Abstract
Planting native groundcover is often recommended to restore the understory of longleaf pine stands in the southeastern United States, but the effectiveness of such restoration activities remains poorly evaluated. We conducted a study in 25-year-old longleaf pine plantation stands in Georgia, USA, to examine the effects of seeding native groundcover on understory characteristics, fire behavior and soil properties. In 2015, four stands were seeded with five warm-season C4 grasses and a legume and four served as controls. In Fall 2020, we sampled the understory and analyzed soils collected from these stands, and in Spring 2021, fire behavior was evaluated. A total of 120 species were recorded in the understory across the stands, with the seeded species average foliar cover of 15%. There were no significant differences in species richness and Shannon diversity index of the seeded and control stands but understory species composition changed significantly. Soil properties and fire behavior during the prescribed fire also did not differ significantly between treatments, however, mean flame residence time was higher in seeded stands (108 s). Agricultural legacies of elevated soil P and old-field indicator species were prominent across stands. Overall, seeding had a minor effect on longleaf pine ecological characteristics in five years.
1. Introduction
Longleaf pine (Pinus palustris Mill.) ecosystems occupied approximately 37 million hectares in the southeastern United States before European settlement but now are reduced to only a fraction of the original extent, making it one of the most imperiled ecosystems in the United States [1,2]. These open-canopied, two-storied systems with distinct overstory and understory layers are among the most species-rich ecosystems in North America, containing high concentrations of endemic, threatened, and endangered species [3]. Most of the species diversity in these ecosystems is contributed by the herbaceous understory vegetation [3,4,5]. Species-rich understory also enhances habitat for local fauna [6] and is a source of fine fuel needed to carry surface fires that sustain these systems [4,7,8]. Groundcover-mediated frequent fire is key to maintaining characteristic open canopy structure, high herbaceous diversity and nutrient cycling in these ecosystems [9,10,11]. However, due to the reduction in their historical extent caused by agricultural land conversion, intensive pine plantations and fire exclusion [12], the species-rich understories in these ecosystems have been replaced, destroyed or degraded. Therefore, the restoration of these ecosystems is currently a high priority for forest management in the southeastern United States [12,13,14], including on the old agricultural lands [15,16].
While restoration of the overstory in these ecosystems can be accomplished by planting and silvicultural management [17,18,19], the restoration of a species-rich understory is a real challenge and is often attempted by thinning the stand and burning the understory at regular intervals, as the initial steps [20]. Additionally, the seeding of native species, such as C4 warm-season grasses and legumes, is also considered either to jumpstart or speed up the restoration process, especially in highly degraded sites such as the sites with agricultural legacy [15,17,21,22,23]. Planting of these native species is also supported by U.S. federal agencies, such as the Natural Resources Conservation Service for restoration purposes [24]. The effectiveness of seeding in restoring longleaf pine understory in sites that have been substantially altered, however, remains unknown. Currently, our understanding of how seeding with native C4 grasses and legumes might alter fire behavior and understory characteristics and their potential for additional effects on soil properties or other ecosystem properties remains limited [25].
Perennial C4 grasses and legumes are the dominant understory vegetation in longleaf pine savanna [26]. In the western outer coastal plain along the Gulf of Mexico and west of central Georgia, the bluestem grasses (Andropogon spp.) dominate the groundcover, whereas in the eastern coastal plains two varieties of wiregrass (Aristida beyrichiana Trin. & Rupr. and Aristida stricta Michx.) are more common [3]. Other than these native warm-season C4 grasses, common groundcover species in frequently burned longleaf pine ecosystems include herbaceous C3 plants belonging to the Asteraceae, Fabaceae, and Poaceae families, as well as some shrubs [10]. In comparison to C3 plants, C4 grasses are generally found in areas with elevated temperature and/or low or moderate precipitation [27]. These grasses also differ in carbon isotope (δ13C) composition: plants with a C3 photosynthetic pathway (C3 plants) have lower, more negative δ13C values than C4 plants [28]. The δ13C ratio of soil organic matter also reveals the contribution of C3 and C4 plants to primary productivity of a community [29]. When the vegetation composition is stable for an ecosystem, the upper soil profile (0–20 cm) demonstrates similar δ13C values as that of the dominant vegetation [30]. In addition, C4 grasses use nitrogen (N), phosphorus (P) and water more efficiently than C3 grasses, making them better adapted to warm, dry and nutrient-limiting conditions [31].
As the dominant component of grasslands and savanna ecosystems, warm-season C4 grasses and legumes serve important functional roles. Positive grass-fire feedback is commonly seen between warm-season C4 grasses and fires, wherein fire promotes the growth and survival of these grasses, and, in turn, these grasses promote frequent fires [32]. C4 grasses decompose slowly, resulting in the rapid accumulation of litter, which contributes to fine fuel during fire [32,33]. Groundcovers consisting mostly of grasses increase the rate of fire spread and decrease flame residence time (heating duration) [11,34], which help reduce encroachment by woody-species that suppress ground fire. Therefore, to restore fire-dependent longleaf pine ecosystems, the reestablishment of a native, diverse groundcover with dominance of perennial grasses, legumes and forbs is a necessary step [35,36].
Fire characteristics along with vegetation and agricultural legacies in old-field sites may influence nutrient cycling in longleaf pine woodlands [37,38]. For example, low-intensity fires may increase the availability of N and other nutrients only over the short term and may not have any significant effect over long-term [39,40,41]. Population of herbaceous legumes increases with frequent fires, increasing N inputs to the soil [42]. Along with fire, P availability regulates N-fixation by legumes [37]. Hence, P might may limit the productivity and growth of these forests through the modification of N-fixation [43]. In some post-agricultural sites that have undergone restoration, P might be elevated for years due to past fertilization while N and C may remain low [38]. Agricultural legacies in the soil such as elevated P might make it difficult to restore the native groundcover because native or non-native undesired species might take the benefit of nutrient rich soil condition and dominate the ecosystem over native species adapted to low-nutrient acidic soil conditions [44,45]. Improved understanding of soil chemistry effects on groundcover seeding success is critical to advance the science and practice for restoration ecology.
In this study, we examined the effects of native groundcover seeding (five warm-season C4 grass and one legume species) on understory characteristics, fire behavior and soil properties in thinned longleaf pine plantation stands. Our specific objectives included evaluating the effects of seeding on: (1) understory richness, diversity and composition; (2) air temperature, rate of fire spread and residence time during prescribed burn; and (3) total soil C, N and P and the changes in the soil isotopic signature of C and N (δ13C and δ15N) over time. We expected that the seeding of native C4 grasses and a legume will change understory species composition in these woodlands with higher species richness and diversity and that these changes will result in higher air temperature, fire rate of spread and lower flame residence time during prescribed fire. Additionally, we expected the groundcover changes to increase soil C, N, δ13C, δ15N and extractable P.
2. Materials and Methods
2.1. Study Site and Experimental Design
The study was conducted in 25-year-old planted longleaf pine stands at The Jones Center at Ichauway in southwest Georgia, USA (31°19′0″ N to 84°20′22″ W). The study area lies in the Dougherty Plain physiographic province in the Gulf of Mexico Coastal Plain in the southeastern United States. The Coastal Plain has a humid subtropical climate, and the study site in southwest Georgia has a mean annual temperature of 19.3 °C, and mean annual precipitation of 1370 mm [38]. The study site has an elevation of 50 m and is an irregular karst plain composed of flat, weekly dissected alluvial deposits over Ocala limestone [46]. Soils are fine to moderately fine textured sands with loamy or clayey subsoils, mostly belonging to the group paleudults, hapludults and some quartzipsamments [47].
The longleaf pine stands were established on the abandoned agricultural lands in 1996. The seedlings were hand planted with a planting density of 988 tree ha−1 at a spacing of 2.74 m × 3.66 m. Before stand establishment, the site was agricultural fields for more than 50 years, when peanut (Arachis hypogaea L.) was grown as the primary crop. The site had been tilled and repeatedly fertilized with phosphorus and potassium.
In 2014, eight plantation stands, 2–4 ha each, were mechanically thinned (third row thinning and removal of smaller trees and those with defects from within remaining rows), while retaining 50% of the original basal area. Due to the presence of pasture grasses such as Bahia grass (Paspalum notatum var. notatum) and Bermuda grass (Cynodon dactylon (L.) Persoon), herbicides were applied before seeding in 2015 [48,49]. All thinned longleaf pine stands were sprayed with 1120 g ha−1 Imazapyr (Arsenal) and 112 g ha−1 Metsulfuron (Escort). Raking was conducted with a grapple rake to clear the rows where trees were removed, and seeding was conducted using a seed drill (Truax Company, New Hope, Minnesota).
A randomized design with two treatments (seeded and control) and four replicates (stands) each was used. In 2015, four stands were seeded with five warm season C4 grasses: wiregrass (Aristida stricta Michx.), little bluestem (Schizachyrium scoparium [Michx.] Nash), yellow Indian grass (Sorghastrum nutans [L.] Nash), lopsided Indian grass (Sorghastrum secundum (Elliott) Nash), and switchgrass (Panicum virgatum L.) and the legume goat’s rue (Tephrosia virginiana (L.) Pers.). Four control stands were left untreated. Stands had been burned on a two-year cycle since 1999. After seeding in 2015, the stands were burned in March 2017, March 2019 and March 2021.
2.2. Data Collection
2.2.1. Stand Structure
Basal area, canopy openness and leaf area index (LAI) of the stands were estimated in 2013 (before thinning), 2014 (after thinning) and 2020 (five years after seeding). Basal area was estimated using a wedge prism with a basal area factor of 2.27 m2 ha−1. Canopy openness and LAI were estimated using Digital Hemispherical Photos obtained using a Nikon Coolpix 4500 camera (Nikon Inc., Melville, NY, USA) equipped with Nikon FC-E8 fisheye lens. Photos were taken in the center of soil sampling plots before sunrise on cloudless mornings in September 2020 [50] and processed using Image J and gap light analyzer 2.0 [51].
2.2.2. Groundcover Sampling
Groundcover sampling was performed in 2016, 2019 and 2020 to obtain the estimates of species richness, species diversity and species composition. In 2016 and 2019, data on the presence or absence of seeded species was collected using 25 1-m2 quadrats located in the rows where seeds were sowed. In 2020, 25 1-m2 quadrats were randomly laid out in each stand, and total foliar cover as well as foliar cover of individual species were visually estimated and assigned to one of the seven percentage cover classes, viz., 0–1%, 1–5%, 5–15%, 15–25%, 25–50%, 50–75% and 75–100% [52].
2.2.3. Fire Behavior
In each stand, we installed 25 thermocouples of 30-gauge chromel-alumel wire with a bead diameter of 0.4 mm (K-type wire, temperature range −260 to 1370 °C; Omega Engineering, Norwalk, Connecticut, USA). Each thermocouple was passed through a protective conduit. Conduit was attached to a metal pole bent at right angle, which was inserted into the ground. Thermocouples were connected to dataloggers (Hobo UX100-14M1or UX120-14M; Onset Computer Corp, Bourne, MA, USA) using K-type wire and connectors [53]. All measurements were taken at 0.5 m height to measure fire temperature above the understory vegetation [54].
A five-point square layout was used to measure rate of spread [55]. Thirteen thermocouples were deployed in the center of each stand, making a five-point square layout; inside the grid, right-angled isosceles triangles were used such that legs were equal to 20 m. Twelve thermocouples were deployed randomly. Thermocouples were installed one day before prescribed burning. The stands were burned in March 2021 using electric pump-driven drip torches on all-terrain vehicles. In each stand, a fire was ignited at the perimeter of the burn area so that fire fronts converged towards the center [56]. Datalogger recorded data at the frequency of 1 Hz.
2.2.4. Soil Nutrients and Isotopic Ratios
Soil samples were collected and analyzed in 2015, 2017 and 2020 to estimate total C, N, P, δ13C and δ15N. Each stand had 7–11 plots depending on the stand area. In each plot, samples were collected from subplots in the center and four cardinal directions (10 m from the center). At all five subplots, a metal ring of ~0.5 m2 was placed to collect fresh leaf litter and forest floor. Undecayed pine litter with needles in fascicles were considered fresh litter, and other leaf litter, pinecones and woody debris were considered forest floor. Soil at 0–10 cm, 10–20 cm and 20–50 cm depths were collected using a soil probe with a slide hammer. Soil in plots was combined by depth. The samples were dried at 70 °C for 48 h, passed through a 2 mm sieve, ground and milled. Approximately 70 (±0.1) mg of soil and 5 (±0.1) mg of plant tissue (leaf litter and forest floor) were sent to the University of California at Davis Lab for total C, total N, δ13C and δ15N analyses. For P analysis, soil samples were air-dried for 2 weeks and passed through a 2 mm sieve. Next, 20 ml of Mehlich 1 extraction solution (0.05 M HCl + 0.0125 M H2SO4) was added to 5 g of soil. The extraction was shaken for 5 minutes and filtered through Whatman 42 filter paper. An aliquot was run through an automated analysis system (Hach Lachat 8500 Series 2 Quick Chem; Hach Company, Loveland, CO, USA) at The Jones Center at Ichauway using the Quick Chem Method 12-115-01-1-N to obtain soil extractable phosphorus [57].
2.3. Statistical Analysis
2.3.1. Understory Characteristics
Both univariate and multivariate analysis were used to examine the differences in understory characteristics between seeded and control plots. The univariate analysis evaluated species richness and diversity, whereas the multivariate analysis examined the difference in community composition due to seeding.
Species richness was calculated as the number of species present in each 1 m2 plot, with mean species richness calculated at the stand level. Species diversity was estimated using Shannon’s Diversity Index, using cover estimates [58]. Linear mixed models were used to test for differences in species richness and diversity between treatments. Fixed effects were treatment and LAI, which was added as a co-variate. Higher LAI means lower canopy openness, which might affect understory characteristics because of lower light availability. Stand (n = 4) was classified as a random effect to account for site variation, and the 25 vegetation plots were nested within stand. The resulting statistical model was as follows:
(Species richness, diversity) ~ B0 + B1∙treatment + B2∙LAI
The lme4 package in the R statistical programming system was used to test the model [59]. The probability of a type-I error was set at α = 0.05 to test hypotheses throughout the analysis [60].
To evaluate species composition, we used non-metric multidimensional scaling (NMDS), permutational ANOVA (PERMANOVA) and indicator species analysis. NMDS was performed using Bray–Curtis distances calculated from the species abundance matrix relative to column totals using 3 dimensions and 100 iterations. To test the hypothesis that seeding warm season grasses and a legume influences plant community composition, PERMANOVA was performed. The analysis was performed using the vegan package for R (Oksanen et al., 2020). Indicator species analysis was performed using the indicspecies package for R [61].
2.3.2. Fire Behavior
We estimated mean air temperature (>40 °C), rate of spread and residence time (heating duration) during the prescribed fire. Fire rate of spread was calculated using a five-point triangle layout [55]. Residence time was calculated as the duration when the air temperature was >40 °C recorded by each thermocouple. Generalized linear mixed models including stand effect as random factors were used to test for treatment effects on air temperature, fire rate of spread and residence time. Because the data were positively skewed, a Gamma probability density function with identity link was used. The resulting model was as follows:
(Air temperature, fire rate of spread, residence time) ~ B0 + B1∙treatment
The lme4 package was used to test the model [59].
2.3.3. Soil Nutrients and Isotopic Ratios
We estimated mean isotopic signature for δ13C and δ15N and nutrient contents for C, N and P. The mean soil δ13C and δ15N ratios were calculated for forest floor, litter layer and mineral soil to examine the differences in their isotopic abundance for treated and control plots. We also analyzed the contribution of grasses and a legume to soil organic C and N inputs in soil by examining the stable isotopes. The IsoError spreadsheet was used to calculate the estimates of source proportional contributions of seeded groundcovers [62].
Linear mixed models (for normal data) and generalized mixed models (for non-normal data) were used to test for an effect on soil properties due to the warm-season grass planting treatment. In the mixed models, treatment, (seeding/control) year and soil depth (including forest floor and litter) were fixed effects, whereas the replications (stands) were random effects. The final model was as follows:
(δ13C, δ15N, C, N, P) ~ B0 + B1∙treatment + B2∙depth + B3∙year
The lme4 package was used to test the model [59]. The ANOVA function from the car package was used to obtain p values and 95% confidence intervals for parameters [63].
3. Results
3.1. Stand Structure
The average basal area of the seeded and control stands was 19.97 m2 ha−1 and 22.11 m2 ha−1, respectively, before thinning in 2014. After thinning, the basal area for the seeded stands and the control stands was 12.29 m2 ha−1 and 10.24 m2 ha−1, respectively, in 2014 and 13.64 m2 ha−1 and 12.82 m2 ha−1, respectively, in 2020. The mean LAI (±standard error) in the seeded and control stands was 1.80 (±0.19) and 2.01 (±0.06) in 2013, 0.999 (±0.04) and 0.808 (±0.05) in 2014 and 1.14 (±0.11) and 1.16 (±0.09) in 2020 for seeded and control plots, respectively. The canopy openness was 31% in both the seeded and control plots.
3.2. Understory Characteristics
3.2.1. Status of Seeded Species
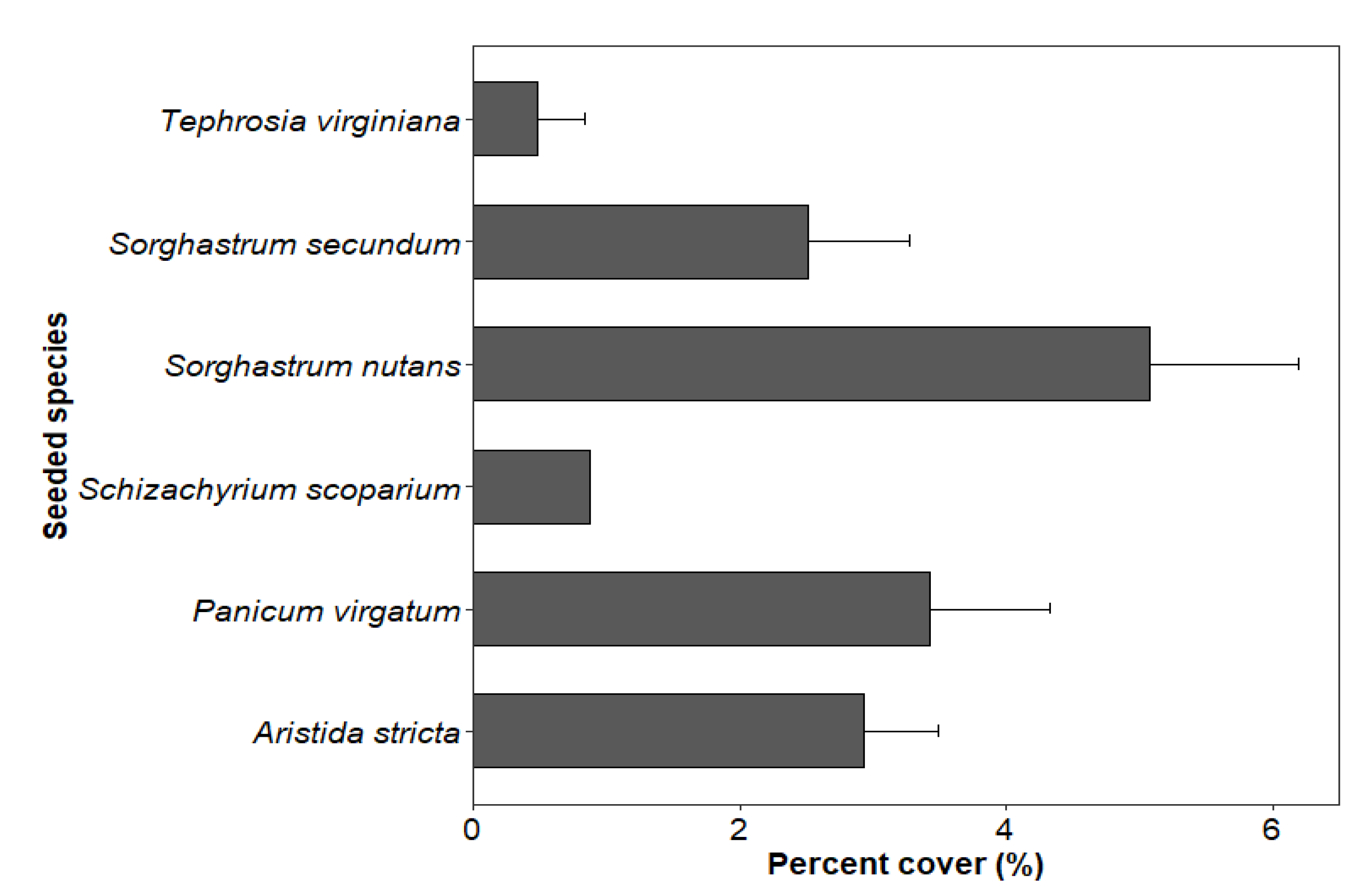
The mean percent cover of seeded species in the seeded treatment was 12.86%, 17.17% and 15.31% in 2016, 2019 and 2020, respectively. However, the percent cover of seeded species was only 1.70% in the control stands in 2020. Among the six planted species, the mean percent cover ± standard error was highest for Sorghastrum nutans (5.08 ± 1.11) followed by Panicum virgatum (3.42 ± 0.90), Aristida stricta (2.93 ± 0.56), Sorghastrum secundm (2.52 ± 0.76), Schizachyrium scoparium (0.88) and Tephrosia virginiana (0.48 ± 0.36) in seeded stands in 2020 (Figure 1).
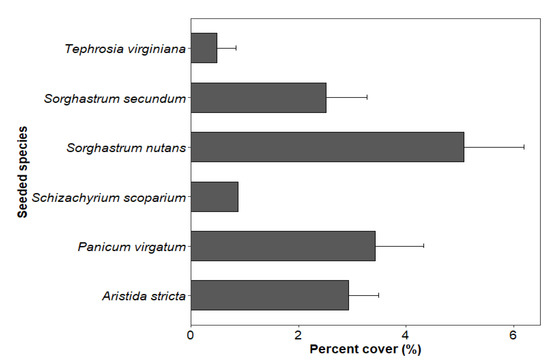
Figure 1.
Percent cover (mean ± standard error) of six groundcover species seeded in four longleaf pine stands in southwest Georgia in 2020 (five years after seeding treatment).
3.2.2. Plant Species Richness and Diversity
A total of 120 species and 46 families were observed across the study stands. The mean species richness in the seeded and control stands was 11.28 (standard error = 0.29) and 10.34 (0.29) species m−2, respectively, which was not a significant difference (p = 0.21). LAI had no effect on species richness (p = 0.63). The random effect (stand) accounted for only 10% of variance in the model, whereas residual variance was 89%. Ninety-seven species occurred in seeded plots, and sand blackberry (Rubus cuneifolius L.H. Bailey) occurred in more plots (93 plots) than any other species. In the control plots, 90 species were found, and the stands were dominated by three-seeded mercury (Acalypha gracilens A. Gray) followed by sand blackberry. Sixty-seven species were common to seeded and control plots. The Shannon Diversity Index ranged from 1.70 to 1.79 in the seeded stands and 1.55 to 1.82 in control stands, with no significant difference (p = 0.40; Table 1).

Table 1.
Understory species richness and diversity (means with standard error in parenthesis) in planted longleaf pine stands that were thinned and seeded with warm-season grasses and a legume. p values are from mixed model analysis.
3.2.3. Community Species Composition
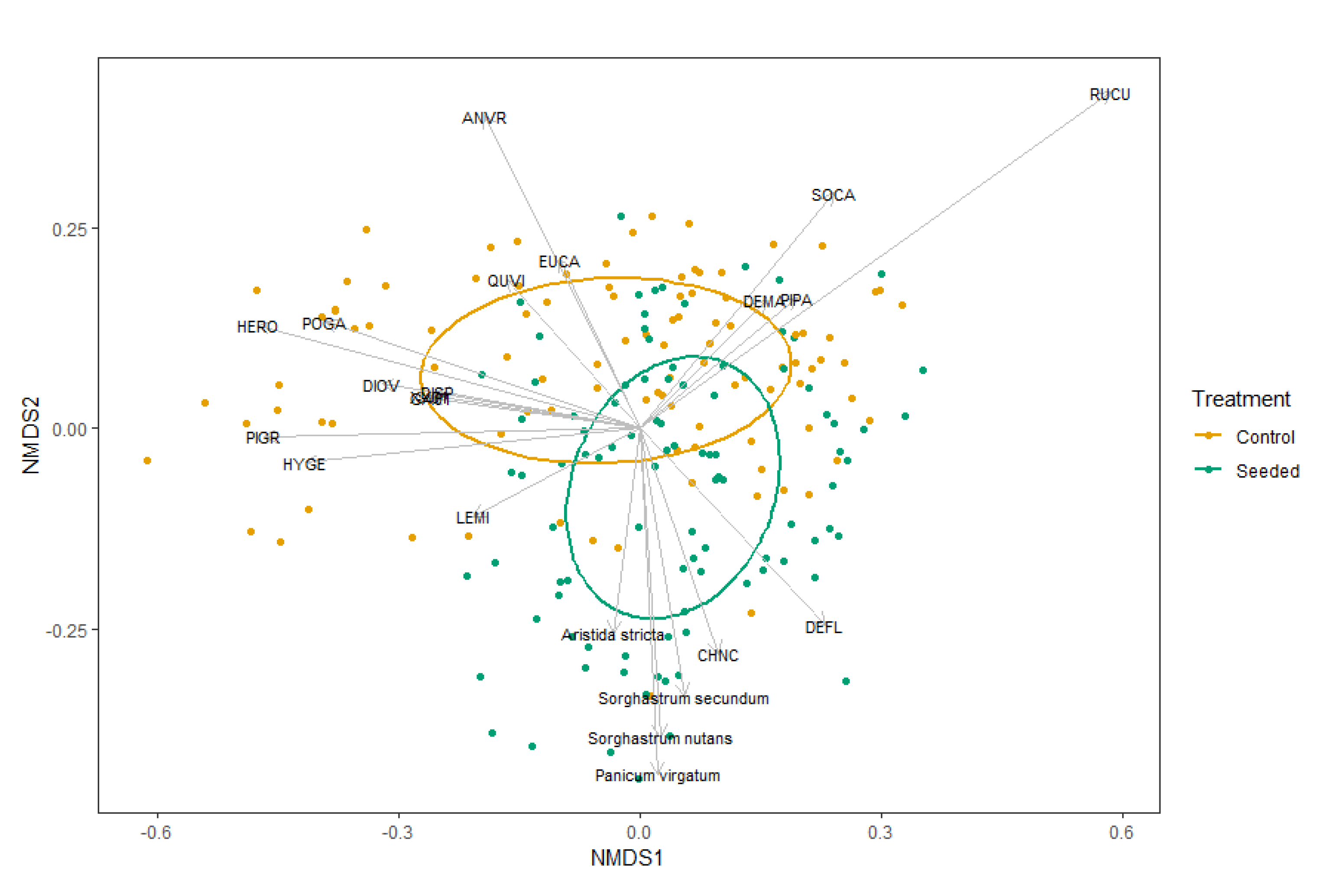
The community species composition varied significantly between the seeded and control stands in abundance (p = 0.001; PERMANOVA test). The NMDS indicated that four seeded grasses, SONU, SOSE, ARST and PAVI, were significant species vectors across the seeded stands (acronyms defined in Figure 2). Another warm-season C4 grass (ANVR) also appeared as a significant species vector across the control stands. The indicator species analysis revealed that nine species, five of them seeded, were classified as indicator species in the seeded stands: SONU, RISC, CHNC, PAVI, SOSE, ARST, DEVI, PAQU and TEVI. Eleven species were classified as indicator species in the control stands: HERO, PIPA, POGA, HYGE, DIOV, PANO, EUCA, PIGR, ANTE, QUVI and GA01.
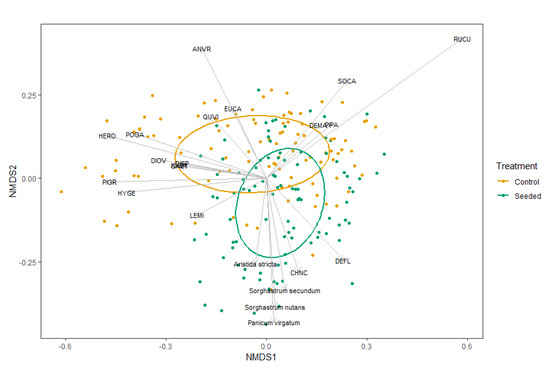
Figure 2.
NMDS based on cover in seeded and control longleaf pine stands with vectors correlating percent cover of each species. Labeled species are highly correlated with Axes 1 or 2 both (p < 0.01). ANVR—Andropogon virginicus; EUCA—Eupatorium capillifolium; QUVI—Quercus virginiana; SOCA—Solidago canadensis; DEMA—Desmodium marilandicum; PIPA—Pinus palustris; RUCU—Rubus cuneifolius; DISP—Dicanthelium sphaerocarpon; POGA—Polygonella gracilis; DIOV—Dicanthelium ovale; PIGR—Pityopsis graminifolia; HYGE—Hypericum gentianides; LEMI—Lechea minor; CHNC—Chamaecrista nictitans; DEFL—Desmodium floridanum; GA01—Gamochaeta species.
3.3. Fire Behavior
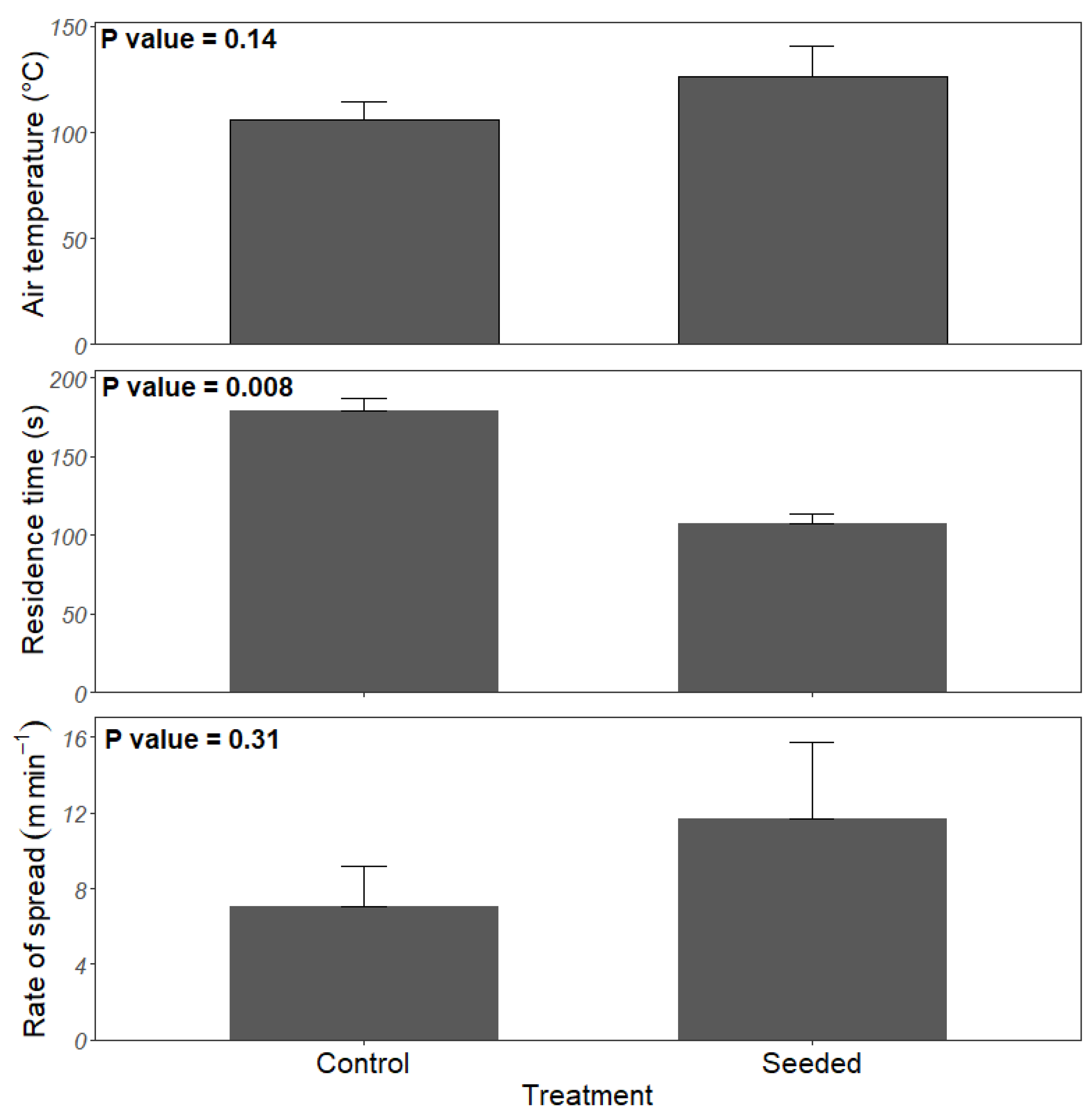
During the prescribed fire, the mean air temperature was 126.8 (standard error = 14) °C in seeded stands and 105.5 (9.0) °C in the control stands, but these values were not significantly different (p = 0.14) (Figure 3). The fire’s rate of spread ranged from 2.97–22.2 m min−1 and 3.18–11.9 m min−1 in the seeded and control stands, respectively. The mean rate of spread in the seeded and control stands was 11.7 (3.98) m min−1 and 7.08 (2.08) m min−1, respectively, which did not differ significantly (p = 0.312) (Figure 3). The residence time ranged from 78 s to 157 s in seeded stands with a mean of 108 (±17) seconds, whereas it ranged from 153 s to 218 s in the control with a mean of 179 (±14.5) seconds (Figure 3).
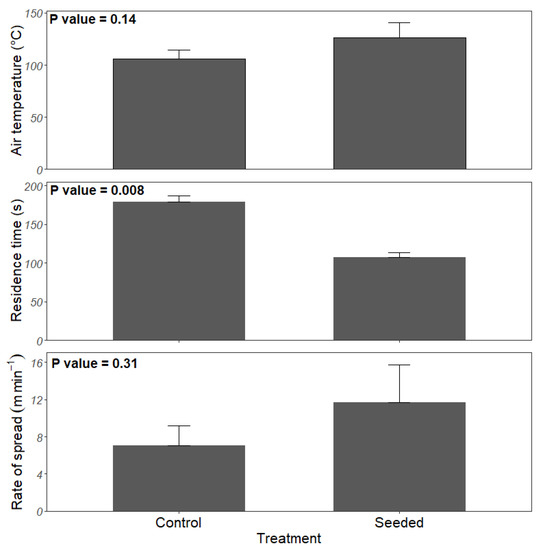
Figure 3.
Mean air temperature at 0.5 m, residence time and rate of spread during prescribed fire in planted longleaf pine stands with experimental warm-season grass and legume seeding in southwest Georgia. Narrow bars represent the standard error.
3.4. Nutrients and Isotopic Signatures Analysis
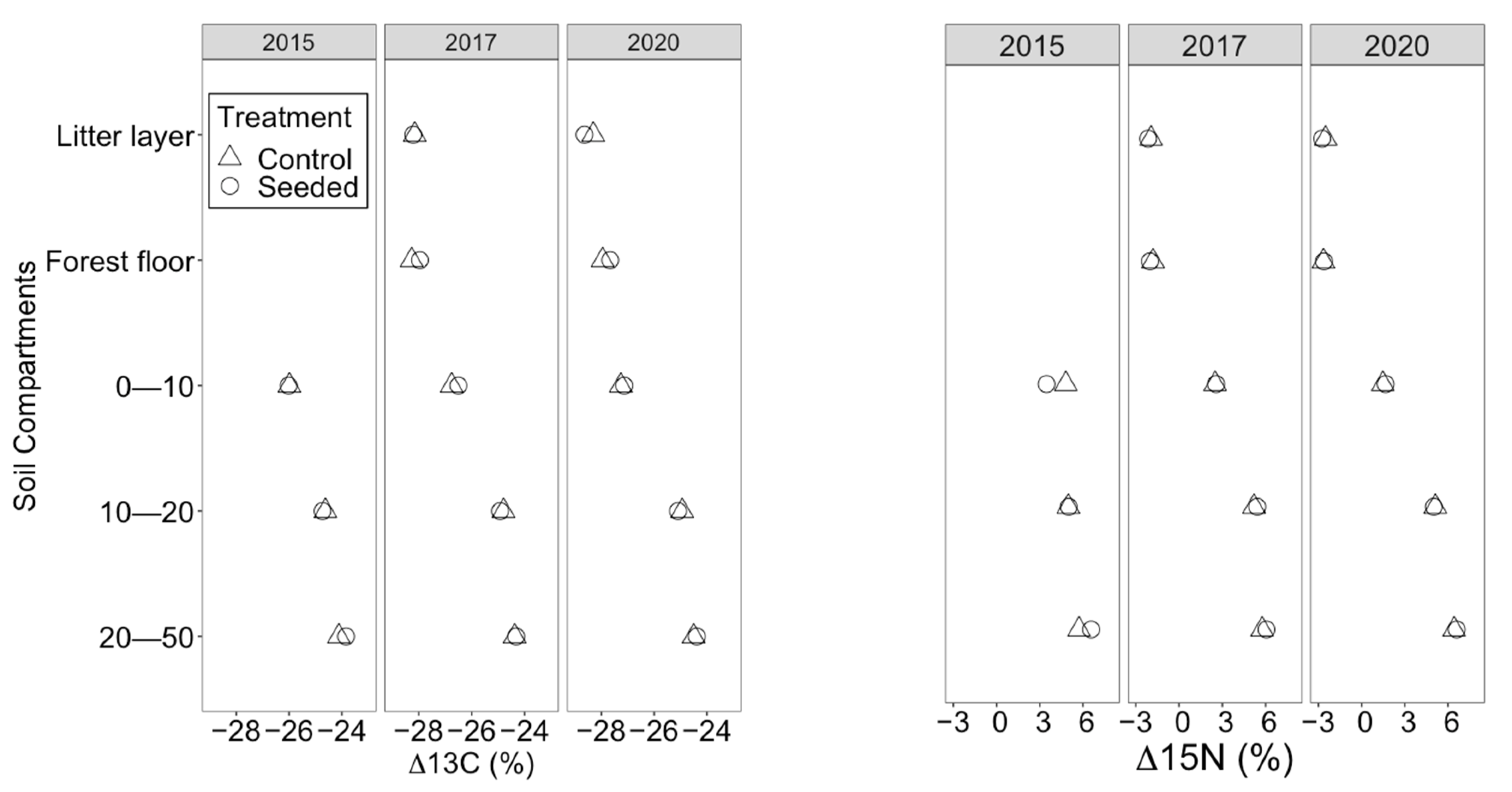
Five years after seeding, mineral soil C (± standard error) and N were similar between the treated (8.4 ± 3.28 g kg−1 C, 0.34 ± 0.1 g kg−1 N) and control stands (6.87 ± 2.47 g kg−1 C, 0.28 ± 0.1 g kg−1 N); however, they differed significantly among the soil depths and over time (Table 2 and Table 3). Soil C and N were highest at a 0–10 cm soil depth, followed by a 10–20 cm soil depth and then the 20–50 cm soil depth (p < 0.05). In 2020, both C and N were higher than in 2017 and 2015. The nutrient content did not differ significantly between 2015 and 2017 (p > 0.05). The soil isotope signatures (δ13C and δ15N) showed no significant changes after the seeding treatment. Nevertheless, they differed significantly among soil depths and over time. In contrast to soil C and N, which decreased with depth, δ13C and δ15N increased significantly with the soil depth (Figure 4). δ13C and δ15N were significantly lower in 2020 than in 2015 and 2017 (Table 2). Seeding warm-season C4 grasses only contributed 0.23% to the total soil organic C, and there was no detectable contribution of the seeded legume to the N content in soil organic matter.

Table 2.
Estimates (mean ± standard error) of C and N in mineral soil (0–50 cm) and organic soil horizon (forest floor and litter) of seeded and control longleaf pine stands in southwest Georgia.

Table 3.
Mixed model and ANOVA results of mineral soil C, N, δ13C and δ15N in seeded and control treatments of planted and thinned longleaf pine stands in 2015, 2017 and 2020. Soil depths analyzed were 0–10 cm, 10–20 cm and 20–50 cm.
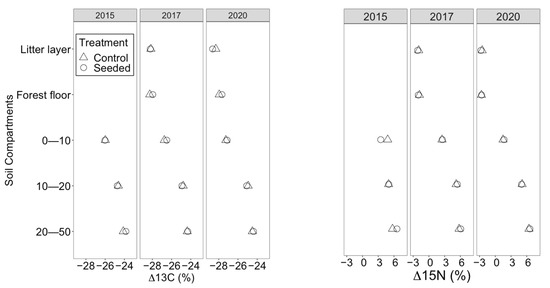
Figure 4.
Mean δ13C and δ15N in seeded and control longleaf pine stands. Three soil depths 0–10 cm, 10–20 cm and 20–50 cm along with plant samples (forest floor and litter) were analyzed. Error bars are removed as they are very small.
There was also no significant difference in organic soil C and N content between seeded and control stands (Table 4). The litter layer had significantly higher C than the forest floor in 2017 and 2020, but the N content differed. In 2017, N was significantly higher in the litter layer, but in 2020, it was significantly higher in the forest floor. Both C and N were significantly higher in 2020 than in 2017 (Table 2). δ13C and δ15N also differed between plant layers. δ13C was significantly higher in forest floor than the litter layer but was similar in 2017 and 2020. In contrast, δ15N was significantly higher in 2017 but did not differ between the forest floor and the litter layer.

Table 4.
Mixed model and ANOVA analysis of plant (litter and forest floor) C, N, δ13C and δ15N in the seeded and control stands of longleaf pine forest in 2015, 2017 and 2020.
Phosphorus Analysis
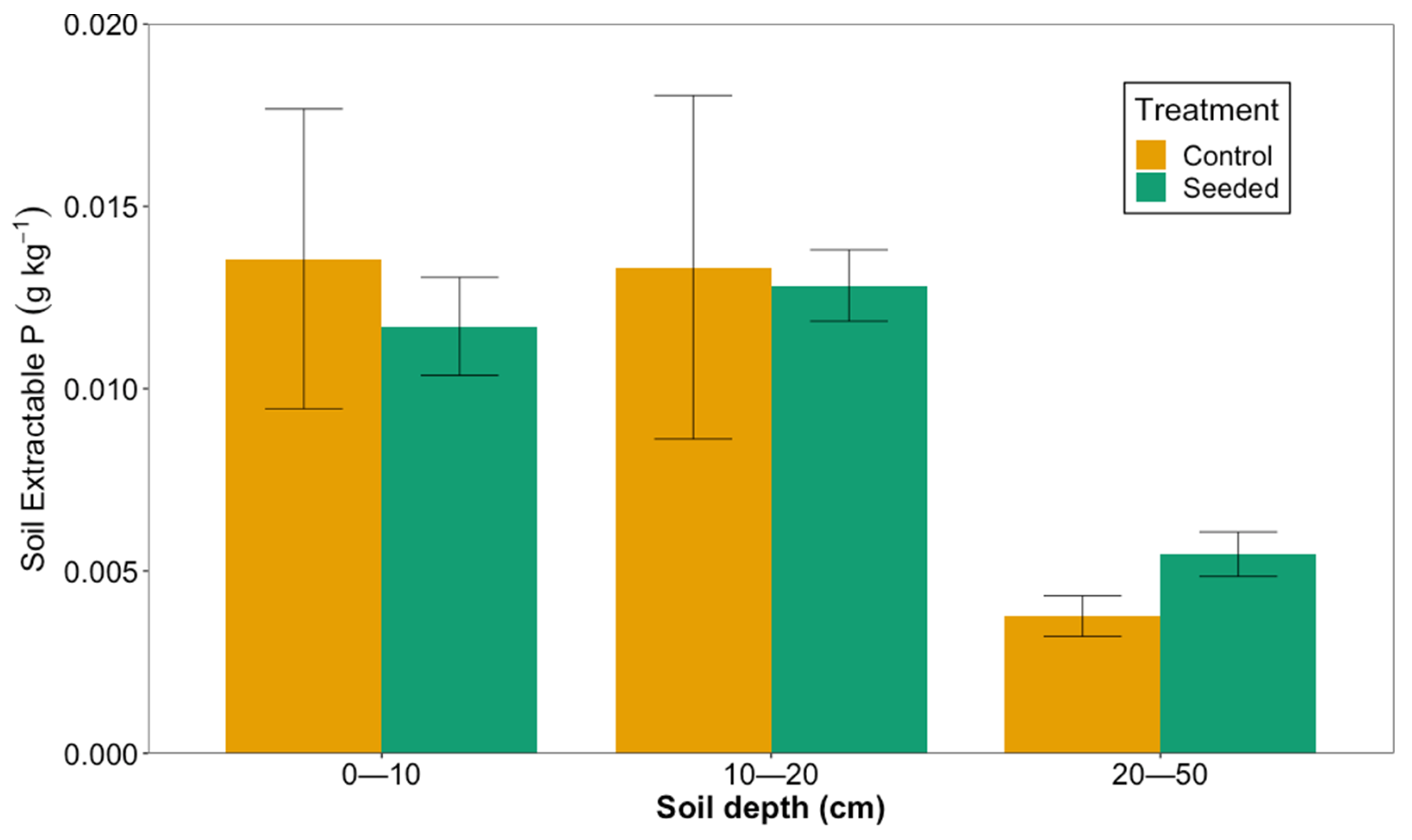
There was no difference in soil-extractable (Mehlich 1) P between the seeded and control stands (generalized mixed model, p = 0.39), but there were differences among soil depths. Soil-extractable P was similar at the 0–10 cm and 10–20 cm soil depths. At the 0–10 cm depth, the mean soil-extractable P (±standard error) was 0.0117 (0.0013) g kg−1 and 0.0136 (0.0041) g kg−1 in the seeded and control stands, respectively. At the 10–20 cm depth, the extractable P was 0.0128 (0.001) g kg−1 in the seeded stands and 0.0133 (0.0047) g kg−1 in the control stands. However, at the 20–50 cm depth, the soil-extractable P was significantly lower than at the two upper soil depths: 0.00546 (0.0006) g kg−1 in the seeded stands and 0.0038 (0.0006) g kg−1 in the control stands (p < 0.05; Figure 5).
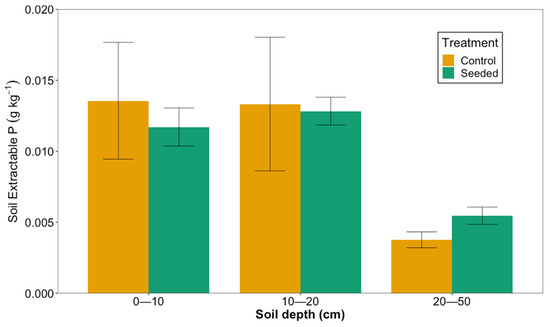
Figure 5.
Soil-extractable P in seeded and control stands at three depths in longleaf pine stands planted on former agricultural fields in southwest Georgia in 2020.
4. Discussion
This study found that seeding of thinned post-agriculture longleaf pine stands by six native species resulted into only a few significant changes in understory, soils or fire characteristics in five years. There was some indication that community species composition in the seeded stands shifted towards greater proportion of seeded warm-season C4 grasses compared to control stands, which were still dominated by C3 plants and non-seeded C4 pasture grasses. Flame residence time was shorter in the seeded stands, possibly signifying the greater presence of the seeded C4 grasses. However, seedings had no effect on understory species richness and diversity, air temperature and rate of spread during prescribed fire or soil nutrients and isotopic signatures.
Restoring a savanna ecosystem requires an amalgamation of several approaches, such as frequent burning, thinning, planting pine, understory seeding and herbicide application based on the objective of restoration [20,64]. In this study, both seeded and control stands received fire every two years and were thinned at the same time. While seeding may hasten the restoration process, just thinning and prescribed fire could promote species richness and diverse species composition in post-agricultural longleaf pine woodlands [65]. In our study as well, it is possible that the effect of these two treatments (thinning and burning) have been more significant than the seeding treatment on species richness, fire and soil characteristics. In addition, it is possible that five years—arguably a modest duration in restoration projects to expect positive outcomes—might not be a long enough time to witness substantial changes in the ecosystem properties of longleaf forests, especially considering the existence of agricultural legacies on the study site [38] and/or that the management of these stands might not have optimized the growth of seeded species. For example, natural longleaf pine woodlands usually have canopy openness of >50% [50], and savanna grasses thrive in high- to full-sun conditions [66]. However, canopy openness in our study’s stands were only about 31%, despite heavy thinning. While thinning guidelines for southern pines recommend removing 30%–45% of the basal area with an approximate residual basal area of ~17 m2 ha−1 for sawtimber management [67], the residual basal area in this study stands was further reduced to 13 m2 ha−1 to encourage groundcover development. Likely due to the low initial tree planting density of 988 ha−1 (400 acre−1), the longleaf pine carried wide branches and crowns in our study stands, contributing to the low canopy openness even after heavy thinning. Further reduction in the residual basal area to allow for greater understory light availability could have resulted in greater groundcover changes.
4.1. Understory Characteristics
While the seeded and control stands did not differ from each other for most response variables, both stand conditions ranked substantially lower when compared to a nearby natural longleaf pine reference site. The total number of species observed in our seeded and control plots (120) was lower than the 134 species reported in nearby natural longleaf pine woodlands [68]. Species richness in our study stands, at the 1 m2 scale, was less than half that that in reference sites [68]. Similarly, Shannon Diversity Index in our seeded and control stands was also lower than reported in natural longleaf pine woodlands, e.g., [69]. Agricultural legacies could be one critical factor that resulted in poor community metrics in our study stands when compared to reference sites. It is well reported that agricultural land use history of a restoration longleaf pine site influences its plant composition by decreasing herbaceous diversity [15,70]. It is also suggested that, in post-agricultural woodlands, transplanting of native seedlings might be a better approach to restore groundcover than seed sowing, although transplanting could be more intensive and expensive than seeding [4]. In a study conducted in a longleaf pine ecosystem of South Carolina, seedlings of 32 native groundcover species were planted. After four years, the mean percent cover of planted species was 25.9%, compared to 14% observed in our seeded plots after five years [71].
Our NMDS ordination clearly showed that the seeded species were significant species vectors across the seeded plots, whereas they were not significant across control plots. Hedman (2000) reported eight indicator species in an intact longleaf pine forest, located about 50 km from our study site, of which two (ARST and TEVI) were indicator species in our seeded plots as well [70]. However, none of the species listed by Hedman (2000) were indicator species in our control plots. Most of the indicator species found in both seeded and control plots were indicative of post-agricultural woodlands; only three of the seeded species were representative of natural woodlands. Brudvig et al. (2013) found 44 indicator species in natural woodlands, of which 3 (TEVI, SOSE, SONU) were indicators found in our seeded stands and none were indicators of control stands. In post-agricultural stands [15]. They found 54 indicator species, out of which 1 (CHNC) was an indicator of our seeded stands and 5 (PIGR, DIOV, HERO, HYGE, ANTE) were indicators of control stands. Hence, our results suggest that community composition of seeded stands showed greater resemblance to natural longleaf pine woodlands than control stands.
4.2. Fire Behavior
In C4 grasslands and savanna, high light availability and low soil N due to frequent burning favor 4 grasses over their 3 counterparts, and in turn these pyrogenic grasses promote fire [72]. Positive feedback exists between these C4 grasses and fire behavior [32]. In this study, however, fire behavior, including air temperature and rate of spread during prescribed fire, was not altered by seeding C4 grasses in the seeded stands. Three reasons might be responsible for this. First, the percent cover of seeded grasses was only 15% in the seeded stands, which is low. Second, the percent cover of C4 grasses was the same in seeded (11.8%) and control (10.6%) stands. Third, stand variation was very high for fire rate of spread and fluctuated greatly within stands.
In our previous study, we found that the mean air temperature during prescribed fire increased from 114 °C to 148 °C with an increase in wiregrass cover from 0 to 100% [53]. In this study, the mean air temperature was 106 °C with the presence of 7% graminoids in control stands and 127 °C in the presence of 9% graminoids in the seeded stands. Mean fire rate of spread in the seeded (0.19 m s−1) and control stands (0.11 m s−1) was low when compared to study by Wragg et al. (2018) [11]. They stated that the stands with only grasses had fire spreading at the rate of 0.8 m s−1. Considering our plots had all functional groups including trees present with no significant difference in percent cover, it is plausible to have a lower rate of spread. Moreover, Wragg et al. (2018) sites did not have trees contributing to a lower rate of speed.
In contrast with air temperature and rate of spread, residence time during prescribed fire was significantly lower in the seeded plots compared to control. Our results are consistent with Loudermilk et al. (2014) who found that grasses tend to decrease flame residence time. Residence time might be the determining factor for understory mortality because increased residence time might kill understory plants that tend to suppress fire, like hardwoods [7,73]. Grasses demonstrate similar heat flux as pine litter with shorter residence time, thus killing hardwoods even at shorter heating duration [74,75]. Although, we did not detect a difference in air temperature during prescribed fire, shorter residence time suggests the positive effect of seeding on fire characteristics of seeded stands. Therefore, the heterogeneity of fuel types (including pine and grass litter) might be important in determining understory characteristics [34].
4.3. Soil Nutrients and Isotopic Ratios
Plant density and composition play an important role in ecosystem soil nutrient cycling [76]. Complementary interactions between C4 grasses and legumes can increase soil C and N accumulations through enhanced N inputs to the soil by legumes and N uptake by C4 grasses, which enables them to expand their root biomass, resulting in increased soil C concentration [35,77]. In the N-limited grasslands of Minnesota, C4 grasses and legumes increased soil C accumulation by 193% and 522%, respectively [35]. In this study, seeding C4 grasses and a legume did not significantly change soil C, N, extractable P, δ13C or δ15N signatures. We did not see any major change due to seeding, which could be because the cover of groundcover species we planted was too low to alter soil properties or that five years is not a long enough time to cause significant changes in soil characteristics [38]. However, response variables did differ significantly between soil depths and over time.
In general, post-agricultural longleaf pine woodlands are characterized by nutrient-limited soils with low soil C and N concentrations [38]. According to Ike (2010) [71], soil C concentration follows the following order: recently abandoned agricultural field < young plantation < older plantation < reference longleaf pine savanna. In contrast, soil P can be substantially higher in post-agricultural woodlands than undisturbed natural woodlands [15]. Our study has similar findings for C, N and P in 0–50 cm soil depth. Soil C concentration of 0.8% in the seeded stands was less than half that in neighboring natural stands (2.19%; [38]). Soil N was also slightly lower in the seeded (0.03%) and control stands (0.029%) of this study compared with typical natural stands (0.06%). Because all stands were on post-agricultural land, they are likely to have less soil C and N than stands with intact native groundcover.
After 14 years of pine plantation growth, the soil-extractable P of 113 kg ha−1 was significantly higher than P of 6 kg ha−1 for never-tilled natural longleaf pine stands at 0–50 cm soil depth [38]. Twenty-five years after longleaf plantation establishment, we found three times greater extractable P (19.6 kg ha−1) than in the never-tilled stand of Markewitz et al. (2002; [38]). This excess P might favor agricultural weeds rather than native savanna groundcover [44]. The study sites were burned every two years after pine plantation establishment and were fertilized annually using phosphorus for decades before that. Hence, the total C and N content was much lower than the nearby longleaf pine woodlands, and soil-extractable P was much higher. However, P was low compared to 14-year-old plantation stands studied by Markewitz et al. (2002; [38]). Because our stands were 25 years old, it is possible that this discrepancy is due to a gradual decline in P in the post-agricultural lands. However, there are contrasting views on the persistence of these agricultural legacies. Soil P usually declines after fertilization ceases; however, it can remain elevated for hundreds of years, making it difficult to restore an ecosystem [15,78]. In comparatively undisturbed forests, there is a decreasing trend of C and N concentration with increasing depth. Our results are consistent with previous studies where soil C, N and P decreased with soil depth and were higher in forest floor and litter [38,79].
The results for δ15N are consistent with Knoepp et al. (2015), who found no change in mineral or organic soil δ13C ratio after the addition of legumes. δ13C and δ15N increased with soil depth in seeded and control stands, which is supported by previous studies [79,80]. An isotope mixing model estimated a contribution of 0.23% soil organic C from the presence of C4 grasses and no contribution from a seeded legume, indicating no significant changes due to seeding. The δ13C ratio of seeded stands was almost 15% less than that of C4 grasses (−12%). However, it was close to the δ13C ratio of C3 plants, which is approximately −26%, indicating that seeding had not changed the soil isotopic signatures [81,82].
It is unclear how much the agricultural legacy in our study affected groundcover restoration, but how to deal with these legacies is a vexing question for restorationists, as it can be difficult to isolate the soil factors that present the greatest barriers to native ground establishment [83]. If such barriers include excesses of soil nutrients, there may be few options available for depleting the nutrients within short timeframes, as studied here. When a range of potential restoration sites are available, to avoid the effects of agricultural legacies, longleaf pine restoration activities should be carried out in sites that have not been cultivated in the past 50–60 years because such sites have greater capacity to sustain native groundcover [70]. Restoration is more likely to succeed if the land use history is known and prescriptions developed to account for its effects during the re-establishment of native species [65]. Site evaluations should include soil chemistry panels [84].
5. Conclusions
This study examined the effects of groundcover seeding on three fundamental ecosystem properties, viz., understory characteristics, soils properties and fire characteristics, of planted longleaf pine stands after five years. Seeding C4 grasses and a legume in post-agricultural woodlands increased their prevalence and changed community species composition in the seeded stands, but no significant differences in species richness, diversity, air temperature and rate of spread during prescribed fire or soil nutrients and isotopic signatures were detected. However, we observed a positive trend in fire behavior with significantly lower fire residence time in the seeded stands. Further increasing canopy openness could create a feedback loop with burning intensity and increase the abundance of these grasses. Agricultural legacies were notable making it slow to restore the ecosystem. For warm season C4 grasses to affect ecosystem properties, they must be present in sufficient abundance, and our study suggests that further long-term research is needed on establishing a thriving native groundcover in the context of a mid-rotation longleaf pine stand.
Author Contributions
Conceptualization, B.B., S.W.B. and A.S.; data curation, B.B.; formal analysis, B.B.; funding acquisition, A.S.; investigation, B.B. and S.T.; methodology, B.B., S.W.B., A.S. and J.G.V.; project administration, A.S.; resources, A.S. and S.T.B.; supervision, S.W.B. and A.S.; validation, S.W.B. and S.T.B.; visualization, B.B.; writing—original draft, B.B.; writing—review and editing, S.W.B., A.S., S.T., J.G.V. and S.T.B. All authors have read and agreed to the published version of the manuscript.
Funding
The work upon which this publication is based was funded partially by the USDA NIFA McIntire Stennis project #1014653 and The Jones Center at Ichauway award # AWD06066.
Data Availability Statement
Data and associated codes are available from the corresponding authors upon reasonable request.
Acknowledgments
We thank Steven Morrone, Katherine A. Russell, Steven B. Jack, Lindsay R. Boring, Lisa M. Giencke, Bryan Cloninger, Scott Smith, Andrew Whelan, O. Stribling Stuber and Mark Melvin for their time, insights and assistance during this study.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Frost, C.C. Four centuries of changing landscape patterns in the longleaf pine ecosystem. In Proceedings of the Tall Timbers Fire Ecology Conference; Tall Timbers Research Station: Tallahassee, FL, USA, 1993; Volume 18, pp. 17–43. [Google Scholar]
- Noss, R.F. Longleaf pine and wiregrass: Keystone components of an endangered ecosystem. Nat. Areas J. 1989, 9, 211–213. [Google Scholar]
- Peet, R.K.; Allard, D.J. Longleaf pine vegetation of the Southern Atlantic and Eastern Gulf Coast Regions: A preliminary classification. In Proceedings of the Tall Timbers Fire Ecology Conference; Tall Timbers Research Station: Tallahassee, FL, USA, 1993; Volume 18, pp. 45–81. [Google Scholar]
- Walker, J.L.; Silletti, A.M. Restoring the ground layer of longleaf pine ecosystems. In The Longleaf Pine Ecosystem; Springer: Berlin/Heidelberg, Germany, 2007; pp. 297–333. [Google Scholar]
- Sharma, A.; Bohn, K.K.; Jose, S.; Miller, D.L. Seed Bank—Vegetation dynamics along a restoration management gradient in pine flatwoods ecosystems of the Florida Gulf Coast. Nat. Areas J. 2018, 38, 26–43. [Google Scholar] [CrossRef]
- Means, D.B. Vertebrate faunal diversity of longleaf pine ecosystems. In The Longleaf Pine Ecosystem; Springer: Berlin/Heidelberg, Germany, 2007; pp. 157–213. [Google Scholar]
- Mitchell, R.J.; Hiers, J.K.; O’Brien, J.; Starr, G. Ecological forestry in the Southeast: Understanding the ecology of fuels. J. For. 2009, 107, 391–397. [Google Scholar]
- Ellair, D.P.; Platt, W.J. Fuel composition influences fire characteristics and understorey hardwoods in pine savanna. J. Ecol. 2013, 101, 192–201. [Google Scholar] [CrossRef]
- Dell, J.E.; Richards, L.A.; O’Brien, J.J.; Loudermilk, E.L.; Hudak, A.T.; Pokswinski, S.M.; Bright, B.C.; Hiers, J.K.; Williams, B.W.; Dyer, L.A. Overstory-derived surface fuels mediate plant species diversity in frequently burned longleaf pine forests. Ecosphere 2017, 8, e01964. [Google Scholar] [CrossRef]
- Kirkman, L.K.; Giencke, L.M. Restoring and managing a diverse ground cover. In Ecological Restoration and Management of Longleaf Pine Forests; CRC Press: Boca Raton, FL, USA, 2017; pp. 207–232. [Google Scholar]
- Wragg, P.D.; Mielke, T.; Tilman, D. Forbs, grasses, and grassland fire behaviour. J. Ecol. 2018, 106, 1983–2001. [Google Scholar] [CrossRef]
- Jose, S.; Jokela, E.J.; Miller, D.L. The Longleaf pine ecosystem: Ecology, silviculture, and restoration. Choice Rev. Online 2006, 44, 1519–1544. [Google Scholar] [CrossRef]
- Kirkman, L.K.; Jack, S.B. Ecological Restoration and Management of Longleaf Pine Forests; CRC Press: Boca Raton, FL, USA, 2017. [Google Scholar]
- Sharma, A.; Cory, B.; McKeithen, J.; Frazier, J. Structural diversity of the longleaf pine ecosystem. For. Ecol. Manag. 2020, 462, 117987. [Google Scholar] [CrossRef]
- Brudvig, L.A.; Grman, E.; Habeck, C.W.; Orrock, J.L.; Ledvina, J.A. Strong legacy of agricultural land use on soils and understory plant communities in longleaf pine woodlands. For. Ecol. Manag. 2013, 310, 944–955. [Google Scholar] [CrossRef]
- Ramsey, C.L.; Jose, S.; Brecke, B.J.; Merritt, S. Growth response of longleaf pine (Pinus palustris Mill.) seedlings to fertilization and herbaceous weed control in an old field in southern USA. For. Ecol. Manag. 2003, 172, 281–289. [Google Scholar] [CrossRef]
- Brockway, D.G. Restoration of Longleaf Pine Ecosystems; USDA Forest Service, Southern Research Station: Asheville, NC, USA, 2005; Volume 83. [Google Scholar]
- Sharma, A. Role of Uneven-Aged Silviculture and the Soil Seed Bank in Restoration of Longleaf Pine-Slash Pine (Pinus Palustris-Pinus Elliottii) Ecosystems. Ph.D. Thesis, University of Florida, Gainesville, FL, USA, 2012. [Google Scholar]
- Sharma, A.; Bohn, K.K.; McKeithen, J.; Singh, A. Effects of conversion harvests on light regimes in a southern pine ecosystem in transition from intensively managed plantations to uneven-aged stands. For. Ecol. Manag. 2019, 432, 140–149. [Google Scholar] [CrossRef]
- Kilgo, J.; Blake, J. Ecology and Management of a Forested Landscape: Fifty Years on the Savannah River Site; USDA Forest Service: Savannah River, New Ellenton, SC, USA, 2005. [Google Scholar]
- Cox, A.C.; Gordon, D.R.; Slapcinsky, J.L.; Seamon, G.S. Understory restoration in longleaf pine sandhills. Nat. Areas J. 2004, 24, 4–14. [Google Scholar]
- Vaughan, E. The Apalachicola Bluffs and Ravines Preserve in Northern Florida: A Longleaf Pine and Wiregrass Restoration Project; University of Minnesota: Minneapolis, MN, USA, 2001. [Google Scholar]
- Hattenbach, M.; Gordon, D.; Seamon, G.; Studenmund, R. Development of directseeding techniques to restore native groundcover in a sandhill ecosystem. In Proceedings of the Longleaf Pine Ecosystem Restoration Symposium: Ecological Restoration and Regional Conservation Strategies, Fort Lauderdale, FL, USA, 12–15 November 1998; pp. 64–70, Longleaf Alliance Report. [Google Scholar]
- U.S. Department of Agriculture Natural Resources Conservation Service [USDA NRCS]. 2017; Longleaf Pine Initiative: Conservation beyond Boundaries. Available online: https://www.nrcs.usda.gov/wps/portal/nrcs/detailfull/national/home/?cid=nrcsdev11_023913 (accessed on 13 April 2017).
- Guo, N.; Degen, A.A.; Deng, B.; Shi, F.; Bai, Y.; Zhang, T.; Long, R.; Shang, Z. Changes in vegetation parameters and soil nutrients along degradation and recovery successions on alpine grasslands of the Tibetan plateau. Agric. Ecosyst. Environ. 2019, 284, 106593. [Google Scholar] [CrossRef]
- McGroddy, M.E.; Daufresne, T.; Hedin, L.O. Scaling of C: N: P stoichiometry in forests worldwide: Implications of terrestrial redfield-type ratios. Ecology 2004, 85, 2390–2401. [Google Scholar] [CrossRef]
- Still, C.J.; Berry, J.A.; Collatz, G.J.; DeFries, R.S. Global distribution of C3 and C4 vegetation: Carbon cycle implications. Glob. Biogeochem. Cycles 2003, 17, 6-1–6-14. [Google Scholar] [CrossRef]
- Basu, S.; Agrawal, S.; Sanyal, P.; Mahato, P.; Kumar, S.; Sarkar, A. Carbon isotopic ratios of modern C3–C4 plants from the Gangetic Plain, India and its implications to paleovegetational reconstruction. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2015, 440, 22–32. [Google Scholar] [CrossRef]
- Boutton, T.W. Stable carbon isotope ratios of organic matter and their use as indicators of vegetation and climate changes. In Mass Spectrometry of Soils; Marcel Dekker: New York, NY, USA, 1996; pp. 47–82. [Google Scholar]
- Balesdent, J.; Girardin, C.; Mariotti, A. Site-Related^(13) C of Tree Leaves and Soil Organic Matter in a Temperate Forest. Ecology 1993, 74, 1713–1721. [Google Scholar] [CrossRef]
- Wedin, D.A. C4 grasses: Resource use, ecology, and global change. Warm-Seas. (C4) Grasses 2004, 45, 15–50. [Google Scholar]
- Wedin, D.A. Species, nitrogen, and grassland dynamics: The constraints of stuff. In Linking Species & Ecosystems; Springer: Berlin/Heidelberg, Germany, 1995; pp. 253–262. [Google Scholar]
- Seastedt, T. Mass, nitrogen, and phosphorus dynamics in foliage and root detritus of tallgrass prairie. Ecology 1988, 69, 59–65. [Google Scholar] [CrossRef]
- Loudermilk, E.L.; Achtemeier, G.L.; O’Brien, J.J.; Hiers, J.K.; Hornsby, B.S. High-resolution observations of combustion in heterogeneous surface fuels. Int. J. Wildland Fire 2014, 23, 1016–1026. [Google Scholar] [CrossRef]
- Fornara, D.A.; Tilman, D. Plant functional composition influences rates of soil carbon and nitrogen accumulation. J. Ecol. 2008, 96, 314–322. [Google Scholar] [CrossRef]
- Pau, S.; Edwards, E.J.; Still, C.J. Improving our understanding of environmental controls on the distribution of C3 and C4 grasses. Glob. Chang. Biol. 2013, 19, 184–196. [Google Scholar] [CrossRef] [PubMed]
- Ament, M.R.; Tierney, J.A.; Hedin, L.O.; Hobbie, E.A.; Wurzburger, N. Phosphorus and species regulate N2 fixation by herbaceous legumes in longleaf pine savannas. Oecologia 2018, 187, 281–290. [Google Scholar] [CrossRef]
- Markewitz, D.; Sartori, F.; Craft, C. Soil change and carbon storage in longleaf pine stands planted on marginal agricultural lands. Ecol. Appl. 2002, 12, 1276–1285. [Google Scholar] [CrossRef]
- Butnor, J.R.; Johnsen, K.H.; Maier, C.A.; Nelson, C.D. Intra-annual variation in soil C, N and nutrients pools after prescribed fire in a mississippi longleaf pine (Pinus palustris Mill.) plantation. Forests 2020, 11, 181. [Google Scholar] [CrossRef]
- Ficken, C.D.; Wright, J.P. Effects of fire frequency on litter decomposition as mediated by changes to litter chemistry and soil environmental conditions. PLoS ONE 2017, 12, e0186292. [Google Scholar] [CrossRef] [PubMed]
- Carter, M.C.; Foster, C.D. Prescribed burning and productivity in southern pine forests: A review. For. Ecol. Manag. 2004, 191, 93–109. [Google Scholar] [CrossRef]
- Hendricks, J.J.; Wilson, C.A.; Boring, L.R. Foliar litter position and decomposition in a fire-maintained longleaf pine-wiregrass ecosystem. Can. J. For. Res. 2002, 32, 928–941. [Google Scholar] [CrossRef]
- Pritchett, W.; Smith, W. Forest fertilization in the US southeast. For. Soils For. Land Manag. 1975, 467–476. [Google Scholar]
- Ledvina, J.; McShea, W.J.; Bourg, N.A.; Herrmann, V.; Akre, T.; Johnson, A.E. Management Regime and Field Age Affect Species Richness and Cover of Native Forbs and Exotic Species in Virginia Grasslands. Ecol. Restor. 2020, 38, 83–93. [Google Scholar] [CrossRef]
- Chapin, F.S., III. The mineral nutrition of wild plants. Annu. Rev. Ecol. Syst. 1980, 11, 233–260. [Google Scholar] [CrossRef]
- Goebel, P.C.; Palik, B.J.; Kirkman, L.K.; Drew, M.B.; West, L.; Pederson, D.C. Forest ecosystems of a Lower Gulf Coastal Plain landscape: Multifactor classification and analysis. J. Torrey Bot. Soc. 2001, 128, 47–75. [Google Scholar] [CrossRef]
- Keys, J.E.; Carpenter, C.A.; Hooks, S.L.; Koenig, F.; McNab, W.H.; Russell, W.; Smith, M.-L. Ecological Units of the Eastern United States: First Approximation; US Department of Agriculture, Forest Service Atlanta: Atlanta, GA, USA, 1995.
- Johnson, R.; Gjerstad, D. Restoring the overstory of longleaf pine ecosystems. In The Longleaf Pine Ecosystem; Springer: Berlin/Heidelberg, Germany, 2007; pp. 271–295. [Google Scholar]
- Hainds, M.J. Establishing longleaf seedlingson agricultural fields and pastures. In Proceedings of the 4th Biennial Regional Longleaf Alliance Conference, Southern Pines, NC, USA, 17–20 November 2002; pp. 69–73. [Google Scholar]
- Battaglia, M.A.; Mou, P.; Palik, B.; Mitchell, R.J. The effect of spatially variable overstory on the understory light environment of an open-canopied longleaf pine forest. Can. J. For. Res. 2002, 32, 1984–1991. [Google Scholar] [CrossRef]
- Frazer, G.W.; Canham, C.D.; Lertzman, K.P. Gap Light Analyzer (GLA), Version 2.0: Imaging Software to Extract Canopy Structure and Gap Light Transmission Indices from True-Colour Fisheye Photographs, Users Manual and Program Documentation; Simon Fraser University: Burnaby, BC, Canada; The Institute of Ecosystem Studies: Millbrook, NY, USA, 1999; p. 36. [Google Scholar]
- Braun-Blanquet, J. PflanzensoziologiePlant Sociology. The Study of Plant Communities; McGraw-Hill Book Company, Inc.: New York, NY, USA; London, UK, 1932. [Google Scholar]
- Bigelow, S.W.; Whelan, A.W. Longleaf pine proximity effects on air temperatures and hardwood top-kill from prescribed fire. Fire Ecol. 2019, 15, 15. [Google Scholar] [CrossRef]
- Butler, B.; Teske, C.; Jimenez, D.; O’Brien, J.; Sopko, P.; Wold, C.; Vosburgh, M.; Hornsby, B.; Loudermilk, E. Observations of energy transport and rate of spreads from low-intensity fires in longleaf pine habitat–RxCADRE 2012. Int. J. Wildland Fire 2015, 25, 76–89. [Google Scholar] [CrossRef]
- Simard, A.J.; Eenigenburg, J.E.; Adams, K.B.; Nissen Jr, R.L.; Deacon, A.G. A general procedure for sampling and analyzing wildland fire spread. For. Sci. 1984, 30, 51–64. [Google Scholar]
- Wade, D.D. A Guide for Prescribed Fire in Southern Forests; US Department of Agriculture, Forest Service, Southern Region: Atlanta, GA, USA, 1989.
- Kuo, S.; Sparks, D.; Page, A.; Helmke, P.; Loeppert, R. Phosphorus. Methods of Soil Analysis. Part 3. Chemical Methods. Madison; Soil Science Society of America, Inc.: Madison, WI, USA; American Society of Agronomy, Inc.: Madison, WI, USA, 1996. [Google Scholar]
- Magurran, A.E. Ecological Diversity and Its Measurement; Princeton University Press: Princeton, NJ, USA, 1988. [Google Scholar]
- Bates, D.M.; Maechler, M.; Bolker, B. Package ‘lme4′ (Version 0.999999-0): Linear Mixed-Effects Models Using S4 Classes. Available online: cran.r-project.org/web/packages/lme4/lme4.pdf (accessed on 10 March 2022).
- Montgomery, D. Design and Analysis of Experiments, 2nd ed.; John Wiley and Sons: New York, NY, USA, 1984. [Google Scholar]
- Cáceres, M.D.; Legendre, P. Associations between species and groups of sites: Indices and statistical inference. Ecology 2009, 90, 3566–3574. [Google Scholar] [CrossRef]
- Phillips, D.L.; Gregg, J.W. Uncertainty in source partitioning using stable isotopes. Oecologia 2001, 127, 171–179. [Google Scholar] [CrossRef]
- Fox, J.; Weisberg, S. An R Companion to Applied Regression; Sage publications: Los Angeles, CA, USA, 2018. [Google Scholar]
- Van Lear, D.H.; Carroll, W.D.; Kapeluck, P.R.; Johnson, R. History and restoration of the longleaf pine-grassland ecosystem: Implications for species at risk. For. Ecol. Manag. 2005, 211, 150–165. [Google Scholar] [CrossRef]
- Brudvig, L.A.; Damschen, E.I. Land-use history, historical connectivity, and land management interact to determine longleaf pine woodland understory richness and composition. Ecography 2011, 34, 257–266. [Google Scholar] [CrossRef]
- Peterson, D.W.; Reich, P.B.; Wrage, K.J. Plant functional group responses to fire frequency and tree canopy cover gradients in oak savannas and woodlands. J. Veg. Sci. 2007, 18, 3–12. [Google Scholar] [CrossRef]
- Farrar, R.M. Thinning longleaf pine on average sites. J. For. 1968, 66, 906–909. [Google Scholar]
- Kirkman, L.K.; Coffey, K.L.; Mitchell, R.J.; Moser, E.B. Ground cover recovery patterns and life-history traits: Implications for restoration obstacles and opportunities in a species-rich savanna. J. Ecol. 2004, 92, 409–421. [Google Scholar] [CrossRef]
- Brockway, D.G.; Lewis, C.E. Long-term effects of dormant-season prescribed fire on plant community diversity, structure and productivity in a longleaf pine wiregrass ecosystem. Forest Ecology and Management. For. Ecol. Manag. 1997, 96, 167–183. [Google Scholar] [CrossRef]
- Hedman, C.W.; Grace, S.L.; King, S.E. Vegetation composition and structure of southern coastal plain pine forests: An ecological comparison. For. Ecol. Manag. 2000, 134, 233–247. [Google Scholar] [CrossRef]
- Aschenbach, T.A.; Foster, B.L.; Imm, D.W. The Initial Phase of a Longleaf Pine-Wiregrass Savanna Restoration: Species Establishment and Community Responses. Restor. Ecol. 2010, 18, 762–771. [Google Scholar] [CrossRef]
- Reed, H.E.; Seastedt, T.R.; Blair, J.M. Ecological consequences of c 4 grass invasion of a c 4 grassland: A dilemma for management. Ecol. Appl. 2005, 15, 1560–1569. [Google Scholar] [CrossRef]
- Gagnon, P.; Harms, K.; Platt, W.; Passmore, H.; Myers, J. Small-Scale Variation in Fuel Loads Differentially Affects Two Co-Dominant Bunchgrasses. PLoS ONE 2012, 7, e29674. [Google Scholar] [CrossRef]
- Simpson, K.J.; Ripley, B.S.; Christin, P.A.; Belcher, C.M.; Lehmann, C.E.; Thomas, G.H.; Osborne, C.P. Determinants of flammability in savanna grass species. J. Ecol. 2016, 104, 138–148. [Google Scholar] [CrossRef]
- Ripley, B.; Visser, V.; Christin, P.-A.; Archibald, S.; Martin, T.; Osborne, C. Fire ecology of C3 and C4 grasses depends on evolutionary history and frequency of burning but not photosynthetic type. Ecology 2015, 96, 2679–2691. [Google Scholar] [CrossRef] [PubMed]
- Tilman, D.; Wedin, D.; Knops, J. Productivity and sustainability influenced by biodiversity in grassland ecosystems. Nature 1996, 379, 718–720. [Google Scholar] [CrossRef]
- Viera-Vargas, M.S.; Souto, C.M.; Urquiaga, S.; Boddey, R.M. Quantification of the contribution of N2 fixation to tropical forage legumes and transfer to associated grass. Soil Biol. Biochem. 1995, 27, 1193–1200. [Google Scholar] [CrossRef]
- McLauchlan, K. The nature and longevity of agricultural impacts on soil carbon and nutrients: A review. Ecosystems 2006, 9, 1364–1382. [Google Scholar] [CrossRef]
- Knoepp, J.D.; Taylor, S.R.; Boring, L.R.; Miniat, C.F. Influence of forest disturbance on stable nitrogen isotope ratios in soil and vegetation profiles. Soil Sci. Soc. Am. J. 2015, 2015, 1470–1481. [Google Scholar] [CrossRef]
- Kitayama, K.; Iwamoto, K. Patterns of natural 15 N abundance in the leaf-to-soil continuum of tropical rain forests differing in N availability on Mount Kinabalu, Borneo. Plant Soil 2001, 229, 203–212. [Google Scholar] [CrossRef]
- Smith2, B.N.; Epsten, S. Two Categories of 1CC/12C Ratios for Higher Plantsl. Plant Physiol. 1971, 47, 380–384. [Google Scholar] [CrossRef]
- Lefroy, R.D.; Blair, G.J.; Strong, W.M. Changes in soil organic matter with cropping as measured by organic carbon fractions and 13C natural isotope abundance. Plant Soil 1993, 155, 399–402. [Google Scholar] [CrossRef]
- Prober, S.M.; Thiele, K.R.; Lunt, I.D. Identifying ecological barriers to restoration in temperate grassy woodlands: Soil changes associated with different degradation states. Aust. J. Bot. 2002, 50, 699–712. [Google Scholar] [CrossRef]
- Prober, S.M.; Lunt, I.D.; Thiele, K.R. Determining reference conditions for management and restoration of temperate grassy woodlands: Relationships among trees, topsoils and understorey flora in little-grazed remnants. Aust. J. Bot. 2002, 50, 687–697. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).