Scanning Electron Microscopy Protocol for Studying Anatomy of Highly Degraded Waterlogged Archaeological Wood
Abstract
1. Introduction
2. Materials and Methods
2.1. Waterlogged Archaeological Wood
2.2. Physical Properties of WAW
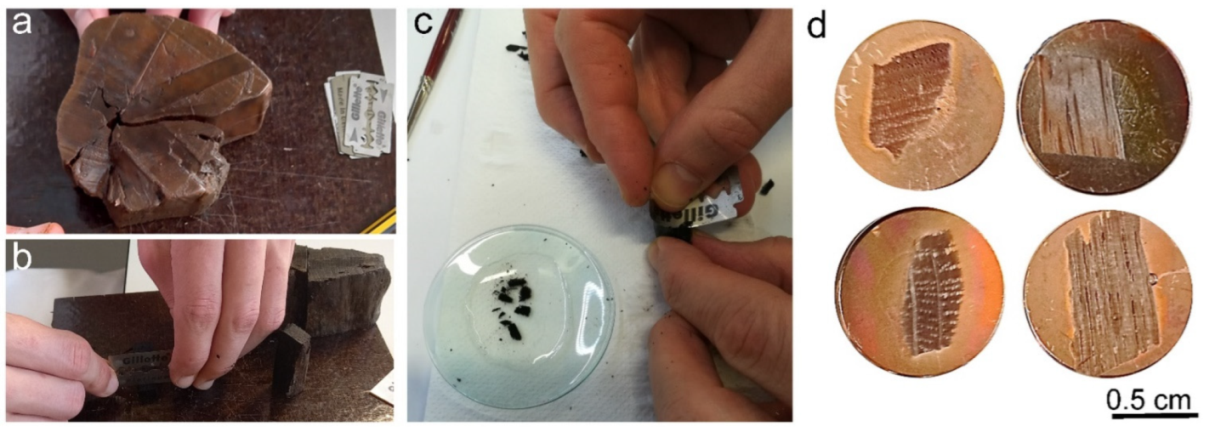
2.3. Scanning Electron Microscopy (SEM) and Energy Dispersive X-ray Spectroscopy (EDX)
2.4. Light Microscopy (LM)
3. Results and Discussion
3.1. Physical Properties of WAW
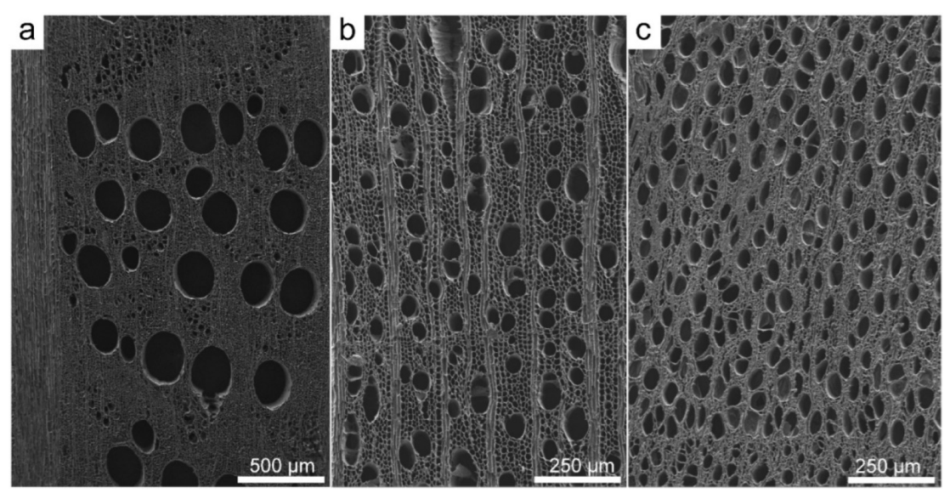
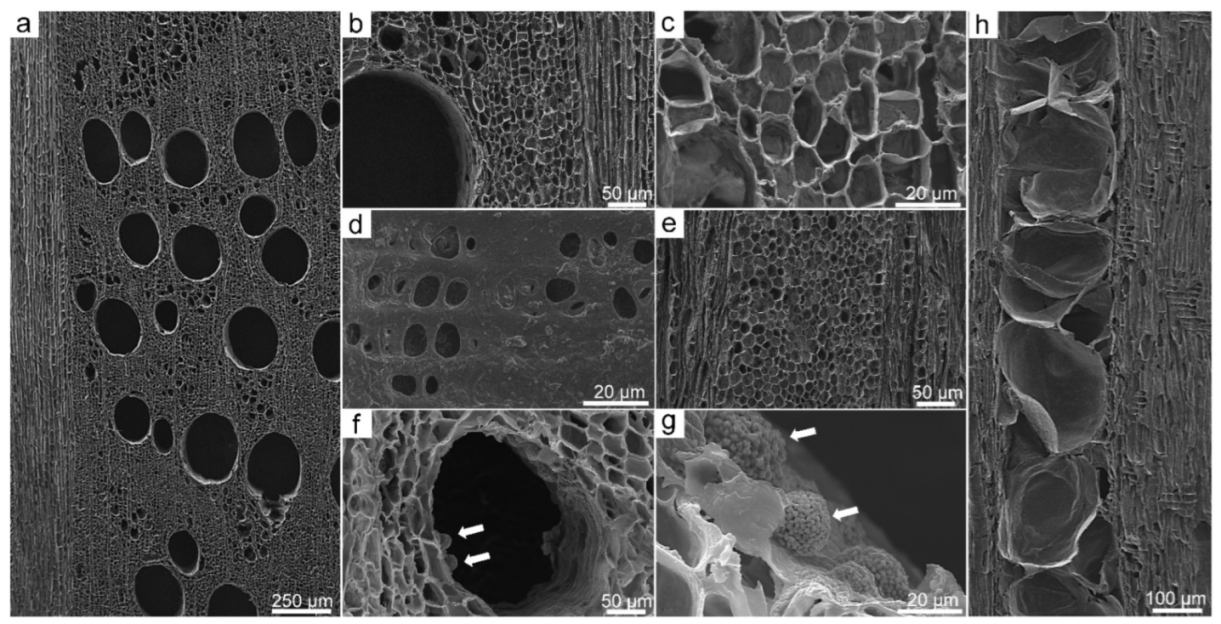
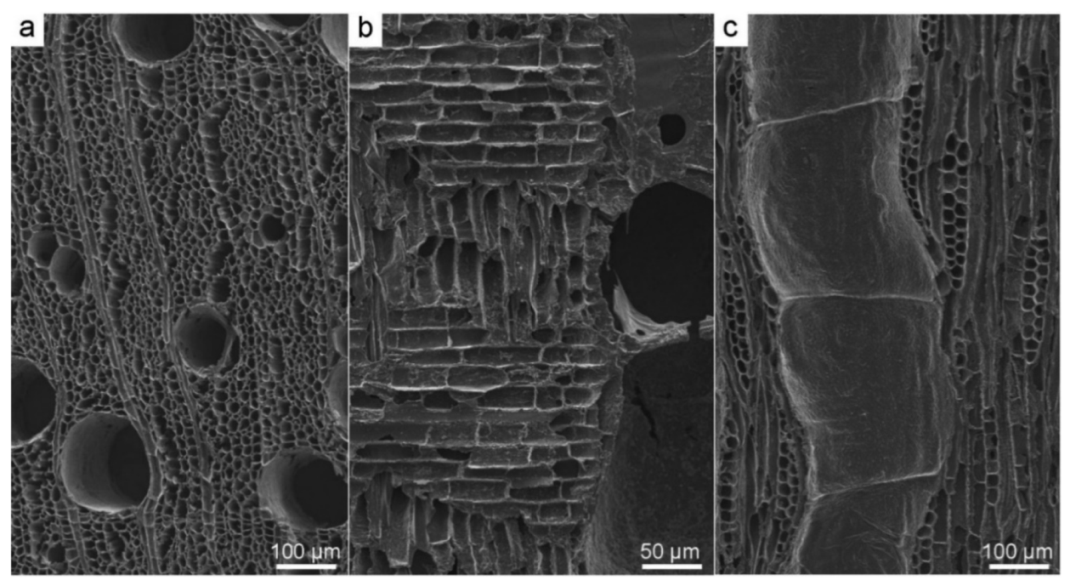
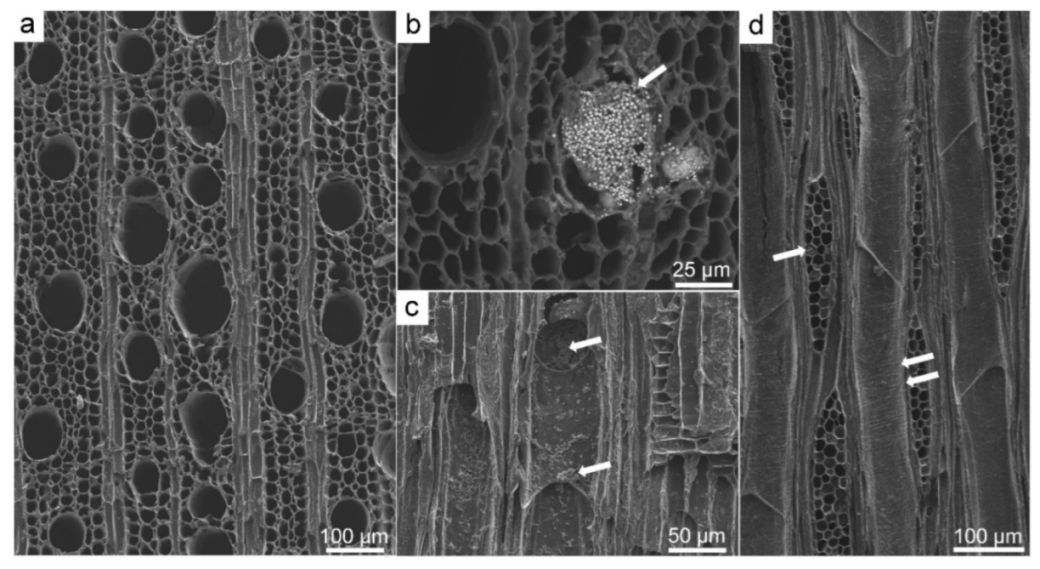
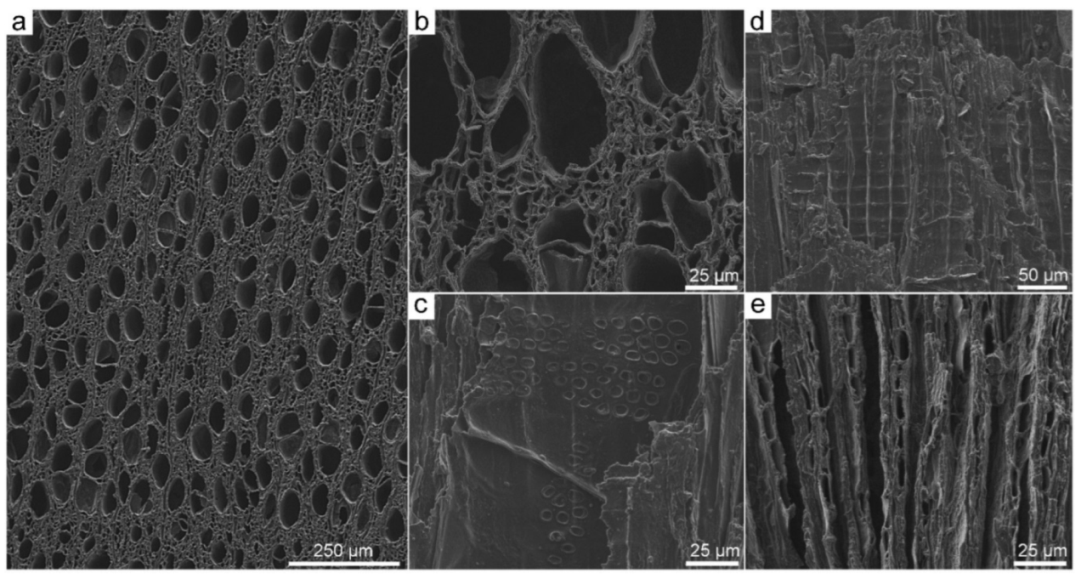
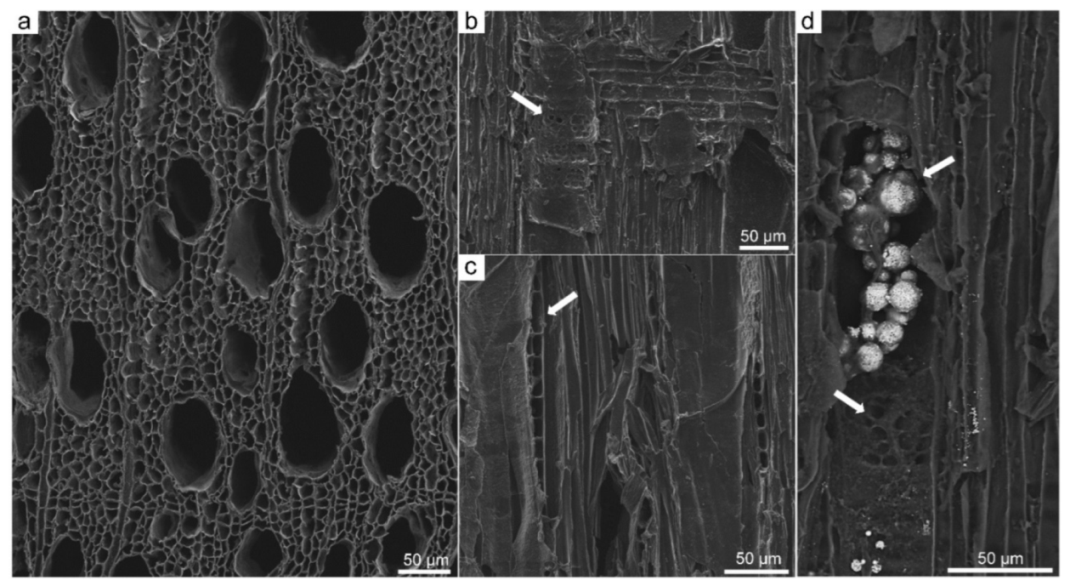
3.2. Scanning Electron Microscopy (SEM)
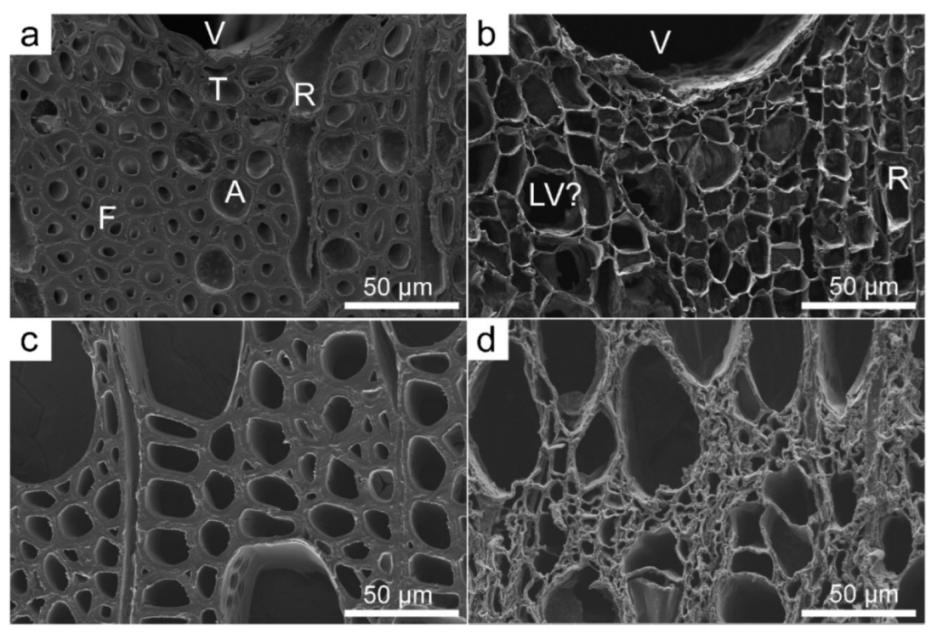
3.3. Application of SEM for Wood Anatomy and Wood Identification
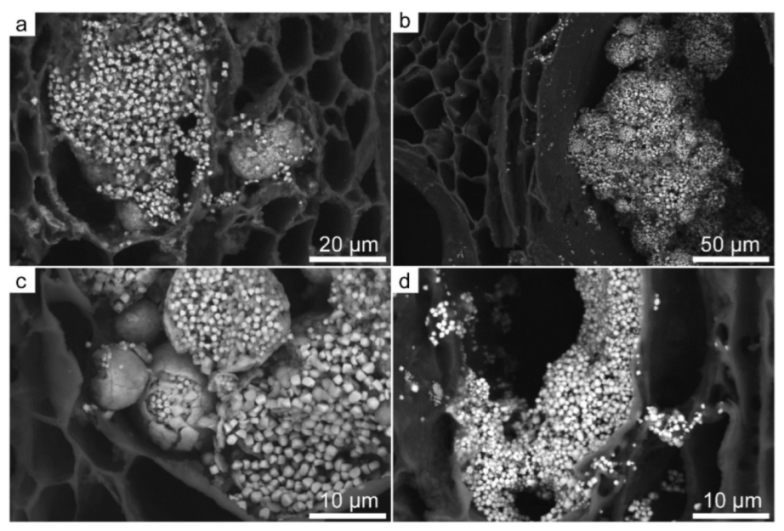
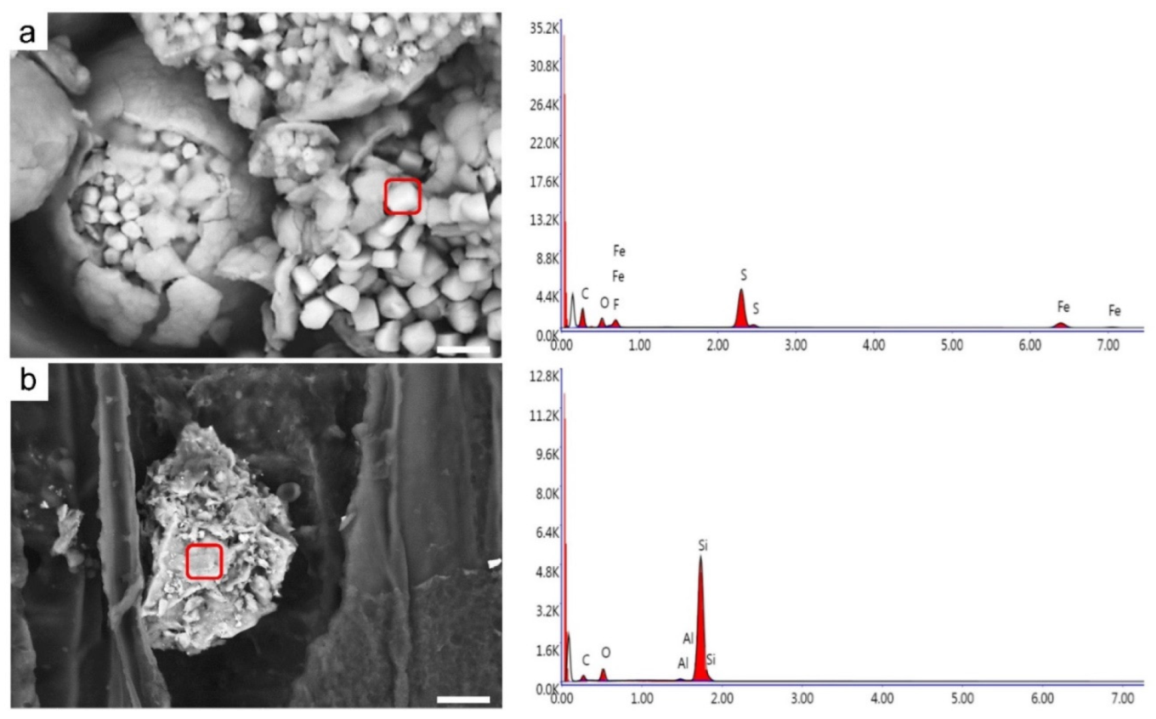
3.4. Mineral Inclusions
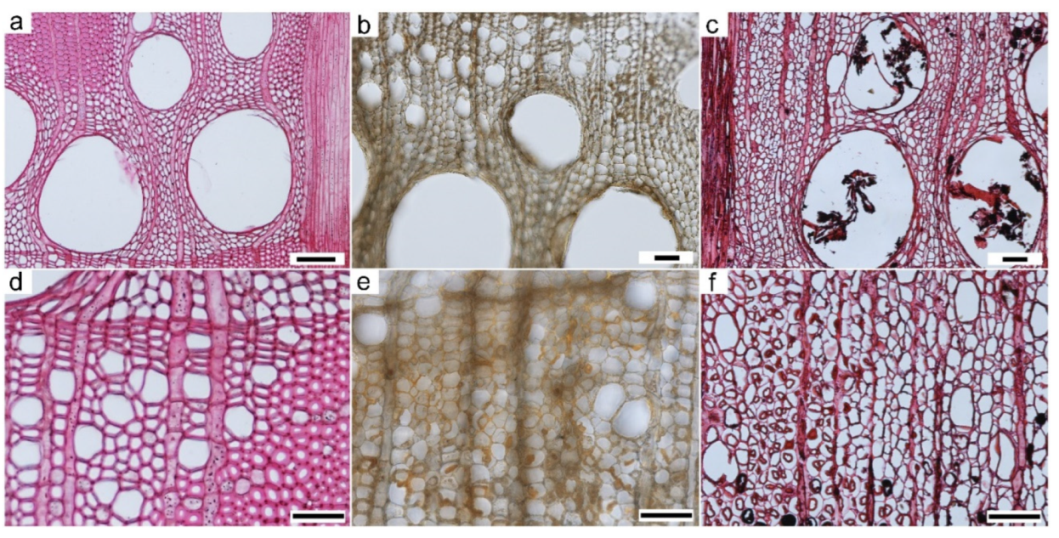
3.5. Light Microscopy (LM)
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Domínguez-Delmás, M.; Rich, S.; Traoré, M.; Hajj, F.; Poszwa, A.; Akhmetzyanov, L.; Groenendijk, P. Tree-ring chronologies, stable strontium isotopes and biochemical compounds: Towards reference datasets to provenance Iberian shipwreck timbers. J. Archaeol. Sci. Rep. 2020, 34, 102640. [Google Scholar] [CrossRef]
- Pearl, J.K.; Keck, J.R.; Tintor, W.; Siekacz, L.; Herrick, H.M.; Meko, M.D.; Pearson, C.L. New frontiers in tree-ring research. Holocene 2020, 30, 923–941. [Google Scholar] [CrossRef]
- Jordan, B.A. Site characteristics impacting the survival of historic waterlogged wood: A review. Int. Biodeterior. Biodegrad. 2001, 47, 47–54. [Google Scholar] [CrossRef]
- Donaldson, L.A.; Singh, A.P. Ultrastructure of Terminalia wood from an ancient Polynesian canoe. IAWA J. 1990, 11, 195–202. [Google Scholar] [CrossRef]
- Singh, A.P.; Nilsson, T.; Daniel, G. Bacterial attack of Pinus sylvestris wood under near-anaerobic conditions. J. Inst. Wood Sci. 1990, 11, 237–249. [Google Scholar]
- Kim, Y.S.; Singh, A.P. Micromorphological characteristics of wood biodegradation in wet environments: A review. IAWA J. 2000, 21, 135–155. [Google Scholar] [CrossRef]
- Björdal, C.G. Microbial degradation of waterlogged archaeological wood. J. Cult. Herit. 2012, 13, S118–S122. [Google Scholar] [CrossRef]
- Singh, A.P.; Kim, Y.S.; Singh, T. Bacterial Degradation of Wood. In Secondary Xylem Biology; Academic Press: Cambridge, MA, USA, 2016; pp. 169–190. [Google Scholar] [CrossRef]
- Singh, A.P.; Hedley, M.E.; Page, D.R.; Han, C.S.; Atisongkroh, K. Microbial decay of CCA-treated cooling tower timbers. IAWA J. 1992, 13, 215–231. [Google Scholar] [CrossRef]
- Singh, A.P.; Kim, Y.S.; Wi, S.G.; Lee, K.H.; Kim, I.J. Evidence of the degradation of middle lamella in a waterlogged archaeological wood. Holzforschung 2003, 57, 115–119. [Google Scholar] [CrossRef]
- Rowell, R.M.; Barbour, J. (Eds.) Archaeological Wood: Properties, Chemistry and Preservation, Advances in Chemistry; American Chemical Society: Washington, DC, USA, 1990; Volume 225. [Google Scholar]
- Kim, Y.S.; Singh, A.P. Ultrastructural aspects of bacterial attacks of a submerged ancient wood. Mokuzai Gakkaishi 1994, 40, 554–562. [Google Scholar]
- Björdal, C.G.; Daniel, G.; Nilsson, T. Depth of burial, an important factor in controlling bacterial decay of waterlogged archaeological poles. Int. Biodeterior. Biodegrad. 2000, 45, 15–26. [Google Scholar] [CrossRef]
- Schmitt, U.; Singh, A.P.; Thieme, H.; Friedrich, P.; Hoffmann, P. Electron microscopic char¬acterization of cell wall degradation of the 400,000-year-old wooden Schöningen spears. Holz Roh-Werkst. 2005, 63, 118–122. [Google Scholar] [CrossRef]
- Singh, A.P.; Kim, Y.S.; Chavan, R.R. Relationship of wood cell wall ultrastructure to bacterial degradation of wood. IAWA J. 2019, 40, 845–870. [Google Scholar] [CrossRef]
- Daniel, G.; Nilsson, T. Developments in the study of soft rot and bacterial decay. In Forest Products Biotechnology; Bruce, A., Palfreymann, J.W., Eds.; Taylor & Francis Ltd.: London, UK, 1998; pp. 37–62. [Google Scholar]
- Klaassen, R.K. Bacterial decay in wooden foundation piles—Patterns and causes: A study of historical pile foundations in the Netherlands. Int. Biodeterior. Biodegrad. 2008, 61, 45–60. [Google Scholar] [CrossRef]
- Björdal, G.C.; Nilsson, T.; Daniel, G. Microbial decay of waterlogged archaeological wood found in Sweden: Applicable to archaeology and conservation. Int. Biodeterior. Biodegrad. 1999, 43, 63–71. [Google Scholar] [CrossRef]
- Billamboz, A. Regional patterns of settlement and woodland developments: Dendroarchaeology in the Neolithic pile-dwellings on Lake Constance (Germany). Holocene. Holocene 2014, 24, 1278–1287. [Google Scholar] [CrossRef]
- Bleicher, N.; Harb, C. Settlement and social organisation in the late fourth millennium BC in Central Europe: The waterlogged site of Zurich-Parkhaus Opéra. Antiquity 2018, 92, 1210–1230. [Google Scholar] [CrossRef]
- Capano, M.; Martinelli, N.; Baioni, M.; Tuna, T.; Bernabei, M.; Bard, E. Is the dating of short tree-ring series still a challenge? New evidence from the pile dwelling of Lucone di Polpenazze (northern Italy). J. Archaeol. Sci. 2020, 121, 105190. [Google Scholar] [CrossRef]
- Čufar, K.; Velušček, A.; Kromer, B. Two Decades of Dendrochronology in the Pile Dwellings of the Ljubljansko Barje, Slovenija. In Chronologie Typologie Ökologie: Festschrift für André Billamboz zum 65. Geburtstag, 1st ed.; Bleicher, N., Ed.; Janus Verlag: Freiburg, Germany, 2013; pp. 35–40. ISBN 978-3-00-042998-9. Available online: http://eprints.gozdis.si/594/ (accessed on 27 November 2021).
- UNESCO. World Heritage List, Prehistoric Pile Dwellings around the Alps. 2021. Available online: https://whc.unesco.org/en/list/1363/> (accessed on 3 November 2021).
- Čufar, K.; Kromer, B.; Tolar, T.; Velušček, A. Dating of 4th millennium BC pile-dwellings on Ljubljansko barje, Slovenia. J. Archaeol. Sci. 2010, 37, 2031–2039. [Google Scholar] [CrossRef]
- Čufar, K.; Tegel, W.; Merela, M.; Kromer, B.; Velušček, A. Eneolithic pile dwellings south of the Alps precisely dated with tree-ring chronologies from the north. Dendrochronologia. 2015, 35, 91–98. [Google Scholar] [CrossRef]
- Tolar, T.; Jacomet, S.; Velušček, A.; Čufar, K. Plant economy at a late Neolithic lake dwelling site in Slovenia at the time of the Alpine Iceman. Veg. Hist. Archaeobotany 2011, 20, 207–222. [Google Scholar] [CrossRef]
- Out, W.A.; Baittinger, C.; Čufar, K.; López-Bultó, O.; Hänninen, K.; Vermeeren, C. Identification of woodland management by analysis of roundwood age and diameter: Neolithic case studies. For. Ecol. Manag. 2020, 467, 118136. [Google Scholar] [CrossRef]
- Čufar, K.; Tišler, V.; Gorišek, Ž. Arheološki les-njegove lastnosti in raziskovalni potencial. Arheol. Vestn. 2002, 53, 69–75, ISSN 0570-8966. Available online: http://eprints.gozdis.si/623/ (accessed on 27 November 2021).
- Čufar, K.; Gričar, J.; Zupančič, M.; Koch, G.; Schmitt, U. Wood anatomy, cell-wall structure and topochemistry of waterlogged archaeological wood aged 5,200 and 4,500 years. IAWA J. 2008, 29, 55–68. [Google Scholar] [CrossRef]
- Wagner, S.; Lagane, F.; Seguin-Orlando, A.; Schubert, M.; Leroy, T.; Guichoux, E.; Orlando, L. High-Throughput DNA sequencing of ancient wood. Mol. Ecol. 2018, 27, 1138–1154. [Google Scholar] [CrossRef]
- Daniel, G. Microscope techniques for understanding wood cell structure and biodegradation. In Secondary Xylem Biology; Academic Press: Cambridge, MA, USA, 2016; pp. 309–343. [Google Scholar]
- High, K.E.; Penkman, K.E.H. Analytical Methods for Assessing Preservation in Waterlogged Archaeological Wood: Their Importance for Site Management Decisions; Research Reports; Historic England: London, UK, 2020; Available online: https://eprints.whiterose.ac.uk/163424/ (accessed on 27 November 2021).
- Powell, K.L.; Pedley, S.; Daniel, G.; Corfield, M. Ultrastructural observations of microbial succession and decay of wood buried at a Bronze Age archaeological site. Int. Biodeterior. Biodegrad. 2001, 47, 165–173. [Google Scholar] [CrossRef]
- Macchioni, N.; Pizzo, B.; Capretti, C.; Pecoraro, E.; Sozzi, L.; Lazzeri, S. New wooden archaeological finds from Herculaneum: The state of preservation and hypothesis of consolidation of the material from the house of the relief of Telephus. Archaeometry 2016, 58, 1024–1037. [Google Scholar] [CrossRef]
- Giachi, G.; Capretti, C.; Donato, I.D.; Macchioni, N.; Pizzo, B. New trials in the consolidation of waterlogged archaeological wood with different acetone-carried products. J. Archaeol. Sci. 2011, 38, 2957–2967. [Google Scholar] [CrossRef]
- Chadwick, A.V.; Berko, A.; Schofield, E.J.; Smith, A.D.; Mosselmans, J.F.W.; Jones, A.M.; Cibin, G. The application of X-ray absorption spectroscopy in archaeological conservation: Example of an artefact from Henry VIII warship, the Mary Rose. J. Non-Cryst. Solids. 2016, 451, 49–55. [Google Scholar] [CrossRef]
- Schweingruber, F.H.; Börner, A. Fossilization, permineralization, coalification, carbonization and wet wood conservation. In The Plant Stem; Springer: Berlin/Heidelberg, Germany, 2018. [Google Scholar] [CrossRef]
- Andrič, M.; Verbič, T.; Lomax, J.; Tolar, T. Človekov vpliv na okolje v prazgodovini: Primer z obrežja Ljubljanice pri Špici (Ljubljana)/Embankment of the Ljubljanica River at Špica (Ljubljana) and human impact on the environment in late prehistory. Arheol. Vestn. 2017, 68, 479–498. [Google Scholar]
- EN 350-2; Durability of Wood and Wood-Based Products—Natural Durability of Solid Wood—Part 2: Guide to Natural Durability and Treatability of Selected Wood Species of Importance in Europe. BSI Standards Publication: London, UK, 1994.
- Humar, M.; Fabčič, B.; Zupančič, M.; Pohleven, F.; Oven, P. Influence of xylem growth ring width and wood density on durability of oak heartwood. Int. Biodeterior. Biodegrad. 2008, 62, 368–371. [Google Scholar] [CrossRef]
- Macchioni, N. Physical characteristics of the wood from the excavations of the ancient port of Pisa. J. Cult. Herit. 2003, 4, 85–89. [Google Scholar] [CrossRef]
- Brunning, R.; Watson, J. Waterlogged Wood: Guidelines on the Recording, Sampling, Conservation, and Curation of Waterlogged Wood; English Heritage: London, UK, 2010. [Google Scholar]
- EN, B.S. 13183-1: 2002; Moisture Content of a Piece of Sawn Timber—Part 1: Determination by Oven Dry Method. BSI Standards Publication: London, UK, 2007.
- Vek, V.; Balzano, A.; Poljanšek, I.; Humar, M.; Oven, P. Improving Fungal Decay Resistance of Less Durable Sapwood by Impregnation with Scots Pine Knotwood and Black Locust Heartwood Hydrophilic Extractives with Antifungal or Antioxidant Properties. Forests 2020, 11, 1024. [Google Scholar] [CrossRef]
- Donato, I.D.; Lazzara, G. Porosity determination with helium pycnometry as a method to characterize waterlogged woods and the efficacy of the conservation treatments. Archaeometry 2012, 54, 906–915. [Google Scholar] [CrossRef]
- Ganguly, S.; Balzano, A.; Petrič, M.; Kržišnik, D.; Tripathi, S.; Žigon, J.; Merela, M. Effects of Different Energy Intensities of Microwave Treatment on Heartwood and Sapwood Microstructures in Norway Spruce. Forests 2021, 12, 598. [Google Scholar] [CrossRef]
- Humar, M.; Balzano, A.; Grbec, S.; Gričar, J.; Kržišnik, D.; Lesar, B.; Vek, V. Investigation of the material resistance and moisture performance of pubescent oak (Quercus pubescens). Holzforschung 2021, 75, 22–36. [Google Scholar] [CrossRef]
- Merela, M.; Thaler, N.; Balzano, A.; Plavčak, D. Optimal surface preparation for wood anatomy research of invasive species by scanning electron microscopy. Drv. Ind. 2020, 71, 117–127. [Google Scholar] [CrossRef]
- Prislan, P.; Koch, G.; Čufar, K.; Gričar, J.; Schmitt, U. Topochemical investigations of cell walls in developing xylem of beech (Fagus sylvatica L.). Holzforschung 2009, 63, 482–490. [Google Scholar] [CrossRef]
- Wagenführ, R. Holzatlas, 4th ed.; Fachbuchverlag: Leipzig, Germany, 2014; pp. 1–688. [Google Scholar]
- Grosser, D.; Teetz, W. Einheimische Nutzhölzer (Loseblattsammlung). Vorkommen, Baum- und Stammform, Holzbeschreibung, Eigenschaften, Verwendung; Centrale Marketinggesellschaft der deutschen Agrarwirtschaft mbH (CMA) 5300 Bonn 2 und Arbeitsgemeinschaft Holz e. V.: Düsseldorf, Germany, 1985. [Google Scholar]
- Zisi, A.; Dix, J.K. Simulating mass loss of decaying waterlogged wood: A technique for studying ultrasound propagation velocity in waterlogged archaeological wood. J. Cult. Herit. 2018, 33, 39–47. [Google Scholar] [CrossRef]
- Björdal, C.G.; Fors, Y. Correlation between sulfur accumulation and microbial wood degradation on shipwreck timbers. Int. Biodeterior. Biodegrad. 2019, 140, 37–42. [Google Scholar] [CrossRef]
- Schweingruber, F.H. Microscopic Wood Anatomy; Swiss Federal Institute of Forestry Research: Birmensdorf, Switzerland, 1978. [Google Scholar]
- Huisman, D.J.; Manders, M.R.; Kretschmar, E.I.; Klaassen, R.K.W.M.; Lamersdorf, N. Burial conditions and wood degradation at archaeological sites in the Netherlands. Int. Biodeterior. Biodegrad. 2008, 61, 33–44. [Google Scholar] [CrossRef]
- Rémazeilles, C.; Meunier, L.; Leveque, F.; Plasson, N.; Conforto, E.; Crouzet, M.; Caillat, L. Post-treatment Study of Iron/Sulfur-containing Compounds in the Wreck of Lyon Saint-Georges 4 (Second Century ACE). Stud. Conserv. 2020, 65, 28–36. [Google Scholar] [CrossRef]
- Fors, Y.; Grudd, H.; Rindby, A.; Jalilehvand, F.; Sandstrom, M.; Cato, I.; Bornmalm, L. Sulfur and Iron Accumulation in Three Marine-Archaeological Shipwrecks in the Baltic Sea: The Ghost, the Crown and the Sword. Sci. Rep. 2014, 4, 4222. [Google Scholar] [CrossRef]
- Rémazeilles, C.; Tran, K.; Guilminot, E.; Conforto, E.; Refait, P. Study of Fe (II) sulphides in waterlogged archaeological wood. Stud. Conserv. 2013, 58, 297–307. [Google Scholar] [CrossRef]
- Broda, M.; Hill, C.A. Conservation of Waterlogged Wood—Past, Present and Future Perspectives. Forests 2021, 12, 1193. [Google Scholar] [CrossRef]
- Canti, M. Environmental Archaeological Evidence: Preservation. Archaeology 2018, 15, 10–29. [Google Scholar]
- Rémazeilles, C.; Leveque, F.; Conforto, E.; Meunier, L.; Refait, P. Contribution of magnetic measurement methods to the analysis of iron sulfides in archaeological waterlogged wood-iron assemblies. Microchem. J. 2019, 148, 10–20. [Google Scholar] [CrossRef]
- Čufar, K.; Merela, M.; Erič, M. A Roman barge in the Ljubljanica river (Slovenia): Wood identification, dendrochronological dating and wood preservation research. J. Archaeol. Sci. 2014, 44, 128–135. [Google Scholar] [CrossRef]
- Balzano, A.; Čufar, K.; De Micco, V. Cell-wall fluorescence highlights the phases of xylogenesis. IAWA J. 2021, 1, 1–10. [Google Scholar] [CrossRef]
- Crestini, C.; El Hadidi, N.M.; Palleschi, G. Characterisation of archaeological wood: A case study on the deterioration of a coffin. Microchem. J. 2009, 92, 150–154. [Google Scholar] [CrossRef]
- Blanchette, R.A. A review of microbial deterioration found in archaeological wood from different environments. Int. Biodeterior. Biodegrad. 2000, 46, 189–204. [Google Scholar] [CrossRef]
- Benedik, A. Mikroskopske Tehnike za Raziskavo Arheološkega Lesa: Diplomsko delo = Microscopic Techniques to Study Archaeological Wood. Bachelor’s Thesis, Biotehniška Fakulteta, Univerza v Ljubljani, Ljubljana, Slovenia, 2021. Available online: https://repozitorij.uni-lj.si/IzpisGradiva.php?lang=slv&id=131105 (accessed on 27 November 2021).
- Lewczuk, B.; Szyryńska, N. Field-Emission Scanning Electron Microscope as a Tool for Large-Area and Large-Volume Ultrastructural Studies. Animals 2021, 11, 3390. [Google Scholar] [CrossRef] [PubMed]
| Wood Taxa | MCmax (%) | WAW | Normal Wood |
|---|---|---|---|
| Density ρ0 (kg/m3) | Density ρ0 (kg/m3) | ||
| Quercus, oak (heartwood) | 747 | 180 | 650 |
| Fraxinus, ash | 517 | 140 | 650 |
| Acer, maple | 795 | 130 | 590 |
| Populus, poplar | 902 | 130 | 410 |
| Salix, willow | 653 | 140 | 400 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balzano, A.; Merela, M.; Čufar, K. Scanning Electron Microscopy Protocol for Studying Anatomy of Highly Degraded Waterlogged Archaeological Wood. Forests 2022, 13, 161. https://doi.org/10.3390/f13020161
Balzano A, Merela M, Čufar K. Scanning Electron Microscopy Protocol for Studying Anatomy of Highly Degraded Waterlogged Archaeological Wood. Forests. 2022; 13(2):161. https://doi.org/10.3390/f13020161
Chicago/Turabian StyleBalzano, Angela, Maks Merela, and Katarina Čufar. 2022. "Scanning Electron Microscopy Protocol for Studying Anatomy of Highly Degraded Waterlogged Archaeological Wood" Forests 13, no. 2: 161. https://doi.org/10.3390/f13020161
APA StyleBalzano, A., Merela, M., & Čufar, K. (2022). Scanning Electron Microscopy Protocol for Studying Anatomy of Highly Degraded Waterlogged Archaeological Wood. Forests, 13(2), 161. https://doi.org/10.3390/f13020161








