Abstract
The aim of this study was to evaluate the antimicrobial activity of wood distillates obtained from Scots pine (Pinus sylvestris) sawdust in order to explore new alternatives for the utilization of wood industry by-products. The distillates were produced by slow pyrolysis thermal conversion in three process phases with increasing temperatures, namely drying, torrefaction and pyrolysis, and three cooling units with different temperatures to condensate the distillates. This yielded nine different liquid fractions. The food-related pathogens, Salmonella, Listeria monocytogenes and Candida albicans, were evaluated for their susceptibility to the distillate fractions using an agar diffusion test. The antimicrobial activity was estimated by measuring the formed inhibition zones after the incubation period. In addition, the minimum inhibitory concentration (MIC) and microbicidic concentration were assayed for a selected fraction (T2) from the torrefaction phase with Bio-screen C. The results indicated that the distillates from the torrefaction and pyrolysis phases had antimicrobial activity against the tested microbes. The MIC value of the T2 fraction for all tested microbes was 0.83% (v/v). Furthermore, the T2 fraction was microbicidic for Salmonella and Listeria strains in 0.83% (v/v) solution and Candida strain in 1.67% (v/v) solution. In conclusion, Scots pine wood distillates obtained from slow pyrolysis have the potential to be developed as antimicrobial agents against pathogenic microbes. Next, research is needed to investigate the chemical composition of the distillates and to assess their safe use.
1. Introduction
Forests cover ca. 35% of the land area of Europe [1]. A large percentage (46%) of European forests are mainly coniferous [1], and Scots pine (Pinus sylvestris), also known as European red pine, is a very common tree species. The conifer zone is located in northern Europe, and thus the largest growing areas of coniferous trees are in Finland and Sweden [1]. Europe is a major exporter of primary wood and wood products, such as paper products [1]. Therefore, the wood industry remains an important industrial sector in Europe. The operations of the wood industry generate significant amounts of various by-products, including sawdust. For example, scenarios for the utilization of wood industry by-products in Finland have been examined and sawdust utilization is mainly seen to be distributed among energy production, biofuels (bioethanol), processing of wood composites, and the pulp and board industries [2]. However, further processing of by-products could also provide an opportunity to refine new products. For example, sawdust can be used as a raw material for the pyrolysis process, and distillates obtained from pyrolysis can offer new recovery targets.
Pyrolysis is a thermochemical process in which organic material is decomposed into solid (biochar), liquid (distillates) and gas products by burning in a low-oxygen atmosphere [3,4]. The pyrolysis can be classified into three sub-classes: slow (conventional), fast, and flash pyrolysis [3,4,5]. In slow pyrolysis, the temperature is increased slowly to approximately 300–500 °C, whereas fast and flash pyrolysis are performed faster and at higher temperatures [4,5]. In addition, the residence time is longer in slow pyrolysis, en-abling the components in the vapour phase to interact with each other and with the formed biochar and distillates [4]. Generally, the particle size of processed material is also larger in the slow pyrolysis process [5,6].
A wood slow pyrolysis process can be theoretically divided into four stages: water evaporation, decomposition of hemicelluloses, decomposition of cellulose and at the end of the process, decomposition of most lignins [4,7]. The decomposition of hemicelluloses at 180–350 °C, and of cellulose at 250–400 °C, results in the formation of carboxylic acids and carbonyl compounds, whereas the decomposition of lignin over a wide temperature range of 250–500 °C forms mainly phenols, the processes taking place partially parallel [8,9]. Thus, during thermal modification of wood materials several degradation processes take place. These include cellulose polymerization degradation, as well as polysaccharide depolymerization [10]. Further, as the thermal treatment intensity increases, carbonyl groups are produced, lignin being the most stable component [10]. However, the degradation processes of wood differ based on the wood species, application of treatments under different conditions and thus reflecting the variation of ensued molecules and proportions of them in the fractions. The chemical composition of the final pyrolysis products depends on the organic starting material and its characteristics, for example the particle size and moisture level, and on the process parameters, such as temperatures and residence times of the different process steps [4,11,12]. We have observed in our earlier studies that pine wood slow pyrolysis liquids are complex organic mixtures with diverse che-mical compositions [13], potentially differing in for their antimicrobial properties.
In order to improve the microbiological safety of food or the food processing environment, all alternative sources for screening food production-related applications should be surveyed. The World Health Organization (WHO) has estimated that Salmonella bacteria are one of the most significant causes of death of all food poisoning agents [14]. Salmonellosis is mainly caused by poor hygiene during food production or processing, and it is the second most common zoonotic disease in the European Union (EU) [15]. Moreover, the European Food Safety Authority (EFSA) has estimated the annual cost of salmonella at 3 billion euros in the EU [15]. Another major concern is Listeria, and its ability to form biofilm in se-veral food processing environments [16,17,18]. Furthermore, eukaryotic yeasts, such as Candida albicans, are spoilage microbes for various food products and beverages, and wild yeasts can contaminate raw ingredients, the surrounding area or manufacturing equipment, causing problems especially for fermented foods by altering their organoleptic properties [19]. As a pathogen, C. albicans can be transmitted via an infected employee to products. In addition to traditional food poisonings and infections, antimicrobial resistance in foodborne microorganisms is also an emerging issue [20].
Wood-based pyrolysis distillates have previously been observed to possess antimicrobial properties against pathogenic microbes [21,22,23]. Besides pyrolysis distillates organic acids and essential oils have shown antimicrobial features. In a study by Bahmani et al. [24], weak organic acids, acetic and propionic acids, were observed to prevent the growth of mold and rot fungi in palm wood samples even with 5% solutions. In addition, plant essential oils, especially lavender oil, lemongrass oil and thyme oil, had antifungal properties against phylogenetically diverse fungi, these including Aspergillus niger, Penicillium commune, Coniophora puteana, Trametes versicolor and Chaetomium globosum in impregnated beech (Fagus orientalis) and pine (Pinus taeda) wood samples [25]. Furthermore, wood-associated polyphenols have been shown to have antimicrobial activity against food spoilage microbes and pathogens [26]. However, studies on the antimicrobial activity of the Scots pine distillates obtained from slow pyrolysis are limited. Therefore, the aim of this study was to evaluate the antimicrobial activity of wood distillates from Scots pine processed by a slow pyrolysis technique. The activity of the distillate fractions was tested against three different pathogens, Salmonella enterica, Listeria monocytogenes and Candida albicans.
2. Materials and Methods
Sawdust raw material samples (n = 6) from Scots pine (Pinus sylvestris) grown in Finland and Sweden [27] were obtained for slow pyrolysis thermal conversion processing. The dust samples were frozen before processing, and warmed up to room temperature before starting the processing. The samples were stored frozen for 1–3 months.
The slow pyrolysis thermal conversion system was designed and constructed at the University of Eastern Finland. The system consisted of a heating chamber controlled with Hotwell software (Hotwell Oy, Mikkeli, Finland) and Siemens Logo hardware (Siemens AG, Munich, Germany), which were used to monitor and adjust the slow pyrolysis process. The distillates were collected by adjusting temperatures in circulation chillers containing oil (120 °C, 60 °C, VWR model 1157 P, VWR International, Vienna, Austria) and water (10 °C, WK1200 Lauda, Lauda-Brinkmann, Delran, NJ, USA). The process scheme is described in our previously published article Salami et al. [28].
The pine dust samples (2.5–3 kg) were packed tightly in the metal holders and placed in the chamber of the slow pyrolysis processing unit (Figure 1a). Nitrogen gas was fed into the tank (3 L/min) to ensure oxygen-free conditions. The temperature of the chamber was slowly increased, and wood distillate samples were collected in three process phases (Table 1). First, the chamber temperature was increased to the drying phase in order to evaporate the water from the biomass. Secondly, the temperature was increased to the torrefaction phase and thirdly, to the pyrolysis phase. The gases and aerosols formed in each phase were released from the chamber into the condensing units and collected into glass bottles (Schott) at three different condensation temperatures, yielding nine different liquid fractions (Figure 1b and Table 1).

Figure 1.
(a) Pine sawdust raw material samples in metal holders before pyrolysis process. (b) Representative distillate fractions from drying (D1, D2, D3), torrefaction (T1, T2, T3) and pyrolysis (P1, P2, P3) phases after pyrolysis process.

Table 1.
Wood distillate fractions from the slow pyrolysis process.
The wood distillates were stored at 0–4 °C until analyzed. The samples were shaken well before the antimicrobial testing because some of the distillates tended to fractionate. The tests were made with distillate samples from six comparable slow pyrolysis processes using at least three replicants per sample.
The activity of the wood distillate samples D1-D3, T1-T3 and P1-P3 to inhibit the growth of Salmonella enterica subsp. enterica serovar Infantis EELA 72 (Gram-bacteria), Listeria monocytogenes ATCC 7644 (Gram+ bacteria) and Candida albicans EELA 188 (eukaryotic yeast) was tested using an agar diffusion method. The Listeria strain was from the American Type Culture Collection and the EELA strains were obtained from the Finnish Food Safety Authority. For the actual assay the samples were diluted to 25%, 10%, 5%, 2%, 1%, 0.2% and 0.1% solutions of acetone:ethanol:water (30:30:40), which was also tested as a solvent control. Salmonella was not tested for in the 0.2% and 0.1% solutions. Furthermore, the antimicrobial activity of acetic acid (100 mg/mL, Merck KGaA, Darmstadt, Germany) was studied with 25%, 10%, 5% and 2% solutions for Listeria and Candida strains, and with 25%, 10% and 5% solutions for the Salmonella strain, because acetic acid is a common compound in pyroligneous liquids known to have antimicrobial properties [23].
The bacterial strains were cultured aerobically on Tryptone Soy agar plates (LabM, Lancashire, Greater Manchester, UK), and the yeast strain on Sabouraud Dextrose agar plates (LabM, Lancashire, Greater Manchester, UK). For the test a colony was transferred from a pure culture plate to the broth solution, and the strains were cultured in Tryptone Soy broth (LabM, Lancashire, Greater Manchester, UK) or Sabouraud Dextrose broth (LabM, Lancashire, Greater Manchester, UK), for 20 h at 37 °C for bacteria and at 30 °C for yeast, before inoculation to 0.6% soft agar (LabM, Lancashire, Greater Manchester, UK) at 45 °C. Turbidity of the inoculated microbial suspension was measured with a spectrophotometer (GENESYS 10 uv, Thermo Electron Corporation, USA) at a wavelength of 625 nm.
The inoculated soft agar containing approximately 107 colony-forming units per millilitre (cfu/mL) of the test microbe was poured on agar plates. Next, solidification wells (Ø 9 mm) were bored into the agar with a sterilized borer. The diluted samples (80 µL) were directly added into the wells of agar plates with Listeria or Candida. For the Salmonella strain, the samples (80 µL) were used to saturate sterile filter-paper discs (Ø 13 mm), which were then placed on the agar plates. Filter-paper discs were used for the Salmonella strain in order to clarify the measurement of formed zones. The inoculated bacterial plates were incubated at 37 °C for 24 h, and the yeast plates at 30 °C for 48 h. After the incubation, the inhibition zones (radius of the well) were measured with a caliper at an accuracy of 0.1 mm. Plates were prepared in triplicate for each experiment.
The antimicrobial activity of the distillate fraction T2 was tested further against the pathogens Salmonella enterica subsp. enterica serovar Typhimurium ATCC 13311, Listeria monocytogenes ATCC 7644 and Candida albicans ATCC 90029 by using an automated incubator and turbidity reader Bioscreen C (Oy Growth Curves Ab Ltd., Helsinki, Finland). The fraction T2 was selected for the test because it yielded the highest amount of distillate in phases having an antimicrobial activity (Table 1). The optical density (OD) of the growth medium was measured, and the results were calculated based on the measured absor-bance values. Growth curves were drawn for each tested microbe, and minimum inhibitory concentrations (MIC) and microbicidic concentrations were determined. The sample was diluted at 50%, 25% and 12.5% in dimethyl sulfoxide (DMSO) (Honeywell Riedel-de Haën, Muskegon, Michigan, USA). In addition, an undiluted sample was tested.
The bacterial strains were cultured aerobically on Tryptone Soy agar plates (LabM, Lancashire, Greater Manchester, UK) and the yeast strain on the Sabouraud Dextrose agar plate (LabM, Lancashire, Greater Manchester, UK). For the test, a colony was transferred from a plate to the broth solution. The bacterial strains were cultured in Tryptone Soy (TS) broth (LabM, Lancashire, Greater Manchester, UK) and the yeast strain in Oxytetracyc-line-Glucose-Yeast Extract (OGYE) broth ((0.5% (w/v) yeast extract (Merck KGaA, Darmstadt, Germany), 2% (w/v) D-(+) glucose anhydrous (MP Biomedicals, Illkirch, France), 0.0001% (w/v) biotin (SERVA Electrophoresis GmbH, Heidelberg, Germany)), at 37 °C for bacteria and at 30 °C for yeast. An overnight culture of each test strain was pelleted, washed, and suspended in physiological saline (0.9% NaCl). The turbidity of the microbial suspension was measured with a spectrophotometer (UV-1600PC, VWR, Leuven, Belgium) at a wavelength of 625 nm. The microbial suspension concentration used in the test was 1 × 106 cfu/mL for bacterial strains and 1 × 103 cfu/mL for the yeast strain.
The antimicrobial analysis was performed in 100-well honeycomb microplates. Each sample was tested in three replicate wells, with each well containing TS- or OGYE-broth medium, 10 µL sample solution and 100 µL microbial cell suspension, achieving a total volume of 300 µL per well. Thus, the final tested solutions of the T2 sample in the Bio-screen test were 3.33% (v/v), 1.67% (v/v), 0.83% (v/v) and 0.42% (v/v). Control samples were also analyzed, including microbe and solvent controls. Microbe controls contained broth medium (200 µL) and 100 µL microbial cell suspension, whereas solvent control contained 10 µL 87.5% DMSO solution instead of the test sample. The plates were incubated for 36 h for bacterial strains and 48 h for the yeast strain, and the OD of each well was measured every 30 min using a wideband filter at 420–580 nm. The plates were shaken for 10 s at low speed before measuring. Three independent experiments were assayed for each tested strain. After the Bioscreen run, an aliquot was taken from the wells that showed no growth and cultured on suitable agar plates in order to determine whether the activity of the sample was microbistatic or microbicidic.
The means and standard deviations of the inhibition zones in the agar diffusion method were calculated from distillate samples from six comparable slow pyrolysis processes. The data were analyzed by the Kruskall-Wallis H test using SPSS software version 25, and p-values less than 0.05 were defined as statistically significant. Significance values were adjusted by the Bonferroni correction for multiple tests. The Kruskall-Wallis H test was used for the statistical analysis because the data did not conform to normal distribution.
The means of the growth curves were calculated for the results of Bioscreen C assays, and the final growth curves were drawn using Microsoft Excel software.
3. Results
3.1. Antimicrobial Activity of the Wood Distillates Assayed by an Agar Diffusion Method
Antimicrobial activity of the wood distillate fractions was tested against the pathogens Salmonella, Listeria monocytogenes and Candida albicans. The results of the antimicrobial assays are presented in Table 2, Table 3 and Table 4. The distillates from the drying phase of the pyrolysis process were not observed to have any antimicrobial activity, whereas the distillates from the torrefaction and pyrolysis phases did show antimicrobial activity.

Table 2.
Inhibition zones of the pyrolysis fractions against Salmonella Infantis.

Table 3.
Inhibition zones of the pyrolysis fractions against Listeria monocytogenes.

Table 4.
Inhibition zones of the pyrolysis fractions against Candida albicans.
The most effective fractions were T1–2 and P1–2 against all the tested microbes. For the Salmonella and Candida strains, the most effective fractions were T1 and P1, whereas T1 and P2 had the strongest effect against the Listeria strain. In the case of Listeria, the inhibitory effects of the torrefaction and pyrolysis distillates were also detected with highly diluted solutions, even with 0.1% solution. Inhibition zones against Salmonella and Candida strains differed significantly (p < 0.05) for fractions T1 and P1, in 25% solutions compared to acetic acid.
The condensation unit appeared to affect the antimicrobial activity. Principally, the activity tended to decrease according to condensation temperature, being highest in unit 1 (120 °C) and lowest in unit 3 (10 °C).
3.2. Antimicrobial Activity of the Selected Distillate Fraction Assayed by Bioscreen C
The results of the antimicrobial activity of the pyrolysis distillate fraction T2 are presented in Figure 2. The MIC value of the sample against Salmonella, Listeria and Candida was 0.83% (v/v). The fraction T2 was observed to be microbicidic for Salmonella and Listeria strains at 0.83% (v/v) concentration, and for Candida strain at 1.67% (v/v). In addition, the 0.42% (v/v) solution inhibited the growth of the microbes effectively for 20 h and at the end of the Bioscreen run. At the time of 36 or 48 h, the inhibition of growth was ca. 68% for Salmonella, ca. 39% for Listeria and ca. 29% for Candida compared to the control.
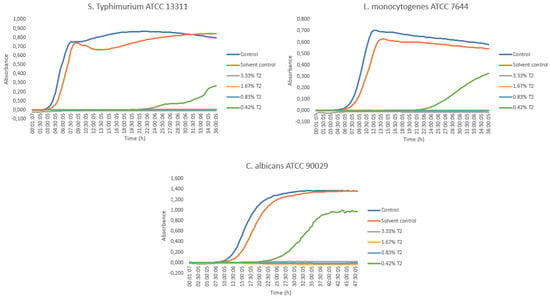
Figure 2.
Growth curves of Salmonella, Listeria and Candida strains for the sample T2 in solution concentrations of 3.33% (v/v) (grey line), 1.67% (v/v) (yellow line), 0.83% (v/v) (light blue line) and 0.42% (v/v) (green line) in Bioscreen C assays. (Control (blue line) = microbe control, Solvent control (red line) = 2.9% DMSO).
4. Discussion
In this study, the antimicrobial activity of Scots pine wood distillates obtained from slow pyrolysis thermal conversion was evaluated utilizing an agar diffusion test and an automated microbiology growth curve analysis system, Bioscreen C. The distillates collected from torrefaction and pyrolysis phases had activity against all the tested microbes, Salmonella, Listeria monocytogenes and Candida albicans. However, the distillates from the drying phase did not have any antimicrobial activity, which was presumably due to the high water content of these fractions. Condensation temperature appeared to affect the antimicrobial activity of the distillates. The fractions were gathered in a row from higher condensation temperature to lower temperature, yielding fractions with decreasing darkness of colour and antimicrobial activity, respectively.
According to our knowledge, studies evaluating the antimicrobial activity of Scots pine wood distillates processed by slow pyrolysis conversion have not previously been reported. However, antimicrobial properties have been assessed from pyrolysis liquids obtained by different pyrolysis processes from various tree species [21,29,30]. For example, pyrolysis oil obtained from Pinus densiflora by a fast pyrolysis process has been shown to have antimicrobial activity against two Salmonella Typhimurium strains, Listeria monocytogenes and Bacillus cereus strains [21,22]. Wei et al. [30] observed that pyroligneous acids prepared from walnut tree branches collected from process temperatures at 230 °C to 370 °C possessed the highest antimicrobial activity, and phenols and organic acids were estimated to be the compounds causing the observed antimicrobial effect. In addition, antimicrobial activity of pyroligenous acids obtained from the wood of Mimosa tenuiflora and Eucalyptus urograndis was evaluated on the yeast Candida albicans, and for both liquids the formed diameter of inhibition halos at concentrations of 20% were from 10.3 to 11.0 mm [29]. Thus, it appears that our results (Table 2, Table 3 and Table 4) are in line with those of previous studies. However, the MIC value of the selected fraction from the torrefaction phase (T2) against all the tested microbes was 0.83% (v/v) (Figure 2), which was higher than the MIC value (0.3125%, v/v) of the pyroligneous acid from softwood mixture for L. monocytogenes in a study by Suresh et al. [23].
The slow pyrolysis thermal processing method, with three phases and three condensation temperatures, was used in our previous study with a different plant material (hemp hurds), with a focus on chemical characterization of the distillates [28]. The chemical composition of wood distillates obtained from the pyrolysis process is very complex, and the liquids may contain hundreds of compounds, which must be characterized with multiple analysis methods in order to obtain a comprehensive characterization. The chemical groups include carboxylic acids, alcohols, aldehydes, ketones, phenols and sugars [4,6,29]. The pyrolysis method and parameters greatly affect the chemical composition of the distillates [4,11,12], and therefore also their antimicrobial properties. It was surprising in this study that there were only modest differences in antimicrobial efficiency between the torrefaction and pyrolysis fractions, although on the basis of our [28] previous studies and also others [31], it can be assumed that their chemical composition is different. This indicates that various compounds are responsible for the antimicrobial effects. Furthermore, there is also typically more water in torrefaction fractions compared to pyrolysis fractions, making them more diluted [28]. We did not analyse the chemical content of the distillate fractions in this study, because our aim was to evaluate the antimicrobial potential of different distillate fractions obtained from slow pyrolysis.
It is an advantage of the slow pyrolysis method that there is a possibility to collect chemically different fractions from the process. Moreover, slow pyrolysis process parameters can be further optimized, for example to yield larger amounts of antimicrobial distillate for a specified pathogen. Process optimization can enable the production of antimicrobial solutions for commercial purposes on an industrial scale and thus their use, for example in the food industry for controlling pathogenic microbes and improving microbial safety.
Based on the results of this study and others, the antimicrobial activity of the tested distillates is a combination of different chemical compounds with possibly different antimicrobial mechanisms. For example, Suresh et al. [23] observed that the antimicrobial effect of pH varied between groups of microbes. Pyroligneous acids at low pH were more effective against fungal strains than against bacterial strains, and the antibacterial efficacy was not only due to high acetic acid concentration [23]. In our study, the antimicrobial activity was stronger in almost all the fractions obtained from the torrefaction and pyrolysis steps than in acetic acid at the same dilution. Acetic acid appeared to be effective only against the Gram-positive microbe Listeria (Table 3). In addition, acetic acid has been observed to have antimicrobial activity against mold and rot fungi in palm wood samples [24]. A combination of the compounds may have the advantage of increasing the wide-spectrum antimicrobial effect of the liquids. Moreover, multi-chemical liquids may lower the probability of the development of resistance of the pathogenic bacteria to the liquids, as proposed by Suresh et al. [23]. However, chemical characterization of the distillates should be performed in order to reveal the underlying antimicrobial mechanisms, and to evaluate their safety if the distillates are utilized as antimicrobial agents in the food industry.
The formed inhibition zones in the agar diffusion method are indicators for estimating the antimicrobial activity of the pyrolysis distillates, and the results can only be compared between the tested samples. According to Holley and Patel [32], many phenolic-based compounds are poorly soluble in water, and in the agar diffusion test, the hydrophobicity of the phenolic compounds may prevent absorption of the compounds into the agar, hus inhibiting the growth of tested microbes. In our study, after checking different kinds of solvent compositions, the composition of acetone:ethanol:water (30:30:40) was selected for all samples to be used in experiments for assessing the antimicrobial activity in the agar diffusion method, although with some samples precipitation was observed. However, in order to maintain the comparability of the tests to compare antimicrobial activity between the distillate fractions, the solvent composition was kept the same during all tests. The analysis method differed when the Salmonella strain was applied because the samples were added to sterile discs, not directly to the wells, which may have affected the formation of inhibition zones. Therefore, the results cannot be compared between Salmonella and the other microbes. Furthermore, in some results, standard deviations were high due to the low number of test samples, and no statistical significances was observed between the samples and the control. As a result of all the above-mentioned factors influencing the tests, the results must be considered preliminary. Presumably, the mechanism of the antimicrobial activity is the sum of many factors, including for example the cell wall composition of the tested microbe and the solubility of the tested liquid. Thus, the activity of the distillates should also be evaluated using another test method, such as by measuring growth curves with Bioscreen C, and with higher numbers of test samples.
Finally, the demonstrated antimicrobial activity is a preliminary assessment of the potential to utilize pine distillates against pathogens in order to improve the microbiological safety of food or the food processing environment. Moreover, with the optimized pyrolysis equipment and using standardized agar test diffusion and Bioscreen C growth curve measurements, the research frame could also be applied to testing the efficacy of wood distillates against multi-resistant nosocomial infectious agents, such as methicillin-resistant Staphylococcus aureus (MRSA), or other pathogens endangering human health. Slow pyrolysis can be a useful biorefining tool to yield the required antimicrobial agents. However, the parameters of the pyrolysis process and the raw materials used both influence the chemical composition of the distillates, and thus also their antimicrobial and toxicological properties. Therefore, antimicrobial studies and safety evaluations, including characterization of chemical composition, should be performed carefully for each individual liquid before applications.
5. Conclusions
The pine wood-based distillates obtained from slow pyrolysis have antimicrobial activity against food-related pathogens, which could offer new possibilities to utilize pine wood by-products in controlling pathogenic microbes and in the future also improving food microbiological safety. Pyrolysis distillates from torrefaction and pyrolysis phases were observed to be more effective than acetic acid in our test which is assumed to be a result of the multi-chemical composition of the distillates. Thus, the combined and synergistic effect of pyrolysis compounds increases their antimicrobial activity. In addition, antimicrobial activity was also observed with highly diluted solutions. For example, MIC value of the fraction T2 for all tested microbes was 0.83% (v/v). Based on our results thermal conversion process could be utilized further in the production of antimicrobial liquids. The next step is to focus on optimizing the process depending on commercial potential which should be evaluated concerning the proposed target application. More research is needed to investigate the chemical composition of the pine wood-based pyrolysis liquids and assess their safe use.
Author Contributions
Conceptualization, J.K. and O.R.; methodology, J.K., O.R., E.K., K.R. (Kati Riekkinen), M.S. and T.V.; laboratory analysis, E.K., K.R. (Kati Riekkinen), M.S. and T.V.; resources, J.K. and O.R.; writing—original draft preparation, K.R. (Kati Riekkinen), K.R. (Kaisa Raninen) and M.S.; writing—review and editing, K.R. (Kati Riekkinen), K.R. (Kaisa Raninen) and J.K.; supervision, J.K. and O.R.; project administration, J.K. and O.R.; funding acquisition, J.K. and O.R. Thermal conversion process design and apparatus construction, M.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by TEKES, nowadays Business Finland. The laboratory experiments were conducted under the project Northern range and raw materials; characteristics and uses of wood-based thermal liquids, NORPYRO (decision number 70024/13).
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors thank Erkki Verkasalo, and Marja Roitto, (Natural Resources Institute Finland, Luke), who organized the collection of raw material samples for this research.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- FOREST EUROPE, 2020: State of Europe’s Forests. 2020. Available online: https://foresteurope.org/wp-content/uploads/2016/08/SoEF_2020.pdf (accessed on 17 March 2022).
- Kunttu, J.; Hurmekoski, E.; Heräjärvi, H.; Hujala, T.; Leskinen, P. Preferable utilisation patterns of wood product industries’ by-products in Finland. For. Policy Econ. 2020, 110, 101946. [Google Scholar] [CrossRef]
- Balat, M. Mechanisms of thermochemical biomass conversion processes. Part 1: Reactions of pyrolysis. Energy Sources Part A 2008, 30, 620–635. [Google Scholar] [CrossRef]
- Mohan, D.; Pittman, C.U.; Steele, P.H. Pyrolysis of wood/biomass for bio-oil: A critical review. Energy Fuels 2006, 20, 848–889. [Google Scholar] [CrossRef]
- Babu, B.V. Biomass pyrolysis: A state-of-the-art review. Biofuels Bioprod. Bioref. 2008, 2, 393–414. [Google Scholar] [CrossRef]
- Balat, M.; Balat, M.; Kirtay, E.; Balat, H. Main routes for the thermo-conversion of biomass into fuels and chemicals. Part 1: Pyrolysis systems. Energy Convers. Manag. 2009, 50, 3147–3157. [Google Scholar] [CrossRef]
- Lingbeck, J.M.; Cordero, P.; O’Bryan, C.A.; Johnson, M.G.; Ricke, S.C.; Crandall, P.G. Functionality of liquid smoke as an all-natural antimicrobial in food preservation. Meat Sci. 2014, 97, 197–206. [Google Scholar] [CrossRef]
- Ramakrishnan, S.; Moeller, P. Liquid smoke: Product of hardwood pyrolysis. Fuel Chem. Div. Prepr. 2002, 47, 366–367. [Google Scholar]
- de Wild, P. Biomass Pyrolysis for Chemicals. Ph.D. Thesis, Proefschrift, Rijksuniversiteit Groningen, Groningen, The Netherlands, 15 July 2011. [Google Scholar]
- Kamperidou, V. Chemical and structural characterization of poplar and black pine wood exposed to short thermal modification. Drv. Ind. 2021, 72, 155–167. [Google Scholar] [CrossRef]
- Akhtar, J.; Amin, S.N. A review on operating parameters for optimum liquid oil yield in biomass pyrolysis. Renew. Sustain. Energy Rev. 2012, 16, 5101–5109. [Google Scholar] [CrossRef]
- Luo, Z.; Wang, S.; Liao, Y.; Zhou, J.; Gu, Y.; Cen, K. Research on biomass fast pyrolysis for liquid fuel. Biomass Bioenergy 2004, 26, 455–462. [Google Scholar] [CrossRef]
- Miettinen, I.; Mäkinen, M.; Vilppo, T.; Jänis, J. Compositional characterization of phase-separated pine wood slow pyrolysis oil by negative-ion electrospray ionization fourier transform ion cyclotron resonance mass spectrometry. Energy Fuels 2015, 29, 1758–1765. [Google Scholar] [CrossRef]
- Havelaar, A.H.; Kirk, M.D.; Torgerson, P.R.; Gibb, H.J.; Hald, T.; Lake, R.J.; Praet, N.; Bellinger, D.C.; de Silva, N.R.; Gargouri, N.; et al. World Health Organization Global Estimates and Regional Comparisons of the Burden of Foodborne Disease in 2010. PLoS Med. 2015, 12, e1001923. [Google Scholar] [CrossRef] [Green Version]
- European Food Safety Authority. The European Union One Health 2018 Zoonoses Report. EFSA J. 2019, 17, 5926. [Google Scholar] [CrossRef] [Green Version]
- Heir, E.; Møretrø, T.; Simensen, A.; Langsrud, S. Listeria monocytogenes strains show large variations in competitive growth in mixed culture biofilms and suspensions with bacteria from food processing environments. Int. J. Food Microbiol. 2018, 275, 46–55. [Google Scholar] [CrossRef]
- Pang, X.; Wong, C.; Chung, H.-J.; Yuk, H.-G. Biofilm formation of Listeria monocytogenes and its resistance to quaternary ammonium compounds in a simulated salmon processing environment. Food Control 2019, 98, 200–208. [Google Scholar] [CrossRef]
- Skowron, K.; Wałecka-Zacharska, E.; Grudlewska, K.; Gajewski, P.; Wiktorczyk, N.; Wietlicka-Piszcz, M.; Dudek, A.; Skowron, K.J.; Gospodarek-Komkowska, E. Disinfectant susceptibility of biofilm formed by Listeria monocytogenes under selected environmental conditions. Microorganisms 2019, 7, 280. [Google Scholar] [CrossRef] [Green Version]
- Hernández, A.; Pérez-Nevado, F.; Ruiz-Moyano, S.; Serradilla, M.J.; Villalobos, M.C.; Martín, A.; Górdoba, M.G. Spoilage yeasts: What are the sources of contamination of foods and beverages? Int. J. Food Microbiol. 2018, 286, 98–110. [Google Scholar] [CrossRef]
- Lai, E.P.C.; Iqbal, Z.; Avis, T.J. Combating antimicrobial resistance in foodborne microorganisms. J. Food Prot. 2016, 79, 321–336. [Google Scholar] [CrossRef]
- Patra, J.K.; Hwang, H.; Choi, J.W.; Baek, K.-H. Bactericidal mechanism of bio-oil obtained from fast pyrolysis of Pinus densiflora against two foodborne pathogens, Bacillus cereus and Listeria monocytogenes. Foodborne Pathog. Dis. 2015, 12, 529–535. [Google Scholar] [CrossRef]
- Patra, J.K.; Das, G.; Choi, J.W.; Baek, K.-H. Antibacterial effects of pyrolysis oil against Salmonella Typhimurium and Escherichia coli. Foodborne Pathog. Dis. 2016, 13, 13–20. [Google Scholar] [CrossRef]
- Suresh, G.; Pakdel, H.; Rouissi, T.; Brar, S.K.; Fliss, I.; Roy, C. In vitro evaluation of antimicrobial efficacy of pyroligneous acid from softwood mixture. Biotechnol. Res. Innov. 2019, 3, 47–53. [Google Scholar] [CrossRef]
- Bahmani, M.; Schmidt, O.; Fathi, L.; Frühwald, A. Environment-friendly short-term protection of palm wood against mould and rot fungi. Wood Mater. Sci. Eng. 2016, 11, 239–247. [Google Scholar] [CrossRef]
- Bahmani, M.; Schmidt, O. Plant essential oils for environment-friendly protection of wood objects against fungi. Maderas Cienc. Y Tecnol. 2018, 20, 325–332. [Google Scholar] [CrossRef]
- Plumed-Ferrer, C.; Väkeväinen, K.; Komulainen, H.; Rautiainen, M.; Smeds, A.; Raitanen, J.-E.; Eklund, P.; Willför, S.; Alakomi, H.-L.; Saarela, M.; et al. The antimicrobial effects of wood-associated polyphenols on food pathogens and spoilage organisms. Int. J. Food Microbiol. 2013, 164, 99–107. [Google Scholar] [CrossRef]
- Verkasalo, E.; Möttönen, V.; Roitto, M.; Vepsäläinen, J.; Kumar, A.; Ilvesniemi, H.; Siwale, W.; Julkunen-Tiitto, R.; Raatikainen, O.; Sikanen, L. Extractives of stemwood and sawmill residues of scots pine (Pinus sylvestris L.) for biorefining in four climatic regions in Finland—Phenolic and resin acid compounds. Forests 2021, 12, 192. [Google Scholar] [CrossRef]
- Salami, A.; Raninen, K.; Heikkinen, J.; Tomppo, L.; Vilppo, T.; Selenius, M.; Raatikainen, O.; Lappalainen, R.; Vepsäläinen, J. Complementary chemical characterization of distillates obtained from industrial hemp hurds by thermal processing. Ind. Crops Prod. 2020, 155, 112760. [Google Scholar] [CrossRef]
- de Souza Araújo, E.; Pimenta, A.S.; Feijó, F.M.C.; Castro, R.V.O.; Fasciotti, M.; Monteiro, T.V.C.; de Lima, K.M.G. Antibacterial and antifungal activities of pyroligneous acid from wood of Eucalyptus urograndis and Mimosa tenuiflora. J. Appl. Microbiol. 2017, 124, 85–96. [Google Scholar] [CrossRef]
- Wei, Q.; Ma, X.; Dong, J. Preparation, chemical constituents and antimicrobial activity of pyroligneous acids from walnut tree branches. J. Anal. Appl. Pyrolysis 2010, 87, 24–28. [Google Scholar] [CrossRef]
- Lu, X.; Jiang, J.; He, J.; Sun, K.; Sun, Y. Effect of pyrolysis temperature on the characteristics of wood vinegar derived from Chinese fir waste: A comprehensive study on its growth regulation performance and mechanism. ACS Omega 2019, 4, 19054–19062. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holley, R.A.; Patel, D. Improvement in shelf-life and safety of perishable foods by plant essential oils and smoke antimicrobials. Food Microbiol. 2005, 22, 273–292. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).