Abstract
Dryland montane forests are important agents for soil and water resource conservation. The growth of these forests under climate warming is strongly affected by local environmental factors. However, how environmental factors impact intra-annual stem growth dynamics across environmental gradients in these regions remains unclear. This work focused on assessing seasonal patterns of stem growth across different elevations and how environmental factors impact stem growth in the Qilian Mountains, northwestern China. The stem growth of 50 Qinghai spruce trees was monitored for two years across an elevation gradient from 2500 m to 3300 m above sea level (a.s.l.). We found that growth initiation occurred later as the elevation increased, and growth commenced when elevation-specific temperature thresholds were reached. However, growth cessation presented large elevational differences: cessation occurred much earlier at low elevations (2500 m and 2700 m a.s.l.). Exceptionally early growth cessation occurred predominantly at 2700 m a.s.l., which was correlated with seasonal drought/insufficient rainfall and low soil moisture occurring since mid-July 2015. Temperature and soil moisture were the key factors governing the daily rate of stem growth in the beginning, rapid growth, and end stages. Overall, due to effects of seasonal drought and low temperature on growth cessation and growth rate, the annual growth of Qinghai spruce was rather low at both low (2500–2700 m a.s.l.) and high (3100–3300 m a.s.l.) elevations; middle elevations (approximately 2900 m a.s.l.) might be the most favorable Qinghai spruce growth. Our results implied that tree growth will likely decline at low elevations and that the optimal elevation for Qinghai spruce growth in northwestern China is expected to shift upward under future climate warming.
1. Introduction
Climate changes, particularly dramatic warming and severe drought events, have occurred around the world [1], most notably in northern China [2,3]. These changes exert a strong influence on forest dynamics and growth [4,5,6]. Therefore, to assess the impacts of future climate change on forest growth, it is crucial to study the climatic forcing of tree growth at annual and seasonal time scales. However, a number of studies about growth-climate relationships were mostly based on tree-ring-based investigations [7,8,9,10]. Such studies do not fully explain the effects of climate conditions on tree growth and do not capture seasonal growth changes and relevant growth-limiting factors. Hence, studies on seasonal growth responses to climate changes are urgently needed, it can help our understanding of tree growth at annual or multiyear time scales.
Elevation, as a natural simulant of future climates [11], has a strong impact on local climate conditions, and seasonal patterns of stem growth and climate–growth relationships may vary with elevation, such as variations in temperature and precipitation [12,13,14]. Many studies have reported that intra-annual stem growth for the same tree species had divergent across elevations; i.e., the growth initiation was linearly delayed and nearly synchronous with increasing elevations, which was primarily controlled by temperature [14,15,16,17]; however, the growth cessation was no consensus across elevations, which was predominately controlled by photoperiod and an earlier cell differentiation [15,18,19], while was influenced by soil moisture deficit in arid and semi-arid regions [17,20,21,22]. Although there is still much uncertainty about intra-annual stem growth at different elevations, how seasonal patterns of stem growth is controlled by climatic and soil conditions at different elevations has rarely been studied in dryland montane forests of northwestern China.
The Qilian Mountains in northwestern China constitute a key nourishing and cherished water source area [23]. Qinghai spruce (Picea crassifolia Kom.) tree growing in this region is a native evergreen conifer growing at an elevation of 2500–3300 m a.s.l. [7,9]. Earlier studies showed that daily and seasonal variation in stem growth of Qinghai spruce trees at the single scale and among tree classes, and that stem growth initiation was governed by air temperature and/or soil temperature [20,23,24,25], and that growth cessation was affected by soil moisture under drought conditions [20]. However, the intra-annual variation in stem growth across elevations remains poor and the environmental factors impacts are not completely understood.
In this study, we hypothesized that there was a uniform intra-annual variation grow pattern at all elevation, i.e., growth initiation was controlled by temperature, while growth cessation was affected by soil moisture at all elevations. To test the above hypothesis, we selected five Qinghai spruce forest plots across elevations in the Qilian Mountains, and we monitored stem diameter and environmental conditions in the growing seasons of 2015 and 2016. The objectives of this study were to: (a) assess the divergent seasonal patterns of stem growth across different elevations, (b) determine the thresholds of environmental factors that determine the seasonal growth patterns, and (c) determine how environmental factors affect the daily stem growth rate at different growth stages. Our results will contribute to improving the understanding of the intra-annual stem growth across elevations and to predicting future forest development in these regions under changing environmental conditions.
2. Materials and Methods
2.1. Study Area
The study was conducted in the Pailugou watershed of the Qilian Mountains, Gansu Province (38°22′–38°35′ N, 100°17′–100°19′ E). The Pailugou watershed covers an area of 2.85 km2; the area has an elevation range of 2500–3800 m a.s.l. and an arid and semiarid continental climate, with a mean annual air temperature of 1.6 °C, a mean annual precipitation of 435.5 mm, a basin evaporation rate of 1081.7 mm·year−1 and a mean annual relative humidity of 60% (the mean during 1994–2014 at the Xishui weather station at 2600 m a.s.l.) [20].
In the Pailugou watershed, montane forests are mainly distributed on shaded or semi-shaded slopes at an elevation of 2500–3300 m a.s.l., and the forest is dominated by Qinghai spruce. The stand density decreases from 2800–2000 trees·ha−1 to only approximately 300 trees·ha−1 with increasing elevation. The soil is thicker and the sand content lower at elevations from 2500 to 2900 m a.s.l., but the soil layer is relatively thin and unevenly distributed at elevations from 3000 to 3300 m a.s.l. [9]. The main shrub community include Salix Gilashanica C. Wang et P. Y. Fu, Caragana jubata (Pall.) Poir., and Potentilla fruticosa L. [26]. The dominant understory herbs are Stipa capillata L., Polygonum viviparum L., and Pedicularis spp. Moreover, moss is widely distributed under Qinghai spruce forests, and the main moss species is Abietinella abietina.
2.2. Experimental Design
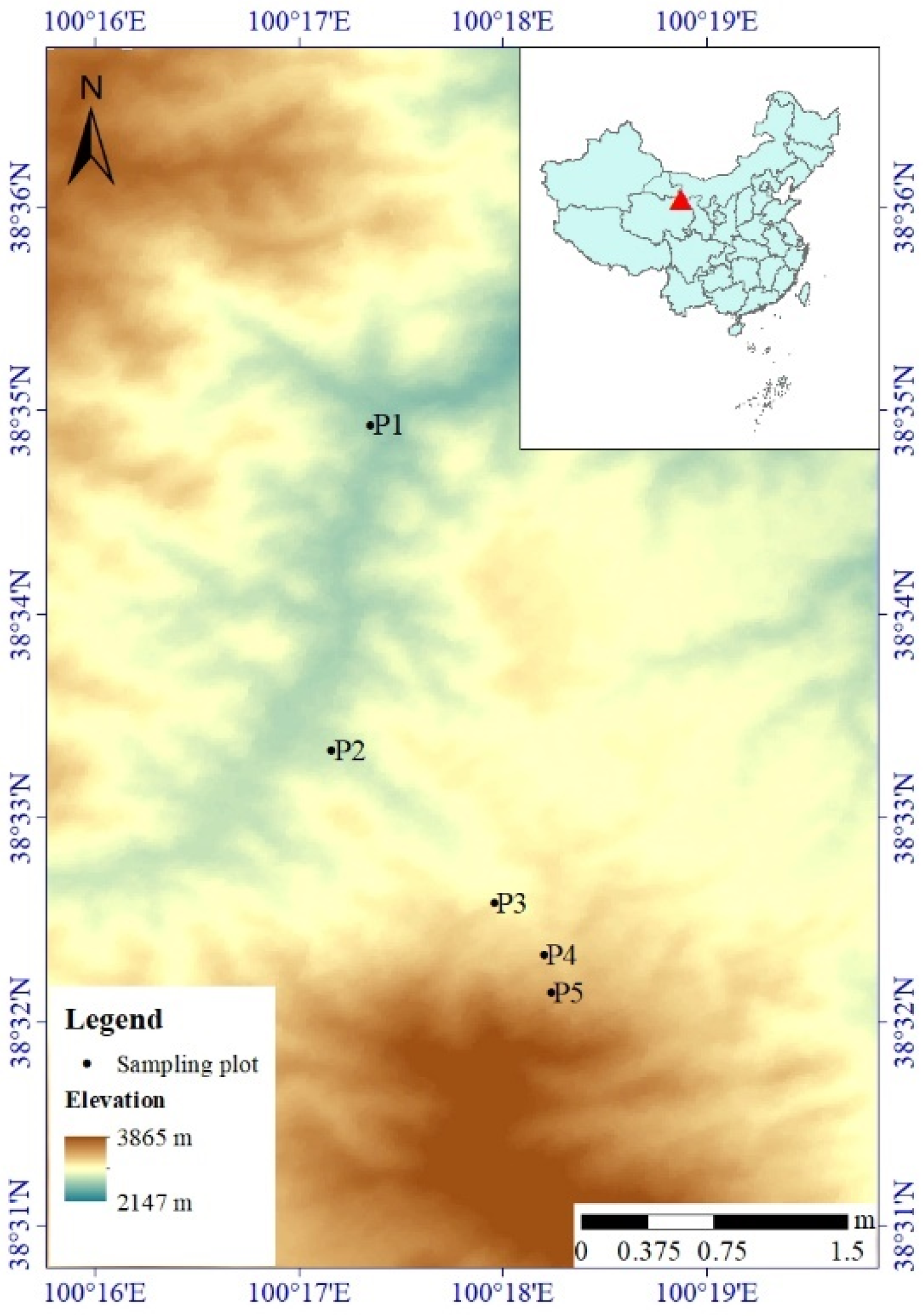
In 2015, five plots (20 m × 20 m in size) were selected across an elevation gradient at specific elevations of 2500 m, 2700 m, 2900 m, 3100 m and 3300 m (Figure 1). The plots at elevations of 2500 m and 3300 m were located in the lower- and upper-forest line ecotones, respectively. All five plots were located on semi-shaded hillsides with similar slope gradients of approximately 27–41°. The soil type of the five plots was a mountainous gray cinnamon soil; this soil texture was considered a loam, with a sand content of 34–58% [9], and the soil thickness was 50–80 cm.
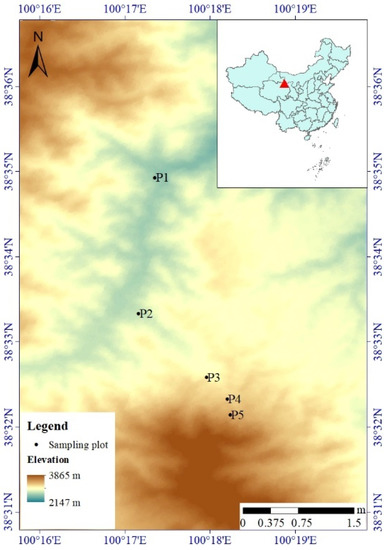
Figure 1.
The digital elevation model (DEM) of the study area showing the locations of Qinghai spruce plots. P1 to P5 represent the locations of five plots at elevations of 2500 m, 2700 m, 2900 m, 3100 m and 3300 m, respectively.
The forest in each plot consisted of Qinghai spruce trees. For each tree in the plots, the diameter at breast height (DBH), tree height, and canopy width were recorded. Basic information concerning elevation, soil depth, aspect, and the slope of each plot was also recorded during field work (Table 1).

Table 1.
Basic characteristics of the five Qinghai spruce plots (mean value ± standard deviation).
2.3. Data Collection
2.3.1. Dendrometer Record Collection
In whole forest, Qinghai spruce trees were divided into three size classes according to their DBH, i.e., large trees (DBH > 22.5 cm), medium trees (12.5 cm < DBH ≤ 22.5 cm) and small trees (4.0 cm < DBH ≤ 12.5 cm) [27]. A total of ten sample trees in each plot were selected, including four large trees, three medium trees and three small trees. The characteristics of sample trees of each size class are shown in Table 2. There was not significant difference of the DBH of sample trees among the five plots (Kruskal–Wallis test, p > 0.05).

Table 2.
Characteristics of sample trees of each size class in the five Qinghai spruce plots (mean value ± standard deviation).
The stem diameter growth of 50 sample trees was measured at 1.3 m above the ground using band dendrometers. The dendrometers (Ecomatik, Munich, Germany), with a resolution of 0.1 mm, consists of a spring and a flexible stainless-steel band with a scale. To minimize disturbance caused by the bark expansion and contraction, the dead bark was lightly removed, and then the dendrometer was installed on the trunk [20]. The scale of the dendrometers varied with trunk changes. The initial stem diameter of individual sample tree was observed on 1 May. Data were collected at approximately 5–10 days intervals to determine whether the stem diameter had changed. The whole experiment was conducted across different elevations from 1 May to 31 October in both 2015 and 2016. Data at elevations of 3100 m and 3300 m, at which the equipment failed in 2016, were eliminated.
The cumulative stem diameter growth (G0, μm) was calculated by Equation (1):
where Di is the daily stem diameter on day i and D0 is the initial stem diameter on 1 May 2015 or 2016.
To minimize the impacts of varying tree size, this study used the mean G of the measured cumulative stem diameter growth (G0) of ten sample trees covering the DBH range in each plot.
2.3.2. Weather and Soil Data Collection
Meteorological data were collected using an automatic weather station (CR3000, Campbell Scientific Inc., Logan, UT, USA) with sensors height of approximately 2 m, installed in an open area at 2700 m a.s.l. Meteorological data included air temperature (HMP115A, Ta (°C)) and precipitation (TE525MM, P (mm)) during the growth periods of 2015 and 2016 (May–October). The elevation gradients of daily temperature and precipitation were calculated using the data from this base weather station. With increasing elevation, the daily P showed an increasing rate of approximately 4.95%·100 m–1 of elevation, while the daily Ta decreased at a rate of approximately 0.58 °C·100 m–1 of elevation in the Pailugou watershed [28,29,30], as shown via Equations (2) and (3) below.
where Pa and Ta are the daily precipitation and daily air temperature at any elevation Ha (m a.s.l.).
In each plot, the volumetric soil moisture content (Ms, %) and soil temperature (Ts, °C) of the 0 to 10 cm, 10 to 20 cm, 20 to 40 cm, and 40 to 60 cm soil layers were continuously monitored using soil moisture and temperature sensors (5-TE, Decagon, Pullman, WA, USA). Data were stored at 10 min intervals in a data logger (EM50, Decagon, Pullman, WA, USA). Since the active roots of Qinghai spruce trees were focused on the 0 to 60 cm soil layer, the weighted averages of Ms and Ts of the 0 to 60 cm soil layer were calculated using the measured Ms and Ts of each soil layers.
2.4. Data Analysis
2.4.1. Seasonal Growth Pattern Assessment
The Gompertz function was used to determine seasonal patterns of stem growth. This function is one of the most commonly used models [18] because of its flexibility and the asymmetrical shape of the resulting curve [31]. In this study, to characterize the complete seasonal growth pattern (May–October), the cumulative stem diameter growth curves of Qinghai spruce trees were fitted using the Gompertz function [18] (Equation (4)):
where Y is the cumulative stem diameter change, A is the upper asymptote, β is the x-axis placement parameter, k is the rate of change parameter, and t is the day of year (DOY).
The parameters of the Gompertz function were estimated by the ordinary least squares method with the MODEL procedure (1stOpt software, 7D-Soft High Technology, Inc., Beijing, China). The use of this model has the advantage of smoothing the measured stem diameter growth records. After smoothing, the daily rate of stem growth (Gr, μm·day−1) curves were obtained by the first-order derivation of the simulated cumulative stem changes.
The mean DBHs and tree heights of ten sampled trees were similar to those of the plots. Therefore, the averages of ten sample trees at each elevation were analyzed. To assess interannual variability, we modeled the averaged stem diameter changes of ten sample trees at each elevation for each year (n = 2). The timing of growth initiation and cessation was determined as DOY when the daily growth rates exceeded the threshold value of 2 μm·day−1, which corresponded to the change in cumulative stem diameter growth [20].
2.4.2. Analysis of the Relationship between Stem Growth and Environmental Factors
Environmental factors have a strong impact on the seasonal patterns of stem growth. Temperature and soil moisture are generally the key influencing factors for initiation and cessation of stem growth [20]. Therefore, we selected the Ta, mean Ts and mean Ms of the 0 to 60 cm soil layers to determine the thresholds of environmental factors for the initiation and cessation of stem growth of Qinghai spruce trees. According to the growth initiation and cessation data, we selected the Ta, Ts and Ms data before and after 4 days (9 days in total) and then performed one-way analysis of variance (ANOVA). If the results of the ANOVA are significant, the environmental factor in question is not at the environmental threshold that governs stem diameter growth; however, if the results of the ANOVA are not significant, the environmental factor is likely to control the threshold of only the stem diameter growth [32].
To better analyze the relationship between environmental factors and stem diameter variation over the growing season (May–October), the dendrometer data for each elevation were divided into three stages according to growth changes: the beginning stage (stage 1), the rapid growth stage (stage 2) and the end stage (stage 3) [20]. The corresponding environmental factors were also divided into three stages. Spearman correlation coefficients between the daily rate of stem growth (Gr) (obtained by using the Gompertz functions) and environmental variables (Ta, Ts, P and Ms) were calculated using the Statistical Product and Service Solutions (SPSS), version 19.0 (IBM Inc., Chicago, IL, USA).
3. Results
3.1. Environmental Variables and Gradients during the Growing Season across Different Elevations
In the Pailugou watershed, which is a mountainous watershed, the annual P mostly occurs in the growing season; moreover, the temporal distribution of P is uneven in the growing season (May–October). For example, at 2700 m a.s.l., the growing seasons P were 76 and 88 rainy days in 2015 and 2016, with total P of 369 mm and 333.7 mm, respectively, 84.2% (2015) and 92.0% (2016) of which had daily P of less than 10 mm. The Ta varied in the ranges of −5.5–22.4 °C and −7.6–23.3 °C, with averages of 10.1 ± 4.9 °C and 10.8 ± 5.8 °C in 2015 and 2016, respectively (Figure 2a,b). Normally, the P increased and the Ta decreased with increasing elevation. There were change rates of 0.58 °C·100 m−1 of elevation for Ta and 4.95%·100 m−1 of elevation for the growing season P when the elevation increased [28,29,30].
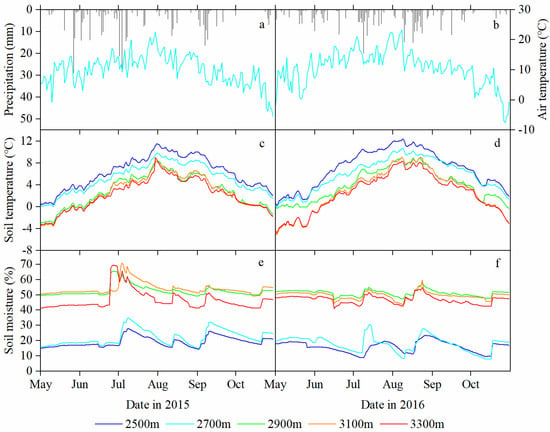
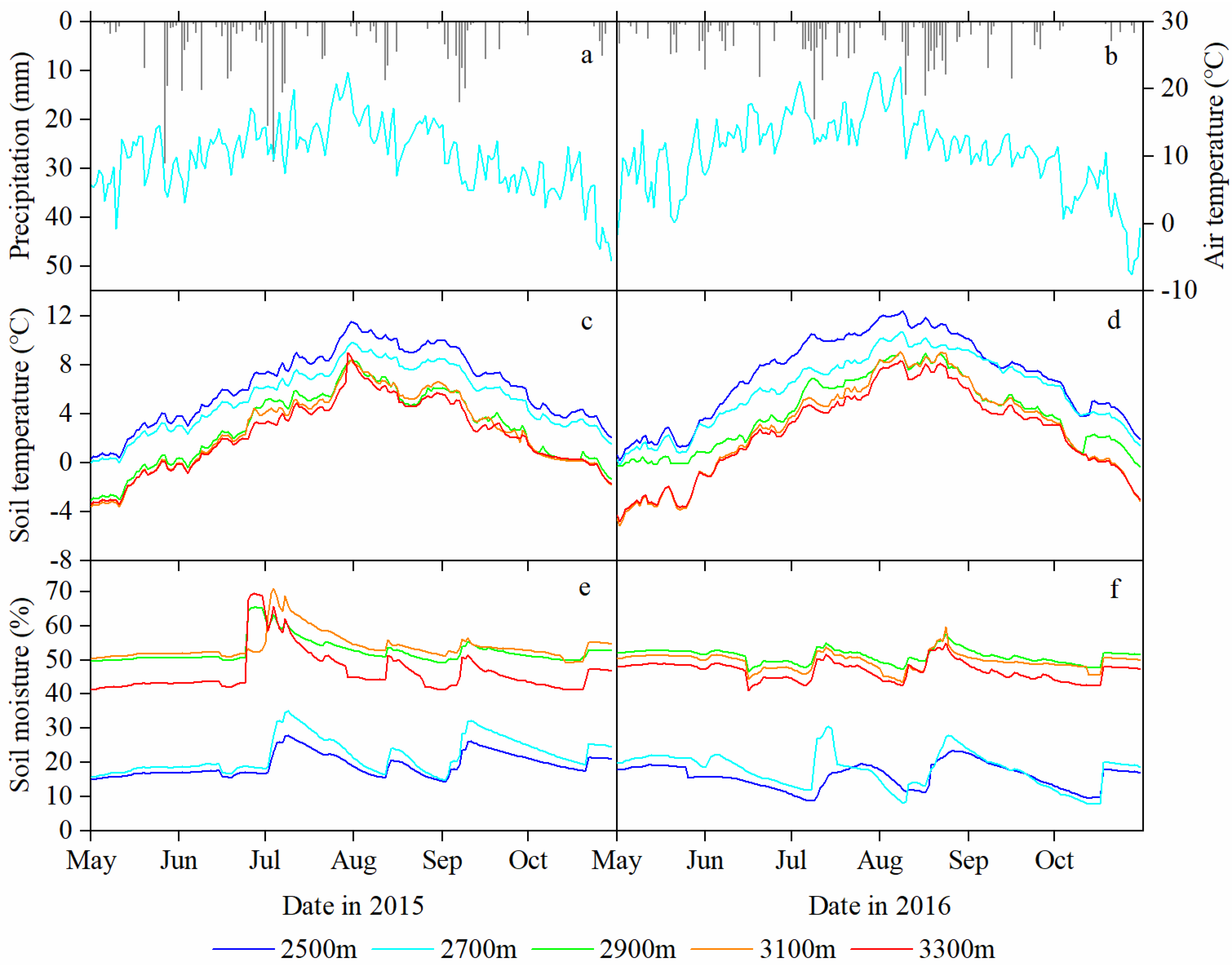
Figure 2.
Daily variation of precipitation (P) and mean air temperature (Ta) at 2700 m a.s.l., and edaphic factors (mean soil temperature (Ts) and volumetric soil moisture content (Ms)) of the 0 to 60 cm soil layer at different elevations in the growing seasons of 2015 (a,c,e) and 2016 (b,d,f).
However, the elevation change pattern of Ts differed significantly from that of Ta, and the pattern of Ms was opposite that of Ts. For the Ts and Ms, there is a critical line at the middle elevation (2900 m a.s.l.), i.e., there is markedly different change ratio both above and below this elevation. In 2015, the Ts was approximately 6.0 °C at low elevations (such as 6.28 ± 2.98 °C and 5.23 ± 2.62 °C at 2500 m and 2700 m a.s.l., respectively), whereas it was approximately 2.5 °C at both middle and high elevations (2.86 ± 2.99 °C, 2.59 ± 3.12 °C and 2.27 ± 2.86 °C at 2900 m, 3100 m and 3300 m a.s.l., respectively) (Figure 2c). The Ms was approximately 20% at low elevations (such as 19.27 ± 3.37% and 22.12 ± 5.03% at 2500 m and 2700 m a.s.l.), respectively, whereas it was approximately 50% at middle and high elevations (52.58 ± 3.57%, 53.97 ± 3.98% and 46.62 ± 6.36% at 2900 m, 3100 m and 3300 m a.s.l., respectively) (Figure 2e). In 2016, there were still Ts and Ms differences of 3 °C and 30% between elevation groups (below and above the elevations of 2900 m) and similar Ts and Ms levels across elevations in the same group (Figure 2d,f).
Temperature and soil moisture remained significantly different both two growing seasons. In May 2015, the Ta and Ts values (2700 m a.s.l., 8.5 °C and 1.6 °C, respectively) were slightly warmer than those in May 2016 (7.1 °C and 1.4 °C, respectively). From mid-July to August 2015, the Ms continued to decline and reached a minimum value for the growing season, which can lead to a deficit in soil water hindering tree growth at 2500 m and 2700 m a.s.l. However, in July and August 2016, a different soil moisture pattern emerged in which the rainfall was uniformly distributed, leading to high levels of soil moisture at 2500 m and 2700 m a.s.l. Such environmental conditions likely promote differences in interannual tree growth.
3.2. Divergent Seasonal Patterns of Stem Diameter Growth with Changes in Elevation
Seasonal patterns of the measured and modeled cumulative stem growth and the daily growth rate for different elevations in both years are shown in Figure 3. The results of the Gompertz function, i.e., the modeled cumulative stem growth data, exhibited remarkable agreement with the measured growth data, with R2 values greater than 0.98. Seasonal patterns of cumulative stem growth at all elevations exhibited an “S” shape in 2015 and 2016. Stem growth at all elevations started in May–June, peaked in July, and finally stopped in August–September.
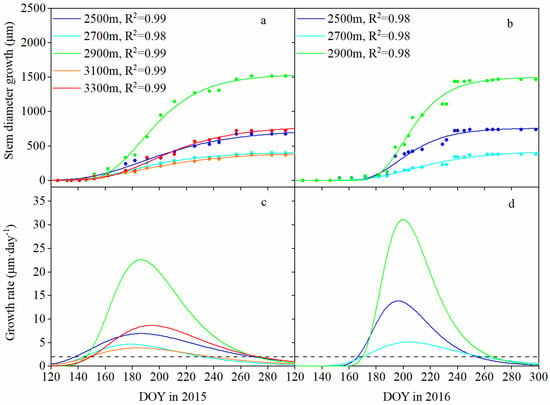
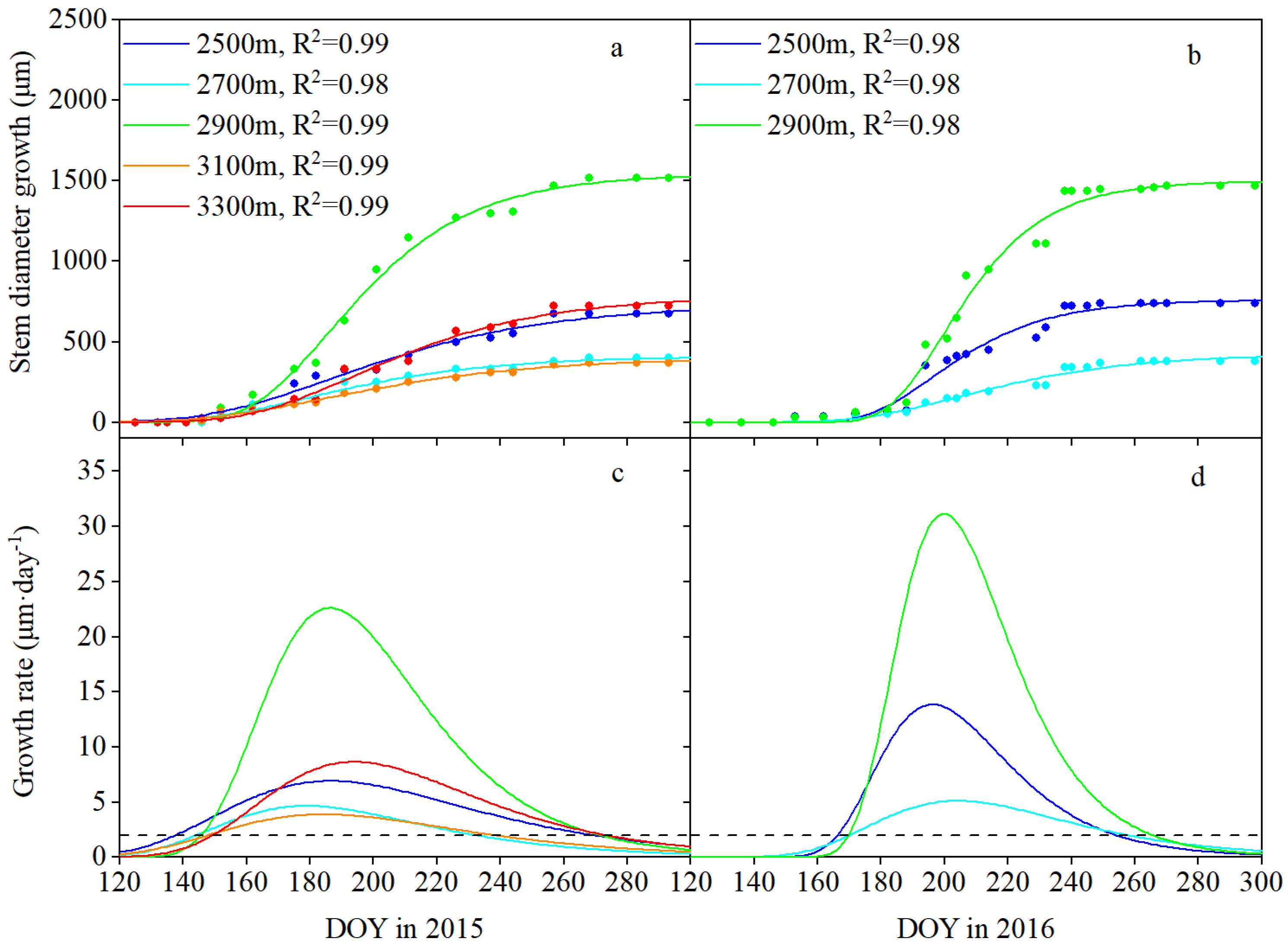
Figure 3.
Gompertz function-modeled curves of the cumulative stem growth (a,b) and daily growth rate (c,d) at different elevations during the growing seasons of 2015 and 2016 (May–October). The dashed lines at the bottom panels indicate the daily growth rate (equal to 2 μm·day−1).
Seasonal patterns at different elevations showed divergent trends (Figure 3). The beginning of the growing season occurred later as the elevation increased; for example, in 2015, the occurrence of growth initiation ranged from May 18 (day of year (DOY) 138) at 2500 m a.s.l. to May 31 (DOY 150) at 3300 m a.s.l. (Table 3), yielding an onset delay of approximately 1.8 days·100 m–1 of elevation (R2 = 0.84, p < 0.05); in 2016, growth initiation at 2500 m a.s.l. occurred 5 days earlier than at other elevations (Table 3). However, there was a significant difference in growth cessation across elevations. Growth cessation occurred much earlier at lower elevations; for example, in 2015, stem growth at 2700 m a.s.l. occurred 35 days earlier than at 2500 m, 2900 m and 3300 m a.s.l.; in 2016, stem growth at low elevations (2500 m and 2700 m a.s.l.) occurred 1 week earlier than it did at middle elevation (2900 m a.s.l.) (Table 3).

Table 3.
Characteristics of seasonal growth patterns at different elevations in 2015 and 2016.
Maximum stem growth at all elevations synchronously occurred in early July (DOY 179–194) in 2015 and in mid-July (DOY 196–204) in 2016 (Table 3). However, the maximum daily growth rate at 2900 m a.s.l. was higher than that at the other elevations, with values of 22.621 μm·day−1 in 2015 and 31.504 μm·day−1 in 2016, which were 2.2–5.8 times those at other altitudes. Accordingly, the cumulative stem diameter increases varied in accordance with a “unimodal” trend with increasing elevation, with a peak at 2900 m a.s.l. (1520 μm in 2015 and 1670 μm in 2016), which was 2.1–4.4 times that at other elevations.
3.3. Thresholds of Environmental Factors for the Seasonal Patterns of Stem Diameter Growth
There is a critical line at 2900 m a.s.l.; i.e., there are markedly different controlling factors for the seasonal patterns of stem diameter growth below or above this elevation. The growth initiation was controlled by air temperature at both low (2500 m and 2700 m a.s.l.) and middle (2900 m a.s.l.) elevations, whereas it was controlled by both air temperature and soil temperature at high elevations (3100 m and 3300 m a.s.l.). The thresholds of air temperature at low elevations ranged from 8.4 °C to 10.7 °C, and the thresholds at both middle and high elevations ranged from 3.5 °C to 6.8 °C for air temperature and from −0.3 °C to −0.1 °C for soil temperature (Table 4).

Table 4.
Thresholds of environmental factors (mean air temperature (Ta), mean soil temperature (Ts) and volumetric soil moisture content (Ms) of the 0 to 60 cm soil layer) for seasonal patterns of stem growth of Qinghai spruce trees in the Pailugou watershed.
The growth cessation at low elevations coincided with the occurrence of high soil temperature (5.33–7.85 °C) and low soil water (21.1–21.5%), whereas water stress was absent at the end of stem growth at both middle and high elevations (Table 4).
3.4. Impacts of Environmental Factors on the Daily Stem Growth Rate
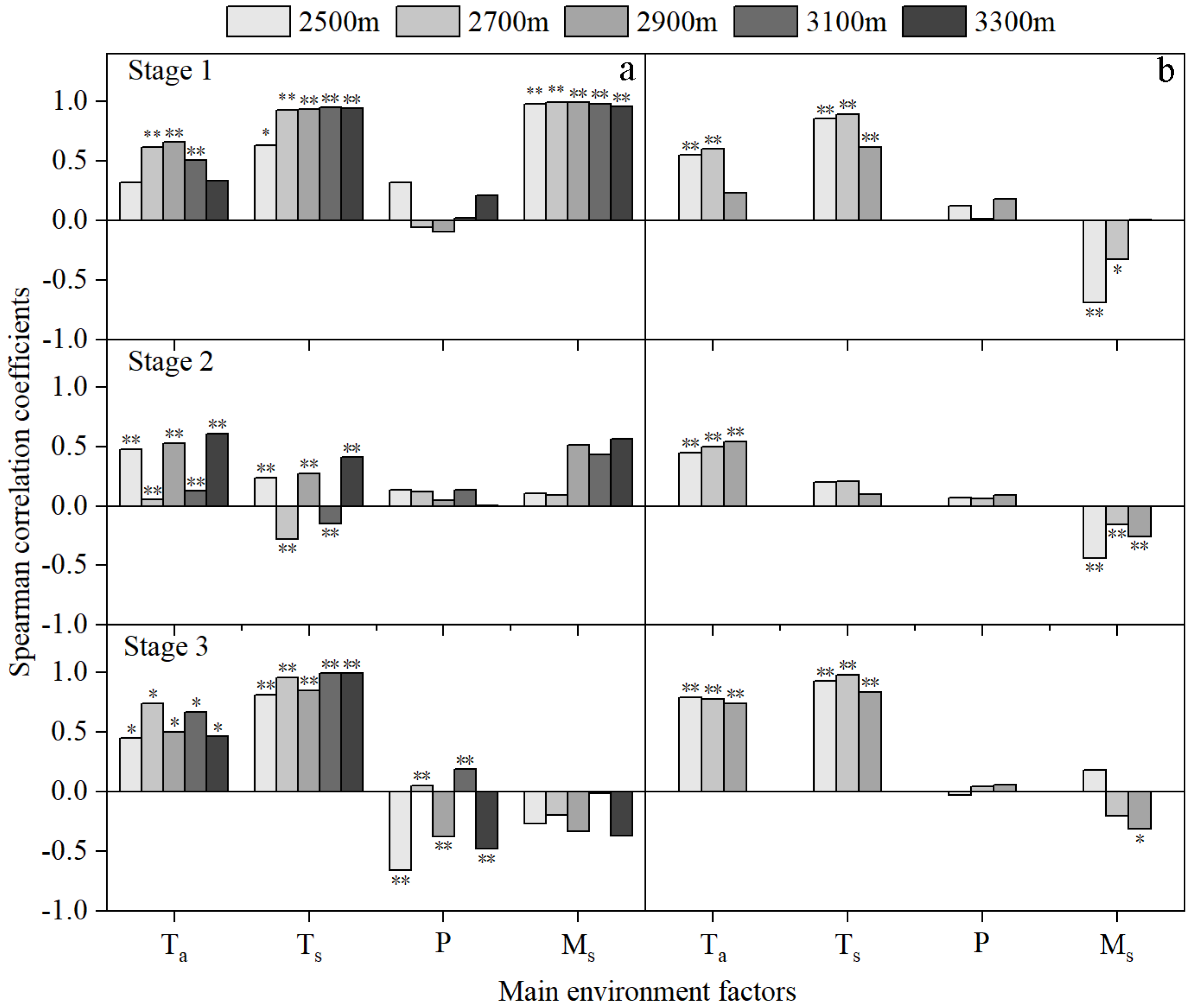
In stages 1 and 3 (i.e., the beginning and end stages), the Gr at all elevations was significantly positively correlated with Ta and Ts, with correlation coefficients greater than 0.4 (Figure 4). There was, however, contrasting data concerning soil moisture in stage 1; the Gr for each elevation was significantly positively correlated with Ms in 2015, with correlation coefficients greater than 0.9, while it was significantly negatively correlated with Ms in 2016. In stage 3 of 2015, Gr was significantly positively correlated with P at 2700 m and 3100 m a.s.l., but it was significantly negatively correlated with P at other elevations.
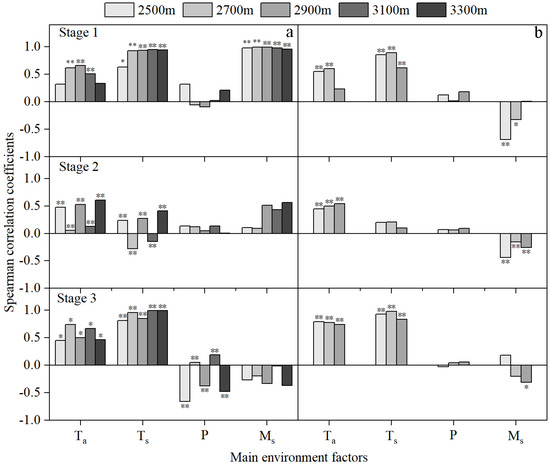
Figure 4.
Spearman correlation coefficients of the daily rate of stem growth (Gr) and air temperature (Ta), precipitation (P), volumetric soil moisture content (Ms) and soil temperature (Ts) at the 0 to 60 cm soil layers in stages 1, 2, and 3 in 2015 (a) and 2016 (b). Values marked ** and * indicate that they are significant at the 0.01 and 0.05 levels, respectively.
In stage 2 (the rapid growth stage), the Gr at all elevations was positively correlated with Ta and P in 2015 and 2016. There was, however, contrasting data concerning Ms; the Gr was positively correlated with Ms in 2015, but it was negatively correlated with Ms in 2016. Similarly, the Gr was significantly negatively correlated with Ts at 2700 m and 3100 m a.s.l. in 2015, but positively correlated with those in 2016 (Figure 4).
4. Discussion
4.1. Impacts of Environmental Factors on Seasonal Growth Patterns across Different Elevations
It was found that the growth initiation occurred later with increasing elevation in the study area. Since temperatures are the most important variables along the elevation gradient [28,29,30], this is due to the lower temperatures at higher elevations. This was consistent with our hypothesis that growth initiation was controlled by temperature at all elevations. Since temperatures can affect cambial activity by increasing the rate of cell division and the number of xylem produced [14,33]. Related studies reported that the growth of Qinghai spruce stems was generally initiated when the mean Ta was maintained above 5 °C or when the mean Ts begin to increase steadily past 0 °C [23,24]. In this study, the growth initiation thresholds of mean Ta and Ts of the 0 to 60 cm soil layer was above 3.5 °C and 0 °C, respectively.
Our study showed a significant difference in growth cessation across elevations, with 35 days earlier at 2700 m a.s.l. compared with the middle and high elevations in 2015. This indicated that the controlling factors of growth cessation were different across elevations. For middle and high elevations, temperatures and soil moisture were very different, but the timing of growth cessation was similar. This implied that the growth cessation was probably governed by photoperiod and cell differentiation [15,18,34]. For low elevations, the growth cessation was mainly affected by soil moisture [20,35,36]. The coexistence of high temperatures and low Ms seemed to trigger the growth cessation at 2700 m a.s.l., leading to a continuous soil water deficit since mid-July. Conversely, the low Ms did not restrict the growth cessation at 2500 m a.s.l., mainly due to the unique microclimate conditions at the bottom of the ditch and approximately 5 m from the river. Thus, the insufficient rainfall and low Ms in August 2015 compared with 2016 induced an earlier cessation of stem growth at low elevations, but not at middle and high elevations.
Several studies reported that the maximum growth rate for conifers in cold environments is synchronized with maximum day length [18,37]; however, some studies reported that it may synchronized not only with maximum day length but also with optimal soil water and maximum solar radiation [20,21,24]. In our study, the maximum growth rate occurred in July at all elevations, which was about 20 days later than the day of maximum daylength. At the summer solstice in both years, the study area experienced insufficient rainfall, low Ms and extended periods of sunny conditions. Although maximum day length coincided with high temperatures and solar radiation, stem growth rate was not at its maximum due to lower Ms. Therefore, the maximum growth rate may synchronize not only maximum day length and suitable weather condition, but also with optimal soil water.
Elevation gradients allow for natural space-for-time/warming experiments to know the mechanism of seasonal growth in response to future climate changes [14,15]. Several studies reported that tree growth at high elevations was sensitive to temperature, and an increasing temperature could increase tree growth at high elevations [38,39]. However, an increasing temperature reduced tree growth at low elevations, mainly due to suffering from temperature-induced drought stress [7,40,41]. In this study, annual stem growth and growing season duration at low elevations were determined by a combination of spring temperature and autumn rainfall, while high elevations were determined by spring temperature. In the context of future warming, a warmer autumn has no effect on stem growth at higher elevations, but lower elevations may lead to drought stress and early cessation of growth. In contrast, spring warming is positive for stem growth in the whole forest zone. When soil temperatures reach above 0 °C, the snow and frozen soil melts, soil moisture content improves [24]. Consequently, soil water is sufficient when growth begins, and temperature is the main factor affecting stem growth. Spring temperature increases, stem growth for the whole forest zone begins earlier, and growing season duration is extended, which is good for tree growth and carbon sequestration of forest ecosystems in semi-arid mountains.
4.2. Impacts of Environmental Factors on Daily Stem Growth across Different Elevations
During the growing season, the daily stem growth was affected by both weather conditions and soil factors [20,23,24], and intra-annual stem growth of several coniferous species had a positive effect on temperature [42,43,44]. In this study, the Gr at all elevations was also positively correlated with Ta and Ts at different growth stages in both 2015 and 2016, except for 2700 m and 3100 m during stage 2 of 2015 (Figure 4). During stage 2, the Ta was high and peaked (Figure 2), and the increase in Ta may have increased soil evaporation and tree transpiration [45]. Moreover, Ms decreased and reached its minimum value; thus, the high temperature and low Ms affected tree growth. In addition, Spearman correlation coefficients between Gr and Ta at 2700 m and 3100 m a.s.l. were lower than they were at 2500 m, 3100 m and 3300 m a.s.l. in stage 2 (Figure 4). These results further confirmed that high temperature caused water stress and led to stem shrinkage [24].
In this study, the Gr at all elevations was positively correlated with Ms in stages 1 and 2 in 2015. These results are in agreement with those of Jiang et al. [21], who found that the stem growth of Platycladus orientalis (L.) Franco was significantly positively related to Ms. However, the Gr was negatively correlated with Ms in stages 1 and 2 in 2016. This interannual difference may be caused by differences in soil conditions. When the soil water content is high due to precipitation, the soil temperature decreases, and root respiration becomes inhibited, which may affect stem growth [23]. Similarly, the Gr at all elevations was negatively correlated with Ms in stage 3 in both 2015 and 2016. This was partly because the soil in the Qinghai spruce plots begins to freeze in late October every year [46]. When the soil is frozen, tree roots are unable to take up water, which may explain the very low Gr, which was less than 2 μm·day−1 in stage 3.
The correlations between the Gr and main environmental factors varied with growth stages (Figure 4). However, quantitative the relationships describing the effects of environmental conditions on stem growth were not available in this study due to limited data. Under changes of environmental conditions in the future, especially the high temperature and drought events, these changes will affect intra-annual stem growth. Therefore, further research should be encouraged to quantify the effects of weather and soil factors on stem growth, and to study the effect of seasonal drought on intra-annual stem growth. These results will provide a basis for further understanding of the controlling mechanisms of growth process.
4.3. Implications for Forest Management
Temperature and soil water availability is the important influencing factors for tree growth in mountain forests [21,32], but high temperatures and drought events will continue to increase in the future [1], which may further affect mountain forests [47,48]. Qinghai spruce is an important tree species for soil and water conservation in the Qilian Mountains. Additionally, thus, it is the important to maintain tree growth of this species. In fact, trees at low elevations were subjected to soil moisture deficit in case of insufficient rainfall, and they had a rather narrow growth. Previous studies also found that Qinghai spruce at low elevations suffer from water deficit, and tree growth decline [7,20]. To mitigate drought stress at low elevation, tree growth can be maintained by appropriately reducing stand density in future forest management. In addition, trees at middle and high elevations may grow faster due to warmer spring temperatures, and the optimal elevations for Qinghai spruce growth is expected to shift upward in the next few decades [49], so the middle and high elevations are more beneficial to the migration and survival of Qinghai spruce.
5. Conclusions
This study on the seasonal patterns of Qinghai spruce growth at different elevations in the Qilian mountains of northwestern China showed that growth initiation occurred later as the elevation increased in both years, which was controlled by temperature; however, growth cessation presented large elevational differences, and cessation occurred much earlier at lower elevations than at higher ones, especially at 2700 m a.s.l. in 2015, which was mainly due to seasonal drought since mid-July. Additionally, there was a “unimodal” trend of annual cumulative growth as the elevation increased, with a peak at 2900 m a.s.l. (1520 μm in 2015 and 1670 μm in 2016), which was 2.1–4.4 times that at other elevations. This difference in stem growth resulted from the varying temperature and soil moisture in the growing season, namely, an insufficient water supply in autumn at low elevations and low spring temperatures at high elevations that collectively may result in a shortened growing season and a rather narrow annual growth at low and high elevations. These results provide a phenological basis for predicting the future adaptation of Qinghai spruce forest in semi-arid area under climate changes.
Author Contributions
Conceptualization, Y.W. (Yanfang Wan), P.Y. and Y.W. (Yanhui Wang); instrument installation, L.Z., X.L. (Xiande Liu) and S.W.; investigation, Y.W. (Yanfang Wan), X.L. (Xiaoqing Li), L.Z., X.L. (Xiande Liu) and S.W.; formal analysis, Y.W. (Yanfang Wan) and X.L. (Xiaoqing Li); writing—original draft preparation, Y.W. (Yanfang Wan); writing—review and editing, Y.W. (Yanfang Wan), P.Y., Y.W. (Yanhui Wang), B.W. and Y.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially supported by the National Natural Science Foundation of China (U21A2005, U20A2085, 41971038, 32171559) and the Central Public-Interest Scientific Institution Basal Research Fund of Chinese Academy of Forestry (CAFYBB2021ZW002, CAFYBB2020QB004).
Data Availability Statement
Not available.
Acknowledgments
We thank Ming Jin, Wenmao Jing, Weijun Zhao, Jian Ma, and other staff of the Academy of Water Resource Conservation Forests of Qilian Mountains in Gansu Province for their assistance in the field work.
Conflicts of Interest
The authors declare no conflict of interest.
References
- IPCC. Climate Change 2014: Mitigation of Climate Change. Contribution of Working Group III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Edenhofer, O., Pichs-Madruga, R., Sokona, Y., Farahani, E., Kadner, S., Seyboth, K., Adler, A., Baum, I., Brunner, S., Eickemeier, P., et al., Eds.; Cambridge University Press: Cambridge, UK, 2014. [Google Scholar]
- Li, J.; Cook, E.R.; D’arrigo, R.; Chen, F.; Gou, X. Moisture variability across China and Mongolia: 1951–2005. Clim. Dyn. 2009, 32, 1173–1186. [Google Scholar] [CrossRef]
- Piao, S.; Ciais, P.; Huang, Y.; Shen, Z.; Peng, S.; Li, J.; Zhou, L.; Liu, H.; Ma, Y.; Ding, Y. The impacts of climate change on water resources and agriculture in China. Nature 2010, 467, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Hof, A.R.; Girona, M.M.; Fortin, M.J.; Tremblay, J.A. Using landscape simulation models to help balance conflicting goals in changing forests. Front. Ecol. Evol. 2021, 9, 818. [Google Scholar] [CrossRef]
- Ameray, A.; Bergeron, Y.; Valeria, O.; Montoro Girona, M.; Cavard, X. Forest carbon management: A review of silvicultural practices and management strategies across boreal, temperate and tropical forests. Curr. For. Rep. 2021, 7, 245–266. [Google Scholar] [CrossRef]
- Andreu, L.; Gutierrez, E.; Macias, M.; Ribas, M.; Bosch, O.; Camarero, J.J. Climate increases regional tree-growth variability in Iberian pine forests. Glob. Chang. Biol. 2007, 13, 804–815. [Google Scholar] [CrossRef]
- Wang, B.; Yu, P.; Zhang, L.; Wang, Y.; Yu, P.; Wang, S. Differential trends of Qinghai spruce growth with elevation in Northwestern China during the recent warming hiatus. Forests 2019, 10, 712. [Google Scholar] [CrossRef] [Green Version]
- Wang, B.; Yu, P.; Yu, Y.; Wang, Y.; Zhang, L.; Wan, Y.; Wang, S.; Liu, X. Trees at a moderately arid site were more sensitive to long-term drought. Forests 2021, 12, 579. [Google Scholar] [CrossRef]
- Zhang, L.; Shi, H.; Yu, P.; Wang, Y.; Pan, S.; Wang, B.; Tian, H. Divergent growth responses to warming between stand-grown and open-grown trees in a dryland montane forest in Northwestern China. Forests 2019, 10, 1133. [Google Scholar] [CrossRef] [Green Version]
- Teets, A.; Fraver, S.; Weiskittel, A.R.; Hollinger, D.Y. Quantifying climate-growth relationships at the stand level in a mature mixed-species conifer forest. Glob. Chang. Biol. 2018, 24, 3587–3602. [Google Scholar] [CrossRef] [PubMed]
- Körner, C. Alpine Plant Life: Functional Plant Ecology of High Mountain Ecosystems; Springer: Berlin/Heidelberg, Germany, 2003. [Google Scholar] [CrossRef]
- Peterson, D.W.; Peterson, D.L. Mountain hemlock growth responds to climatic variability at annual and decadal time scales. Ecology 2001, 82, 3330–3345. [Google Scholar] [CrossRef]
- Takahashi, K. Effects of altitude and competition on growth and mortality of the conifer Abies sachalinensis. Ecol. Res. 2010, 25, 801–812. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, B.; Deslauriers, A.; Achim, B. Intra-annual stem radial increment response of Qilian juniper to temperature and precipitation along an altitudinal gradient in Northwestern China. Trees 2015, 29, 25–34. [Google Scholar] [CrossRef]
- Moser, L.; Fonti, P.; Buntgen, U.; Esper, J.; Luterbacher, J.; Franzen, J.; Frank, D. Timing and duration of European larch growing season along altitudinal gradients in the Swiss Alps. Tree Physiol. 2010, 30, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Saderi, S.; Rathgeber, C.B.K.; Rozenberg, P.; Fournier, M. Phenology of wood formation in larch (Larix decidua Mill.) trees growing along a 1000-m elevation gradient in the French Southern Alps. Ann. For. Sci. 2019, 76, 89. [Google Scholar] [CrossRef]
- Gao, J.; Yang, B.; He, M.; Shishov, V. Intra-annual stem radial increment patterns of Chinese pine, Helan Mountains, Northern Central China. Trees Struct. Funct. 2019, 33, 751–763. [Google Scholar] [CrossRef]
- Duchesne, L.; Houle, D.; D’Orangeville, L. Influence of climate on seasonal patterns of stem increment of balsam fir in a boreal forest of Québec, Canada. Agric. For. Meteorol. 2012, 108–114. [Google Scholar] [CrossRef]
- Zhang, J.; Gou, X.; Pederson, N.; Zhang, F.; Niu, H.; Zhao, S.; Wang, F. Cambial phenology in Juniperus przewalskii along different altitudinal gradients in a cold and arid region. Tree Physiol. 2018, 38, 840–852. [Google Scholar] [CrossRef]
- Wan, Y.; Yu, P.; Li, X.; Wang, Y.; Wang, B.; Yu, Y.; Zhang, L.; Liu, X.; Wang, S. Seasonal pattern of stem diameter growth of Qinghai spruce in the Qilian Mountains, northwestern China. Forests 2020, 11, 494. [Google Scholar] [CrossRef]
- Jiang, Y.; Wang, B.; Dong, M.; Huang, Y.; Wang, M.; Wang, B. Response of daily stem radial growth of Platycladus orientalis to environmental factors in a semi-arid area of North China. Trees 2015, 29, 87–96. [Google Scholar] [CrossRef]
- Ziaco, E.; Biondi, F. Tree growth, cambial phenology, and wood anatomy of limber pine at a Great Basin (USA) mountain observatory. Trees Struct. Funct. 2016, 30, 1507–1521. [Google Scholar] [CrossRef] [Green Version]
- Wang, B.; Chen, T.; Xu, G.; Liu, X.; Wang, W.; Wu, G.; Zhang, Y. Alpine timberline population dynamics under climate change: A comparison between Qilian juniper and Qinghai spruce tree species in the middle Qilian Mountains of Northeast Tibetan Plateau. Boreas 2016, 45, 411–422. [Google Scholar] [CrossRef]
- Tian, Q.; He, Z.; Xiao, S.; Peng, X.; Ding, A.; Lin, P. Response of stem radial growth of Qinghai spruce (Picea crassifolia) to environmental factors in the Qilian Mountains of China. Dendrochronologia 2017, 44, 76–83. [Google Scholar] [CrossRef]
- Li, X.; Liu, X.; Wang, L.; Yu, P.; Niu, Y.; Wang, S.; Wan, Y. Diameter structure and its effect on radial growth of Picea crassifolia forest in the Qilian Mountains. Arid Zone Res. 2017, 34, 1117–1123. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, X.; Li, G.; Wang, S.; Zhao, W.; Ma, J. Phenology of five shrub communities along an elevation gradient in the Qilian Mountains, China. Forests 2018, 9, 58. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Yu, P.T.; Wang, Y.H.; Wang, S.L.; Liu, X.D. Biomass change of middle aged forest of Qinghai spruce along an altitudinal gradient on the north slope of Qilian Mountains. Sci. Silva. Sin. 2015, 51, 4–10. [Google Scholar] [CrossRef]
- Wang, J.; Chang, X.; Ge, S.; Miao, M.; Chang, Z.; Zhang, H. Vertical distribution of the vegetation and water and heat conditions of Qilian Mountain (northern slope). J. Northwest For. Coll. 2001, 16, 1–3. [Google Scholar]
- Wang, J.; Wang, Y.; Wang, S.; Yu, P.; Zhang, X.; Ge, S. A preliminary study on the precipitation variation of complex watershed on forestry and grasses of Qilian Mountains. For. Res. 2006, 19, 416–422. [Google Scholar]
- Yang, W.; Wang, Y.; Webb, A.; Li, Z.; Tian, X.; Han, Z.; Wang, S.; Yu, P. Influence of climatic and geographic factors on the spatial distribution of Qinghai spruce forests in the dryland Qilian Mountains of Northwest China. Sci. Total Environ. 2018, 612, 1007–1017. [Google Scholar] [CrossRef] [PubMed]
- Zeide, B. Analysis of growth equations. For. Sci. 1993, 39, 594–616. [Google Scholar] [CrossRef]
- Liu, X.; Wang, C.; Zhao, J. Seasonal drought effects on intra-annual stem growth of Taiwan Pine along an elevational gradient in subtropical China. Forests 2019, 10, 1128. [Google Scholar] [CrossRef] [Green Version]
- Deslauriers, A.; Morin, H. Intra-annual tracheid production in balsam fir stems and the effect of meteorological variables. Trees 2005, 19, 402–408. [Google Scholar] [CrossRef] [Green Version]
- Oladi, R.; Pourtahmasi, K.; Eckstein, D.; Brauning, A. Seasonal dynamics of wood formation in Oriental beech (Fagus orientalis Lipsky) along an altitudinal gradient in the Hyrcanian forest, Iran. Trees 2011, 25, 425–433. [Google Scholar] [CrossRef]
- Levanič, T.; Gričar, J.; Gagen, M.; Jalkanen, R.; Loader, N.J.; McCarroll, D.; Oven, P.; Robertson, I. The climate sensitivity of Norway spruce (Picea abies (L.) Karst) in the Southeastern European Alps. Trees 2008, 23, 169–180. [Google Scholar] [CrossRef] [Green Version]
- Oberhuber, W.; Gruber, A.; Kofler, W.; Swidrak, I. Radial stem growth in response to microclimate and soil moisture in a drought-prone mixed coniferous forest at an inner Alpine site. Eur. J. Res. 2014, 133, 467–479. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rossi, S.; Deslauriers, A.; Anfodillo, T.; Morin, H.; Saracino, A.; Motta, R.; Borghetti, M. Conifers in cold environments synchronize maximum growth rate of tree-ring formation with day length. New Phytol. 2006, 170, 301–310. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Jiang, Y.; Dong, M.; Kang, M.; Yang, H. Relationship between the radial growth of Picea meyeri and climate along elevations of the Luyashan Mountain in North-Central China. For. Ecol. Manag. 2012, 265, 142–149. [Google Scholar] [CrossRef]
- Salzer, M.W.; Hughes, M.K.; Bunn, A.G.; Kipfmueller, K.F. Recent unprecedented tree-ring growth in bristlecone pine at the highest elevations and possible causes. Proc. Natl. Acad. Sci. USA 2009, 106, 20348–20353. [Google Scholar] [CrossRef] [Green Version]
- Barber, V.A.; Juday, G.P.; Finney, B.P. Reduced growth of Alaskan white spruce in the twentieth century from temperature-induced drought stress. Nature 2000, 405, 668–673. [Google Scholar] [CrossRef]
- Liu, H.; Williams, A.P.; Allen, C.; Guo, D.; Wu, X.; Anenkhonov, O.; Liang, E.; Sandanov, D.; Yin, Y.; Qi, Z.; et al. Rapid warming accelerates tree growth decline in semi-arid forests of Inner Asia. Glob. Chang. Biol. 2013, 19, 2500–2510. [Google Scholar] [CrossRef]
- Tardif, J.; Flannigan, M.; Bergeron, Y. An analysis of the daily radial activity of 7 boreal tree species: Northwestern Quebec. Environ. Monit. Assess. 2001, 67, 141–160. [Google Scholar] [CrossRef]
- Deslauriers, A.; Morin, H.; Urbinati, C. Daily weather response of balsam fir (Abies balsamea (L.) Mill.) stem radius increment from dendrometer analysis in the boreal forests of Qubec (Canada). Trees 2003, 17, 477–484. [Google Scholar] [CrossRef]
- Zweifel, R.; Zimmermann, L.; Zeugin, F.; Newbery, D.M. Intra-annual radial growth and water relations of trees: Implications towards a growth mechanism. J. Exp. Bot. 2006, 57, 1445–1459. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Way, D.A.; Sage, R.F. Elevated growth temperatures reduce the carbon gain of black spruce (Picea mariana (Mill.) BSP). Glob. Chang. Biol. 2008, 14, 624–636. [Google Scholar] [CrossRef]
- Ren, L.; Wang, S.; Yu, P.; Wang, Y.; Zhang, X.; Wang, B.; Liu, X.; Jin, M. Seasonal change and numerical simulation of the frozen soil under two types of vegetation in Qilian Mountains. For. Res. 2016, 29, 596–602. [Google Scholar] [CrossRef]
- Achim, A.; Moreau, G.; Coops, N.C.; Axelson, J.N.; Barrette, J.; Bédard, S.; Byrne, K.E.; Caspersen, J.; Dick, A.R.; D’Orangeville, L.; et al. The changing culture of silviculture. For. Int. J. For. Res. 2021, cpab047. [Google Scholar] [CrossRef]
- Pappas, C.; Bélanger, N.; Bergeron, Y.; Blarquez, O.; Chen, H.; Comeau, P.G.; Grandpré, L.D.; Delagrange, S.; DesRochers, A.; Diochon, A.; et al. Smartforests Canada: A network of monitoring plots for forest management under environmental change. In Climate-Smart Forestry in Mountain Regions; Springer: Cham, Switzerland, 2022; pp. 521–543. [Google Scholar] [CrossRef]
- Liang, E.; Wang, Y.; Piao, S.; Lu, X.; Camarero, J.; Zhu, H.; Zhu, L.; Ellison, A.; Ciais, P.; Penuelas, J. Species interactions slow warming-induced upward shifts of treelines on the Tibetan Plateau. Proc. Natl. Acad. Sci. USA 2016, 113, 4380–4385. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).