Environment Controls Seasonal and Daily Cycles of Stem Diameter Variations in Portuguese Oak (Quercus faginea Lambert)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site and Data Collection
2.2. Data Analysis
3. Results
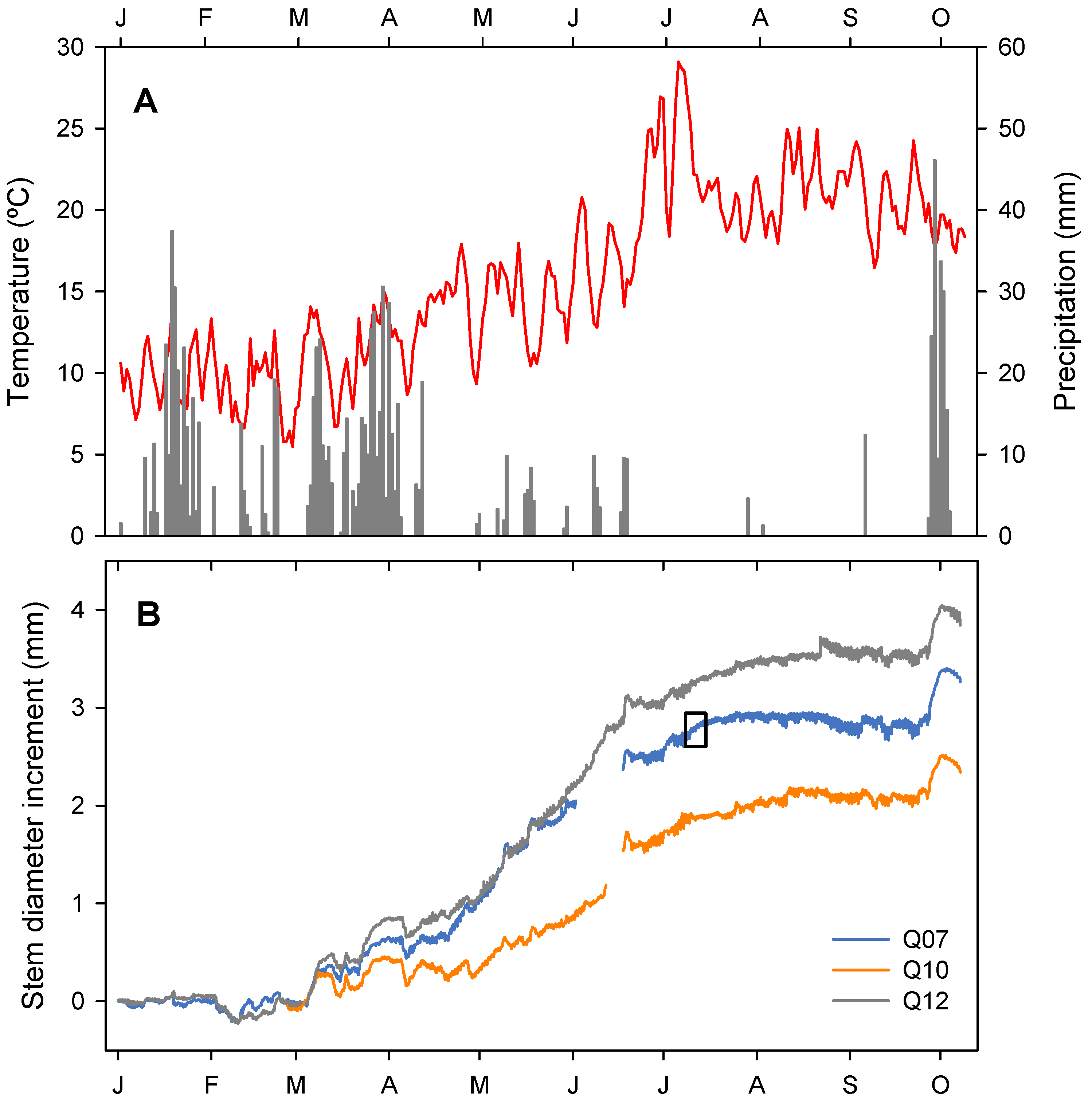
3.1. Climatic Conditions of the Study Year
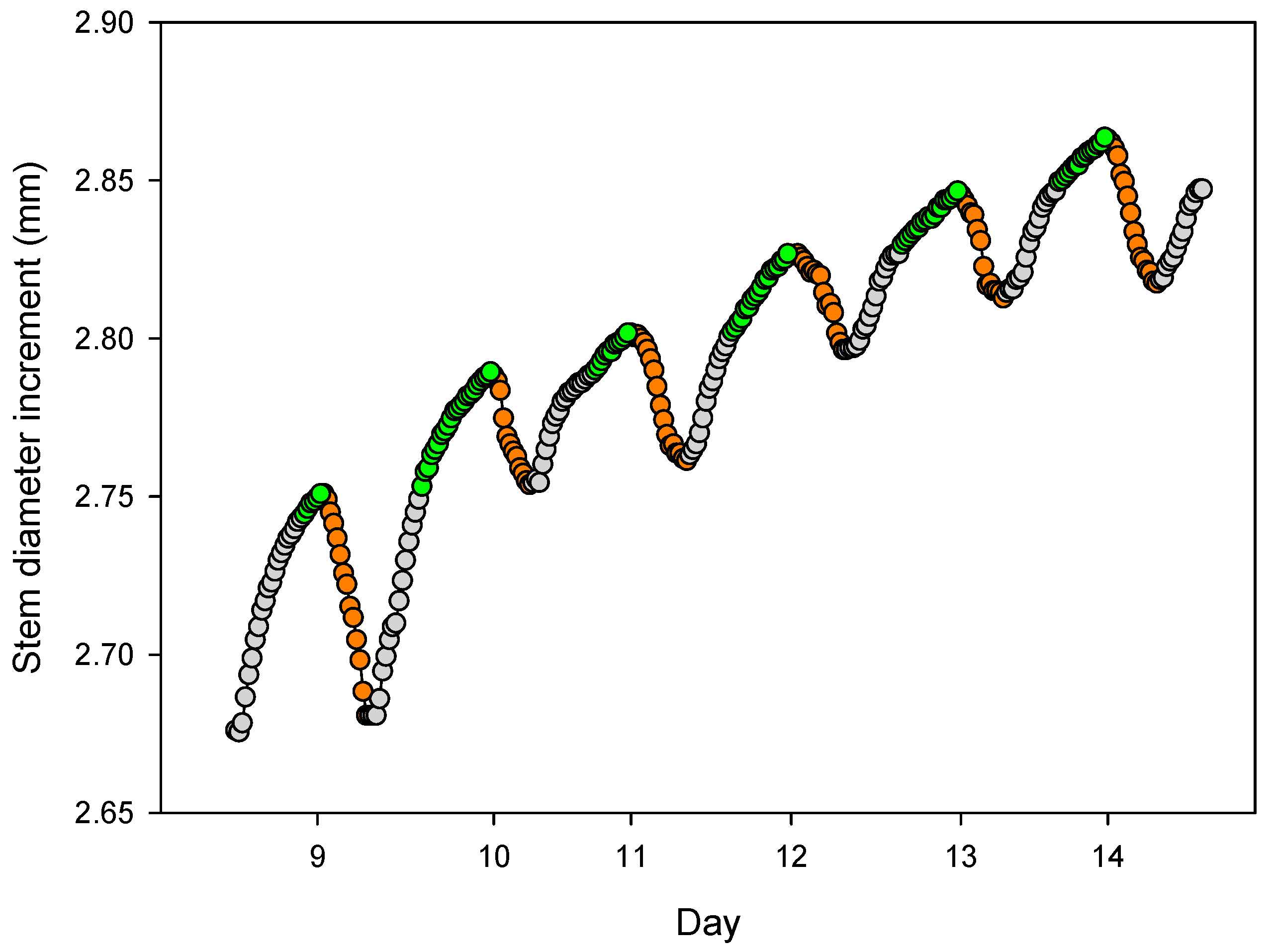
3.2. Stem Diameter Variation
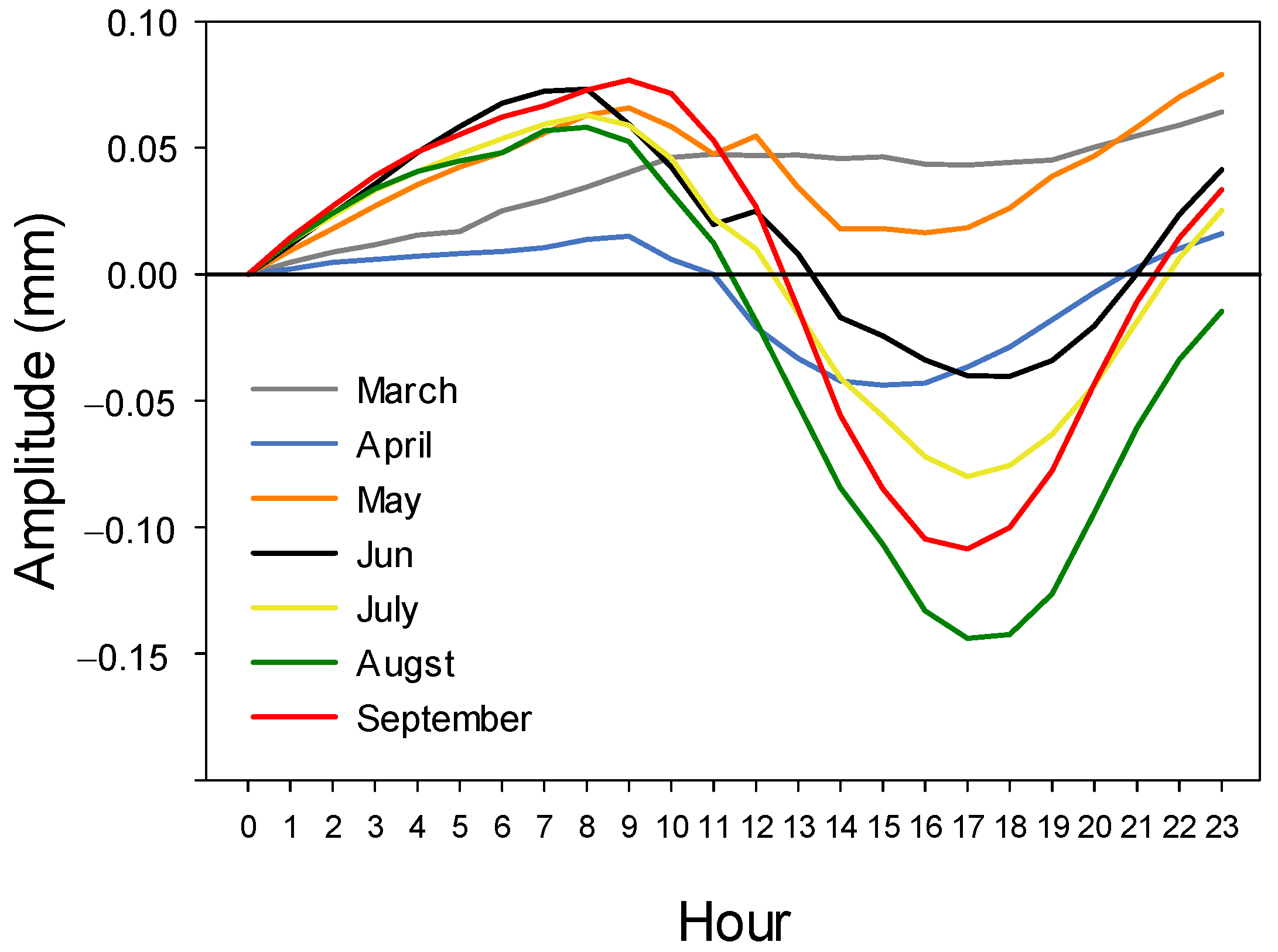
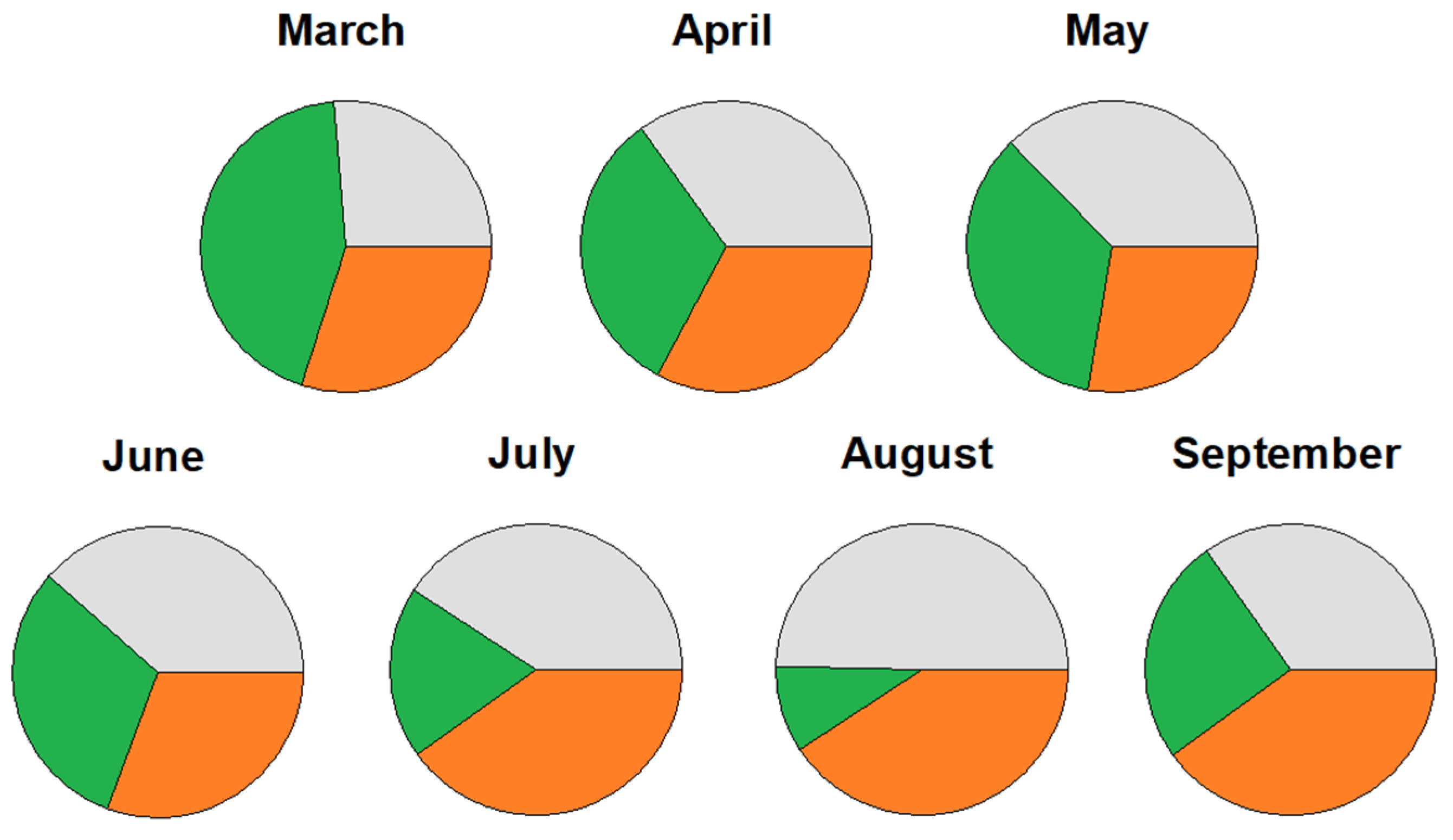
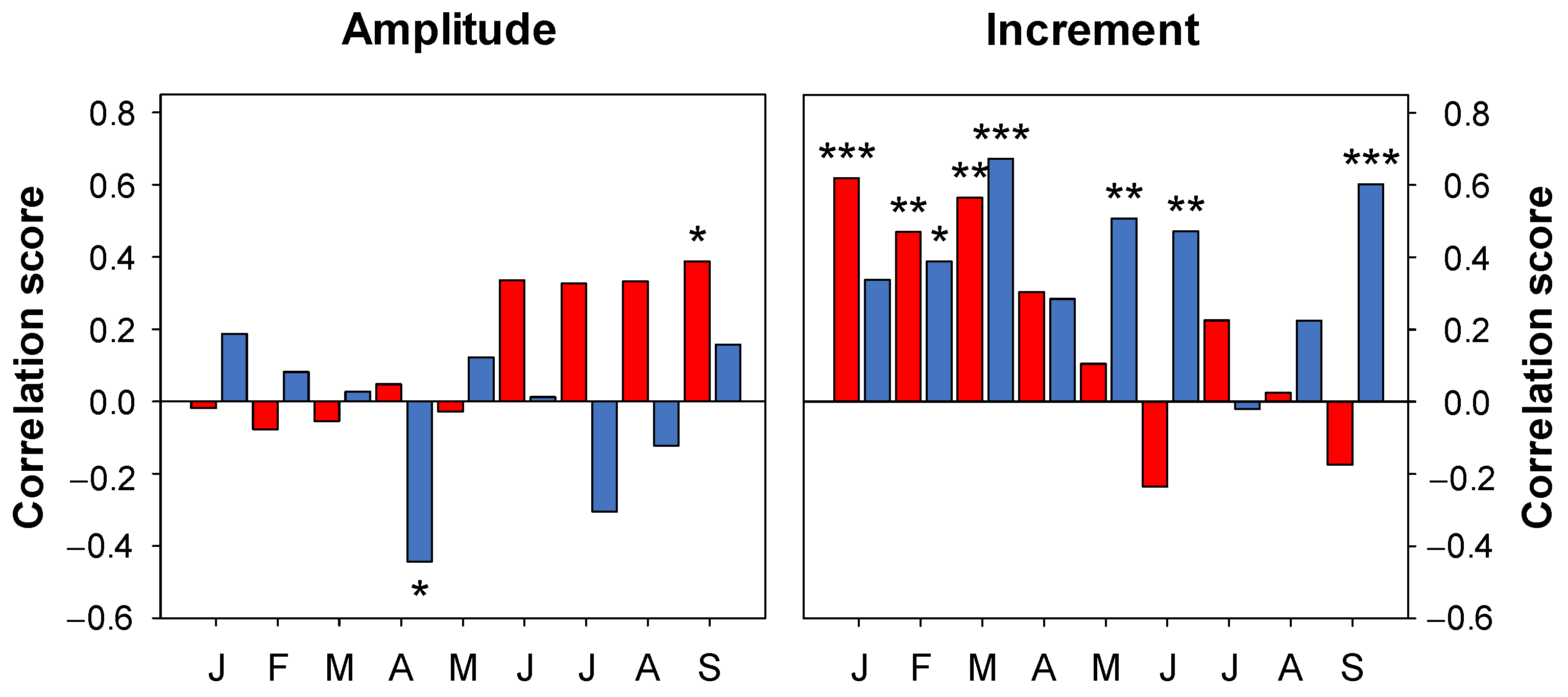
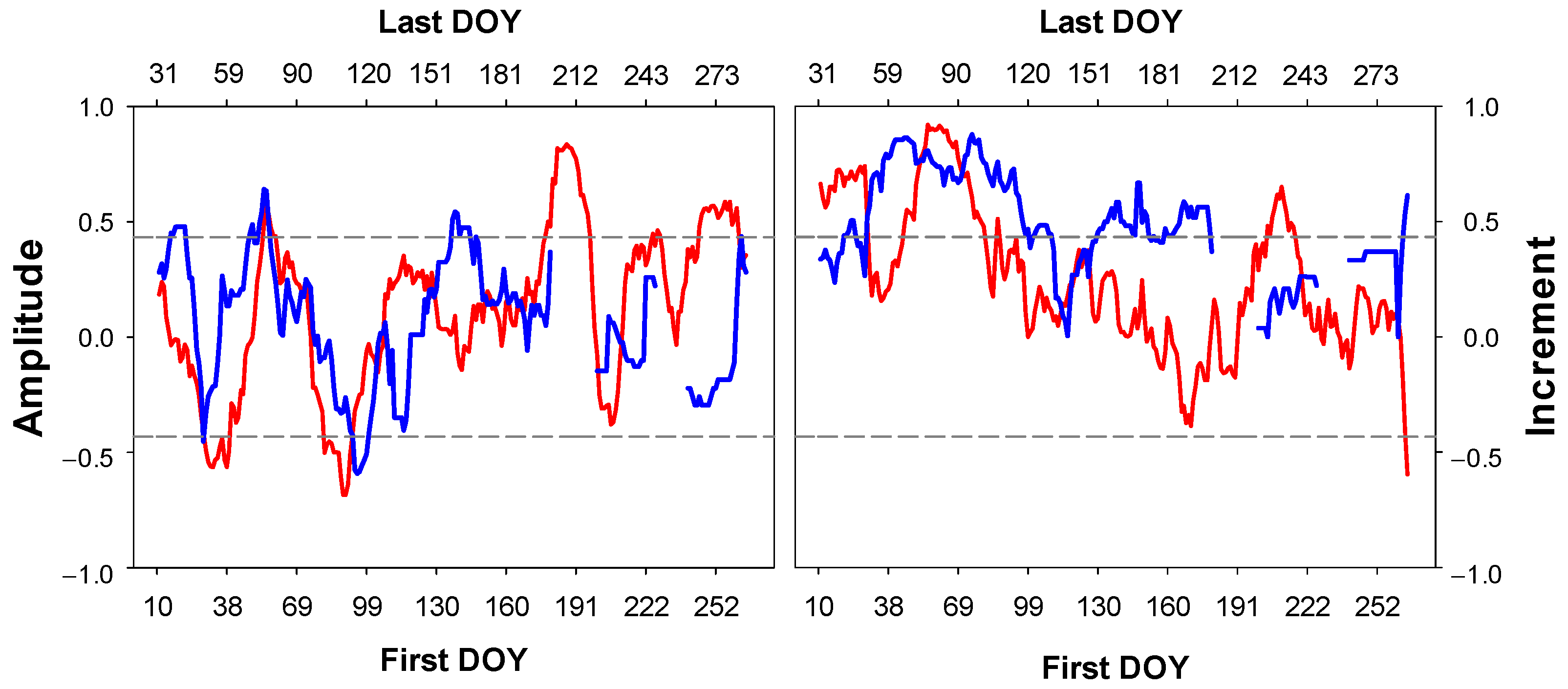
3.3. Climatic Signal
4. Discussion
4.1. Seasonal Pattern
4.2. Daily Cycles and Its Ecophysiological Meaning
4.3. Climate Response
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fritts, H.C. Tree Rings and Climate; Academic Press: London, UK, 1976. [Google Scholar]
- Huang, J.-G.; Ma, Q.; Rossi, S.; Biondi, F.; Deslauriers, A.; Fonti, P.; Liang, E.; Mäkinen, H.; Oberhuber, W.; Rathgeber, C.B.K.; et al. Photoperiod and temperature as dominant environmental drivers triggering secondary growth resumption in Northern Hemisphere conifers. Proc. Natl. Acad. Sci. USA 2020, 117, 20645–20652. [Google Scholar] [CrossRef]
- Delpierre, N.; Lireux, S.; Hartig, F.; Camarero, J.J.; Cheaib, A.; Čufar, K.; Cuny, H.E.; Deslauriers, A.; Fonti, P.; Gričar, J.; et al. Chilling and forcing temperatures interact to predict the onset of wood formation in Northern Hemisphere conifers. Glob. Chang. Biol. 2019, 25, 1089–1105. [Google Scholar] [CrossRef]
- Rossi, S.; Deslauriers, A.; Anfodillo, T.; Morin, H.; Saracino, A.; Motta, R.; Borghetti, M. Conifers in cold environments synchronize maximum growth rate of tree-ring formation with day length. New Phytol. 2006, 170, 301–310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vieira, J.; Carvalho, A.; Campelo, F. Tree growth under climate change: Evidence from xylogenesis timings and kinetics. Front. Plant Sci. 2020, 11, 90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deslauriers, A.; Morin, H.; Urbinati, C.; Carrer, M. Daily weather response of balsam fir (Abies balsamea (L.) Mill.) stem radius increment from dendrometer analysis in the boreal forests of Québec (Canada). Trees 2003, 17, 477–484. [Google Scholar] [CrossRef]
- Zweifel, R.; Zimmermann, L.; Zeugin, F.; Newbery, D.M. Intra-annual radial growth and water relations of trees: Implications towards a growth mechanism. J. Exp. Bot. 2006, 57, 1445–1459. [Google Scholar] [CrossRef] [Green Version]
- Drew, D.D.M.; Downes, G.M. The use of precision dendrometers in research on daily stem size and wood property variation: A review. Dendrochronologia 2009, 27, 159–172. [Google Scholar] [CrossRef]
- Gutiérrez, E.; Campelo, F.; Camarero, J.J.; Ribas, M.; Muntán, E.; Nabais, C.; Freitas, H. Climate controls act at different scales on the seasonal pattern of Quercus ilex L. stem radial increments in NE Spain. Trees 2011, 25, 637–646. [Google Scholar] [CrossRef]
- Perämäki, M.; Nikinmaa, E.; Sevanto, S.; Ilvesniemi, H.; Siivola, E.; Hari, P.; Vesala, T. Tree stem diameter variations and transpiration in Scots pine: An analysis using a dynamic sap flow model. Tree Physiol. 2001, 21, 889–897. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zweifel, R.; Drew, D.M.; Schweingruber, F.; Downes, G.M. Xylem as the main origin of stem radius changes in Eucalyptus. Funct. Plant Biol. 2014, 41, 520–534. [Google Scholar] [CrossRef] [Green Version]
- Oberhuber, W.; Sehrt, M.; Kitz, F. Hygroscopic properties of thin dead outer bark layers strongly influence stem diameter variations on short and long time scales in Scots pine (Pinus sylvestris L.). Agric. For. Meteorol. 2020, 290, 108026. [Google Scholar] [CrossRef]
- De Swaef, T.; De Schepper, V.; Vandegehuchte, M.W.; Steppe, K. Stem diameter variations as a versatile research tool in ecophysiology. Tree Physiol. 2015, 35, 1047–1061. [Google Scholar] [CrossRef] [PubMed]
- Irvine, J.; Grace, J. Continuous measurements of water tensions in the xylem of trees based on the elastic properties of wood. Planta 1997, 202, 455–461. [Google Scholar] [CrossRef]
- Scholz, F.C.; Bucci, S.J.; Goldstein, G.; Meinzer, F.C.; Franco, A.C.; Miralles-Wilhelm, F. Temporal dynamics of stem expansion and contraction in savanna trees: Withdrawal and recharge of stored water. Tree Physiol. 2008, 28, 469–480. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deslauriers, A.; Rossi, S.; Anfodillo, T. Dendrometer and intra-annual tree growth: What kind of information can be inferred? Dendrochronologia 2007, 25, 113–124. [Google Scholar] [CrossRef] [Green Version]
- Zweifel, R.; Sterck, F.; Braun, S.; Buchmann, N.; Eugster, W.; Gessler, A.; Häni, M.; Peters, R.L.; Walthert, L.; Wilhelm, M.; et al. Why trees grow at night. New Phytol. 2021, 231, 2174–2185. [Google Scholar] [CrossRef]
- Čermák, J.; Kučera, J.; Bauerle, W.L.; Phillips, N.; Hinckley, T.M. Tree water storage and its diurnal dynamics related to sap flow and changes in stem volume in old-growth Douglas-fir trees. Tree Physiol. 2007, 27, 181–198. [Google Scholar] [CrossRef]
- Sevanto, S.; Vesala, T.; Peramaki, M.; Nikinmaa, E. Time lags for xylem and stem diameter variations in a Scots pine tree. Plant Cell Environ. 2002, 25, 1071–1077. [Google Scholar] [CrossRef]
- Zweifel, R.; Item, H.; Hasler, R.; Häsler, R. Link between diurnal stem radius changes and tree water relations. Tree Physiol. 2001, 21, 869–877. [Google Scholar] [CrossRef] [Green Version]
- Zweifel, R.; Item, H.; Häsler, R. Stem radius changes and their relation to stored water in stems of young Norway spruce trees. Trees 2000, 15, 50–57. [Google Scholar] [CrossRef] [Green Version]
- Vieira, J.; Rossi, S.; Campelo, F.; Freitas, H.; Nabais, C. Seasonal and daily cycles of stem radial variation of Pinus pinaster in a drought-prone environment. Agric. For. Meteorol. 2013, 180, 173–181. [Google Scholar] [CrossRef] [Green Version]
- King, G.; Fonti, P.; Nievergelt, D.; Büntgen, U.; Frank, D. Climatic drivers of hourly to yearly tree radius variations along a 6 °C natural warming gradient. Agric. For. Meteorol. 2013, 168, 36–46. [Google Scholar]
- Arias, P.; Bellouin, N.; Coppola, E.; Jones, R.; Krinner, G.; Marotzke, J.; Vaishali, N.; Matthew, P.; Plattner, G.-K.; Joeri, R.; et al. IPCC Climate Change 2021: The Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change. Masson-Delmotte, V., Zhai, P., Pirani, A., Connors, S.L., Péan, C., Berger, S., Caud, N., Chen, Y., Eds.; Cambridge University Press: Cambridge, UK, 2021; p. 3949. [Google Scholar]
- Jacob, D.; Petersen, J.; Eggert, B.; Alias, A.; Christensen, O.B.; Bouwer, L.M.; Braun, A.; Colette, A.; Déqué, M.; Georgievski, G.; et al. EURO-CORDEX: New high-resolution climate change projections for European impact research. Reg. Environ. Chang. 2014, 14, 563–578. [Google Scholar] [CrossRef]
- Peguero-Pina, J.J.; Sisó, S.; Sancho-Knapik, D.; Diaz-Espejo, A.; Flexas, J.; Galmés, J.; Gil-Pelegrín, E. Leaf morphological and physiological adaptations of a deciduous oak (Quercus faginea Lam.) to the Mediterranean climate: A comparison with a closely related temperate species (Quercus robur L.). Tree Physiol. 2016, 36, 287–299. [Google Scholar] [CrossRef] [Green Version]
- Colangelo, M.; Camarero, J.J.; Borghetti, M.; Gazol, A.; Gentilesca, T.; Ripullone, F. Size matters a lot: Drought-affected Italian oaks are smaller and show lower growth prior to tree death. Front. Plant Sci. 2017, 8, 135. [Google Scholar] [CrossRef] [Green Version]
- Mediavilla, S.; Escudero, A. Stomatal responses to drought at a Mediterranean site: A comparative study of co-occurring woody species differing in leaf longevity. Tree Physiol. 2003, 23, 987–996. [Google Scholar] [CrossRef]
- De Dios, R.S.; Benito-Garzón, M.; Sainz-Ollero, H. Present and future extension of the Iberian submediterranean territories as determined from the distribution of marcescent oaks. Plant Ecol. 2009, 204, 189–205. [Google Scholar] [CrossRef]
- Sousa, V.B.; Cardoso, S.; Pereira, H. Age trends in the wood anatomy of Quercus faginea. IAWA J. 2014, 35, 293–306. [Google Scholar] [CrossRef]
- Direcção-Geral Do Território Uso E Ocupação Do Solo Em Portugal Continental—Análise Temática I. 2020. Available online: https://www.dgterritorio.gov.pt/sites/default/files/publicacoes/folheto_cos_lq.pdf (accessed on 16 January 2022).
- Capelo, J.; Catry, F. A distribuição do carvalho-portugês em Portugal. In Árvores E Florestas De Potugal—Os Carvalhais, Um Património A Conservar; Silva, J.S., Ed.; Público/FLAD/LPN: Lisbon, Portugal, 2007; p. 94. [Google Scholar]
- Vieira, J.; Campelo, F.; Rossi, S.; Carvalho, A.; Freitas, H.; Nabais, C. Adjustment capacity of maritime pine cambial activity in drought-prone environments. PLoS ONE 2015, 10, e0126223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Campelo, F.; Ribas, M.; Gutiérrez, E. Plastic bimodal growth in a Mediterranean mixed-forest of Quercus ilex and Pinus halepensis. Dendrochronologia 2021, 67, 125836. [Google Scholar] [CrossRef]
- Garcia-Forner, N.; Vieira, J.; Nabais, C.; Carvalho, A.; Martínez-Vilalta, J.; Campelo, F. Climatic and physiological regulation of the bimodal xylem formation pattern in Pinus pinaster saplings. Tree Physiol. 2019, 39, 2008–2018. [Google Scholar] [CrossRef] [PubMed]
- Campelo, F.; Gutiérrez, E.; Ribas, M.; Sánchez-Salguero, R.; Nabais, C.; Camarero, J.J. The facultative bimodal growth pattern in Quercus ilex—A simple model to predict sub-seasonal and inter-annual growth. Dendrochronologia 2018, 49, 77–88. [Google Scholar] [CrossRef]
- Camarero, J.J.; Rubio-Cuadrado, Á.; Gazol, A. Climate windows of intra-annual growth and post-drought recovery in Mediterranean trees. Agric. For. Meteorol. 2021, 308–309, 108606. [Google Scholar] [CrossRef]
- Pérez-De-Lis, G.; Olano, J.M.; Rozas, V.; Rossi, S.; Vázquez-Ruiz, R.A.; García-González, I. Environmental conditions and vascular cambium regulate carbon allocation to xylem growth in deciduous oaks. Funct. Ecol. 2017, 31, 592–603. [Google Scholar] [CrossRef] [Green Version]
- Downes, G.; Beadle, C.; Worledge, D. Daily stem growth patterns in irrigated Eucalyptus globulus and E. nitens in relation to climate. Trees 1999, 14, 102–111. [Google Scholar] [CrossRef]
- Van der Maaten, E.; van der Maaten-Theunissen, M.; Smiljanić, M.; Rossi, S.; Simard, S.; Wilmking, M.; Deslauriers, A.; Fonti, P.; von Arx, G.; Bouriaud, O. dendrometeR: Analyzing the pulse of trees in R. Dendrochronologia 2016, 40, 12–16. [Google Scholar] [CrossRef]
- Rossi, S.; DesLauriers, A.; Anfodillo, T.; Carraro, V. Evidence of threshold temperatures for xylogenesis in conifers at high altitudes. Oecologia 2007, 152, 1–12. [Google Scholar] [CrossRef]
- Gričar, J.; Zupančič, M.; Čufar, K.; Koch, G.; Schmitt, U.; Oven, P. Effect of local heating and cooling on cambial activity and cell differentiation in the stem of norway spruce (Picea abies). Ann. Bot. 2006, 97, 943–951. [Google Scholar] [CrossRef] [Green Version]
- Vieira, J.; Rossi, S.; Campelo, F.; Freitas, H.; Nabais, C. Xylogenesis of Pinus pinaster under a Mediterranean climate. Ann. For. Sci. 2014, 71, 71–80. [Google Scholar] [CrossRef] [Green Version]
- Begum, S.; Nakaba, S.; Yamagishi, Y.; Oribe, Y.; Funada, R. Regulation of cambial activity in relation to environmental conditions: Understanding the role of temperature in wood formation of trees. Physiol. Plant. 2013, 147, 46–54. [Google Scholar] [CrossRef] [PubMed]
- González-González, B.D.; García-González, I.; Vázquez-Ruiz, R.A. Comparative cambial dynamics and phenology of Quercus robur L. and Q. pyrenaica Willd. in an Atlantic forest of the northwestern Iberian Peninsula. Trees 2013, 27, 1571–1585. [Google Scholar] [CrossRef]
- Forner, A.; Aranda, I.; Granier, A.; Valladares, F. Differential impact of the most extreme drought event over the last half century on growth and sap flow in two coexisting Mediterranean trees. Plant Ecol. 2014, 215, 703–719. [Google Scholar] [CrossRef] [Green Version]
- Grossiord, C.; Forner, A.; Gessler, A.; Granier, A.; Pollastrini, M.; Valladares, F.; Bonal, D. Influence of species interactions on transpiration of Mediterranean tree species during a summer drought. Eur. J. For. Res. 2015, 134, 365–376. [Google Scholar] [CrossRef] [Green Version]
- Brodribb, T.J.; Holbrook, N.M. Changes in leaf hydraulic conductance during leaf shedding in seasonally dry tropical forest. New Phytol. 2003, 158, 295–303. [Google Scholar] [CrossRef]
- Salleo, S.; Nardini, A.; Gullo, M.L.; Ghirardelli, L. Changes in stem and leaf hydraulics preceding leaf shedding in Castanea Sativa L. Biol. Plant. 2002, 45, 227–234. [Google Scholar] [CrossRef]
- Corcuera, L.; Camarero, J.; Gil-Pelegrín, E. Effects of a severe drought on growth and wood anatomical properties of Quercus faginea. IAWA J. 2004, 25, 185–204. [Google Scholar] [CrossRef]
- Sass-Klaassen, U.; Sabajo, C.R.; Ouden, J.D. Vessel formation in relation to leaf phenology in pedunculate oak and European ash. Dendrochronologia 2011, 29, 171–175. [Google Scholar] [CrossRef]
- Abe, H.; Nakai, T.; Utsumi, Y.; Kagawa, A. Temporal water deficit and wood formation in Cryptomeria japonica. Tree Physiol. 2003, 23, 859–863. [Google Scholar] [CrossRef] [Green Version]
- Albuixech, J.; Camarero, J.; Montserrat-Marti, G. Dinámica estacional del crecimiento secundario y anatomía del xilema en dos Quercus mediterráneos que coexisten. For. Syst. 2012, 21, 9–22. [Google Scholar] [CrossRef] [Green Version]
- Bingre, P.; Damasceno, P. Biologia e ecologia das florestas de carvalho-portugês. In Árvores E Florestas De Potugal—Os Carvalhais, Um Património A Conservar; Silva, J., Ed.; Público/FLAD/LPN: Lisbon, Portugal, 2007; pp. 15–46. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vieira, J.; Campelo, F.; Nabais, C. Environment Controls Seasonal and Daily Cycles of Stem Diameter Variations in Portuguese Oak (Quercus faginea Lambert). Forests 2022, 13, 170. https://doi.org/10.3390/f13020170
Vieira J, Campelo F, Nabais C. Environment Controls Seasonal and Daily Cycles of Stem Diameter Variations in Portuguese Oak (Quercus faginea Lambert). Forests. 2022; 13(2):170. https://doi.org/10.3390/f13020170
Chicago/Turabian StyleVieira, Joana, Filipe Campelo, and Cristina Nabais. 2022. "Environment Controls Seasonal and Daily Cycles of Stem Diameter Variations in Portuguese Oak (Quercus faginea Lambert)" Forests 13, no. 2: 170. https://doi.org/10.3390/f13020170
APA StyleVieira, J., Campelo, F., & Nabais, C. (2022). Environment Controls Seasonal and Daily Cycles of Stem Diameter Variations in Portuguese Oak (Quercus faginea Lambert). Forests, 13(2), 170. https://doi.org/10.3390/f13020170






