Introducing N2-Fixing Tree Species into Eucalyptus Plantation in Subtropical China Alleviated Carbon and Nitrogen Constraints within Soil Aggregates
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Site
2.2. Soil Sampling
2.3. Soil Aggregate Separation
2.4. Physicochemical Analyses of Litterfall and Soil
2.5. Soil Microbial Biomass Analysis
2.6. Analysis of Soil Extracellular Enzyme Activity
2.7. Data Calculation and Analysis
3. Results
3.1. Plant and Bulk Soil Characteristics
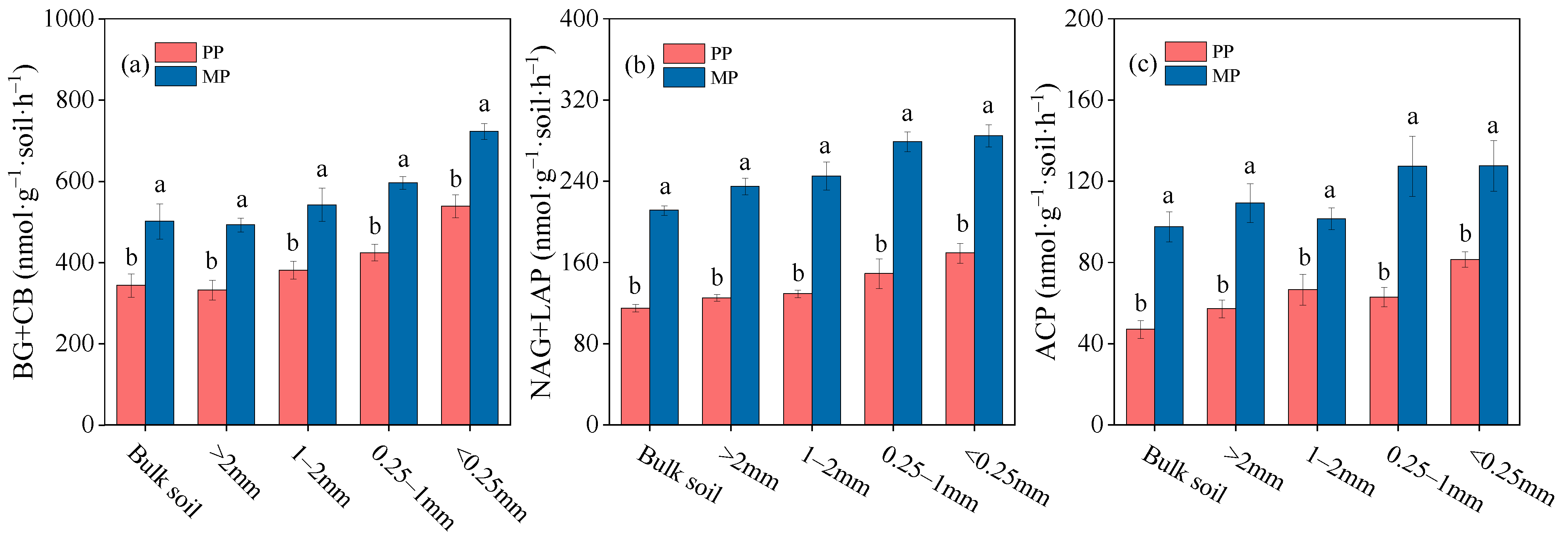
3.2. Soil Enzyme Activity within Bulk Soil and Aggregates
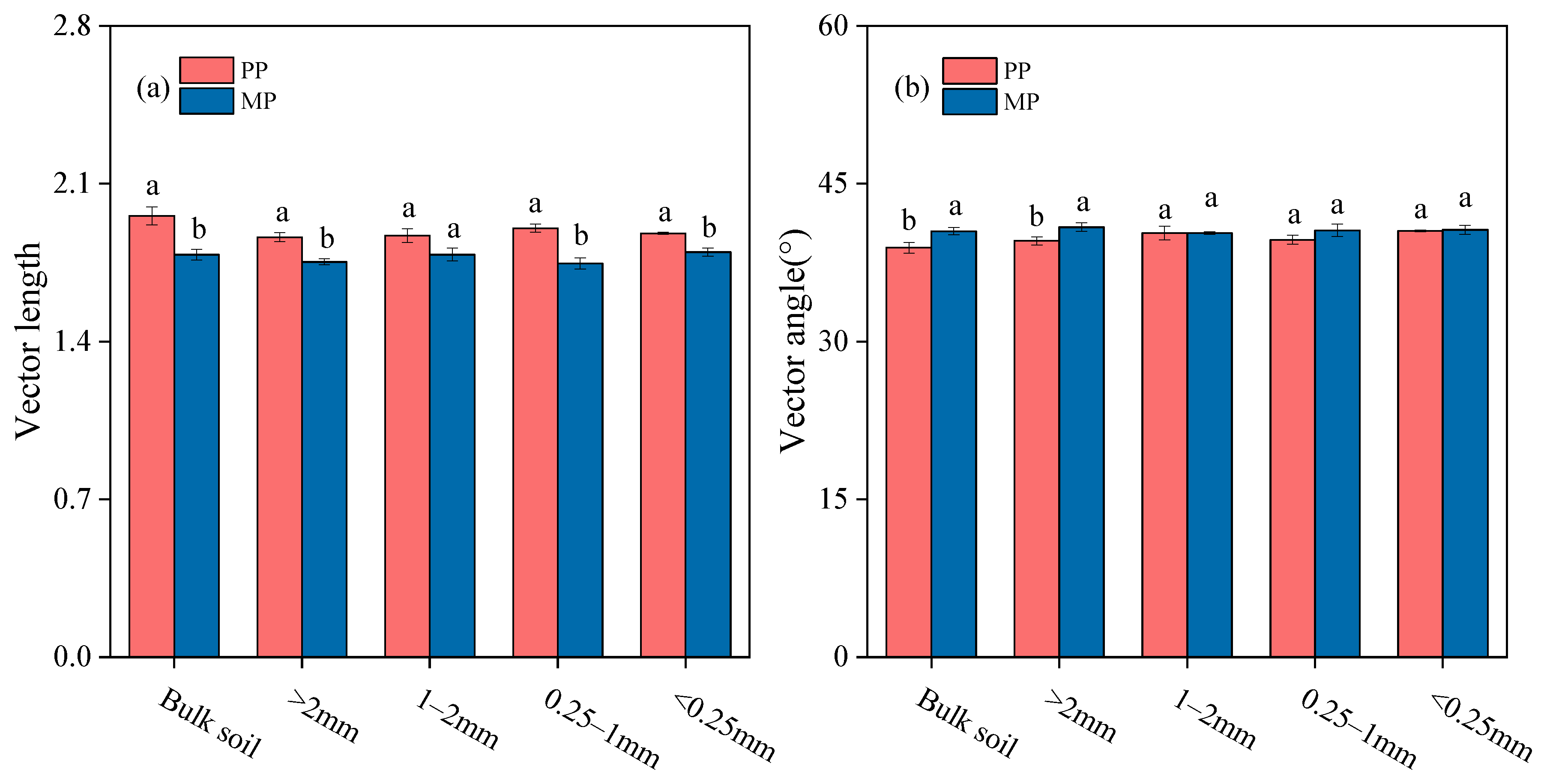
3.3. Soil Enzyme Stoichiometry within Bulk Soil and Aggregates
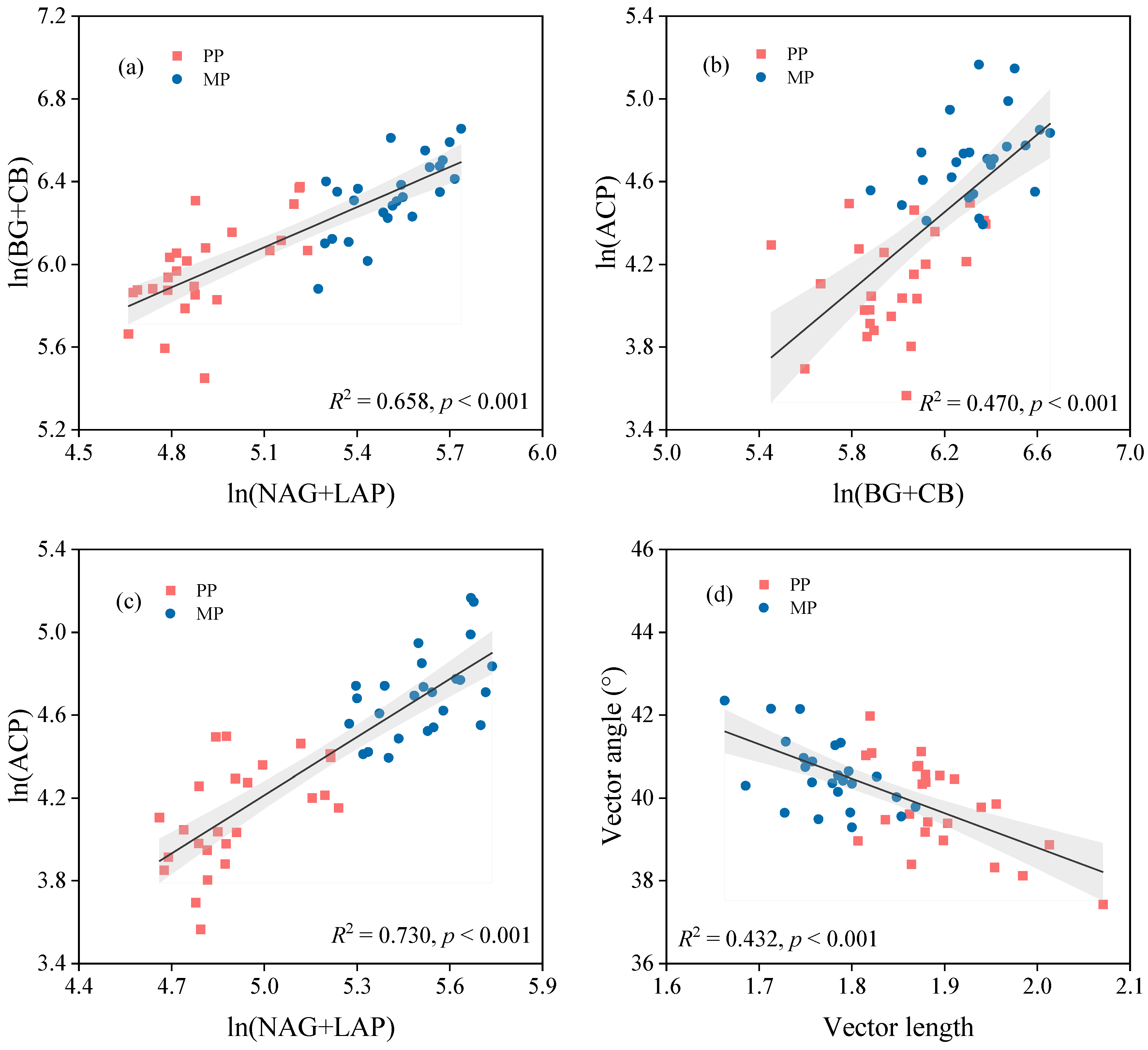
3.4. Factors Influencing Soil Enzyme Activities and their Stoichiometry
4. Discussion
4.1. Effect of Introducing N2-Fixing Species on EEA within Soil Aggregates
4.2. Introducing N2-Fixing Species into Eucalyptus Plantations Alleviates C and N Limitation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Salazar, S.; Sánchez, L.E.; Alvarez, J.; Valverde, A.; Galindo, P.; Igual, J.M.; Peix, A.; Santa-Regina, I. Correlation among soil enzyme activities under different forest system management practices. Ecol. Eng. 2011, 37, 1123–1131. [Google Scholar] [CrossRef]
- Waring, B.G.; Weintraub, S.R.; Sinsabaugh, R.L. Ecoenzymatic stoichiometry of microbial nutrient acquisition in tropical soils. Biogeochemistry 2013, 117, 101–113. [Google Scholar] [CrossRef]
- Cui, Y.; Bing, H.; Fang, L.; Jiang, M.; Shen, G.; Yu, J.; Wang, X.; Zhu, H.; Wu, Y.; Zhang, X. Extracellular enzyme stoichiometry reveals the carbon and phosphorus limitations of microbial metabolisms in the rhizosphere and bulk soils in alpine ecosystems. Plant Soil 2019, 458, 7–20. [Google Scholar] [CrossRef]
- Deng, L.; Peng, C.; Huang, C.; Wang, K.; Liu, Q.; Liu, Y.; Hai, X.; Shangguan, Z. Drivers of soil microbial metabolic limitation changes along a vegetation restoration gradient on the Loess Plateau, China. Geoderma 2019, 353, 188–200. [Google Scholar] [CrossRef]
- Li, J.; Shangguan, Z.; Deng, L. Dynamics of soil microbial metabolic activity during grassland succession after farmland abandonment. Geoderma 2020, 363, 114167. [Google Scholar] [CrossRef]
- Zhou, L.; Liu, S.; Shen, H.; Zhao, M.; Xu, L.; Xing, A.; Fang, J. Soil extracellular enzyme activity and stoichiometry in China’s forests. Funct. Ecol. 2020, 34, 1461–1471. [Google Scholar] [CrossRef]
- Sinsabaugh, R.L.; Lauber, C.L.; Weintraub, M.N.; Ahmed, B.; Allison, S.D.; Crenshaw, C.; Contosta, A.R.; Cusack, D.; Frey, S.; Gallo, M.E.; et al. Stoichiometry of soil enzyme activity at global scale. Ecol. Lett. 2008, 11, 1252–1264. [Google Scholar] [CrossRef]
- Qiu, X.; Peng, D.; Tian, H.; Wang, H.; Liu, X.; Cao, L.; Li, Z.; Cheng, S. Soil ecoenzymatic stoichiometry and microbial resource limitation driven by thinning practices and season types in Larix principis-rupprechtii plantations in North China. For. Ecol. Manag. 2021, 482, 118880. [Google Scholar] [CrossRef]
- Xu, Z.; Yu, G.; Zhang, X.; He, N.; Wang, Q.; Wang, S.; Wang, R.; Zhao, N.; Jia, Y.; Wang, C. Soil enzyme activity and stoichiometry in forest ecosystems along the North-South Transect in eastern China (NSTEC). Soil Biol. Biochem. 2017, 104, 152–163. [Google Scholar] [CrossRef]
- Cui, Y.; Fang, L.; Guo, X.; Wang, X.; Zhang, Y.; Li, P.; Zhang, X. Ecoenzymatic stoichiometry and microbial nutrient limitation in rhizosphere soil in the arid area of the northern Loess Plateau, China. Soil Biol. Biochem. 2018, 116, 11–21. [Google Scholar] [CrossRef]
- Zheng, H.; Liu, Y.; Chen, Y.; Zhang, J.; Li, H.; Wang, L.; Chen, Q. Short-term warming shifts microbial nutrient limitation without changing the bacterial community structure in an alpine timberline of the eastern Tibetan Plateau. Geoderma 2020, 360, 113985. [Google Scholar] [CrossRef]
- Tapia-Torres, Y.; Elser, J.J.; Souza, V.; García-Oliva, F. Ecoenzymatic stoichiometry at the extremes: How microbes cope in an ultra-oligotrophic desert soil. Soil Biol. Biochem. 2015, 87, 34–42. [Google Scholar] [CrossRef]
- Kivlin, S.N.; Treseder, K.K. Soil extracellular enzyme activities correspond with abiotic factors more than fungal community composition. Biogeochemistry 2013, 117, 23–37. [Google Scholar] [CrossRef]
- Ruamps, L.S.; Nunan, N.; Chenu, C. Microbial biogeography at the soil pore scale. Soil Biol. Biochem. 2011, 43, 280–286. [Google Scholar] [CrossRef]
- Kong, A.Y.; Scow, K.M.; Cordova-Kreylos, A.L.; Holmes, W.E.; Six, J. Microbial community composition and carbon cycling within soil microenvironments of conventional, low-input, and organic cropping systems. Soil Biol. Biochem. 2011, 43, 20–30. [Google Scholar] [CrossRef] [PubMed]
- Qiao, L.; Li, Y.; Song, Y.; Zhai, J.; Wu, Y.; Chen, W.; Liu, G.; Xue, S. Effects of Vegetation Restoration on the Distribution of Nutrients, Glomalin-Related Soil Protein, and Enzyme Activity in Soil Aggregates on the Loess Plateau, China. Forests 2019, 10, 796. [Google Scholar] [CrossRef]
- Wang, R.; Dorodnikov, M.; Yang, S.; Zhang, Y.; Filley, T.R.; Turco, R.F.; Zhang, Y.; Xu, Z.; Li, H.; Jiang, Y. Responses of enzymatic activities within soil aggregates to 9-year nitrogen and water addition in a semi-arid grassland. Soil Biol. Biochem. 2015, 81, 159–167. [Google Scholar] [CrossRef]
- Allison, S.D.; Jastrow, J.D. Activities of extracellular enzymes in physically isolated fractions of restored grassland soils. Soil Biol. Biochem. 2006, 38, 3245–3256. [Google Scholar] [CrossRef]
- Wei, Y.; Wang, Z.; Zhang, X.; Yang, H.; Liu, X.; Liu, W. Enzyme Activities and Microbial Communities in Subtropical Forest Soil Aggregates to Ammonium and Nitrate-Nitrogen Additions. J. Resour. Ecol. 2017, 8, 258–267. [Google Scholar]
- Qin, S.; Hu, C.; He, X.; Dong, W.; Cui, J.; Wang, Y. Soil organic carbon, nutrients and relevant enzyme activities in particle-size fractions under conservational versus traditional agricultural management. Appl. Soil Ecol. 2010, 45, 152–159. [Google Scholar] [CrossRef]
- Liang, Q.; Chen, H.; Gong, Y.; Yang, H.; Fan, M.; Kuzyakov, Y. Effects of 15 years of manure and mineral fertilizers on enzyme activities in particle-size fractions in a North China Plain soil. Eur. J. Soil Biol. 2014, 60, 112–119. [Google Scholar] [CrossRef]
- Yang, G.; Wen, M.; Deng, Y.; Su, X.; Jiang, D.; Wang, G.; Chen, Y.; Chen, G.; Yu, S. Occurrence patterns of black water and its impact on fish in cutover areas of Eucalyptus plantations. Sci. Total Environ. 2019, 693, 133393. [Google Scholar] [CrossRef] [PubMed]
- Asante, P.; Armstrong, G.W.; Adamowicz, W.L. Carbon sequestration and the optimal forest harvest decision: A dynamic programming approach considering biomass and dead organic matter. J. For. Econ. 2011, 17, 3–17. [Google Scholar] [CrossRef]
- González-García, S.; Moreira, M.T.; Feijoo, G. Environmental aspects of eucalyptus based ethanol production and use. Sci. Total Environ. 2012, 438, 1–8. [Google Scholar] [CrossRef] [PubMed]
- LeBauer, D.S.; Treseder, K.K. Nitrogen limitation of net primary productivity in terrestrial ecosystems is globally distributed. Ecology 2008, 89, 371–379. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, S.; Xiang, W.; Wang, X.; Xiao, W.; Chen, L.; Li, S.; Sun, H.; Deng, X.; Forrester, D.I.; Zeng, L.; et al. Effects of stand age, richness and density on productivity in subtropical forests in China. J. Ecol. 2019, 107, 2266–2277. [Google Scholar] [CrossRef]
- Ouyang, S.; Xiang, W.; Gou, M.; Chen, L.; Lei, P.; Xiao, W.; Deng, X.; Zeng, L.; Li, J.; Zhang, T.; et al. Stability in subtropical forests: The role of tree species diversity, stand structure, environmental and socio-economic conditions. Glob. Ecol. Biogeogr. 2020, 30, 500–513. [Google Scholar] [CrossRef]
- Huang, X.; Liu, S.; Wang, H.; Hu, Z.; Li, Z.; You, Y. Changes of soil microbial biomass carbon and community composition through mixing nitrogen-fixing species with Eucalyptus urophylla in subtropical China. Soil Biol. Biochem. 2014, 73, 42–48. [Google Scholar] [CrossRef]
- Huang, X.; Liu, S.; You, Y.; Wen, Y.; Wang, H.; Wang, J. Microbial community and associated enzymes activity influence soil carbon chemical composition in Eucalyptus urophylla plantation with mixing N2-fixing species in subtropical China. Plant Soil 2016, 414, 199–212. [Google Scholar] [CrossRef]
- You, Y.; Xu, H.; Wu, X.; Zhou, X.; Tan, X.; Li, M.; Wen, Y.; Zhu, H.; Cai, D.; Huang, X. Native broadleaf tree species stimulate topsoil nutrient transformation by changing microbial community composition and physiological function, but not biomass in subtropical plantations with low P status. For. Ecol. Manag. 2020, 477, 118491. [Google Scholar] [CrossRef]
- Wang, S.; Li, T.; Zheng, Z. Effects of tea plantation age on soil aggregate-associated C- and N-cycling enzyme activities in the hilly areas of Western Sichuan, China. Catena 2018, 171, 145–153. [Google Scholar] [CrossRef]
- Schutter, M.E.; Dick, R.P. Microbial Community Profiles and Activities among Aggregates of Winter Fallow and Cover-Cropped Soil. Soil Sci. Soc. Am. J. 2002, 66, 142–153. [Google Scholar] [CrossRef]
- Nelson, D.W.; Sommers, L.E. Total Carbon, Organic Carbon, and Organic Matter. In Methods of Soil Analysis; American Society of Agronomy and Soil Science Society of American: Madison, WI, USA, 1996; pp. 916–1010. [Google Scholar]
- Gallaher, R.N.; Weldon, C.O.; Boswell, F.C. A Semiautomated Procedure for Total Nitrogen in Plant and Soil Samples. Soil Sci. Soc. Am. J. 1976, 40, 887–889. [Google Scholar] [CrossRef]
- Murphy, J.; Riley, J.P. A modified single solution method for the determination of phosphate in natural waters. Anal. Chim. Acta 1962, 27, 678–681. [Google Scholar] [CrossRef]
- Vance, E.D.; Brookes, P.C.; Jenkinson, D.S. An extraction method for measuring soil microbial biomass C. Soil Biol. Biochem. 1987, 19, 703–707. [Google Scholar] [CrossRef]
- Ruiz, R.; Nickel, B.; Koch, N.; Feldman, L.C.; Haglund, R.F., Jr.; Kahn, A.; Family, F.; Scoles, G. Dynamic scaling, island size distribution, and morphology in the aggregation regime of submonolayer pentacene films. Phys. Rev. Lett. 2003, 91, 136102. [Google Scholar] [CrossRef]
- German, D.P.; Weintraub, M.N.; Grandy, A.S.; Lauber, C.L.; Rinkes, Z.L.; Allison, S.D. Optimization of hydrolytic and oxidative enzyme methods for ecosystem studies. Soil Biol. Biochem. 2011, 43, 1387–1397. [Google Scholar] [CrossRef]
- Looby, C.I.; Treseder, K.K. Shifts in soil fungi and extracellular enzyme activity with simulated climate change in a tropical montane cloud forest. Soil Biol. Biochem. 2018, 117, 87–96. [Google Scholar] [CrossRef]
- Fattet, M.; Fu, Y.; Ghestem, M.; Ma, W.; Foulonneau, M.; Nespoulous, J.; Le Bissonnais, Y.; Stokes, A. Effects of vegetation type on soil resistance to erosion: Relationship between aggregate stability and shear strength. Catena 2011, 87, 60–69. [Google Scholar] [CrossRef]
- Zhong, X.; Li, J.; Li, X.; Ye, Y.; Liu, S.; Xu, G.; Ni, J. Early effect of soil aggregates on enzyme activities in a forest soil with simulated N deposition elevation. Acta Ecol. Sin. 2015, 35, 1422–1433. (In Chinese) [Google Scholar]
- Sinsabaugh, R.L.; Hill, B.H.; Follstad Shah, J.J. Ecoenzymatic stoichiometry of microbial organic nutrient acquisition in soil and sediment. Nature 2009, 462, 795–798. [Google Scholar] [CrossRef] [PubMed]
- Moorhead, D.L.; Sinsabaugh, R.L.; Hill, B.H.; Weintraub, M.N. Vector analysis of ecoenzyme activities reveal constraints on coupled C, N and P dynamics. Soil Biol. Biochem. 2016, 93, 1–7. [Google Scholar] [CrossRef]
- Cusack, D.F.; Silver, W.L.; Torn, M.S.; Burton, S.D.; Firestone, M.K. Changes in microbial community characteristics and soil organic matter with nitrogen additions in two tropical forests. Ecology 2011, 92, 621–632. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Zhou, W.; Liang, G.; Sun, J.; Wang, X.; He, P. Distribution of soil nutrients, extracellular enzyme activities and microbial communities across particle-size fractions in a long-term fertilizer experiment. Appl. Soil Ecol. 2015, 94, 59–71. [Google Scholar] [CrossRef]
- Li, M.; You, Y.; Tan, X.; Wen, Y.; Yu, S.; Xiao, N.; Shen, W.; Huang, X. Mixture of N2-fixing tree species promotes organic phosphorus accumulation and transformation in topsoil aggregates in a degraded karst region of subtropical China. Geoderma 2022, 413, 115752. [Google Scholar] [CrossRef]
- Dong, W.; Zhang, X.; Liu, X.; Fu, X.; Chen, F.; Wang, H.; Sun, X.; Wen, X. Responses of soil microbial communities and enzyme activities to nitrogen and phosphorus additions in Chinese fir plantations of subtropical China. Biogeosciences 2015, 12, 5537–5546. [Google Scholar] [CrossRef]
- Sun, X.; Zhao, J.; You, Y.; Jianxin Sun, O. Soil microbial responses to forest floor litter manipulation and nitrogen addition in a mixed-wood forest of northern China. Sci. Rep. 2016, 6, 19536. [Google Scholar] [CrossRef]
- Jing, X.; Chen, X.; Tang, M.; Ding, Z.; Jiang, L.; Li, P.; Ma, S.; Tian, D.; Xu, L.; Zhu, J.; et al. Nitrogen deposition has minor effect on soil extracellular enzyme activities in six Chinese forests. Sci. Total Environ. 2017, 607–608, 806–815. [Google Scholar] [CrossRef]
- Luo, Y.; Su, B.; Currie, W.S.; Dukes, J.S.; Finzi, A.; Hartwig, U.; Hungate, B.; McMurtrie, R.E.; Oren, R.; Parton, W.J.; et al. Progressive nitrogen limitation of ecosystem responses to rising atmospheric carbon dioxide. Bioscience 2004, 54, 731–739. [Google Scholar] [CrossRef]
- Fatemi, F.R.; Fernandez, I.J.; Simon, K.S.; Dail, D.B. Nitrogen and phosphorus regulation of soil enzyme activities in acid forest soils. Soil Biol. Biochem. 2016, 98, 171–179. [Google Scholar] [CrossRef]
- Marklein, A.R.; Houlton, B.Z. Nitrogen inputs accelerate phosphorus cycling rates across a wide variety of terrestrial ecosystems. New Phytol. 2012, 193, 696–704. [Google Scholar] [CrossRef] [PubMed]
- Pereira, A.P.D.A.; Araujo, A.S.F.; Santana, M.C.; Lima, A.Y.V.; Araujo, V.L.V.P.D.; Verma, J.P.; Cardoso, E.J.B.N. Enzymatic stoichiometry in tropical soil under pure and mixed plantations of eucalyptus with N2-fixing trees. Sci. Agric. 2022, 80, e20210283. [Google Scholar] [CrossRef]
- Nasto, M.K.; Alvarez-Clare, S.; Lekberg, Y.; Sullivan, B.W.; Townsend, A.R.; Cleveland, C.C. Interactions among nitrogen fixation and soil phosphorus acquisition strategies in lowland tropical rain forests. Ecol. Lett. 2014, 17, 1282–1289. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Ai, Z.; Liang, C.; Wang, G.; Liu, G.; Xue, S. How microbes cope with short-term N addition in a Pinus tabuliformis forest-ecological stoichiometry. Geoderma 2019, 337, 630–640. [Google Scholar] [CrossRef]
- Dong, C.; Wang, W.; Liu, H.; Xu, X.; Zeng, H. Temperate grassland shifted from nitrogen to phosphorus limitation induced by degradation and nitrogen deposition: Evidence from soil extracellular enzyme stoichiometry. Ecol. Indic. 2019, 101, 453–464. [Google Scholar] [CrossRef]
- Gallo, M.; Amonette, R.; Lauber, C.; Sinsabaugh, R.L.; Zak, D.R. Microbial community structure and oxidative enzyme activity in nitrogen-amended north temperate forest soils. Microb. Ecol. 2004, 48, 218–229. [Google Scholar] [CrossRef]
- Allison, S.D.; Treseder, K.K. Warming and drying suppress microbial activity and carbon cycling in boreal forest soils. Glob. Chang. Biol. 2008, 14, 2898–2909. [Google Scholar] [CrossRef]
- Zhou, X.; Chen, C.; Wang, Y.; Xu, Z.; Han, H.; Li, L.; Wan, S. Warming and increased precipitation have differential effects on soil extracellular enzyme activities in a temperate grassland. Sci. Total Environ. 2013, 444, 552–558. [Google Scholar] [CrossRef]
- Yang, Y.; Liang, C.; Wang, Y.; Cheng, H.; An, S.; Chang, S.X. Soil extracellular enzyme stoichiometry reflects the shift from P- to N-limitation of microorganisms with grassland restoration. Soil Biol. Biochem. 2020, 149, 107928. [Google Scholar] [CrossRef]
- Lagomarsino, A.; Grego, S.; Kandeler, E. Soil organic carbon distribution drives microbial activity and functional diversity in particle and aggregate-size fractions. Pedobiologia 2012, 55, 101–110. [Google Scholar] [CrossRef]
- Nannipieri, P.; Giagnoni, L.; Renella, G.; Puglisi, E.; Ceccanti, B.; Masciandaro, G.; Fornasier, F.; Moscatelli, M.C.; Marinari, S. Soil enzymology: Classical and molecular approaches. Biol. Fertil. Soils 2012, 48, 743–762. [Google Scholar] [CrossRef]
- Li, W.T.; Liu, M.; Jiang, C.Y.; Wu, M.; Chen, X.F.; Ma, X.Y.; Li, Z.P. Changes in soil aggregate-associated enzyme activities and nutrients under long-term chemical fertilizer applications in a phosphorus-limited paddy soil. Soil Use Manag. 2017, 33, 25–33. [Google Scholar] [CrossRef]
- Fanin, N.; Fromin, N.; Buatois, B.; Hättenschwiler, S. An experimental test of the hypothesis of non-homeostatic consumer stoichiometry in a plant litter-microbe system. Ecol. Lett. 2013, 16, 764–772. [Google Scholar] [CrossRef] [PubMed]
- Shen, F.; Wu, J.; Fan, H.; Liu, W.; Guo, X.; Duan, H.; Hu, L.; Lei, X.; Wei, X. Soil N/P and C/P ratio regulate the responses of soil microbial community composition and enzyme activities in a long-term nitrogen loaded Chinese fir forest. Plant Soil 2018, 436, 91–107. [Google Scholar] [CrossRef]
- Santos, F.M.; Balieiro, F.D.C.; Fontes, M.A.; Chaer, G.M. Understanding the enhanced litter decomposition of mixed-species plantations of Eucalyptus and Acacia mangium. Plant Soil 2017, 423, 141–155. [Google Scholar] [CrossRef]
- Chen, X.; Han, X.; Lu, X.; Yan, J.; Biswas, A.; Zou, W. Long-term continuous cropping affects ecoenzymatic stoichiometry of microbial nutrient acquisition: A case study from a Chinese Mollisol. J. Sci. Food Agric. 2021, 101, 6338–6346. [Google Scholar] [CrossRef]
- Xu, M.; Li, W.; Wang, J.; Zhu, Y.; Feng, Y.; Yang, G.; Zhang, W.; Han, X. Soil ecoenzymatic stoichiometry reveals microbial phosphorus limitation after vegetation restoration on the Loess Plateau, China. Sci. Total Environ. 2022, 815, 152918. [Google Scholar] [CrossRef]
- Inagaki, M.; Kamo, K.; Miyamoto, K.; Titin, J.; Jamalung, L.; Lapongan, J.; Miura, S. Nitrogen and phosphorus retranslocation and N:P ratios of litterfall in three tropical plantations: Luxurious N and efficient P use by Acacia mangium. Plant Soil 2010, 341, 295–307. [Google Scholar] [CrossRef]
- Liu, J.; Yang, Z.; Dang, P.; Zhu, H.; Gao, Y.; Ha, V.N.; Zhao, Z. Response of soil microbial community dynamics to Robinia pseudoacacia L. afforestation in the loess plateau: A chronosequence approach. Plant Soil 2017, 423, 327–338. [Google Scholar] [CrossRef]
- Zeng, Q.; Lal, R.; Chen, Y.; An, S. Soil, Leaf and Root Ecological Stoichiometry of Caragana korshinskii on the Loess Plateau of China in Relation to Plantation Age. PLoS ONE 2017, 12, e0168890. [Google Scholar] [CrossRef]
- Peng, X.; Wang, W. Stoichiometry of soil extracellular enzyme activity along a climatic transect in temperate grasslands of northern China. Soil Biol. Biochem. 2016, 98, 74–84. [Google Scholar] [CrossRef]
- Peri, P.L.; Gargaglione, V.; Pastur, G.M. Dynamics of above- and below-ground biomass and nutrient accumulation in an age sequence of Nothofagus antarctica forest of Southern Patagonia. For. Ecol. Manag. 2006, 233, 85–99. [Google Scholar] [CrossRef]
- Zhang, W.; Xu, Y.; Gao, D.; Wang, X.; Liu, W.; Deng, J.; Han, X.; Yang, G.; Feng, Y.; Ren, G. Ecoenzymatic stoichiometry and nutrient dynamics along a revegetation chronosequence in the soils of abandoned land and Robinia pseudoacacia plantation on the Loess Plateau, China. Soil Biol. Biochem. 2019, 134, 1–14. [Google Scholar] [CrossRef]
- Sinsabaugh, R.L.; Follstad Shah, J.J. Integrating resource utilization and temperature in metabolic scaling of riverine bacterial production. Ecology 2010, 91, 1455–1465. [Google Scholar] [CrossRef] [PubMed]
- Ushio, M.; Balser, T.C.; Kitayama, K. Effects of condensed tannins in conifer leaves on the composition and activity of the soil microbial community in a tropical montane forest. Plant Soil 2012, 365, 157–170. [Google Scholar] [CrossRef]
- Wang, X.; Cui, Y.; Wang, Y.; Duan, C.; Niu, Y.; Sun, R.; Shen, Y.; Guo, X.; Fang, L. Ecoenzymatic stoichiometry reveals phosphorus addition alleviates microbial nutrient limitation and promotes soil carbon sequestration in agricultural ecosystems. J. Soils Sediments 2021, 22, 536–546. [Google Scholar] [CrossRef]
- Zhu, X.; Liu, M.; Kou, Y.; Liu, D.; Liu, Q.; Zhang, Z.; Jiang, Z.; Yin, H. Differential effects of N addition on the stoichiometry of microbes and extracellular enzymes in the rhizosphere and bulk soils of an alpine shrubland. Plant Soil 2020, 449, 285–301. [Google Scholar] [CrossRef]
- Wang, W.; Qiu, L.; Zu, Y.; Su, D.; An, J.; Wang, H.; Zheng, G.; Sun, W.; Chen, X. Changes in soil organic carbon, nitrogen, pH and bulk density with the development of larch (Larix gmelinii) plantations in China. Glob. Chang. Biol. 2011, 17, 2657–2676. [Google Scholar]
- Yan, T.; Lü, X.; Zhu, J.; Yang, K.; Yu, L.; Gao, T. Changes in nitrogen and phosphorus cycling suggest a transition to phosphorus limitation with the stand development of larch plantations. Plant Soil 2017, 422, 385–396. [Google Scholar] [CrossRef]
- Chen, H.; Li, D.; Zhao, J.; Xiao, K.; Wang, K. Effects of nitrogen addition on activities of soil nitrogen acquisition enzymes: A meta-analysis. Agric. Ecosyst. Environ. 2018, 252, 126–131. [Google Scholar] [CrossRef]
- Zhao, J.; Zeng, Z.; He, X.; Chen, H.; Wang, K. Effects of monoculture and mixed culture of grass and legume forage species on soil microbial community structure under different levels of nitrogen fertilization. Eur. J. Soil Biol. 2015, 68, 61–68. [Google Scholar] [CrossRef]
- Feng, J.; Wu, J.; Zhang, Q.; Zhang, D.; Li, Q.; Long, C.; Yang, F.; Chen, Q.; Cheng, X. Stimulation of nitrogen-hydrolyzing enzymes in soil aggregates mitigates nitrogen constraint for carbon sequestration following afforestation in subtropical China. Soil Biol. Biochem. 2018, 123, 136–144. [Google Scholar] [CrossRef]




| Stand Type | Elevation (m) | Slope (°) | Age (Years) | SD (Trees∙hm−2) | DBH (cm) | TH (m) |
|---|---|---|---|---|---|---|
| PP | 232 | 24 | 15 | 580 ± 23 | 20.43 ± 1.32 | 24.82 ± 0.93 |
| MP | 241 | 22 | 15 | - | - | - |
| Eucalyptus urophylla | - | - | - | 300 ± 14 | 21.22 ± 1.56 | 24.65 ± 2.31 |
| Acacia mangium | - | - | - | 310 ± 31 | 15.81 ± 1.08 | 20.82 ± 1.69 |
| Sample | Item | Stand Type | |
|---|---|---|---|
| PP | MP | ||
| Litter | Amount (kg·hm−2·years−1) | 4907.19 ± 127.25 b | 8434.85 ± 199.90 a |
| C:Nlitter | 50.47 ± 1.34 a | 37.74 ± 1.09 b | |
| Root | Biomass (kg·hm−2) | 1124.08 ± 60.82 a | 995.67 ± 42.78 a |
| Soil | SOC (g·kg−1) | 15.54 ± 0.36 b | 19.01 ± 0.79 a |
| TN (g·kg−1) | 1.23 ± 0.06 b | 1.72 ± 0.09 a | |
| C:Nsoil | 12.74 ± 0.70 a | 11.16 ± 0.63 a | |
| TP (g·kg−1) | 0.23 ± 0.01 a | 0.22 ± 0.01 a | |
| C:Psoil | 67.21 ± 3.65 b | 89.04 ± 4.75 a | |
| N:Psoil | 5.36 ± 0.47 b | 8.00 ± 0.29 a | |
| NH4+-N (mg·kg−1) | 28.80 ± 1.43 a | 24.82 ± 0.94 a | |
| NO3−-N (mg·kg−1) | 5.51 ± 0.13 b | 12.26 ± 0.65 a | |
| pH | 4.67 ± 0.06 b | 5.22 ± 0.12 a | |
| MWD (mm) | 1.66 ± 0.01 b | 1.98 ± 0.04 a | |
| Stand Type | Aggregate Size | REAIC | REAIN | REAIP | REACI |
|---|---|---|---|---|---|
| PP | >2 mm | 0.84 ± 0.05 c | 0.91 ± 0.01 b | 0.89 ± 0.04 b | 0.88 ± 0.02 c |
| 1–2 mm | 0.97 ± 0.04 bc | 0.94 ± 0.04 b | 1.03 ± 0.09 b | 0.98 ± 0.03 bc | |
| 0.25–1 mm | 1.08 ± 0.07 b | 1.07 ± 0.07 ab | 0.99 ± 0.05 b | 1.05 ± 0.05 b | |
| <0.25 mm | 1.37 ± 0.06 a | 1.23 ± 0.06 a | 1.29 ± 0.10 a | 1.29 ± 0.05 a | |
| MP | >2 mm | 0.90 ± 0.02 c | 0.94 ± 0.02 b | 0.96 ± 0.05 a | 0.93 ± 0.02 b |
| 1–2 mm | 1.00 ± 0.07 bc | 0.97 ± 0.03 b | 0.91 ± 0.08 a | 0.96 ± 0.06 b | |
| 0.25–1 mm | 1.10 ± 0.04 b | 1.11 ± 0.04 a | 1.12 ± 0.08 a | 1.11 ± 0.05 a | |
| <0.25 mm | 1.33 ± 0.04 a | 1.13 ± 0.02 a | 1.13 ± 0.09 a | 1.20 ± 0.03 a |
| Variables | Lambda-A | Lambda-B | p | F |
|---|---|---|---|---|
| NO3−-N | 66.5 | 66.5 | 0.002 | 95.2 |
| LF | 61.9 | 1.2 | 0.110 | 2.2 |
| N:Psoil | 58.4 | 0.1 | 0.792 | 0.2 |
| C:Nlitter | 58.0 | 0.2 | 0.592 | 0.4 |
| TN | 55.1 | 5.5 | 0.002 | 9.2 |
| MBP | 51.4 | <0.1 | 0.958 | <0.1 |
| MBN | 46.6 | 0.2 | 0.662 | 0.4 |
| C:Psoil | 44.9 | 1.2 | 0.134 | 2.1 |
| SOC | 40.6 | 0.2 | 0.710 | 0.3 |
| MBC | 35.3 | 1.1 | 0.114 | 2.1 |
| MWD | 33.3 | 0.5 | 0.380 | 0.9 |
| MBC:MBP | 26.6 | 2.5 | 0.018 | 4.4 |
| pH | 19.6 | 0.2 | 0.734 | 0.3 |
| MBC:MBN | 18.4 | 0.2 | 0.750 | 0.3 |
| Variables | Lambda-A | Lambda-B | p | F |
|---|---|---|---|---|
| LF | 38.1 | 38.1 | 0.002 | 29.6 |
| C:Nlitter | 36.7 | 0.4 | 0.726 | 0.3 |
| NO3−-N | 34.5 | 1.1 | 0.396 | 0.8 |
| N:Psoil | 27.1 | 0.5 | 0.618 | 0.4 |
| MWD | 21.1 | 0.8 | 0.542 | 0.6 |
| TN | 20.1 | 0.5 | 0.672 | 0.4 |
| MBN | 18.2 | 0.5 | 0.708 | 0.3 |
| MBP | 15.1 | 0.4 | 0.778 | 0.3 |
| MBC:MBP | 14.0 | 2.7 | 0.136 | 2.2 |
| C:Psoil | 13.6 | 0.3 | 0.768 | 0.2 |
| pH | 13.0 | 0.6 | 0.628 | 0.5 |
| C:Nsoil | 10.5 | 2.0 | 0.162 | 1.6 |
| MBC:MBN | 9.1 | 1.0 | 0.420 | 0.8 |
| SOC | 7.8 | 0.2 | 0.822 | 0.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, J.; Huang, X.; Su, X.; Zhang, W.; Gao, G.; You, Y. Introducing N2-Fixing Tree Species into Eucalyptus Plantation in Subtropical China Alleviated Carbon and Nitrogen Constraints within Soil Aggregates. Forests 2022, 13, 2102. https://doi.org/10.3390/f13122102
Yan J, Huang X, Su X, Zhang W, Gao G, You Y. Introducing N2-Fixing Tree Species into Eucalyptus Plantation in Subtropical China Alleviated Carbon and Nitrogen Constraints within Soil Aggregates. Forests. 2022; 13(12):2102. https://doi.org/10.3390/f13122102
Chicago/Turabian StyleYan, Jinliu, Xueman Huang, Xiaoyan Su, Wen Zhang, Guannv Gao, and Yeming You. 2022. "Introducing N2-Fixing Tree Species into Eucalyptus Plantation in Subtropical China Alleviated Carbon and Nitrogen Constraints within Soil Aggregates" Forests 13, no. 12: 2102. https://doi.org/10.3390/f13122102
APA StyleYan, J., Huang, X., Su, X., Zhang, W., Gao, G., & You, Y. (2022). Introducing N2-Fixing Tree Species into Eucalyptus Plantation in Subtropical China Alleviated Carbon and Nitrogen Constraints within Soil Aggregates. Forests, 13(12), 2102. https://doi.org/10.3390/f13122102




