Growth Rhythm Analysis of Young Stand and Selection of Superior Families in Choerospondias axillaris
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Setting Area
2.2. Experimental Design and Materials
2.3. Data Analysis
3. Results
3.1. Growth Traits Showed Significant Differences in Various Families and Superior Families Selection
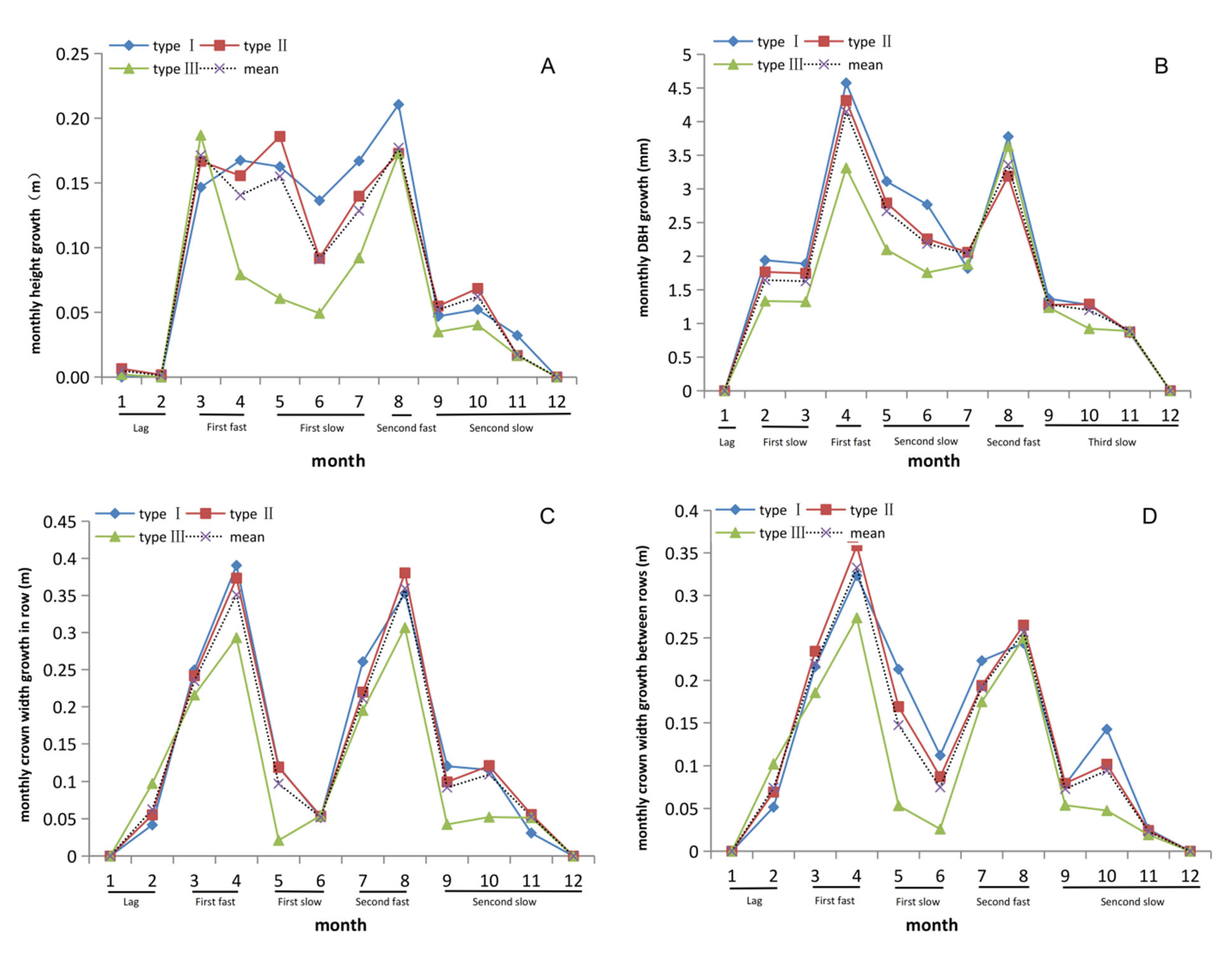
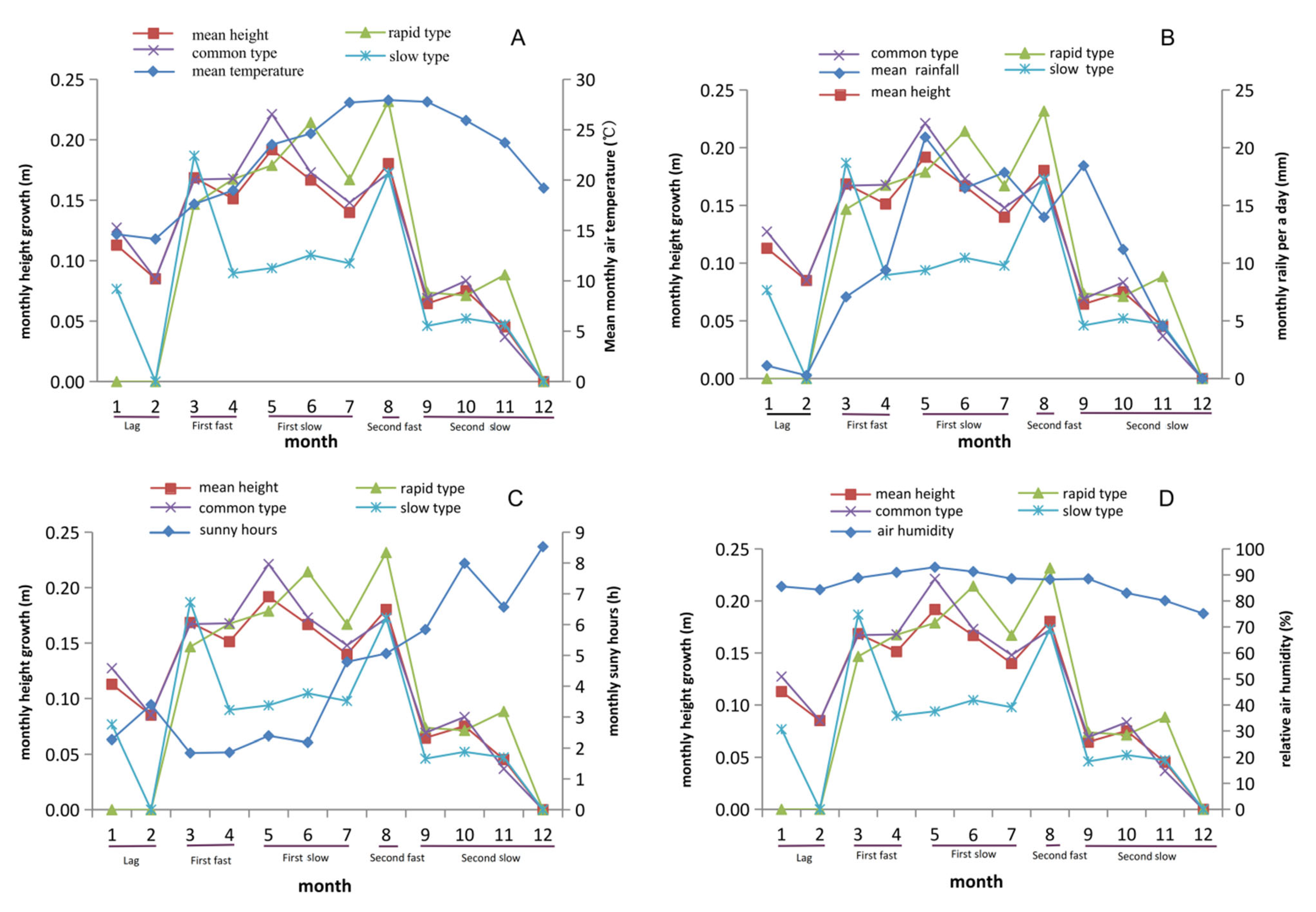
3.2. Two Obvious Peaks Occurred in Tree Height Growth in One Year
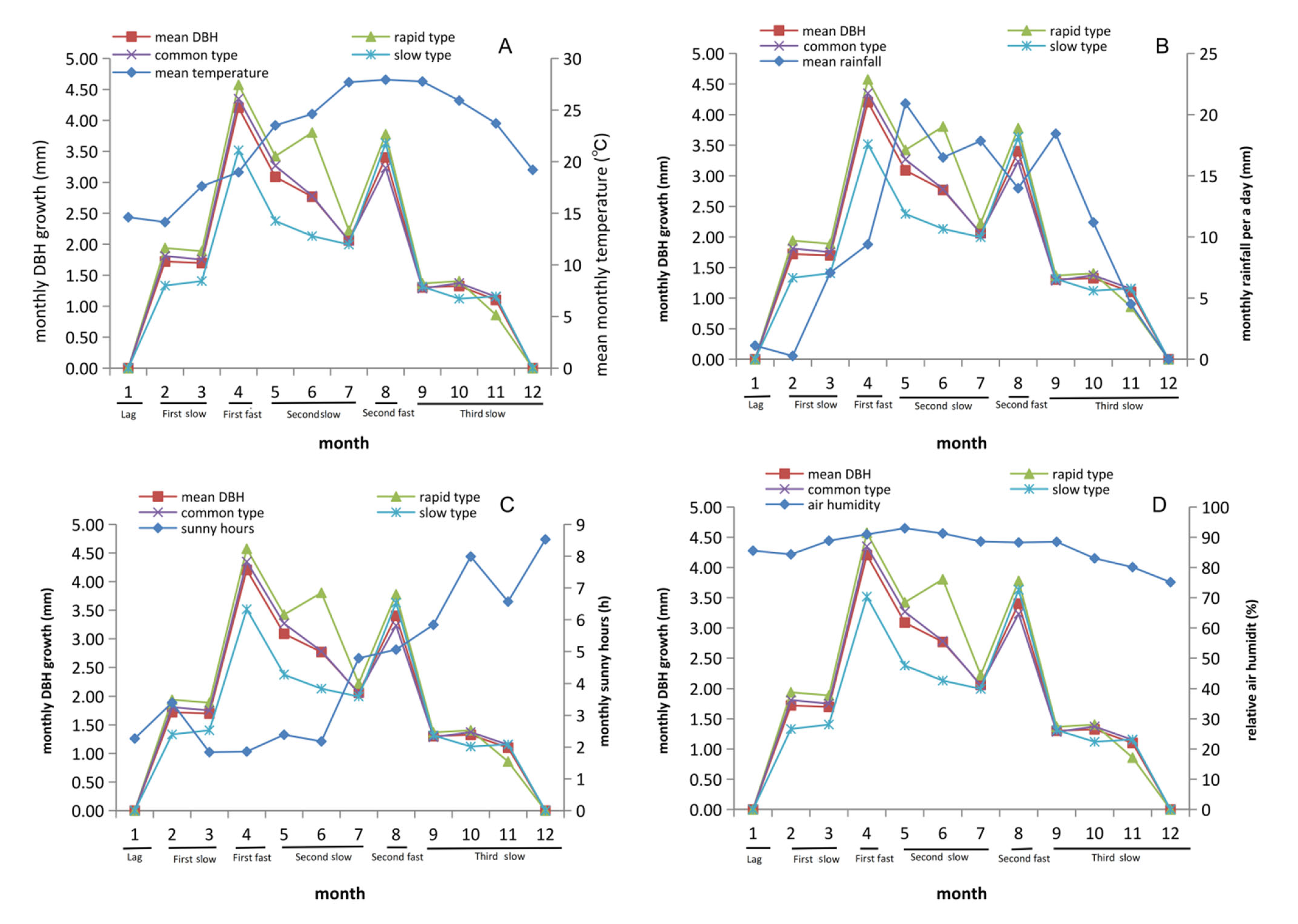
3.3. DBH Stablely Increased from February to August in C. axillaris Families
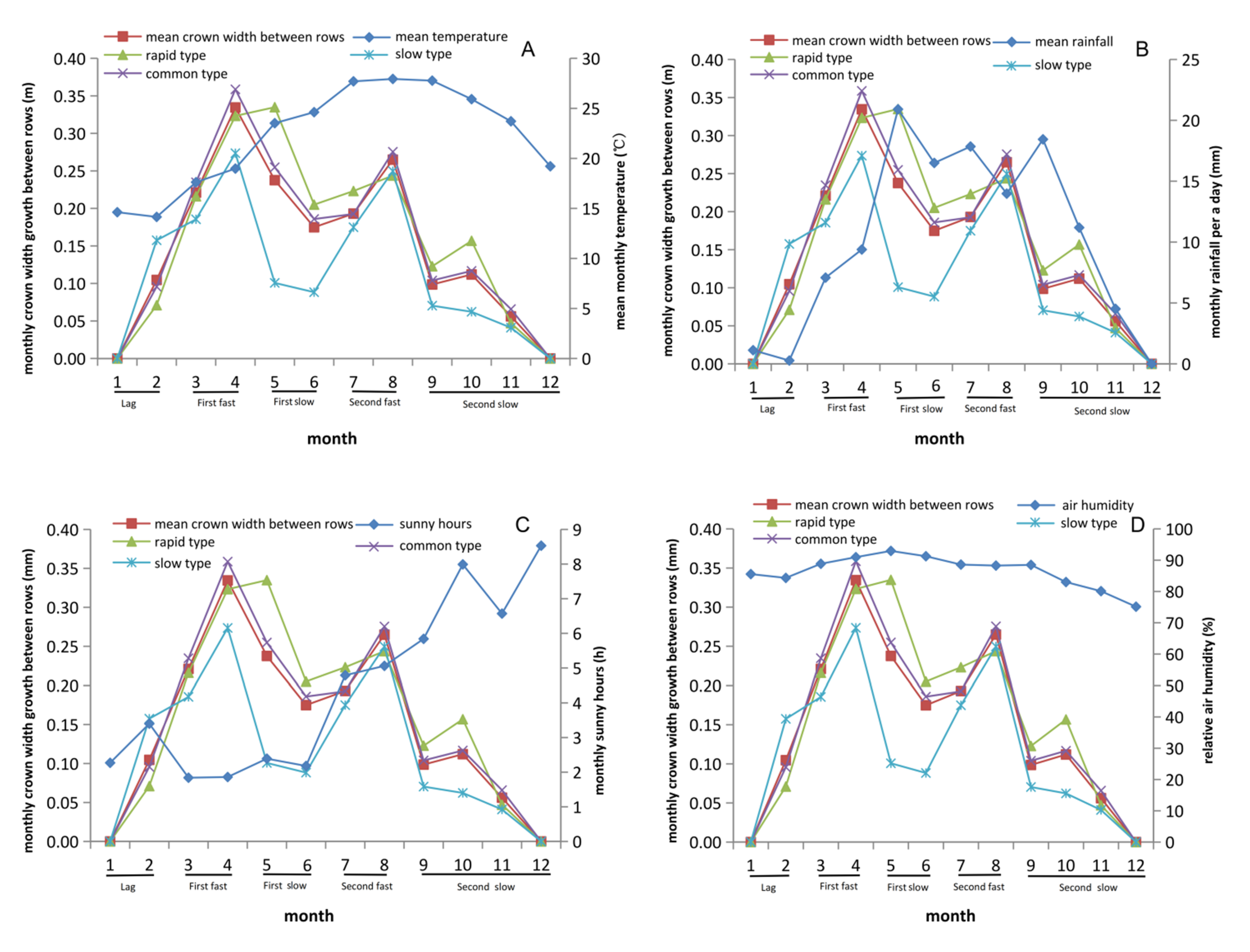
3.4. Growth Rhythm of Crown Width within Row Showed Differences with Crown Width between Rows
3.5. Rainfall and Sunshine Hours Significantly Affect Growth Traits
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhu, H. A review on the research of Choerospondias Axillaris. Guangxi For. Sci. 2005, 34, 122–126. [Google Scholar]
- The Editorial Committee of Dendrology (Southern Edition). Dendrology; China Forestry Press: Beijing, China, 1994; pp. 580–587. [Google Scholar]
- Hau, C.H. Tree seed predation on degraded hillsides in Hong Kong. For. Eco. Manag. 1997, 99, 215–221. [Google Scholar]
- Liu, X.; Ding, Y.; Chen, M.; Wen, F.; Xie, X. Research and manufacturing of Choerospondias Axillaris juice. Soft Drink. Industry. 1995, 39, 14–16. [Google Scholar]
- Bu, C. Analysis of nutritional components and processing utilization of Choerospondias Axillaris fruit. Wild Plants China 1992, 11, 32–36. [Google Scholar]
- Gautam, K.H. The sweet and sour tale of la psi-domesticating and commercialzing Choerpondias axillaris. Agro-For. Today 1997, 9, 12–16. [Google Scholar]
- Li, M. By Mongolia Qiwei Pills progress of science. North. Pharm. 2011, 8, 70–71. [Google Scholar]
- Zeng, F. Studies on the growth laws of Choerosponsias axillaries Natural Forests. J. Fujian For. Sci Technol. 2001, 28, 13–15. [Google Scholar]
- Huang, Y.; Zhuang, X. Seedling Raising Techniques of Native Tree Species in South China; China Forestry Press: Beijing, China, 2007; pp. 158–159. [Google Scholar]
- Luo, W.; Guiping, H.E.; Chen, Y.; Sun, H.; Feng, J.; Zhang, J.; Jin, Q. A study on variation of growing traits of young Choerospondias axillaris forest in its geographic provenances and its provenance selection. Acta Agri. Univ. Jiangxiensis 2007, 29, 365–371. [Google Scholar]
- Wang, T.; Tigerstedt, P.M. A variation of growth rhythm among families and correlation between growth rhythm and growth rate in Betula pendula Roth. Scand. J. For. Res. 1993, 8, 489–497. [Google Scholar] [CrossRef]
- Huang, J.; Guo, J.; Zeng, J. Early selection of Betula alnoides clones and their growth rhythm in Western Yunan of China. For. Res. 2017, 30, 518–524. [Google Scholar]
- Lai, M.; Sun, X.; Zhang, S. Growth rhythm of young Larix kaempferi×Larix olgensis hybrid. J. Northeast. Univ. 2014, 42, 13–17, 33. [Google Scholar]
- Zhou, X.; Zhou, W.; Lin, W.; Zhou, P.; Chen, X. Observation and analysis on growth rhythms of Toona sinensis of 14 provenances. J. South China Agri. Univ. 2016, 37, 90–96. [Google Scholar]
- Wang, X.; Zhang, R.; Xu, Z.; Cheng, Y.; Wu, J.; Zhou, J. Study on growth rhythm of different Zelkova schneideriana provenances at seedling stage. J. Cent. South Univ. For. Technol. 2013, 33, 37–40. [Google Scholar]
- Cao, J.; Fang, L. Studies on observation of growth rhythm of young Betula luminifera. For. Res. 2006, 19, 142–145. [Google Scholar]
- Lukkarinen, A.J.; Ruotsalainen, S.; Nikkanen, T.; Peltola, H. The growth rhythm and height growth of seedlings of siberian (Larix sibirica Ledeb.) and dahurian (Larix gmelinii Rupr.) larch provenances in greenhouse conditions. Silva Fenn. 2009, 43, 5–20. [Google Scholar] [CrossRef]
- Lukkarinen, A.J.; Ruotsalainen, S.; Peltola, H.; Nikkanen, T. Annual growth rhythm of Larix sibirica and Larix gmelinii provenances in a field trial in southern Finland. Scand. J. For. Res. 2013, 28, 518–532. [Google Scholar] [CrossRef]
- Danusevicius, D.; Gabrilavicius, R. Variation in juvenile growth rhythm among Picea abies provenances from the Baltic states and the adjacent regions. Scand. J. For. Res. 2001, 16, 305–317. [Google Scholar] [CrossRef]
- Zhang, L.; Han, Y.; Liu, Z.; Meng, Y.; Liu, Y.; Kong, D. Study on the growth rhythm of Tapiseia sinensis at seedling stage. Guangdong Agri. Sci. 2018, 45, 47–51. [Google Scholar]
- Qin, G.; Song, Y.; Qiao, Y.; Yu, Z.; Peng, L. Annual height growth of salix matsudana at nursery stage and its gray correlation. J. Northeast For. Univ. 2019, 47, 42–45. [Google Scholar]
- Kuang, L.; Deng, X.; Chen, S.; Qu, W.; Zhaxi, Q.; Chen, X. Growth rhythm of Zenia insignis seedlings from four provenances. J. South China Agri. Univ. 2014, 35, 98–101, 107. [Google Scholar]
- Peng, Y.; Huang, Z.; Shen, W.; Zhu, J.; Hao, H. Analysis on growth rhythm and biomass increment of an introduced variety of Alnus formosana. J. of Central South Univ. of For. Technol. 2014, 34, 7–12. [Google Scholar]
- Li, P.; Que, Q.; Wu, L.; Zhu, Q.; Chen, X. Growth rhythms of Toona ciliate seedlings from different provenances. J. South China Agri. Univ. 2017, 38, 96–102. [Google Scholar]
- Lan, H.; Huang, X.; Luo, J.; Arnold, R.J. Genetic variation in growth and stem straightness in Eucalyptus saligna trials in Fujian. Aust. For. 2012, 75, 163–174. [Google Scholar]
- Wu, H.; Duan, A.; Zhang, J. Long-term Growth Variation and Selection of Geographical Provenances of Cunninghamia lanceolata (Lamb.) Hook. Forests 2019, 10, 876. [Google Scholar] [CrossRef]
- Hiraoka, Y.; Miura, M.; Fukatsu, E.; Iki, T.; Yamanobe, T.; Kurita, M.; Isoda, K.; Kubota, M.; Takahashi, M. Time trends of genetic parameters and genetic gains and optimum selection age for growth traits in sugi (Cryptomeria japonica) based on progeny tests conducted throughout Japan. J. For. Res. 2019, 24, 303–312. [Google Scholar] [CrossRef]
- Hayatgheibi, H.; Fries, A.; Kroon, J.; Wu, H.X. Genetic analysis of lodgepole pine (Pinus contorta) solid-wood quality traits. Can. J. For. Res. 2017, 47, 1303–1313. [Google Scholar] [CrossRef]
- Zhang, F.; Ya, H.; Li, X.; Mao, C.; Yang, T.; Li, G.; Wu, Y. Growth dynamics of Hevea brasiliensis clones and correlation with meteorological factors. J. Northwest For. Univ. 2019, 34, 91–97. [Google Scholar]
- Dong, Q.; Zhou, Y.; Zhang, H.; Cao, S.; Liu, X.; Zhao, X. Introduction experiment of Quercus rubra in Yulin Sandy Land. Prot. For. Sci. Technol. 2018, 11, 13–14, 50. [Google Scholar]
- Yang, X.; Xu, J.; Zhu, Y.; Shen, L.; Li, G.; Wu, S.; Luo, Y.; Liu, J.; Chen, L. Growth rhythm for half-sib families of young Pinus kesiya plantations in Southern Yunnan, China. J. Trop. Subtrop. Bot. 2019, 27, 399–407. [Google Scholar]
- Pumijumnong, N.; Eckstein, D. Reconstruction of pre-monsoon weather conditions in northwestern Thailand from the tree-ring widths of Pinus merkusii and Pinus kesiya. Trees-Struct. Funct. 2011, 25, 125–133. [Google Scholar] [CrossRef]
- Fan, G.; Liu, Y.; Lai, X. Analysis of woody plants phenology variation characteristics in China. J. Meteoro. Sci. 2012, 32, 68–73. [Google Scholar]
- Skrøppa, T.; Steffenrem, A. Performance and Phenotypic Stability of Norway Spruce Provenances, Families, and Clones Growing under Diverse Climatic Conditions in Four Nordic Countries. Forests 2021, 12, 230. [Google Scholar] [CrossRef]
- Mckenney, D.; Davis, W.J.; Turnbull, J.W. Impact of Australian Tree Species Selection Research in China: An Economic Perspective. For. Ecol. Manag. 1993, 60, 59–76. [Google Scholar] [CrossRef]
- Huang, S.; Xie, W. Practical SAS Programming and Forestry Experiment Data Analysis; South China University of Technology Press: Guangzhou, China, 2001; pp. 247–268. [Google Scholar]
- Kung, F.H. Estimating Parent Effects in Full-sib Progeny Tests Following Use of an Irregular Mating Design. Silvae Genet. 1978, 27, 196–200. [Google Scholar]
- Wang, L. Dynamic Study on Species Height Growth of Evergreen Broad-Leaved Trees of Tiantong in Zhejiang; Nanjing Normal University: Nanjing, China, 2014; pp. 1–52. [Google Scholar]
- Yang, L. Study on Leaves Phenology in Spring of Evergreen Broad-Leaved Forest in Tiantong, Zhejiang; East China Normal University: Shanghai, China, 2009; pp. 1–85. [Google Scholar]
- Liu, W.; Li, D.; Ji, Q.; Chen, H.; Lai, S.; Gen, S.; Yun, X.; Chen, Q. Leaf nitrogen allocation of evergreen and deciduous broad-leaved tree species and their relationships with photo-synthetic capacity in the two habitats. Ecol. Sci. 2015, 34, 1–8. [Google Scholar]
- Surayothee, W.; Buajan, S.; Fu, P.; Pumijumnong, N.; Fan, Z.; Panthi, S.; Finnegan, P.M.; Zhang, Y.; Chen, Y.; Tor-ngern, P.; et al. Growth-Climate Relationships and Long-Term Growth Trends of the Tropical Forest Tree Choerospondias axillaris (Anacardiaceae) in East-Central Thailand. Forests 2021, 12, 1655. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, D.; Guo, Z. Diameter growth of three tree species in the lower subtropical climate. Acta Ecol. Sin. 1999, 19, 939–943. [Google Scholar]
- Ouédraogo, D.-Y.; Mortier, F.; Gourlet-Fleury, S.; Freycon, V.; Picard, N. Slow-growing species cope best with drought: Evidence from long-term measurements in a tropical semi-deciduous moist forest of Central Africa. J. Ecol. 2013, 101, 1459–1470. [Google Scholar] [CrossRef]
- Breitsprecher, A.; Bethel, J.S. Stem-growth periodicity of trees in a tropicalwet forest of Costa Rica. Ecology 1990, 71, 1156–1164. [Google Scholar] [CrossRef]
- Wu, C.; Jiang, B.; Yuan, W.; Shen, A.; Yang, S.; Yao, S.; Liu, J. On the Management of Large-Diameter Trees in China’s Forests. Forests 2020, 11, 111. [Google Scholar] [CrossRef]
- Zhou, Y. Relative Contribution of Growing Season Length and Amplitude to Long-Term Trend and Interannual Variability of Vegetation Productivity over Northeast China. Forests 2020, 11, 112. [Google Scholar] [CrossRef]

| Growth Survey Month | Average Daily Temperature (°C) | Daily Maximum Temperature (°C) | Daily Minimum Temperature (°C) | Rainfall (mm) | Sunshine Hours (h) | Daily Air Humidity (%) |
|---|---|---|---|---|---|---|
| 2017.7 | 28.05 | 36.20 | 23.30 | 151.80 | 145.50 | 83.17 |
| 2017.8 | 28.13 | 38.40 | 21.70 | 260.40 | 230.70 | 81.67 |
| 2017.9 | 27.48 | 36.20 | 25.40 | 25.00 | 182.20 | 85.08 |
| 2017.10 | 23.31 | 33.80 | 24.30 | 52.40 | 216.10 | 78.56 |
| 2017.11 | 18.99 | 28.70 | 20.90 | 42.70 | 79.20 | 81.77 |
| 2017.12 | 14.71 | 24.10 | 15.30 | 0.10 | 180.60 | 66.28 |
| 2018.1 | 13.19 | 25.00 | 17.00 | 57.8 | 100.10 | 82.13 |
| 2018.2 | 13.80 | 29.00 | 16.90 | 4.20 | 112.40 | 76.46 |
| 2018.3 | 13.80 | 29.00 | 1.60 | 8.60 | 112.40 | 76.46 |
| 2018.4 | 19.23 | 30.10 | 5.30 | 49.40 | 124.70 | 82.62 |
| 2018.5 | 21.59 | 31.40 | 7.90 | 53.60 | 70.60 | 84.32 |
| 2018.6 | 27.29 | 32.42 | 24.30 | 39.19 | 4.78 | 27.29 |
| 2018.7 | 27.29 | 35.70 | 21.80 | 587.80 | 143.40 | 85.23 |
| 2018.8 | 27.28 | 36.20 | 22.90 | 213.40 | 181.90 | 87.96 |
| 2018.9 | 26.30 | 35.70 | 25.20 | 153.80 | 165.30 | 86.02 |
| 2018.10 | 22.15 | 32.30 | 21.70 | 23.70 | 147.50 | 80.89 |
| 2018.11 | 19.67 | 29.50 | 20.40 | 16.90 | 131.40 | 85.88 |
| 2018.12 | 14.62 | 28.40 | 18.70 | 6.50 | 70.50 | 85.56 |
| 2019.1 | 14.62 | 28.40 | 5.20 | 11.20 | 70.50 | 85.56 |
| 2019.2 | 14.15 | 26.20 | 5.40 | 1.60 | 105.50 | 84.34 |
| 2019.3 | 17.62 | 29.30 | 8.80 | 85.00 | 51.40 | 88.86 |
| 2019.4 | 18.98 | 29.80 | 10.90 | 140.90 | 57.60 | 90.97 |
| 2019.5 | 23.52 | 33.20 | 15.80 | 292.80 | 71.80 | 92.97 |
| 2019.6 | 24.62 | 35.20 | 16.80 | 379.50 | 67.70 | 91.27 |
| 2019.7 | 27.70 | 36.10 | 21.40 | 339.10 | 143.70 | 88.60 |
| 2019.8 | 27.94 | 37.90 | 23.50 | 237.70 | 156.80 | 88.28 |
| 2019.9 | 27.77 | 37.50 | 23.20 | 350.40 | 180.90 | 88.50 |
| 2019.10 | 25.92 | 35.50 | 16.90 | 145.50 | 239.80 | 83.02 |
| 2019.11 | 23.71 | 34.80 | 15.10 | 27.10 | 203.60 | 80.11 |
| 2019.12 | 19.21 | 30.50 | 11.30 | 0.00 | 255.90 | 75.14 |
| Family Codes | Quantity of Family | Provenance | Latitude (N) | Longitude (E) | Altitude (m) |
|---|---|---|---|---|---|
| 1–4 | 4 | Hunan Anhua | 28°24′ | 111°12′ | 650 |
| 5–9 | 5 | Guangdong Longchuan | 24°12′ | 115°18′ | 500 |
| 10–40, 52–77 | 57 | Guangdong Raoping | 23°43′ | 116°59′ | 30 |
| 41–47 | 7 | Guangdong Lianshan | 24°35′ | 112°05′ | 360 |
| 48–51 | 4 | Guangxi Quanzhou | 25°55′ | 112°05′ | 200 |
| Families | Height (m) | DBH (mm) | Mean Volume (cm3·tree−1) | Crown Width in Rows (m) | Crown Width between Rows (m) |
|---|---|---|---|---|---|
| 15 | 3.97 ± 1.43 ABCDEFG | 50.21 ± 21.34 ABCDE | 5763 ± 64 A | 2.88 ± 1.05 ABCDE | 2.93 ± 1.05 ABCDEF |
| 76 | 4.03 ± 1.18 ABCDEF | 52.07 ± 19.43 ABC | 5720 ± 57 AB | 3.12 ± 0.89 ABCD | 3.16 ± 0.70 ABCDEF |
| 56 | 4.33 ± 0.29 A | 55.98 ± 9.94 A | 5459 ± 16 ABC | 3.27 ± 0.50 ABC | 3.47 ± 0.76 AB |
| 49 | 4.13 ± 0.94 ABC | 53.09 ± 14.83 AB | 5440 ± 40 ABCD | 3.16 ± 0.65 ABCD | 3.30 ± 0.73 ABCD |
| 17 | 3.88 ± 1.16 ABCDEFG | 51.20 ± 19.56 ABCD | 5380 ± 49 ABCDE | 3.36 ± 1.22 AB | 3.38 ± 0.98 ABC |
| 5 | 3.91 ± 1.16 ABCDEFG | 47.77 ± 20.3 ABCDEFG | 5170 ± 66 ABCDEF | 2.97 ± 0.53 ABCDE | 3.16 ± 0.67 ABCDEF |
| 48 | 4.02 ± 1.31 ABCDEF | 48.51 ± 18.07 ABCDEF | 5109 ± 55 ABCDEFG | 3.14 ± 0.94 ABCD | 3.19 ± 0.90 ABCDEF |
| 18 | 4.08 ± 1.00 ABCD | 50.24 ± 14.60 ABCDE | 4958 ± 47 ABCDEFG | 3.02 ± 0.71 ABCDE | 3.04 ± 0.85 ABCDEF |
| 71 | 3.88 ± 0.93 ABCDEFG | 50.28 ± 17.61 ABCDE | 4942 ± 39 ABCDEFG | 2.93 ± 0.92 ABCDE | 2.94 ± 0.94 ABCDEF |
| 12 | 4.06 ± 0.89 ABCDE | 50.69 ± 14.39 ABCDE | 4909 ± 33 ABCDEFG | 3.17 ± 0.67 ABCD | 3.27 ± 0.79 ABCDE |
| 62 | 3.67 ± 1.43 BCDEFGHIJ | 50.97 ± 15.94 ABCD | 4872 ± 42 ABCDEFG | 3.02 ± 0.79 ABCDE | 3.00 ± 1.00 ABCDEF |
| ⋮ | ⋮ | ⋮ | ⋮ | ⋮ | ⋮ |
| 4 | 3.15 ± 0.55 IJ | 37.23 ± 10.32 GF | 1980 ± 11 H | 2.56 ± 0.73 DE | 2.51 ± 0.70 F |
| 75 | 3.19 ± 0.41 HIJ | 36.16 ± 9.50 GF | 1856 ± 26 H | 2.60 ± 0.34 CDE | 2.56 ± 0.51 EF |
| 29 | 3.11 ± 0.50 J | 35.80 ± 11.52 G | 1854 ± 13 H | 2.37 ± 0.73 E | 2.53 ± 0.77 F |
| Mean | 3.66 ± 0.84 | 44.34 ± 14.65 | 3552 ± 3304 | 2.87 ± 0.79 | 2.91 ± 0.81 |
| F value | 2.78 | 2.32 | 2.74 | 1.99 | 2.33 |
| Types | Family Codes | Height (m·a−1) | DBH (mm·a−1) | Mean Volume (cm3·a−1·tree−1) | Crown Width in Rows (m·a−1) | Crown Width between Rows (m·a−1) |
|---|---|---|---|---|---|---|
| Ⅰ | 15, 76, 56, 49, 17, 5, 48, 18, 71, 12, 62 | 1.33 ± 0.36 | 16.87 ± 5.74 | 5247 ± 324 | 1.03 ± 0.28 | 1.05 ± 0.29 |
| Ⅱ | 73, 24, 11, 9, 46, 63, 52, 10, 45, 42, 47, 68, 36, 50, 67, 32, 25, 51, 41, 16, 20, 64, 31, 65, 55, 59, 54, 40, 44, 35, 33, 28, 2, 30, 6, 66, 74, 34, 22, 43, 7, 8, 23, 72, 14, 1, 39, 77, 3 | 1.23 ± 0.27 | 14.81 ± 4.88 | 3540 ± 49 | 0.96 ± 0.27 | 0.97 ± 0.28 |
| Ⅲ | 61, 69, 37, 21, 13, 60, 27, 38, 70, 57, 19, 53, 26, 58, 4, 75, 29 | 1.15 ± 0.21 | 13.50 ± 3.86 | 2532 ± 339 | 0.91 ± 0.23 | 0.90 ± 0.23 |
| Mean | 1.22 ± 0.28 | 14.78 ± 4.88 | 3562 ± 917 | 0.96 ± 0.26 | 0.97 ± 0.27 | |
| Growth Stage | Month | Growth Level in One Month (m) | ||
|---|---|---|---|---|
| Rapid Growth Type Ⅰ | Mean Growth Type Ⅱ | Slow Growth Type Ⅲ | ||
| Lag-growing stage | January–February | 0.00 ± 0.00 B | 0.12 ± 0.04 C | 0.08 ± 0.06 D |
| First fast-growing stage | March–May | 0.16 ± 0.11 A | 0.18 ± 0.13 A | 0.13 ± 0.14 B |
| First slow-growing stage | June–July | 0.19 ± 0.14 A | 0.16 ± 0.10 B | 0.10 ± 0.08 BC |
| Second fast-growing stage | August | 0.23 ± 0.18 A | 0.17 ± 0.12 A | 0.17 ± 0.10 A |
| Second slow-growing stage | September–December | 0.08 ± 0.08 B | 0.07 ± 0.06 C | 0.05 ± 0.04 CD |
| Growth Stage | Month | Growth Level in One Month (m) | ||
|---|---|---|---|---|
| Rapid Growth Type Ⅰ | Mean Growth Type Ⅱ | Slow Growth Type Ⅲ | ||
| Lag-growing stage | January | 0.00 ± 0.00 E | 0.00 ± 0.00 E | 0.00 ± 0.00 D |
| First slow-growing stage | February–March | 1.91 ± 0.98 CD | 1.78 ± 1.06 C | 1.37 ± 0.64 BC |
| First fast-growing stage | April | 4.58 ± 3.24 A | 4.35 ± 1.97 A | 3.52 ± 1.61 A |
| Second slow-growing stage | May–July | 3.14 ± 1.72 BC | 2.67 ± 1.75 C | 2.17 ± 1.54 B |
| Second fast-growing stage | August | 3.78 ± 2.25 AB | 3.23 ± 1.97 B | 3.63 ± 1.83 A |
| Third slow-growing stage | September–December | 1.20 ± 0.74 DE | 1.28 ± 1.28 D | 1.20 ± 0.95 CD |
| Growth Stage | Month | Growth Level in One Month (m) | ||
|---|---|---|---|---|
| Rapid Growth Type Ⅰ | Mean Growth Type Ⅱ | Slow Growth Type Ⅲ | ||
| Lag-growing stage | January–February | 0.07 ± 0.06 B | 0.08 ± 0.09 C | 0.24 ± 0.34 B |
| First fast-growing stage | March–April | 0.32 ± 0.15 A | 0.31 ± 0.15 A | 0.25 ± 0.13 A |
| First slow-growing stage | May–June | 0.24 ± 0.16 B | 0.18 ± 0.14 B | 0.12 ± 0.10 B |
| Second fast-growing stage | July–August | 0.31 ± 0.20 A | 0.31 ± 0.22 A | 0.25 ± 0.17 A |
| Second slow-growing stage | September–December | 0.12 ± 0.12 B | 0.11 ± 0.11 BC | 0.07 ± 0.07 B |
| Growth Stage | Month | Growth Level in One Month (m) | ||
|---|---|---|---|---|
| Rapid Growth Type Ⅰ | Average Growth Type Ⅱ | Slow Growth Type Ⅲ | ||
| Lag-growing stage | January–February | 0.07 ± 0.06 C | 0.10 ± 0.10 D | 0.16 ± 0.21 B |
| First fast-growing stage | March–April | 0.27 ± 0.09 A | 0.30 ± 0.14 A | 0.23 ± 0.10 A |
| First slow-growing stage | May–June | 0.27 ± 0.22 B | 0.23 ± 0.18 C | 0.10 ± 0.08 B |
| Second fast-growing stage | July–August | 0.23 ± 0.14 AB | 0.23 ± 0.13 B | 0.21 ± 0.12 A |
| Second slow-growing stage | September–December | 0.12 ± 0.12 C | 0.10 ± 0.11 D | 0.06 ± 0.05 B |
| Factors |
Mean Monthly Air Temperature |
Mean Monthly Highest Air Temperature |
Mean Monthly Lowest Air Temperature |
Monthly Rainfall |
Monthly Sunny Hours |
Relative Air Humidity |
|---|---|---|---|---|---|---|
| Height | 0.2625 | 0.3098 | 0.2473 | 0.1418 | 0.4155 * | 0.1927 |
| DBH | 0.2093 | 0.2618 | 0.1882 | 0.0866 | 0.4018 * | 0.1535 |
| Volume | 0.2830 | 0.3608 | 0.2404 | 0.0282 | 0.5842 *** | −0.0426 |
| Crown width in rows | 0.2940 | 0.3606 | 0.2569 | 0.1140 | 0.5021 ** | 0.0709 |
| Crown width between rows | 0.2966 | 0.3536 | 0.2672 | 0.1190 | 0.4564 ** | 0.1293 |
| Factors |
Mean Monthly Air Temperature |
Mean Monthly Highest Air Temperature |
Mean Monthly Lowest Air Temperature |
Monthly Rainfall |
Monthly Sunny Hours |
Relative Air Humidity |
| Monthly height growth | 0.1183 | 0.0704 | 0.1167 | 0.4679 ** | −0.4203 * | 0.2619 |
| Monthly DBH growth | 0.3082 | 0.2396 | 0.3269 | 0.6056 *** | −0.3239 | 0.3657 |
| Monthly volume | 0.4521 ** | 0.3942 * | 0.4776 ** | 0.5359 ** | −0.1779 | 0.6901 *** |
| Monthly crown width between tree | −0.2136 | −0.2157 | −0.2282 | −0.0499 | −0.3049 | 0.0211 |
| Monthly crown width between row | −0.1891 | −0.2121 | −0.2017 | −0.0265 | −0.4608 ** | 0.1825 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, G.; Xu, J.; Li, J.; Lu, C.; Lu, H.; Mai, B.; Luo, M.; Fan, C. Growth Rhythm Analysis of Young Stand and Selection of Superior Families in Choerospondias axillaris. Forests 2022, 13, 2145. https://doi.org/10.3390/f13122145
Li G, Xu J, Li J, Lu C, Lu H, Mai B, Luo M, Fan C. Growth Rhythm Analysis of Young Stand and Selection of Superior Families in Choerospondias axillaris. Forests. 2022; 13(12):2145. https://doi.org/10.3390/f13122145
Chicago/Turabian StyleLi, Guangyou, Jianmin Xu, Juan Li, Canzhang Lu, Haifei Lu, Baoying Mai, Mingdao Luo, and Chunjie Fan. 2022. "Growth Rhythm Analysis of Young Stand and Selection of Superior Families in Choerospondias axillaris" Forests 13, no. 12: 2145. https://doi.org/10.3390/f13122145
APA StyleLi, G., Xu, J., Li, J., Lu, C., Lu, H., Mai, B., Luo, M., & Fan, C. (2022). Growth Rhythm Analysis of Young Stand and Selection of Superior Families in Choerospondias axillaris. Forests, 13(12), 2145. https://doi.org/10.3390/f13122145





