceae). Agrociencia 2012, 46, 481–487. [Google Scholar]
- West, W.; Baldwin, S.; Rich, T.C.G. Pollen viability and size in British Centaurium Hill and Gentianella Moench (Gentianaceae) taxa. Grana 2014, 53, 111–116. [Google Scholar] [CrossRef]
- Atlagić, J.; Terzić, S.; Marjanović-Jeromela, A. Staining and fluorescent microscopy methods for pollen viability determination in sunflower and other plant species. Ind. Crops Prod. 2012, 35, 88–91. [Google Scholar] [CrossRef]
- Dafni, A.; Firmage, D. Pollen viability and longevity: Practical, ecological and evolutionary implications. In Pollen and Pollination; Dafni, A., Hesse, M., Pacini, E., Eds.; Springer: Vienna, Austria, 2000; pp. 113–132. ISBN 978-3-7091-7248-3. [Google Scholar]
- Nepi, M.; Franchi, G. Cytochemistry of mature angiosperm pollen. Syst. Evol. 2000, 222, 45–62. [Google Scholar] [CrossRef]
- Alexander, L. Ploidy level influences pollen tube growth and seed viability in interploidy crosses of Hydrangea macrophylla. Front. Plant Sci. 2020, 11, 100. [Google Scholar] [CrossRef]
- Báez, P.; Riveros, M.; Lehnebach, C. Viability and longevity of pollen of Nothofagus species in South Chile. N. Z. J. Bot. 2002, 40, 671–678. [Google Scholar] [CrossRef]
- Marcellán, O.N.; Camadro, E.L. The viability of asparagus pollen after storage at low temperatures. Sci. Hortic. 1996, 67, 101–104. [Google Scholar] [CrossRef]
- Munhoz, M.; Da Luz, C.F.P.; Meissner Filho, P.E.; Barth, O.M.; Reinert, F. Viabilidade polínica de Carica papaya L.: Uma comparação metodológica. Rev. Bras. Bot. 2008, 31, 209–214. [Google Scholar] [CrossRef]
- Aguila, Y.; Jiménez, M.; García, Y. Pollen Viability in Taro (Colocasia esculenta (L.) Schott) in Cuba. Agrisost 2018, 24, 33–38. [Google Scholar]
- Mazzeo, A.; Palasciano, M.; Gallotta, A.; Camposeo, S.; Pacifico, A.; Ferrara, G. Amount and quality of pollen grains in four olive (Olea europaea L.) cultivars as affected by ‘on’ and ‘off’ years. Sci. Hortic. 2014, 170, 89–93. [Google Scholar] [CrossRef]
- Zulkarnain, Z. Assessment of pollen viability and germination in Swainsona formosa (G. Don) J. Thomson. Biospecies 2019, 12, 49–54. [Google Scholar]
- Beck-Pay, S.L. Optimisation of pollen viability tests for Acacia podalyriifolia and two ploidys of Acacia mearnsii. S. Afr. J. Bot. 2012, 78, 285–289. [Google Scholar] [CrossRef]
- Sousa, A.; Santos, M.; Pelacani, C.; Santos, F. Testing culture media for pollen germination of Elaeis guineensis Jacq. (oil palm, Arecaceae). Bot. J. Linn. Soc. 2016, 182, 536–542. [Google Scholar] [CrossRef]
- Nogueira, P.V.; Coutinho, G.; Pio, R.; Da Silva, D.F.; Zambon, C.R. Establishment of growth medium and quantification of pollen grains and germination of pear tree cultivars. Rev. Ciê. Agron. 2016, 47, 380–386. [Google Scholar] [CrossRef][Green Version]
- Burke, I.; Wilcut, J.; Allen, N. Viability and In Vitro Germination of Johnsongrass (Sorghum halepense) Pollen. Weed Technol. 2007, 21, 23–29. [Google Scholar] [CrossRef]
- Wang, Z.; Ge, Y.; Scott, M.; Spangenberg, G. Viability and longevity of pollen from transgenic and nontransgenic Tall Fescue (Festuca arundinacea) (Poaceae) plants. Am. J. Bot. 2004, 91, 523–530. [Google Scholar] [CrossRef]
- Duro, A.; Piccione, V.; Zampino, D. Air quality biomonitoring through pollen viability of Fabaceae. Environ. Monit. Assess. 2013, 185, 3803–3817. [Google Scholar] [CrossRef]
- Ishida, K.; Hiura, T. Pollen fertility and flowering phenology in an androdioecious tree, Fraxinus lanuginosa (Oleaceae), in Hokkaido, Japan. Int. J. Plant Sci. 1998, 159, 941–947. [Google Scholar] [CrossRef]
- Kremer, D.; Jemrić, T. Pollen germination and pollen tube growth in Fraxinus pennsylvanica. Biologia 2006, 61, 79–83. [Google Scholar] [CrossRef]
- Matsuda, H.; Higuchi, H. Effects of temperature and medium composition on pollen germination of ‘Bengal’ and ‘Chakrapat’ lychee (Litchi chinensis Sonn.) in vitro. Trop. Agri. Dev. 2013, 57, 120–125. [Google Scholar]
- Fragallah, S.; Lin, S.; Li, N.; Ligate, E.; Chen, Y. Effects of sucrose, boric acid, pH, and incubation time on in vitro germination of pollen and tube growth of chinese fir (Cunnighamial lanceolata L.). Forests 2019, 10, 102. [Google Scholar] [CrossRef]
- Aslantaş, R.; Prilak, L. Storage of strawberry pollen. Acta Agro. 2001, 567, 117–121. [Google Scholar] [CrossRef]
- El Kadri, N.; Ben Mimoun, M. In vitro germination of different date palm (Phoenix dactylifera L.) pollen sources from southern tunisia under the effect of three storage temperatures. Int. J. Fruit Sci. 2020, 20, S1519–S1529. [Google Scholar] [CrossRef]
- Mesnoua, M.; Roumani, M.; Salem, A. The effect of pollen storage temperatures on pollen viability, fruit set and fruit quality of six date palm cultivars. Sci. Hortic. 2018, 236, 279–283. [Google Scholar] [CrossRef]
- Du, G.; Xu, J.; Gao, C.; Lu, J.; Li, Q.; Du, J.; Lv, M.; Sun, X. Effect of low storage temperature on pollen viability of fifteen herbaceous peonies. Biotechnol. Rep. 2019, 21, e00309. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Gómez, P.; Gradziel, T.M.; Ortega, E.; Dicenta, F. Low temperature storage of almond pollen. Hortic. Sci. 2002, 37, 691–692. [Google Scholar] [CrossRef]
- Shekari, A.; Nazeri, V.; Shokrpour, M. Pollen viability and storage life in Leonurus cardiaca L. J. Appl. Res. Med. Aromat. Plants 2016, 3, 101–104. [Google Scholar] [CrossRef]
- Raquin, C.; Brachet, S.; Jeandroz, S.; Vedel, F.; Frascaria-Lacoste, N. Combined analysis of microsatellite and RAPD markers demonstrate possible hybridization between Fraxinus excelsior L. and Fraxinus angustifolia Vahl. For. Gen. 2002, 9, 111–114. [Google Scholar]
- Wang, S.; Xie, B.; Yin, L.; Duan, L.; Li, Z.; Eneji, A.E.; Tsuji, W.; Tsunekawa, A. Increased UV-B radiation affects the viability, reactive oxygen species accumulation and antioxidant enzyme activities in maize (Zea mays L.) pollen. Photochem. Photobiol. 2010, 86, 110–116. [Google Scholar] [CrossRef]
- Hidvégi, S.; Rácz, F.; Hadi, G.; Gesztesi, L.; Tóth, Z. Effect of UV-radiation on the pollen viability of some parental lines of hybrid maize. Cer. Res. Commun. 2009, 37, 349–352. [Google Scholar]
- Schueler, S.; Schlnzen, K.H.; Scholz, F. Viability and sunlight sensitivity of oak pollen and its implications for pollen-mediated gene flow. Trees 2005, 19, 154–161. [Google Scholar] [CrossRef]
- Bohrerova, Z.; Bohrer, G.; Cho, K.D.; Bolch, M.A.; Linden, K.G. Determining the viability response of pine pollen to atmospheric conditions during long-distance dispersal. Ecol. Appl. 2009, 19, 656–667. [Google Scholar] [CrossRef] [PubMed]
- Ge, Y.; Fu, C.; Bhandari, H.; Bouton, J.; Brummer, E.C.; Wang, Z.-Y. Pollen viability and longevity of switchgrass (Panicum virgatum L.). Crop Sci. 2011, 51, 2698–2705. [Google Scholar] [CrossRef]
| Experiment 1 | Experiment 2 | Experiment 3 | ||||
|---|---|---|---|---|---|---|
| Day | Night | Day | Night | Day | Night | |
| Temperature | 10 °C | 5 °C | 12 °C | 7 °C | 20 °C | 12 °C |
| Relative humidity | 65% | 80% | 65% | 80% | 60% | 80% |
| UV radiation | on | off | on | off | on | off |
| 0% | 5% | 10% | 15% | ||
|---|---|---|---|---|---|
| 5% | <0.001 * | ||||
| Sucrose | 10% 15% 20% | <0.001 * | <0.001 * | ||
| <0.001 * | 0.084 | 0.005 * | |||
| <0.001 * | 0.131 | <0.001 * | 0.008 * | ||
| 0 ppm | 20 ppm | 100 ppm | 200 ppm | ||
| 20 ppm | 0.008 * | ||||
| Boric acid | 100 ppm 200 ppm 1000 ppm | 0.059 | 0.554 | ||
| 0.034 * | 0.694 | 0.694 | |||
| 0.524 | 0.001 * | 0.007 * | 0.004 * | ||
| 0 ppm | 100 ppm | 300 ppm | 500 ppm | ||
| 100 ppm | 0.190 | ||||
| Calcium nitrate | 300 ppm 500 ppm 1000 ppm | 0.880 | 0.190 | ||
| <0.001 * | <0.001 * | <0.001 * | |||
| <0.001 * | <0.001 * | <0.001 * | 0.720 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Buchner, L.; Eisen, A.-K.; Šikoparija, B.; Jochner-Oette, S. Pollen Viability of Fraxinus excelsior in Storage Experiments and Investigations on the Potential Effect of Long-Range Transport. Forests 2022, 13, 600. https://doi.org/10.3390/f13040600
Buchner L, Eisen A-K, Šikoparija B, Jochner-Oette S. Pollen Viability of Fraxinus excelsior in Storage Experiments and Investigations on the Potential Effect of Long-Range Transport. Forests. 2022; 13(4):600. https://doi.org/10.3390/f13040600
Chicago/Turabian StyleBuchner, Lisa, Anna-Katharina Eisen, Branko Šikoparija, and Susanne Jochner-Oette. 2022. "Pollen Viability of Fraxinus excelsior in Storage Experiments and Investigations on the Potential Effect of Long-Range Transport" Forests 13, no. 4: 600. https://doi.org/10.3390/f13040600
APA StyleBuchner, L., Eisen, A.-K., Šikoparija, B., & Jochner-Oette, S. (2022). Pollen Viability of Fraxinus excelsior in Storage Experiments and Investigations on the Potential Effect of Long-Range Transport. Forests, 13(4), 600. https://doi.org/10.3390/f13040600








