Abstract
Protection of Norway spruce stands using anti-attractants was tested during an outbreak of bark beetles (Ips typographus) in their spring flight. The aims of this study were as follows: (1) to test the proposed experimental design for tree protection; (2) to evaluate height-specific alternatives for dispenser installation on trees; and (3) to evaluate the efficiency of tree protection measures using anti-attractants under bark beetle infestation and drought stress. The experiment was conducted at the forest edges adjacent to recent clearcuts on 10 blocks in the eastern Czech Republic. Each block had three adjacent experimental areas, with 20 trees growing in two rows at the recently cut forest edge (10 trees per row). In front of a block in each of the three areas, four pheromone traps were installed. The treatment area was protected by anti-attractants. The second area served as a so-called switch area, where beetles from the treatment area, as the outflux redirected from the anti-attractant, would start new attacks if not caught in nearby pheromone traps. The third area was a control. We attached anti-attractant tube dispensers on each tree trunk of the treated area at two heights. The results suggest a redirecting effect of anti-attractants, pushing beetles into the switch area and causing subsequent attacks, which was greater than in areas containing treated trees. There was no difference between two dispensers placed at 1 and 8 m height and both at 1 m. A switching effect of beetle attacks occurring outside of the treated areas was observed. Mounting anti-attractant dispensers on tree trunks at one low position above the ground can be substantially less labour-intensive and as efficient as positioning them at two different heights. For areas affected by severe drought and extremely dense bark beetle populations, the use of anti-attractants did not prove effective.
1. Introduction
Large areas of productive Norway spruce (Picea abies (L.) Karst.) forests in the Czech Republic are affected by severe climate-driven outbreaks of Eurasian spruce bark beetle (Ips typographus L.) [1,2]. Ips typographus attacks the whole trunk of mature spruce trees [3] adjacent to previous infestations or trees located predominantly on sun-exposed forest edges recently created by wind or sanitary logging [4]. During its most biologically active period in April–September, which fluctuates depending on weather conditions, I. typographus can produce up to three generations per year [5].
Methods of spruce stand protection vary in their scope and approach, ranging from conventional sanitary or salvage cutting of infested trees [6] to arrangements of pheromone trap barriers [7], trap trees, and mass trapping [6,8,9,10,11]. However, the biological and economic efficiency of trapping to substantially reduce I. typographus populations has been questioned [6,12,13]. The use of pheromone traps for mass beetle catching is constrained by the “spillover effect”, as described in [9,11,14]. The construction of commercial dispensers ensures strong pest attraction to the pheromone, potentially attracting more beetles than the traps are able to catch. Thus, attracted insects may attack trees adjacent to the traps.
New methods of bark beetle control based on the use of anti-attractants were pioneered in North America and Europe [15,16,17], along with the push-and-pull strategy. The repelling effect (push) of anti-attractants on trees is combined with aggregated pheromone traps on nearby clearcuts (pull) [18,19,20]. Several active anti-attractant compounds have been identified for I. typographus [21]. The first compound is verbenone, metabolised from the host compound alpha-pinene or by the conversion of the main pheromone component of I. typographus, cis-verbenol [22]. The second group comprises non-host volatiles (NHVs): trans-conophthorin, an important synergistic compound found in the bark of broad-leaf trees [23]; green leaf volatiles (GLVs; 1-hexanol, (Z)-3-hexen-1-ol, and (E)-2-hexen-1-ol) detected in non-host birch (Betula spp.) and aspen (Populus tremula L.) [24]; and C8 alcohols (3-octanol and 1-octen-3-ol) emitted from the bark of these tree species. According to [25], the combination of these seven compounds can produce an active inhibition radius (AIR) of 2–4 m. In addition, 1,8-cineol, a new active anti-attractant compound, showed field activity [26] with more precise spatial action than verbenone due to an inhibition of the pheromone component cis-verbenol at the antenna single-sensillum level [27]. Recently, a number of other oxygenated monoterpenes of host tree origin has been reported [28] as physiologically active anti-attractants, including trans-thujan-4-ol [29,30,31]. The term “repellent” is not used here, as the behavioural data (above) do not support a movement away from odour source, as originally defined [32].
The effect of anti-attractants on tree mortality has been evaluated mainly in mountainous or boreal landscapes under normal weather conditions [21,33,34,35]. Experiments were performed with wick-based anti-attractant dispensers containing verbenone [33,34] or a combination of verbenone, conophthorin, and GLVs [21,34,35]. The experimental design often involves mounting dispensers with anti-attractants at different heights on tree trunks, e.g., at 2 and 3–6 m above the ground. Installing dispensers at 3–6 m above the ground is time-consuming. Alternately, dispensers can be located on poles in gaps of the forest grid, as was done in a protection experiment on spruce stands severely affected by diffuse sanitary felling [33]. The conventional experimental design involves establishing adjacent pairs of treatment and control plots at forest edges adjacent to recent clearcuts [21,34]. An alternative approach is to experimentally evaluate tree protection efficiency on a much larger scale by considering the stand surrounding the treatment plot at the forest edge as the control and measuring the geographical positions of attacked trees in and around the treatment area [21].
Analyses of data obtained under neighbouring pairwise positioning of the plots indicated that beetle-caused tree mortality was 35 to 76% lower in treatment plots than control areas [21,34]. Jakuš et al. [21] recorded increasing attack intensity in a 15–30 m swath along the border of treated areas at the forest edge compared to the landscape average. We observed a similar pattern in pilot experiments conducted in spruce stands during a bark beetle outbreak in the Moscow region in Russia. Anti-attractants can have the effect of pushing bark beetles outside of treated areas onto neighbouring trees in a non-controlled way, causing an undesirable result for forest management [21]. Similar consequences of a push-and-pull system used for protecting Douglas fir (Pseudotsuga menziesii (Mirb.) Franco) against Douglas fir beetle (Dendroctonus pseudotsugae Hopkins) were reported in [18]. According to that study, increased tree mortality observed outside of the treated areas was triggered by the “spillover effect” of suppression traps.
In this study, the quantitative relationships between the number of trees infested by bark beetles and the use of anti-attractants under bark beetle infestation were experimentally explored. The working hypothesis was that the inhibition effects of an anti-attractant blend would push the beetles outside of the treated area and that placing one double dose at 1 m would have the same effect as placing one at 1 m and one at 8 m. The aims of the study were as follows: (1) to test the proposed alternative experimental design for tree protection; (2) to evaluate height-specific alternatives for dispenser installation on trees; and (3) to evaluate the efficiency of tree protection using anti-attractants under bark beetle infestation and drought stress by measuring effects on a larger scale around treatments.
2. Materials and Methods
2.1. Description of Study Area
The experiment was conducted from 19 April 2018 to 2 July 2018 in mature spruce stands (Table 1) in the Potštát Forest district located in the north-eastern part of the Czech Republic (49.670319, 17.545289). The area spans an administrative district of military forest and farms and has served as the Czech army’s training area since 1946. The topography is rolling, with elevation ranging from 500 to 650 m. Average annual air temperature ranges from 5 to 6 °C. Average daily temperature in the growing season (April–September) does not exceed 12 °C [36]. Average annual precipitation ranges from 700 to 800 mm/year [37]. Monoculture spruce, characterised by low static stability, are subject to frequent wind damage. The windstorm of 1991 triggered a chain of permanent bark beetle outbreaks, which, intensified by climate change, caused substantial forest decline in the area. Military training activities limit the implementation of forest management and pest control practices in Potštát. In 2018, bark beetles attacked an extensive area of spruce stands. At the same time, severe drought, which affected the whole Central European region [2,38], caused large-scale forest dieback, continuing into 2019 (Table 1). In the year preceding the experiment, sanitary felling, which removed dead and infested trees growing in the stands used in this study, reached 24 to 130 m3/ha. Sanitary felling intensity in the forest district was 55 m3/ha.

Table 1.
Summary of experimental block parameters. Age refers to mean age of spruce trees in each block. Percentage of spruce shows prevalence of P. abies in stand structure. Altitude refers to mean elevation of each of 10 blocks.
2.2. Dispensers
To protect stand edges against I. typographus attack, tube dispensers with an anti-attractant blend (Fytofarm Ltd., Bratislava, Slovak Republic) were used in the experiment. Dispensers contained a combination of verbenone (Vn), 1,8-cineol (Ci), racemic trans-conophthorin (tC), and GLV (1-hexanol) in a loading ratio of Vn:Ci:tC:GLV of 60:40:0.2:15, as determined by the producer. The content of the customized dispensers was developed based on a modified mixture of compounds used to compose the IT-REP experimental lure, an anti-attractant blend that was proved to have inhibition efficacy in field experiments in Slovakia and Sweden [21]. The dispensers release 50 mg/day of the blend for 8 weeks, as stated by the manufacturer. A commercial wick pheromone lure, IT Ecolure Extra (Fytofarm Ltd., Bratislava, Slovak Republic), was used in the traps. We did not renew the dispensers during the summer since their nominal release duration of 2 months was enough to capture the tree protection effects during the period of maximum bark beetle attack intensity.
2.3. Experimental Design
The experiments were performed in mono-dominant Norway spruce stands. Treatments were hierarchically designed, which means that each treatment area was adjacent to an untreated “switch” area and a nearby control area (treatment + switch + control = block) (Figure 1). Experimental blocks were located at the northern edge of a fresh salvage cut of trees attacked the previous year, processed by harvester, and removed before the start of the experiment. The last phase of processing attacked trees took place a few weeks before the start of the experiment. All investigated trees and surrounding plots had no signs of beetle attack before the experiment. We kept the distance between the opposite edges of each clearcut twice as large as the mean stand tree height. The close range of the three areas was intended to reduce large spatial variability in beetle density, forest edge orientation, and site and stand conditions typical for severe I. typographus outbreaks [39]. The distance between experimental blocks was more than 50 m.
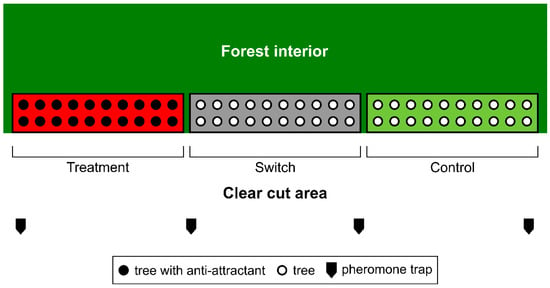
Figure 1.
Arrangement of three experimental areas in a block, each with 20 trees in two lines parallel to stand edge. Total number of treated trees on 10 blocks was 200, for a grand total of 600 trees in the experimental areas of the study.
In contrast to the original experimental designs for tree protection [21,34], the suggested alternative incorporates a “switch” area in the centre of each experimental block, serving as a buffer zone between treatment and control areas (Figure 1). This area was introduced to quantify the bark beetle “switching” behaviour observed in our previous study of anti-attractants [21], thus extending the scale for the quantification of effects by the treatment. Each block had three adjacent areas—treatment, switch, and control—with two rows of adjacent trees in each area and 10 trees per row (total of 20 trees per treatment area) (Figure 1). Anti-attractants were mounted on the 20 trees growing in the treatment area. The distance between trees ranged from 1 to 10 m. Untreated trees in the switch and control areas differed in terms of proximity to the treatment area. The switch area adjacent to the latter served to capture the potential bark beetle redirection outflux from the treatment area presumed to be caused by anti-attractant inhibition effects.
According to the experimental design, each treated tree was protected by dispensers attached to the north side of the trunk. To test for the effects of the attachment height on tree protection efficiency, we used two dispenser placements (experimental variants): In variant A, one dispenser was fixed on the trunk at ~1.2 m height and another one at 8 m height. In variant B, two dispensers were installed at ~1.2 m. In this experiment, we aimed to optimize the method of anti-attractant application. In contrast to our previous studies, in which we found that mounting anti-attractants at two heights in mountainous conditions was time-consuming and challenging [21,34], here, we set out to determine whether single-height dispenser application, if effective, could substantially reduce the labour involved in pheromone beetle trapping.
The two dispenser placement variants were, for practical reasons (lack of sufficiently long forest edges with similar structure), applied in separate 5 + 5 blocks. Each height-specific variant was implemented on 100 trees, i.e., 20 trees per 5 blocks dedicated to a given variant. Out of 60 trees growing in each block, 20 trees were treated with anti-attractants (variant treatment) (Figure 1). The distance between experimental blocks varied from 200 m to several hundred meters. Variants A and B alternated between blocks.
Four pheromone-baited traps were installed in the salvage cut area 25 m from the forest edge of each block parallel to the stand. We used black window-slot traps (Ridex Ltd., Vrbno Pod Pradědem, Czech Republic). The distance between traps was approximately 15 m. Trees were monitored for signs of bark beetle infestation, and the pheromone traps at the forest edges were checked at intervals of ~2–7 days depending on the weather (Figure 2). In the course of the experiment, beetle-infested trees were gradually subjected to sanitary cutting as part of the conventional local forest management practices implemented in the experimental blocks.
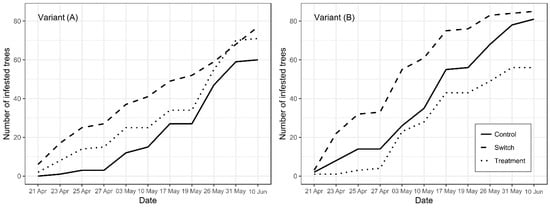
Figure 2.
Mean cumulated trees attacked per block in treatment areas (baited with anti-attractant dispensers), “switch areas” (unbaited but adjacent to baited trees), and control areas (unbaited and separated from treatment) in variants (A) (2 spaced dispensers/tree) and variant (B) (2 joined dispensers /tree).
2.4. Statistical Analyses
A mixed-effect model was developed to statistically evaluate the tree protection effects of anti-attractants. The choice of model resulted from the hierarchical experimental design [40]. In the hierarchical (nested) design, each level of the nested predictor is uniquely associated with only one level of the higher-level predictor [41]. Since each block contained either variant A or variant B, this factor was fitted as a fixed effect. The second-level predictors for the purpose of nested analysis (three areas: treatment, switch, and control) were defined as a “random” component although experimentally, these factors were fixed. The response variable was the number of trees infested by bark beetles (I. typographus) 3 weeks after the beginning of the experiment. The dependent variable, represented by counts or frequencies, was fitted with Poisson distribution, which was corrected for the reduction in mean errors of estimated coefficients and test statistics due to overdispersion [42].
Correlation analysis between catches in pheromone traps near the plots and infested trees on the plots was performed separately for variants A and B for the whole duration of the experiment. The cumulative number of captured I. typographus individuals on inspection days was analysed in relation to the total number of infested trees in the area at the same time. All analyses were conducted in R software [43] using the nlme package [44].
To assess the magnitude of difference in infested trees between experimental areas and to examine this in relation to the effects of anti-attractants reported in other studies (i.e., a small meta-review), we performed a formal calculation of effect sizes [45,46].
3. Results
The number of infested trees in each variant increased quickly, with the lines for variant B (pair of dispensers at 1 m) being slightly steeper (Figure 2). During the whole experiment, the most attacked trees were located in the switch area regardless of the variant. A relatively lower number of bark-beetle-infested trees protected by anti-attractants was observed in variant B. In particular, we observed a relatively high numbers of infested trees in the treatment area, where the intensity of successful colonization attempts approached that in the switch area (Figure 2a). By contrast, in variant B, the differences in intensity in bark-beetle-infested trees between areas were more pronounced during the whole reporting period. Until 27 April 2018, the increase in the number of infested trees in the area treated with anti-attractants was the lowest not only in variant B (Figure 2b) but also areas of both A and B variants. In other words, spruce trees treated with two dispensers installed at 1.2 m were infested least.
In 3 weeks, approximately 30% of the trees were infested. During this period, statistically significant differences in the number of dead trees were observed, where “switch” areas had most attacks (Figure 3). However, there were no differences between anti-attractant treated areas and control areas. Later, large sister-brood swarming started (Figure 4), and all plots were severely infested. A 30-day swarming period did not lead to any statistically significant differences in the number of infested trees between experimental variants A and B (GLMM df = 1; n = 30; p > 0.33; Table 2). Later, large sister-brood swarming started (Figure 4), and all plots were severely infested.
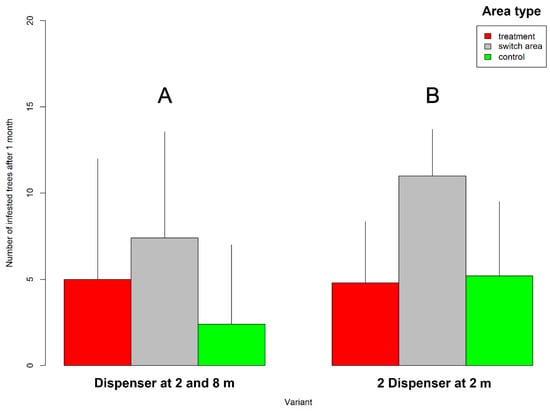
Figure 3.
Interaction effects of height-specific dispenser placement (variant (A), 2 spaced dispensers/tree; variant (B), 2 joined dispensers/tree) and experimental area (treatment, switch, and control) on average number of trees infested by spruce bark beetle 1 month after initiating experiment. Vertical lines represent upper 95% confidence intervals calculated from linear mixed-effect model (Table 2).
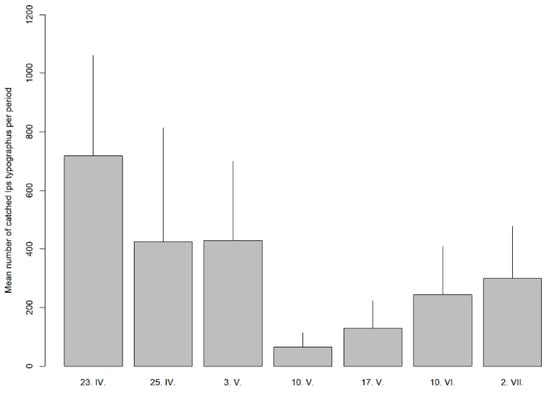
Figure 4.
Mean number of captured spruce bark beetles per trap from 23 April (23. IV) to 2 July 2. VII).

Table 2.
Analysis of variance of linear mixed-effect model. Dependent variable was numbers of infested trees, with experimental area as nested factor; LME model-fitting function in nlme package in R was used. Significance of factors was determined at usual α levels: * α < 0.05 and *** α < 0.
The highest number of infested trees was recorded in the switch area (mean 9 trees; Figure 3), followed by the treatment area (4 trees) and the control area (3 trees). These area-specific differences in the numbers of infested trees were statistically significant (GLMM df = 2; n = 30; p < 0.05) (Table 2).
Height of dispenser placement (variants A and B) and experimental area showed no interaction in terms of mean number of infested trees by the nested model (GLMM df = 2; n = 30; p > 0.61) (Table 2). In other words, dispenser placement similarly affected infestations in the three areas. A significant correlation between the number of infested trees in the area and catches in pheromone traps in both variants was found (correlation coefficients: 0.62 for variant A and 0.70 for variant B) (Figure 5).
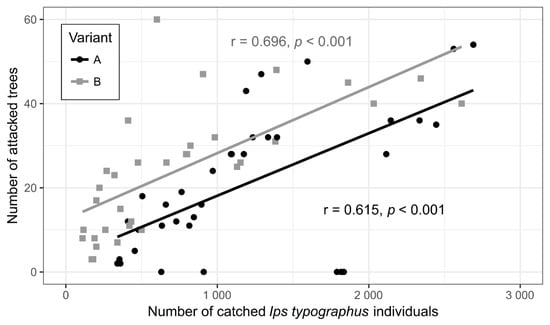
Figure 5.
Correlation analysis between catches of Ips typographus individuals in pheromone traps near plots and infested trees on plots in treatment variants A and B. Cumulative number of captured I. typographus individuals on inspection days was analysed in relation to total number of infested trees in the area on the same day.
The differences of infestation in experimental areas were of a large magnitude (d > 1) when comparing switch to control in both A and B variants (Table 3, rows 2 and 4). In contrast, there was no reduction in attacks in treatment compared to control (Table 3, rows 1 and 3).

Table 3.
Effect size dependency of height-specific dispenser placement (variants A and B) and area (treatment, switch, control) on mean number of trees infested by spruce bark beetle 1 month after initiation of experiment and effect sizes estimated from 32 other studies (2000–2012) and later studies.
4. Discussion
The main result of the experiment is that the effect of a switch of beetle attacks caused mortality from the anti-attractant-treated area to the adjacent switch area, which at the end underwent more attacks. Placing two anti-attractant dispensers at two heights or at one height produced similar anti-attractant effects. In terms of attacks switching within the blocks, there was no difference between the anti-attractant-treated area and the control area located farther away. Thus, the treatment did not prove to be efficient at protecting spruce trees during the whole study period.
4.1. Experimental Results and “Switch” Effect
The experimental design was an area of blocks with three adjacent areas: treatment, switch, and control. In similar experiments in the past, a system of only treatment and control areas was used [21,34]. Our results confirm a strong switch effect, i.e., the effect of pushing beetle attacks outside the treated areas, which was first described in [21]. Pheromone traps were installed parallel to all experimental blocks (Figure 1), and there were very high I. typographus catches (Figure 4). This means we cannot be sure what the results would be if we used traps only in sections treated with anti-attractants.
The spillover effect, as observed in [18] during push-and-pull experiments with D. pseudotsuagae, could be responsible for the infestation of trees growing in the switch and control areas. The presence of pheromone-baited traps nearby could have drawn dispersing adult beetles to the study area. However, uncertainty about the source of attacking bark beetles prevents us from drawing an unequivocal conclusion since the insects might have flown from places other than anti-attractant areas before attempting colonization on trees located in the switch zone. Anti-aggregation pheromones or anti-attractants signal to dispersing beetles that a tree is fully occupied and that they should search for another host. This does not necessarily mean that they are more likely to colonize individual trees adjacent to treated areas. Nevertheless, experiments in Sweden [21] and our pilot experiments in the Moscow region showed the presence of a switch effect even in the absence of pheromone traps in treated areas.
The current system of using three experimental areas for evaluation may be a more appropriate way to describe the process than the simpler pair of plots (treatment and control). Another possible solution would be to use treated areas and geographical coordinates of infested trees at the patch, habitat, and landscape scale, as done in the later part of the study mentioned above [21]. The level of development of GIS and RM technology nowadays may allow the application of a more elaborate evaluation more conveniently than before. The switch effect could potentially be a problem for practical application of anti-attractants, as mentioned in [21]. Pushing beetles outside treated areas, thus leaving the problem to neighbours, is not desirable. However, in a pilot application on a larger scale [35], the switch effect was not observed. Potential problems could be solved by appropriately locating the protected area (line). In the case of American bark beetles, two strategies have been applied that could possibly eliminate the switch effect: (1) uniform spraying with small dispensers [49,50] and (2) deploying fewer but stronger dispensers arranged in a grid [51]. The grid design was also used for I. typographus in forest damaged by severe salvage cutting, with no observable switch effect [33].
The differences in efficacy between the two anti-attractant application variants are not statistically significant. The results show a visible trend towards the greater efficiency of variant B (installation at one height). If further attempts confirm this result, it would be appropriate to apply anti-attractants at one height. In addition, based on our current knowledge, it is appropriate to use the practically more convenient variant B. Such application would significantly reduce the labour involved. However, the result would only be valid for the spring swarming, as studied here. Previous work [33] showed low effectiveness of anti-attractants in the summer. All successful applications of anti-attractants took place in mountain or boreal conditions during spring swarming [21,34]. We do not have sufficient data to determine the effectiveness in the summer swarming period.
4.2. Effect of Drought and High Bark Beetle Population
The results do not show statistically significant differences between treated and control parts of the forest edges. In previous experiments where treatment areas had significantly fewer attacked trees, switch areas were not used [21,34]. On the other hand, those studies were performed in different conditions, and for Norway spruce close to a natural forest (NP Šumava, Czech Republic; TANAP, Slovakia; and Spiš, Slovakia), a strong switch effect was not observed. The switch effect was first observed in an experiment in Småland (Sweden) production forest [21]. The concept of a push-and-pull system assumes that bark beetle populations disperse among host trees at an attack density below the threshold required to successfully overcome the host defence [18]. The observed very strong switch effect and low efficiency of treatment may have been caused by a very large population of I. typographus in experimental locations, as shown in Table 1 and Figure 5. In addition, spruce defence was diminished by long-term drought and a climatically extreme drought in 2018 [2].
4.3. Effect of Pheromone Traps and Push-Pull System
A correlation between the number of infested trees in the area and catches in pheromone traps in both variants (Figure 5) was expected and is in agreement with previous works [52,53]. The relatively high tree mortality (Table 1) and switch effect (Figure 3) could also be explained by the effect of limited pheromone trap barriers used at landscape scale (no other intensive use of pheromone traps). The four traps used in our experimental blocks could attract beetles from a larger area under heavy outbreak conditions, as described in [11]. When the use of pheromone traps on I. typographus was successful, large numbers of traps were used [7,54]. Thus, our experimental design (Figure 1) and landscape conditions may not have allowed us to fully assess the impact of pheromone traps in a potential push-pull system. Future experiments performed at plots with medium or low bark beetle population density should investigate the effects of the push-pull system on pheromone trap catches prior to tree infestation. Gillette et al. [20] showed that in the case of a push-and-pull system for Dendroctonus ponderosae, the use of anti-attractants did not influence the catches in pheromone traps. They also found no differences between only push and push-pull systems. This is an opposite result to the theoretic assumption of the push-pull effect described in [55]. Other studies using a push-and-pull system for Dendroctonus ponderosae used baited traps on trees instead of pheromone traps. In [56], a stronger effect of verbenone application and a relatively small effect of baited trap trees was reported. It seems that the effect of a combination of pheromone traps and anti-attractants is not clear. In the case of I. typographus outbreaks with less intensive use of pheromone traps, we should test the use of only anti-attractants without nearby pheromone traps. Vandygriff et al. [57] showed that treatment with anti-attractants provided little or no additional protection when compared with the use of baited trees only. Positive results from the use a push-and-pull system were shown in [58].
4.4. Limitations of the Study
The time and number of infested trees removed from the treatment, switch, and control areas were not recorded. Unfortunately, in the case of large bark beetle outbreak and the associated logistic pressure of salvage cutting, it is impossible to arrange for the cutting of attacked trees on experimental plots just after attack. Sanitary cutting could potentially affect the clarity of the experiment since the total amount and spatial distribution of anti-attractant emission would be modified by the removal of trees carrying dispensers. Nevertheless, a phased pattern of sanitary cutting provided enough time to complete the experiment within the scheduled timeframe, which coincided with the first peak period (spring flight) of beetle colonization activity. By the end of 2018, the study area was severely disturbed forest, with almost all mature spruce stands killed or fragmented by beetle attacks.
Another limitation of the study is that we used data from the first 3 weeks for statistical analysis rather than from the entire experimental period. Later, statistical analysis did not show any significant results. This may have been caused by a large effect of sister-brood swarming or by different periods of removing attacked trees from experimental sites by salvage cutting. The limited length of the experimental forest edges at hand prevented us from investigating the switch effect on both sides of the treated plots.
4.5. Research Needs
Anti-attractants are still not in operational use for the protection of spruce stands against I. typographus. A review of 32 experiments published up to 2011 indicated that anti-attractants were similarly active in tree protection for both I. typographus and D. ponderosae, with an effect size (Cohen’s d) of around −1 or one standard deviation lower tree kill in treatment compared to control (Table 3, row 5) [16]; a similar value was found in a later 7-year extensive study [59]. There was a large variation between studies and years; however, one important factor might have been the switching effect with high beetle populations [60]. Comparing the largest effect sizes (around −1 in studies of 2000–2011; Table 3, row 5) [16] to those of recent studies in North America (2015–2020), in many cases, they are largely similar (Table 3, rows 6 and 17). Interestingly, the recent successful papers include three additional species of Dendroctonus and not only D. ponderosae [47,48,49,61]. The development of dispensers [47,49] as well as the development of blends, such as the addition of NHV compounds to verbenone, may provide good [47] or even dramatic [48] improvement of protection effects. Another way would be more intensive use of cineol, which has more precise action over distances [26,27], and thujanol, which has a stronger effect on females [31] in comparison to verbenone.
For I. typographus, at the current stage of development, anti-attractants could be used to protect spruce stands early in the season near known attack spots when beetles are leaving their overwintering sites, and their dispersal is limited by lower temperatures and shorter day length [21]. Anti-attractants are ineffective at stand edge segments that are either damaged by freshly windthrown or broken trees or wedged between windthrown areas [35]. Our result indicates that anti-attractants are not effective in helping to protect forest stands in areas heavily affected by drought and extremely high bark beetle populations. We would recommend their use as part of a push-pull system or as part of a more complex system of bark beetle management in natural mountain or boreal spruce forests at active (fresh) forest edges not affected by wind damage with low or medium bark beetle populations in the spring.
In order to improve push-pull methods of spruce stand protection for I. typographus, we should act in the following ways:
- Increase the effectiveness of anti-attractant lures (by incorporating newer compounds that cause less switching [26,27,31] or, better, longer-lasting dispensers);
- Optimize the spatial layout by not placing anti-attractants on all trees and only applying them at lower height;
- Optimize the area of anti-attractant application by using spatial tools (tree health diagnostics, remote sensing, and GIS) and methods on the scale from sniffer dogs [62] to UAVs to satellites;
- Test the possibility of increasing the effectiveness of anti-attractants in summer conditions;
- Test the possibility of using anti-attractants in areas with intensive use of mass trapping or pheromone trap barriers (push-pull effect) and in areas without them (only anti-attractants).
5. Conclusions
The main result of the experiment is the high damage to the second experimental area, i.e., that in between pure treatment and control, confirming the switching effect of beetle attacks adjacent to treated areas. It is possible to use anti-attractant dispensers at only one low height, which would reduce labour costs. The use of anti-attractants is not effective in areas affected by severe drought and extremely high bark beetle populations.
Author Contributions
Conceptualization, R.J., R.M., M.B., J.K., A.J. and F.S.; methodology, R.J., R.M., M.B. and F.S.; investigation, R.J., R.M., J.K., M.B., A.M. and F.S.; resources, R.J., M.B. and F.S.; data curation, R.M. and F.S.; writing—original draft preparation, R.J., A.M., N.K. and F.S.; writing—review and editing, R.J., R.M., M.B., J.K., N.K., A.J. and F.S.; visualization, R.M., A.M. and F.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by grants: “Building up an excellent scientific team and its spatiotechnical background focused on mitigation of the impact of climatic changes to forests from the level of a gene to the level of a landscape at the FFWS CULS Prague”, No. CZ.02.1.01/0.0/0.0/15_003/0000433 financed by OP RDE; “Factors of Norway spruce survival during large-scale disturbance in NP Šumava”, No. A_21_09 funded by the Internal Grant Agency FFWS CULS in Prague; “Development of integrated modern and innovative diagnostic and protection methods of spruce stands with the use of semiochemicals and methods of molecular biology”, No. QK1910480 financed by the Ministry of Agriculture of Czech Republic and the Slovak Research and Development Agency under contract APVV-19-0606 and APVV-15-0761.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
The authors wish to thank the military forests and farms of the Czech Republic for providing data and supporting the experimental work.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Toth, D.; Maitah, M.; Maitah, K.; Jarolínová, V. The Impacts of Calamity Logging on the Development of Spruce Wood Prices in Czech Forestry. Forests 2020, 11, 283. [Google Scholar] [CrossRef]
- Hlásny, T.; Zimová, S.; Merganičová, K.; Štěpánek, P.; Modlinger, R.; Turčáni, M. Devastating Outbreak of Bark Beetles in the Czech Republic: Drivers, Impacts, and Management Implications. For. Ecol. Manag. 2021, 490, 119075. [Google Scholar] [CrossRef]
- Gonzalez, R.; Grégoire, J.-C.; Drumont, A.; De Windt, N. A Sampling Technique to Estimate Within-Tree Populations of Pre-Emergent Ips typographus (Col., Scolytidae). J. Appl. Entomol. 1996, 120, 569–576. [Google Scholar] [CrossRef]
- Kautz, M.; Schopf, R.; Ohser, J. The “Sun-Effect”: Microclimatic Alterations Predispose Forest Edges to Bark Beetle Infestations. Eur. J. For. Res. 2013, 132, 453–465. [Google Scholar] [CrossRef]
- Doležal, P.; Sehnal, F. Effects of Photoperiod and Temperature on the Development and Diapause of the Bark Beetle Ips typographus. J. Appl. Entomol. 2007, 131, 165–173. [Google Scholar] [CrossRef]
- Wermelinger, B. Ecology and Management of the Spruce Bark Beetle Ips typographus—A Review of Recent Research. For. Ecol. Manag. 2004, 202, 67–82. [Google Scholar] [CrossRef]
- Jakuš, R. A Method for the Protection of Spruce Stands Against Ips typographus by the Use of Barriers of Pheromone Traps in North-Eastern Slovakia. Anz. Für Schädlingskunde Pflanzenschutz Umweltschutz 1998, 71, 152–158. [Google Scholar] [CrossRef]
- Bakke, A.; Sæther, T.; Kvamme, T. Mass Trapping of the Spruce Bark Beetle Ips typographus: Pheromone and Trap Technology; Norsk Institutt for Skogforskning: Ås, Norway, 1983; ISBN 978-82-7169-299-5. [Google Scholar]
- Vité, J.P. The European Struggle to Control Ips typographus—Past, Present and Future. Ecography 1989, 12, 520–525. [Google Scholar] [CrossRef]
- Weslien, J. Effects of Mass Trapping on Ips typographus (L.) Populations. J. Appl. Entomol. 1992, 114, 228–232. [Google Scholar] [CrossRef]
- Niemeyer, H. Integrated Bark Beetle Control: Experiences and Problems in Northern Germany. In Proceedings: Integrating Cultural Tactics into the Management of Bark Beetle and Reforestation Pests; Vallombrosa, Italy, 1–3 September 1996; U.S. Department of Agriculture, Forest Service, Northeastern Forest Experiment Station: Radnor, PA, USA, 1997; pp. 80–86. [Google Scholar]
- Faccoli, M.; Stergulc, F. Damage Reduction and Performance of Mass Trapping Devices for Forest Protection against the Spruce Bark Beetle, Ips typographus (Coleoptera Curculionidae Scolytinae). Ann. For. Sci. 2008, 65, 309. [Google Scholar] [CrossRef]
- Zahradník, P.; Zahradníková, M. The Efficacy of a New Pheromone Trap Setup Design, Aimed for Trapping Ips typographus (Coleoptera, Curculionidae, Scolytinae). Šumar. List 2015, 139, 185–186. [Google Scholar]
- Vité, J.P. Erfahrungen und Erkenntnisse zur akuten Gefahrdung des mitteleuropaischen Fichtenwaldes durch Kaferbefall. Allg. Forstz. 1984, 29, 249–254. [Google Scholar]
- Ross, D.W.; Daterman, G.E. Efficacy of an Antiaggregation Pheromone for Reducing Douglas-Fir Beetle, Dendroctonus pseudotsugae Hopkins (Coleoptera: Scolytidae), Infestation in High Risk Stands. Can. Entomol. 1995, 127, 805–811. [Google Scholar] [CrossRef]
- Schlyter, F. Semiochemical Diversity in Practice: Antiattractant Semiochemicals Reduce Bark Beetle Attacks on Standing Trees—A First Meta-Analysis. Psyche J. Entomol. 2012, 2012, e268621. [Google Scholar] [CrossRef]
- Seybold, S.J.; Bentz, B.J.; Fettig, C.J.; Lundquist, J.E.; Progar, R.A.; Gillette, N.E. Management of Western North American Bark Beetles with Semiochemicals. Annu. Rev. Entomol. 2018, 63, 407–432. [Google Scholar] [CrossRef] [PubMed]
- Ross, D.W.; Daterman, G.E. Reduction of Douglas-Fir Beetle Infestation of High-Risk Stands by Antiaggregation and Aggregation Pheromones. Can. J. For. Res. 1994, 24, 2184–2190. [Google Scholar] [CrossRef]
- Borden, J.H.; Birmingham, A.L.; Burleigh, J.S. Evaluation of the Push-Pull Tactic against the Mountain Pine Beetle Using Verbenone and Non-Host Volatiles in Combination with Pheromone-Baited Trees. For. Chron. 2006, 82, 579–590. [Google Scholar] [CrossRef]
- Gillette, N.E.; Mehmel, C.J.; Mori, S.R.; Webster, J.N.; Wood, D.L.; Erbilgin, N.; Owen, D.R. The Push-Pull Tactic for Mitigation of Mountain Pine Beetle (Coleoptera: Curculionidae) Damage in Lodgepole and Whitebark Pines. Environ. Entomol. 2012, 41, 1575–1586. [Google Scholar] [CrossRef]
- Schiebe, C.; Blaženec, M.; Jakuš, R.; Unelius, C.R.; Schlyter, F. Semiochemical Diversity Diverts Bark Beetle Attacks from Norway Spruce Edges. J. Appl. Entomol. 2011, 135, 726–737. [Google Scholar] [CrossRef]
- Birgersson, G.; Leufvén, A. The Influence of Host Tree Response to Ips typographus and Fungal Attack on Production of Semiochemicals. Insect Biochem. 1988, 18, 761–770. [Google Scholar] [CrossRef]
- Zhang, Q.-H.; Schlyter, F. Olfactory Recognition and Behavioural Avoidance of Angiosperm Nonhost Volatiles by Conifer-Inhabiting Bark Beetles. Agric. For. Entomol. 2004, 6, 1–20. [Google Scholar] [CrossRef]
- Zhang, Q.-H.; Birgersson, G.; Zhu, J.; Löfstedt, C.; Löfqvist, J.; Schlyter, F. Leaf Volatiles from Nonhost Deciduous Trees: Variation by Tree Species, Season and Temperature, and Electrophysiological Activity in Ips typographus. J. Chem. Ecol. 1999, 25, 1923–1943. [Google Scholar] [CrossRef]
- Zhang, Q.-H.; Schlyter, F. Redundancy, Synergism, and Active Inhibitory Range of Non-Host Volatiles in Reducing Pheromone Attraction in European Spruce Bark Beetle Ips typographus. Oikos 2003, 101, 299–310. [Google Scholar] [CrossRef]
- Andersson, M.N.; Larsson, M.C.; Blazenec, M.; Jakus, R.; Zhang, Q.-H.; Schlyter, F. Peripheral Modulation of Pheromone Response by Inhibitory Host Compound in a Beetle. J. Exp. Biol. 2010, 213, 3332–3339. [Google Scholar] [CrossRef] [PubMed]
- Binyameen, M.; Jankuvová, J.; Blaženec, M.; Jakuš, R.; Song, L.; Schlyter, F.; Andersson, M.N. Co-Localization of Insect Olfactory Sensory Cells Improves the Discrimination of Closely Separated Odour Sources. Funct. Ecol. 2014, 28, 1216–1223. [Google Scholar] [CrossRef]
- Kalinová, B.; Břízová, R.; Knížek, M.; Turčáni, M.; Hoskovec, M. Volatiles from Spruce Trap-Trees Detected by Ips typographus Bark Beetles: Chemical and Electrophysiological Analyses. Arthropod-Plant Interact. 2014, 8, 305–316. [Google Scholar] [CrossRef]
- Blažytė-Čereškienė, L.; Apšegaitė, V.; Radžiutė, S.; Mozūraitis, R.; Būda, V.; Pečiulytė, D. Electrophysiological and Behavioural Responses of Ips typographus (L.) to Trans-4-Thujanol—A Host Tree Volatile Compound. Ann. For. Sci. 2016, 73, 247–256. [Google Scholar] [CrossRef]
- Schiebe, C.; Unelius, C.R.; Ganji, S.; Binyameen, M.; Birgersson, G.; Schlyter, F. Styrene, (+)-Trans-(1R,4S,5S)-4-Thujanol and Oxygenated Monoterpenes Related to Host Stress Elicit Strong Electrophysiological Responses in the Bark Beetle Ips typographus. J. Chem. Ecol. 2019, 45, 474–489. [Google Scholar] [CrossRef]
- Jirošová, A.; Kalinová, B.; Modlinger, R.; Jakuš, R.; Unelius, C.R.; Blaženec, M.; Schlyter, F. Anti-Attractant Activity of (+)-Trans-4-Thujanol for Eurasian Spruce Bark Beetle Ips typographus: Novel Potency for Females. Pest Manag. Sci. 2022, 78, 1992–1999. [Google Scholar] [CrossRef]
- Miller, J.; Siegert, P.; Amimo, F.; Walker, E. Designation of chemicals in terms of the locomotor responses they elicit from insects: An update of Dethier et al. (1960). J. Econ. Entomol. 2009, 102, 2056–2060. [Google Scholar] [CrossRef]
- Jakuš, R.; Dudová, A. Experimental Use of Aggregation and Anti-Aggregation Pheromones against the Spruce Bark Beetle (Ips typographus) in Decaying Spruce Stands with a Lower Level of Canopy Closure. J. For. Sci. 1999, 45, 525–531. [Google Scholar]
- Jakuš, R.; Schlyter, F.; Zhang, Q.-H.; Blaženec, M.; Vaverčák, R.; Grodzki, W.; Brutovský, D.; Lajzová, E.; Turčáni, M.; Bengtsson, M.; et al. Overview of Development of an Anti-Attractant Based Technology for Spruce Protection against Ips typographus: From Past Failures to Future Success. Anz. Für Schädlingskunde 2003, 76, 89–99. [Google Scholar] [CrossRef]
- Jakuš, R.; Blaženec, M.; Vojtěch, O. Use of anti-attractants in specific conditions of protected areas. Folia Oecol. 2011, 38, 46–51. [Google Scholar]
- Vojenský Újezd Libavá. Available online: https://www.vojujezd-libava.cz/vismo/dokumenty2.asp?u=9342&id_org=9342&id=3381 (accessed on 11 December 2022).
- Tolasz, R.; Míková, T.; Valerıánová, A.; Voženílek, V. Climate Atlas of Czechia, 1st ed.; Czecia Český Hydrometeoro-Logický Ústav, Univerzita Palackého Praha: Olomouc, Czech Republic, 2007; p. 255. ISBN 978-80-86690-26-1. [Google Scholar]
- Buras, A.; Rammig, A.; Zang, C.S. Quantifying Impacts of the 2018 Drought on European Ecosystems in Comparison to 2003. Biogeosciences 2020, 17, 1655–1672. [Google Scholar] [CrossRef]
- Wichmann, L.; Ravn, H.P. The Spread of Ips typographus (L.) (Coleoptera, Scolytidae) Attacks Following Heavy Windthrow in Denmark, Analysed Using GIS. For. Ecol. Manag. 2001, 148, 31–39. [Google Scholar] [CrossRef]
- Pekár, S.; Brabec, M. Moderní Analýza Biologických Dat; MUNI Press: Brno, Czech Republic, 2012; ISBN 978-80-210-5812-5. [Google Scholar]
- Schielzeth, H.; Nakagawa, S. Nested by Design: Model Fitting and Interpretation in a Mixed Model Era. Methods Ecol. Evol. 2013, 4, 14–24. [Google Scholar] [CrossRef]
- Pekár, S.; Brabec, M. Modern Analysis of Biological Data; MUNI Press: Brno, Czech Republic, 2016; ISBN 978-80-210-8019-5. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing. 2022. Available online: https://www.r-project.org/ (accessed on 11 December 2022).
- Pinheiro, J.; Bates, D.; DebRoy, S.; Sarkar, D.; Heisterkamp, S.; Van Willigen, B.; Ranke, J.; R Core Team. Nlme: Linear and Nonlinear Mixed Effects Models. 2022. Available online: https://cran.r-project.org/web/packages/nlme/nlme.pdf (accessed on 11 December 2022).
- Maher, J.M.; Markey, J.C.; Ebert-May, D. The Other Half of the Story: Effect Size Analysis in Quantitative Research. CBE—Life Sci. Educ. 2013, 12, 345–351. [Google Scholar] [CrossRef]
- Nakagawa, S.; Cuthill, I.C. Effect Size, Confidence Interval and Statistical Significance: A Practical Guide for Biologists. Biol. Rev. 2007, 82, 591–605. [Google Scholar] [CrossRef]
- Fettig, C.J.; Munson, A.S.; Reinke, M.; Mafra-Neto, A. A Novel Semiochemical Tool for Protecting Pinus contorta from Mortality Attributed to Dendroctonus ponderosae (Coleoptera: Curculionidae). J. Econ. Entomol. 2015, 108, 173–182. [Google Scholar] [CrossRef]
- Hansen, E.M.; Munson, A.S.; Blackford, D.C.; Graves, A.D.; Coleman, T.W.; Baggett, L.S. 3-Methylcyclohex-2-En-1-One for Area and Individual Tree Protection against Spruce Beetle (Coleoptera: Curculionidae: Scolytinae) Attack in the Southern Rocky Mountains. J. Econ. Entomol. 2017, 110, 2140–2148. [Google Scholar] [CrossRef]
- Foote, G.G.; Fettig, C.J.; Ross, D.W.; Runyon, J.B.; Coleman, T.W.; Gaylord, M.L.; Graves, A.D.; McMillin, J.D.; Mortenson, L.A.; Mafra-Neto, A. A Biodegradable Formulation of MCH (3-Methylcyclohex-2-En-1-One) for Protecting Pseudotsuga menziesii from Dendroctonus pseudotsugae (Coleoptera: Curculionidae) Colonization. J. Econ. Entomol. 2020, 113, 1858–1863. [Google Scholar] [CrossRef] [PubMed]
- Gillette, N.E.; Mehmel, C.J.; Webster, J.N.; Mori, S.R.; Erbilgin, N.; Wood, D.L.; Stein, J.D. Aerially Applied Methylcyclohexenone-Releasing Flakes Protect Pseudotsuga Menziesii Stands from Attack by Dendroctonus pseudotsugae. For. Ecol. Manag. 2009, 257, 1231–1236. [Google Scholar] [CrossRef]
- Gillette, N.E.; Erbilgin, N.; Webster, J.N.; Pederson, L.; Mori, S.R.; Stein, J.D.; Owen, D.R.; Bischel, K.M.; Wood, D.L. Aerially Applied Verbenone-Releasing Laminated Flakes Protect Pinus contorta Stands from Attack by Dendroctonus ponderosae in California and Idaho. For. Ecol. Manag. 2009, 257, 1405–1412. [Google Scholar] [CrossRef]
- Weslien, J.; Annila, E.; Bakke, A.; Bejer, B.; Eidmann, H.H.; Narvestad, K.; Nikula, A.; Ravn, H.P. Estimating Risks for Spruce Bark Beetle (Ips typographus (L.)) Damage Using Pheromone-baited Traps and Trees. Scand. J. For. Res. 1989, 4, 87–98. [Google Scholar] [CrossRef]
- Faccoli, M.; Stergulc, F. Ips typographus (L.) Pheromone Trapping in South Alps: Spring Catches Determine Damage Thresholds. J. Appl. Entomol. 2004, 128, 307–311. [Google Scholar] [CrossRef]
- Weslien, J. Monitoring Ips typographus (L.) Populations and Forecasting Damage. J. Appl. Entomol. 1992, 114, 338–340. [Google Scholar] [CrossRef]
- Cook, S.M.; Khan, Z.R.; Pickett, J.A. The Use of Push-Pull Strategies in Integrated Pest Management. Annu. Rev. Entomol. 2007, 52, 375–400. [Google Scholar] [CrossRef]
- Lindgren, B.S.; Borden, J.H. Displacement and Aggregation of Mountain Pine Beetles, Dendroctonus ponderosae (Coleoptera: Scolytidae), in Response to Their Antiaggregation and Aggregation Pheromones. Can. J. For. Res. 1993, 23, 286–290. [Google Scholar] [CrossRef]
- Vandygriff, J.C.; Rasmussen, L.A.; Rineholt, J.F. A Novel Approach to Managing Fuelwood Harvest Using Bark Beetle Pheromones. West. J. Appl. For. 2000, 15, 183–188. [Google Scholar] [CrossRef]
- Borden, J.H.; Chong, L.J.; Earle, T.J.; Huber, D.P.W. Protection of Lodgepole Pine from Attack by the Mountain Pine Beetle, Dendroctonus ponderosae (Coleoptera: Scolytidae) Using High Doses of Verbenone in Combination with Nonhost Bark Volatiles. For. Chron. 2003, 79, 685–691. [Google Scholar] [CrossRef][Green Version]
- Perkins, D.L.; Jorgensen, C.L.; Rinella, M.J. Verbenone Decreases Whitebark Pine Mortality Throughout a Mountain Pine Beetle Outbreak. For. Sci. 2015, 61, 747–752. [Google Scholar] [CrossRef]
- Raffa, K.F.; Andersson, M.N.; Schlyter, F. Chapter One—Host Selection by Bark Beetles: Playing the Odds in a High-Stakes Game. In Advances in Insect Physiology; Beetles, P.B., Tittiger, C., Blomquist, G.J., Eds.; Academic Press: Cambridge, MA, USA, 2016; Volume 50, pp. 1–74. [Google Scholar]
- Fettig, C. Efficacy of SPLAT® Verb for Protecting Individual Pinus contorta, Pinus ponderosa, and Pinus lambertiana from Mortality Attributed to Dendroctonus ponderosae. J. Entomol. Soc. Br. Columbia 2016, 113, 11–20. [Google Scholar]
- Vošvrdová, N.; Johansson, A.; Turčáni, M.; Jakuš, R.; Tyšer, D.; Schlyter, F.; Modlinger, R. Dogs trained to recognise a bark beetle pheromone locate recently attacked spruces better than human experts. For. Ecol. Manag. 2023, 528, 120626. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).