Abstract
Knowledge acquisition on the response of tree species to drought in the Mediterranean hotspot is an important step to guide adaptation strategies to climate change impacts, e.g., assisted migration. We assessed the resilience components—i.e., resistance, recovery, and resilience—to drought in 2003 in five provenances of maritime pine planted in four common gardens in Sardinia, and analysed the possible influence of climate variables on these indices. The provenances showed differences in growth rate but not in the components of resilience. Among the provenances, Corsica was the most productive, while Tuscany was the least. One of the two provenances from Sardinia (Limbara) showed good performance in terms of tree growth in the comparatively drier site. The resilience components were influenced by prevailing environmental conditions at the common garden sites. In the relatively drier sites, trees showed the lowest resistance but the highest recovery values. However, two sites, which had the lowest stand density, showed the opposite trend during the drought year, probably due to moderate thinning. Predictive models showed different probability in the response of resilience components to climate variables. Resistance and resilience had a similar pattern, both being positively related to temperature, while recovery showed an opposite trend. The models’ results indicate a noticeable adaptation of maritime pine to the drought conditions of Sardinia, though the age factor should be considered as well. Despite only minor differences among provenances being found, environmental conditions and management practices at the common gardens were important in determining tree growth patterns. This study suggests that the provenance of Corsica may provide appropriate material for forest plantations in Mediterranean conditions with mitigation purposes.
1. Introduction
Forest ecosystems and their management are at the forefront of reducing the negative impacts caused by climate change [1]. Forests are closely connected with the carbon cycle, absorbing CO2, and representing one of the major carbon sinks in Europe [2]. However, along with increasing temperature, warming-related disturbances are also becoming more frequent and severe [3], with significant impacts on forest health and tree vitality [4,5,6]. The Mediterranean area is considered particularly vulnerable to extreme events [7]. Due to the recurrent drought spells and heat waves, the net primary production of Mediterranean forests may decrease, altering the long-term carbon balances and reversing the role of forests from carbon sinks to carbon sources [8]. In response to disturbance pressure, forest tree species will have to adapt to changing local environmental conditions. Alternatively, tree populations will have to migrate to environments matching their present climate. However, tree species and their populations are genetically adapted to the historical local climate conditions where they occur, and migration over long distances requires several generations [9]. However, climate change and extreme events may result in large-scale tree mortality due to the maladaptation of local species or populations.
A shift from a carbon-centric vision to a climate-smart approach is, therefore, warranted in the Mediterranean regions (such as marginal mountain systems) to account for increasing disturbances and to integrate adaptation and mitigation strategies for the continuous provisioning of forest functions and services [10,11]. Assisted migration, which consists of relocating species or populations used for reforestation to colder environments in anticipation of climate warming over tree life span [12], represents a climate-smart management option for preserving forest ecosystem functions and services. Indeed, the rapid pace of climate change exceeds the capacity of tree species to migrate towards other geographic areas [13,14]. Although less risky than aided species range expansion, assisted population migration requires the support of solid scientific bases to minimise risks in its implementation [5]. Despite an increasing interest in adopting assisted population migration measures, the evidence base for the suitability of this practice is currently lacking for most species and specific areas, such as the Mediterranean. Common garden experiments, consisting of plantations with trees of the same species from different geographical origins in the same environmental conditions, aimed at understanding intraspecific variations in responses to disturbances, may provide evidence of adaptation bounds and lags between the trees in each seed zone and the prevailing climate conditions.
Resistance, recovery, and resilience are useful indicators for assessing the vulnerability of forest tree species to natural disturbances and to support the application of assisted population migration. This is because “resistance” is defined as the ability of an ecosystem to maintain its functions during the disturbance without losses; “recovery” represents the capacity to recover relative to the damage that occurred during the disturbance; and “resilience” is the capacity of a system to absorb disturbance and reorganize, while undergoing change to still retain essentially the same function, structure, and feedbacks [15,16,17]. Several studies performed in common gardens used the dendrochronological techniques to define these indices [18,19,20,21], utilising tree rings as suitable archives of information on climate as they are strongly influenced by climate conditions [22]. Tree ring studies allow the observation and comparison of responses to disturbances among forest populations [23], highlighting the intraspecific strategies developed by different species or provenances as response to the stress conditions. For example, in common gardens of the Pacific Northwest region of the USA, populations of Pseudotsuga menziesii var. menziesii (Mirb.) Franco from regions with cold winters showed high resistance to drought and freezing, while populations from regions with dry summers displayed high resistance to drought only [19]. In Austria, populations of Larix decidua Mill. from the northern distribution limit were more resistant to drought than populations from the southern fringe [20]. In a provenance trial in Germany, the growth response to drought in Pinus sylvestris L., based on tolerance indices of resistance, recovery, and resilience, indicated a better adaptation to the water stress of the provenance in Bosnia Herzegovina and of local provenances compared to other provenances [18]. Although the Mediterranean basin is strongly threatened by global warming, studies focused on the sensitivity of different conifer tree provenances to changing climate conditions are rather limited [21].
Pinus pinaster Ait. (maritime pine) has been used widely for soil protection and dune stabilisation because of its tolerance to salt spray and fast growth, even in poor soils [24]. Its range is strongly fragmented, extending from the sea level in France, Spain, Portugal, and Italy to 2000 m a.s.l. in Morocco (the southernmost limit). Thus, maritime pine grows under different environmental conditions, which may trigger the development of different intraspecific strategies [25]. Secondary growth is sensitive to precipitation occurring during the growing season [26], and summer drought is its principal limiting factor [27]. Populations of maritime pine from dry sites showed relatively higher resistance to drought-induced xylem cavitation [28] and a greater allocation of biomass to roots [29] and to water-loss resistant needles [30]. However, mesic ecotypes may also tolerate drought stress through a fine stomatal control [31]. Taking advantage of four common gardens in Sardinia, the second largest island in the Mediterranean Sea, we studied the resilience components and the influence of climate variables on resistance, recovery, and resilience in maritime pine from five provenances. Our hypothesis was that differences in the adaptive capacity to drought stress among provenances in the four common gardens are stronger when resistance, recovery, and resilience indices are measured at harsher common garden sites. We assumed that local provenances from Sardinia are better adapted to the present common garden conditions. The objectives of the present study were: (i) assess whether differences in the growth response to drought stress occur between common garden sites; (ii) identify provenances with higher growth tolerance to drought events, if any; (iii) determine if provenances from local origins with drier climatic conditions are more adapted to drought events than other provenances; (iv) understand which climate variables could have the largest impact on resilience components.
2. Materials and Methods
2.1. Study Sites
In 1979, five provenances of maritime pine were bred in the forest nursery of Montes, in Sardinia. The provenances used were one from Corsica, one from Tuscany, two from Sardinia (Limbara and Telti), and one from Portugal; for more details see [31,32]. In early 1981, the seedlings were planted with 2.5 m spacing in Sardinia Island in four experimental field sites: Montarbu, Montes, Uatzo, and Usinavà (Figure 1). At each site, all provenances were planted within five 25-tree square plots, and the arrangement of plots were randomized within five (Montarbu, Montes and Usinavà) or three (Uatzo) blocks. Usinavà had a lower altitude and DMI index than Uatzo, Montarbu, and Montes (Table 1). Except for Montarbu, all the sites were thinned and were previously surveyed through dendrometric measures at 7, 16, and 20 years since planting [31,32,33].
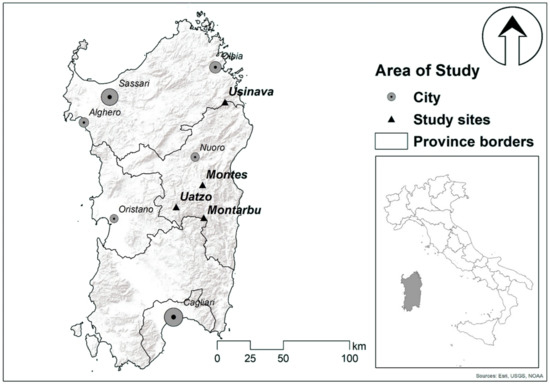
Figure 1.
Location of the four common gardens in Sardinia.

Table 1.
Stand attributes of the four common gardens (experimental sites). Number of trees per ha, basal area, and tree volume (calculated according to the national forest inventory equations [34]) are referred to the year of last field sampling (2018), and De Martonne Index (DMI) for each site for the period of study 1988–2018.
2.2. Field Sampling and Dendrochronological Analysis
Diameter at breast height (DBH) and tree height were collected in spring 2018, when trees were 37 years old (since transplanting of seedlings). At the same time, two wood cores were taken from 8–10 healthy trees per provenance, at each common garden. The trees were chosen randomly, excluding the margin of the sites in each block. Cores were taken at 1.3 m height and at an angle of 120° from each other [35] using the Pressler increment borer. Cores were glued onto wooden mounts, air-dried, and progressively sanded until the tree rings were clearly visible. Subsequently, the tree ring widths were measured to the nearest 0.001 mm, using LINTAB measuring system (Rinntech, Heidelberg, Germany) and TSAP-Win program, applying as a validity threshold Gleichläufigkeit ≥ 60 [36] for cross-dating. The accuracy of cross-dating was checked using COFECHA software [37]. However, to avoid distorting the chronology, because tree rings show curvature at the stem center [38], the common period among the samples, i.e., 1988–2017, was selected. Assuming that the stem can be a regular circumference, tree ring widths were averaged yearly for each tree and the BAI (Basal Area Increment, cm2 yr−1) was calculated through bai.out in “DplR” package [39], obtaining a biologically significant measurement of tree growth [40].
2.3. Climate Data, Identification of Drought Years
Climate data, including precipitation, minimum, maximum, and mean temperatures, were obtained by the nearest weather stations to common gardens (<25 km). We used monthly cumulative precipitation and potential evapotranspiration data based on Thornthwaite (1948) [41] to calculate the non-dimensional, multi-scalar standardised precipitation–evapotranspiration index (SPEI) for six-month windows (SPEI6) with R-package SPEI [42]. Following [43,44,45], the drought years were identified when the SPEI6 value in August (SPEI6AUG) was less than −1. The SPEI in August with a scale of six months was considered to cover climatic conditions during the active period of growth (March-August). To characterise the water supply in the four common gardens and at the sites of origin of the five provenances, the De Martonne Index was calculated as reported in Equation (1) [46]:
where PCPyear represents the annual mean precipitation in mm and Tyear is the mean annual temperature in °C. According to De Martonne’s classification, the sites with higher values of DMI have a higher water supply, and sites with lower DMI values are more xeric. The different climatic types are reported in Table 2.
DMI = PCPyear/(Tyear + 10)

Table 2.
Classification of the different climatic types according to the De Martonne index (DMI) [47].
Climate data at the sites of origin of the five provenances were obtained from the National Aeronautics and Space Administration (NASA) Langley Research Center (LaRC) Prediction of Worldwide Energy Resource (POWER) Project funded through the NASA Earth Science/Applied Science Program (https://power.larc.nasa.gov/) for the period 1960–1990 (accessed on 6 September 2022).
2.4. Resilience Components
To quantify the response of maritime pine provenances to drought in 2003, the resilience components proposed by [23], namely resistance (Rt), resilience (Rs), and recovery (Rc), were calculated according to the Equations (2), (3), and (4), respectively:
where BAIDr is the BAI value during the drought year; BAIPostDr is the mean value of BAI during the three post-drought years, and the BAIPreDr is the mean value of BAI during the three pre-drought years. Values of the indices equal to 1 indicate that trees did not suffer from stress-induced growth reduction during drought (Rt), or that they recovered the growth performance as before the disturbance (Rs). Values of Rc < 1 suggests the persistence of disturbance-induced stress, while the Rc value ≥ 1 indicates an immediate regeneration in growth from a drought stress. It is not possible to calculate this index when the intensity of disturbance is stronger to cause the death of trees. In this case the BAIDr is equal to 0 preventing the evaluation of the index.
Rt = BAIDr/BAIPreDr
Rs = BAIPostDr/BAIPreDr
Rc = BAIPostDr/BAIDr
2.5. Statistical Analysis
All analyses were done using R software version 4.1.2 (R core team 2021). Differences in climate conditions, tree growth rates, and resilience components were assessed through the Kruskal-Wallis’s test, due to the heteroscedasticity of data, which did not meet the normality assumption. When the Kruskal-Wallis’s test was rejected, the Conover test in “DescTools” package [48] was used for the comparisons between groups. We used a p-value ≤ 0.05 as a significant threshold. To determine what and how the climate and dendromentric variables might influence the resilience components, the Generalized Linear Model (GLM) were built for each index (i.e., for resistance, resilience, and recovery) using the Gamma error family with the inverse link function. The minimum and maximum temperatures, precipitation, SPEI6AUG, DBH, and height of trees were used as predictors. First, the predictors with low multicollinearity were obtained with vifstep function in “usdm” R package [49] with a threshold ≥ 10. Afterwards, the several models were elaborated for each index, with non-multicollinearity predictors. Therefore, these models were compared with the step function (“stats” package) using the backward method, and the best model was selected with the lowest Akaike’s Information Criterion (AIC) for each model and for each index. Finally, for each model selected we calculated the R-squared with the rsq function in “rsq” package [50].
3. Results
3.1. Climate Conditions and Drought Years
From the comparison of the De Martonne index across the common gardens, Usinavà was significantly drier than the other sites (see Figure S1), given its comparatively higher temperature and lower precipitation–especially during the summer period (Figure 2). According to the De Martonne classification, Usinavà has a semi-humid climate, while the other sites show comparatively good water supply and a very humid climate (Table 2). In general, in all common gardens, November and December were the months with the highest precipitation, followed by January and February. Considering the average values, Montes showed the highest mean annual precipitation (924 mm yr−1). Montarbu showed a less pronounced dry period when compared to the rest of the sites (Figure 2). Overall, the driest years common to all sites were 2003 and 2017 (Figure 3a). The year 2017 was excluded from the analysis of resilience components because the tree ring time chronologies terminated in 2017 and the post-drought data were not available for the resilience and recovery calculation. The value of SPEI6AUG in 2003 ranged between −1.37 in Montes to −1.56 in Montarbu, and −1.60 in Uatzo and Usinavà. Nevertheless, Usinavà and Montes also experienced drought years before 2003. In Usinavà, previous drought years were 1994, 1999, and 2001; in Montes, previous drought years were 1994, 2000, and 2001. The severity of drought in these years was similar between these two sites, except for 1994, when drought was more pronounced in Usinavà than in Montes (Figure 3a).
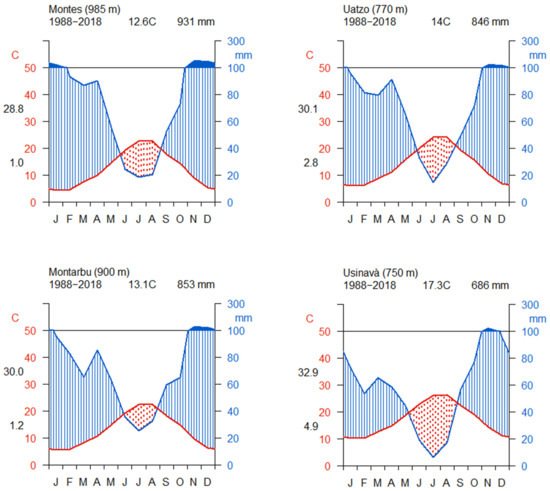
Figure 2.
Walter and Lieth climate diagrams from 1988 to 2018 in the four common gardens. Daily maximum average temperature of the hottest month and daily minimum average temperature of the coldest month are labelled in black at the left margin of the diagram. The red line shows the mean temperature, while the total precipitation is reported in blue. The average temperature and the average precipitation are reported on top of the graphics. The red area represents the drought period.
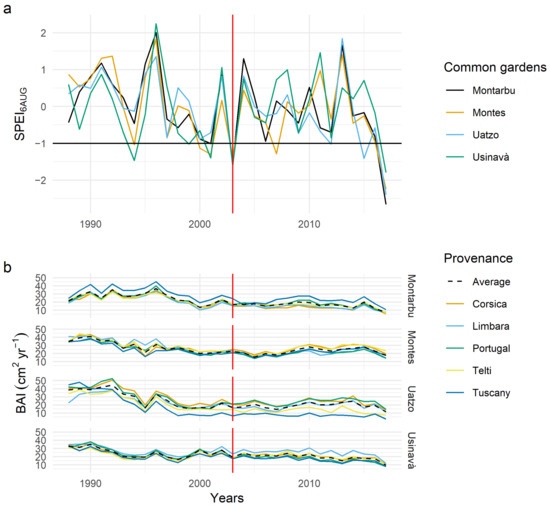
Figure 3.
(a) SPEI value for August calculated on a timescale of six months to cover the growing period. The drought years were identified when this value was lower than −1. (b) Annual growth pattern (BAI) of the five provenances and the mean for each common garden. The red vertical line indicates the year 2003.
3.2. Climate Conditions of the Sites and Provenance Origin
DMI values at the sites of provenance origin showed good water supply and a humid to semi-humid climate (Table 2), except for Telti and Limbara. Sardinia was confirmed as a relatively xeric site because the DMI value was significantly lower than DMI values of the other sites (Table 3).

Table 3.
Climate data at the locations of origin of the provenances (annual mean for the period 1960–1990).
3.3. Growth Rates and Trends
In all sites, tree growth decreases over time (Figure 3b). The trees growth was different among the sites (Table S1). Mean values of BAI (±SD) were significantly higher in Montes (25.74 ± 7.92 cm2 yr−1) than in the other common gardens (Table S2), i.e., Uatzo (22.30 ± 11.06 cm2 yr−1), Usinavà (22.07 ± 6.14 cm2 yr−1), and Montarbu, which recorded the lowest value (20.94 ± 7.92 cm2 yr−1). Trees in all common gardens showed a reduction in stem radial growth during the drought year compared with the previous three years (Figure 3b), except for Montes. The most important growth reduction was recorded in Usinavà, followed by Uatzo and Montarbu.
In Montes, no significant differences among provenances were observed (Table S3). In Montarbu, Tuscany displayed a significantly higher growth rate than the other maritime pine provenances, except for Portugal. In Uatzo, conversely, Tuscany had a significantly lower growth rate than Portugal, Corsica, and Telti. Furthermore, in Usinavà, Limbara had a significantly higher growth rate than Tuscany, which was the provenance with the least growth rate (Figure 3b). The same provenance showed differences in tree growth across common gardens. Indeed, Tuscany showed the highest growth rate in Montarbu, with significant differences from Usinavà and Uatzo sites. Limbara showed significantly lower growth rate in Montarbu than in Usinavà and Montes. Telti, conversely, showed a higher growth rate in Montes than in other sites. Corsica had a significantly lower growth rate in Montarbu than in Uatzo and Montes, where growth rates were similar. Portugal did not show significant differences in growth rate across common gardens.
3.4. Resilience Components at Common Gardens and for Provenances
The resilience components differed among the sites (Table S4). Usinavà and Uatzo had lower resistance values but higher recovery values than Montes and Montarbu. Usinavà showed lower resilience values than Montarbu and Uatzo (Table S5). The resilience components index of provenances did not show differences between each other when growing in the same common garden, but the same provenance differed in resilience components across common gardens, except for Telti. In the case of resistance, provenances showed lower values in Usinavà than in other common gardens (Table S6). Corsica in Usinavà differed from the same provenance in Montes. While Limbara in Usinavà differed from the same provenance in Montarbu. Again, Tuscany in Usinavà showed lower resistance values than the same provenance in Montarbu and Montes (Figure 4), with Portugal showing similar patterns but differing from Uatzo.
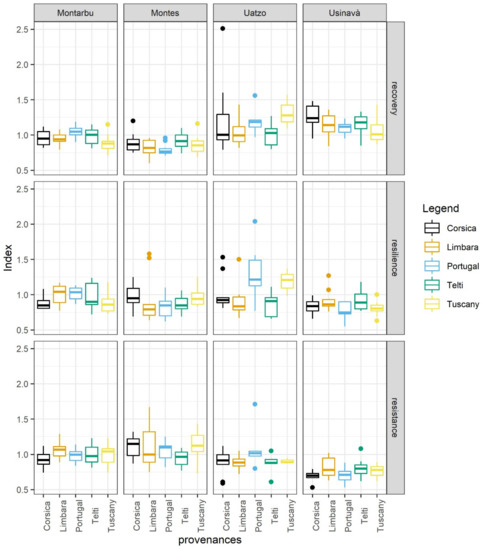
Figure 4.
The resilience components for the five provenances grown in the four common gardens.
Both Corsica and Limbara showed higher recovery values in Usinavà than in Montes. Tuscany had higher recovery values in Uatzo than in Montes and Montarbu. Portugal showed lower recovery values in Montes than in the other common gardens (Table S7). Finally, the only difference observed among provenances for resilience values was for Portugal (Table S8), which showed higher resilience in Uatzo than in Usinavà, resulting in the highest and lowest values, respectively, in absolute terms.
3.5. Resilience Components and Climate Variables
Multicollinearity issues (vifstep function) prevented precipitation to be included in the model construction. The structure of models was different for each index. The best model for resistance did not include DBH and SPEI6AUG; the best model for resilience and recovery did not include DBH and maximum temperatures (Table 4). Tree height had a significantly negative influence on the resilience components, i.e., higher tree height was associated with a lower probability to have high values of resilience indices. Furthermore, temperatures might have significant effects on resilience indices, though in different ways. Increases in minimum temperatures were associated with a higher probability to have high values of resistance, but a high probability to have low recovery values. The SPEI6AUG covariate showed the similar effects in resilience and recovery models but with slight effect on resilience.

Table 4.
Models selected for each index. Values for each predictor represent the estimates and the standard errors (SE).
4. Discussion
4.1. Differences in Tree Growth among Provenances Is Influenced by Site Conditions More than Seed Sources
Notable differences in tree growth occurred across the common garden sites (see also [51]) though only Usinavà showed markedly distinct climatic conditions, i.e., relatively dry (Table 1). Maritime pine had higher stem radial growth in Montes than in the other common gardens, which could be associated with the comparatively higher availability of water [27] and nutrient resources [32] in this site. Versace et al., [51] found differences among these common gardens in terms of growth–climate responses (including sensitivity to summer drought), suggesting a greater dependency of tree growth on site conditions than on seed sources. However, trees in Montarbu had the lowest mean value of BAI, which could be related to a disturbance event affecting the site that caused high mortality in 1987 (see [32]) and the absence of any thinning operations reducing tree density [33]. In many studies on pine trees [52,53], thinning was found to promote tree growth through the reduction in competition for resources and the improvement of water availability [54]. Therefore, the negative impact of drought events on forest plantations in dry landscapes and poor soils of Mediterranean environments, as it is the case of Sardinia Island, can be consistently buffered through thinning operations, in combination with management practices to mitigate wildfire severity and pest risk, in turn increasing forest resilience to natural disturbances [55]. Nevertheless, despite thinning being considered a management strategy to cope with drought stress [56], the positive effects may vanish over time [57,58]. Changes in microclimate, due to the increase in wind speed below canopy [59] and higher incidence of solar radiation on topsoil [60], may cause an increase in temperature, leading to an increase in evapotranspiration rate and, in turn, of fire risk. Disturbance-induced tree mortality might have also contributed to determining the current variation in tree density across common gardens [61]. As climate change drives increased drought in the Mediterranean region, management practices conferring drought tolerance in trees, such as thinning, are becoming increasingly important, though the effect of thinning intensity and type on the relationship between tree height and stem diameter (i.e., slenderness) should be considered carefully.
Thus, site-specific microclimate conditions were probably the cause of differences in tree growth among the four common gardens (see also [51]), as in the case of Montes and Usinavà, in 2003. Despite the similar tree density (Table 1) in Montes, trees in this area showed relatively higher growth rates during the drought year in comparison with the previous three years; conversely, in Usinavà, trees had a lower growth rate during the drought year than in the three years preceding the drought. Competition for resource availability, namely water, may make pine trees growing in dense stands more vulnerable to drought conditions than trees growing in sparse stands, particularly in Mediterranean semi-arid conditions [62,63]. Indeed, similar tree densities but different growth rates, functional traits, or health conditions might stem from site-specific environmental conditions [64]. The greater altitude and North exposition provided greater moisture and more shade to the Montes site–the contrary to Usinavà. In addition, differences in microclimate conditions between Montes and Usinavà [32] and in the soil traits and geomorphologic characteristics might have affected tree growth potential. Sandy soils with low water retention might, indeed, have impaired tree growth in Usinavà more than in Montes, which had less permeable soils, regardless of the provenance. High levels of phenotypic plasticity modulate growth performances and adaptation processes in maritime pine, according to the environment where it grows [65].
Different carbon allocations between mesic and xeric provenances may contribute to explain the differences in growth rates among provenances [29,30]. In the relatively warmer sites (i.e., Uatzo and Usinavà), trees from Tuscany showed lower growth rates than those from Sardinia (xeric ecotypes)–probably due to comparatively more mesic habits (Table 3), which might favour preferential allocation of carbon to drought-sensitive juvenile needles after stress events [30,66], which are more frequent in these sites. This carbon allocation strategy allows trees to compete in favourable environmental conditions, which would explain the higher growth rates of Tuscany in Montarbu in comparison with more xeric provenances–this common garden being characterised by less pronounced drought in the growth period (Figure 2). Furthermore, lower growth rates of xeric provenances could be caused by the preferential allocation of biomass to belowground plant compartments (i.e., roots), which would translate into less growth aboveground, as observed in xeric seed sources of this species by [29]. Lower growth rates of Tuscany in warmer sites could also be related to abundant fruiting, as found by [32]. Indeed, although this provenance showed the lowest growth rates, among the same five provenances tested here, it was the one with the highest percentage of plants that had mature strobili [32]. Higher growth rates of the local provenances in the dry site (i.e., Limbara in Usinavà) and in the site that experienced more frequent drought years during the growth period (i.e., both Sardinian provenances in Montes) might suggest good adaptation to local environmental conditions in these provenances. Finally, results of this study confirm observations made by [31,32,33] in the same common gardens, i.e., provenances in Montes showing minor differences in growth and Tuscany displaying the lowest growth rate among the five provenances.
4.2. Environemtal Conditions and Tree Height Matter on Resilience Components
The decreasing in trees growth is an important factor, having a negative impact on resilience components. Decreased tree growth could be a symptom of tree stress, approaching threshold values. Thus, environmental circumstances at the common gardens were the main drivers determining resilience components. Indeed, in the same site, the five provenances did not show differences in response to tree growth to drought. Climate conditions differed among the four common gardens, with Montes being the moistest and Usinavà the warmest, and Montarbu being the site in which drought in the growth period was the least pronounced compared to the other common gardens (Figure 2). Similarly, [67] observed that the tested provenances of maritime pine growing in dry sites showed less resistance to drought but higher recovery values than those provenances growing in mesic conditions (Figure 4).
Several studies found that the severity of disturbances may strongly influence the response capacity of trees [68]. Accordingly, trees in Usinavà were less resistant than those in other common gardens, as drought stress in 2003 was comparatively more pronounced in this site. In contrast, trees in Montes showed relatively higher resistance–this location being less affected by drought. Serra-Maluquer et. al, [69] found that past events caused a reduction in resistance to extreme drought in P. halepensis, P. nigra, and P. sylvestris in northern Spain. These authors suggested an increased vulnerability in pine trees to drought after successive drought episodes. Consecutive years of drought may reduce soil water reserves, with pine trees becoming increasingly dependent on moisture from deeper soil horizons [70], possibly increasing water stress in shallow soils and dry conditions. Differences between Montes and Usinavà, and between the same provenances grown in these sites, could be the result of unlike soil depths and cumulative drought events, as years before the drought event of 2003 tended to be drier in Usinavà than in Montes (e.g., 1994, Figure 3b). Indeed, in Montes, despite the SPEI6AUG values in the three years after 2003 being like those recorded in Uatzo and Usinavà, trees showed low recovery (Figure 4). Indeed, 2000 and 2001 were relatively dry years (Figure 3a). It is worth noting that the inconsistency regarding the selection and characterization of the climatic conditions in pre- and post-drought periods represents a limitation when using these indices [71].
Interestingly, Portugal was the only provenance showing differences in resilience across the four common gardens. This provenance was more resilient in Uatzo than in Usinavà, probably because drought in the latter site was relatively more pronounced in the years before 2003. de La Mata et. al, [30] observed that provenances from mesic origins (as Portugal; Table 3), which are more vulnerable to drought stress [66], may invest greater amounts of biomass to juvenile needles than to stem growth. Higher resilience in Uatzo could be associated with higher photosynthetic activity of further juvenile needles [72]. It is worth noting that, in Usinavà, the two provenances from Sardinia showed higher resistance than the other provenances. Xeric ecotypes of pine species may develop a larger root system that is able to explore greater soil volumes and deeper soil layers [73], and they may have needles that are more resistant to water loss and adapted to cope with recurrent dry years.
An increase in tree height corresponded to a lower probability to have high values of resilience indices. Indeed, in tall trees, water supply to the canopy can be limited by the water travel distance between roots and leaves. The higher the trees the lower the water potentials (higher energy required per quantity of water) required to transport water through the plant [74], which may translate into higher tension and xylem cavitation with a consequent loss of conduit functionality, causing in turn a decrease in resistance, resilience, and recovery. Serra-Maluquer, et. al, [69] observed, for instance, that resistance, recovery, and resilience in pine species differed across drought events, so that larger trees were significantly less resistant and resilient than smaller ones. Height growth can be reduced–relative to diameter growth–by thinning, in turn decreasing the risk of drought-induced xylem embolism, therefore providing a measure for stand adaptation towards dry conditions [56]. However, trees of a large size may gain an advantage during warmer and wetter conditions in terms of water use [75]. It is worth noting that functional parameters may return rapidly to pre-stress levels [76], while growth legacies will persist over time. As an example, increased root biomass–as a memory from a previous drought–may help increase resistance and recovery to subsequent stress events, because these allow greater exploration of the soils and better access to the groundwater.
4.3. Temperature as a Proxy to Assess Resilience Components
The model’s results highlighted the important role of temperature during drought stress [77], because temperature was included in the best models for all resilience components. Zas et. al, [21] suggested a trade-off between resistance and recovery in maritime pine, as found in the model results. In the present study, in the recovery model, an increase in minimum temperatures corresponded to a greater probability to have lower recovery, while the opposite was true for the resistance model. In brief, when the temperature increased, the ratio between tree growth in the years after drought and that during the drought year decreased (Rc; Equation (4)); whereas the ratio between tree growth during the drought year and that in the years before drought increased (Rt; Equation (2)). The recovery model explains the endogenous processes that bring the system back to a balance [78]. In our case, trees experienced extreme drought stress 22 years after transplanting, when they were still in the process of becoming progressively more adjusted to drought, for instance, through changes in xylem conduit traits and plant water relations [79]. Evidence of adjustments was somewhat underlined in the comparison between resilience and recovery models. The influence of SPEI6AUG on these indices was similar, though less pronounced on resilience than on recovery (Table 4). Post-drought “ecological memory” [80,81] and the elongation of xylogenesis due to higher minimum temperatures [82] might support a positive relationship between minimum temperature and growth resilience, influencing resilience trajectories of pine plantation responses to subsequent drought events. Commonly, negative drought legacies on tree growth are expected due to impaired root functioning and altered stomatal behaviour [83,84]. Eventually, severe drought may cause tree mortality [85], as well as epigenetic modifications in pine tree populations [86].
4.4. Common Gardens as a Tool for Implementing Climate-Smart Forestry
Although population-level variations in maritime pine affected only tree growth and not resilience components, the present study provides important information for both productive tree plantations and assisted migration measures regarding this species. Maritime pine from Tuscany was the least productive provenance and, as such, it should be excluded from reforestation programs—at least in conditions like those predominant in Sardinia. In contrast, Corsica showed the greatest tree growth. In addition, the latter provenance has a good stem form [9,87], which makes Corsica a good candidate in reforestation programs aimed at producing high quality wood, and thus supporting forest chains in the Mediterranean environment. Limbara showed good tree growth as well as good resistance and resilience to drought in the relatively drier common garden site. Therefore, this provenance from Sardinia could be considered in reforestation programs with climate mitigation pledges and for local genome conservation [88]. Portugal, despite its good tree growth, should not be considered for assisted migration activities of this species, as this provenance showed lower resilience in the relatively drier site and to extreme drought events. Considering the dynamic feature of climate change and its negative impacts on forest resources, common gardens represent important tools to assess the effects of climate on tree growth. Findings carried out from common garden experiments will become helpful for promoting climate-smart forestry strategies, such as forest adaptation and mitigation, to promote forest health and high-quality timber production.
5. Conclusions
This study helps expand our knowledge on the behaviour of maritime pine provenances from mesic and xeric origins planted in Mediterranean environments, bearing in mind that common garden experiments are limited in the Italian Peninsula and Sardinia Island in comparison with those established in the Iberian Peninsula and southwestern France. Our hypothesis that differences in the adaptive capacity to drought stress among provenances are stronger when resistance, recovery, and resilience indices are measured in harsher environmental conditions, and that local provenances from Sardinia are better adapted to local common garden conditions, was confirmed.
Although the provenance factor did not influence the resilience components and only influenced the growth rate, this study highlighted the importance of establishing common gardens and long-term experiments to tackle future climate change. As progeny trials allow us to study the adaptive phenomena and the adaptive potential of different provenances, common gardens are essential to direct correct forest planning for tree species in hotspot areas for climate change, such as the Mediterranean region. In view of this, marginal populations (and experimental sites) at the boundary of the range may become important, in addition to core populations and trials established within the center of the range. Long-term experiments are, indeed, needed to improve our understanding of resilience trajectories to drought events, considering the lack of information on the cumulative effects of seasonality, frequency, and intensity of stress events, including interactions with heat waves and other extreme events [89].
Since Maritime pine may develop strategies to cope with drought stress over time (and already in the juvenile stage), it would be appropriate to use the same indices tested in this study across subsequent stages of the tree life span and compare a wider collection of provenances. However, additional research would be needed to provide a better understanding of differences in the tree growth of maritime pine provenances in response to thinning treatments, including the comparative analysis of root system development; indeed, [29] found differences between mesic and xeric origins of this species in the juvenile stage. Nonetheless, information provided by this study may help develop appropriate climate-smart forestry measures suitable for this species in Mediterranean environments.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/f13121986/s1, Figure S1: De Martonne index in the period 1988-2018 in each site. The lines indicate which one sites differ among each other and the asterisk the significance level: *** p < 0.001; ** p < 0.01; * p < 0.05; Table S1: Kruskal wallis results for the BAI among the sites. among provenances and among provenances in the different sites; Table S2: Conover test results for the BAI in the different sites; Table S3: Conover test results for the BAI of provenances in different sites (P × S); Table S4: Results of Kruskal wallis for resilience components among the sites, provenances and among the provenances in sites (Sites × Provenances); Table S5: Conover test results for the resilience components among the sites; Table S6: Conover test results for provenances in different sites for resistance index; Table S7: Conover test results for recovery index among the provenances in different sites; Table S8: Conover test results for the resilience index among the provenances in different sites.
Author Contributions
Conceptualization C.L., S.A. and R.T., Formal analysis C.L., S.A. and G.S., Methodology C.L., S.A. and G.S., Supervision M.M. and R.T., Writing—original draft C.L., S.A. and G.S., Writing—review & editing C.L., S.A., G.S., M.M. and R.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
We are grateful to Andrea Tani, Barbara Mariotti, Alberto Maltoni, Fabio Salbitano, and Alberto Pierguidi (University of Firenze) for providing basic information on the provenance trials, and Matteo Mura (University of Sassari), Cesar Alvites, Fabrizio Caraglia (University of Molise) for assistance during field work. We are also grateful to Soraya Versace (University of Molise) for supporting the tree ring widths measurement. Gavino Palmas, Manuela Manca and personnel of FoReSTAS (Regione Sardegna) are acknowledged for providing help and logistic support in field campaigns.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bastin, J.-F.; Finegold, Y.; Garcia, C.; Mollicone, D.; Rezende, M.; Routh, D.; Zohner, C.M.; Crowther, T.W. Comment on “The Global Tree Restoration Potential”. Science 2019, 366, 76–79. [Google Scholar] [CrossRef] [PubMed]
- FOREST EUROPE, 2020: State of Europe’s Forests 2020. Available online: https://foresteurope.org/wp-content/uploads/2016/08/SoEF_2020.pdf (accessed on 8 September 2022).
- IPCC. Climate Change 2021: The Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; DLR: Cologne, Germany, 2021. [Google Scholar]
- Allen, C.D.; Macalady, A.K.; Chenchouni, H.; Bachelet, D.; McDowell, N.; Vennetier, M.; Kitzberger, T.; Rigling, A.; Breshears, D.D.; Hogg, E.H.; et al. A Global Overview of Drought and Heat-Induced Tree Mortality Reveals Emerging Climate Change Risks for Forests. For. Ecol. Manag. 2010, 259, 660–684. [Google Scholar] [CrossRef]
- Williams, M.I.; Dumroese, R.K. Preparing for Climate Change: Forestry and Assisted Migration. J. For. 2013, 111, 287–297. [Google Scholar] [CrossRef]
- Locosselli, G.M.; Brienen, R.J.W.; Leite, M.S.; Gloor, M.; Krottenthaler, S.; Oliveira, A.A.; Barichivich, J.; Anhuf, D.; Ceccantini, G.; Schöngart, J.; et al. Global Tree-Ring Analysis Reveals Rapid Decrease in Tropical Tree Longevity with Temperature. Proc. Natl. Acad. Sci. USA 2020, 117, 33358–33364. [Google Scholar] [CrossRef]
- Vogel, J.; Paton, E.; Aich, V.; Bronstert, A. Increasing Compound Warm Spells and Droughts in the Mediterranean Basin. Weather Clim. Extrem. 2021, 32, 100312. [Google Scholar] [CrossRef]
- Ciais, P.; Reichstein, M.; Viovy, N.; Granier, A.; Ogée, J.; Allard, V.; Aubinet, M.; Buchmann, N.; Bernhofer, C.; Carrara, A.; et al. Europe-Wide Reduction in Primary Productivity Caused by the Heat and Drought in 2003. Nature 2005, 437, 529–533. [Google Scholar] [CrossRef]
- González-Martínez, S.C.; Alía, R.; Gil, L. Population Genetic Structure in a Mediterranean Pine (Pinus pinaster Ait.): A Comparison of Allozyme Markers and Quantitative Traits. Heredity 2002, 89, 199–206. [Google Scholar] [CrossRef]
- Nabuurs, G.-J.; Verkerk, P.J.; Schelhaas, M.-J.; González Olabarria, J.R.; Trasobares, A.; Cienciala, E. Climate-Smart Forestry: Mitigation Impacts in Three European Regions; European Forest Institute: Joensuu, Finland, 2018; ISBN 9789525980530. Available online: https://efi.int/sites/default/files/files/publication-bank/2018/efi_fstp_6_2018.pdf (accessed on 8 September 2022).
- Bowditch, E.; Santopuoli, G.; Binder, F.; del Río, M.; la Porta, N.; Kluvankova, T.; Lesinski, J.; Motta, R.; Pach, M.; Panzacchi, P.; et al. What Is Climate-Smart Forestry? A Definition from a Multinational Collaborative Process Focused on Mountain Regions of Europe. Ecosyst. Serv. 2020, 43, 101113. [Google Scholar] [CrossRef]
- Hewitt, N.; Klenk, N.; Smith, A.L.; Bazely, D.R.; Yan, N.; Wood, S.; Maclellan, J.I.; Lipsig-mumme, C.; Henriques, I. Taking Stock of the Assisted Migration Debate. Biol. Conserv. 2011, 144, 2560–2572. [Google Scholar] [CrossRef]
- Woodall, C.W.; Zhu, K.; Westfall, J.A.; Oswalt, C.M.; D’Amato, A.W.; Walters, B.F.; Lintz, H.E. Assessing the Stability of Tree Ranges and Influence of Disturbance in Eastern US Forests. For. Ecol. Manag. 2013, 291, 172–180. [Google Scholar] [CrossRef]
- Liang, Y.; Duveneck, M.J.; Gustafson, E.J.; Serra-Diaz, J.M.; Thompson, J.R. How Disturbance, Competition, and Dispersal Interact to Prevent Tree Range Boundaries from Keeping Pace with Climate Change. Glob. Chang. Biol. 2018, 24, e335–e351. [Google Scholar] [CrossRef] [PubMed]
- Westman, W.E. Measuring the Inertia and Resilience of Ecosystems. Bioscience 1978, 28, 705–710. [Google Scholar] [CrossRef]
- Folke, C.; Carpenter, S.R.; Walker, B.; Scheffer, M.; Chapin, T.; Rockström, J. Resilience Thinking: Integrating Resilience, Adaptability and Transformability. Ecol. Soc. 2010, 15, 20–28. [Google Scholar] [CrossRef]
- Lake, P.S. Resistance, Resilience and Restoration. Ecol. Manag. Restor. 2013, 14, 20–24. [Google Scholar] [CrossRef]
- Taeger, S.; Zang, C.; Liesebach, M.; Schneck, V.; Menzel, A. Impact of Climate and Drought Events on the Growth of Scots Pine (Pinus Sylvestris L.) Provenances. For. Ecol. Manag. 2013, 307, 30–42. [Google Scholar] [CrossRef]
- Bansal, S.; Harrington, C.A.; Bradley, J.; Clair, S. Tolerance to Multiple Climate Stressors: A Case Study of Douglas-Fir Drought and Cold Hardiness Tolerance to Multiple Climate Stressors: A Case Study of Douglas-Fir Drought and Cold Hardiness. Ecol. Evol. 2016, 6, 2074–2083. [Google Scholar] [CrossRef]
- George, J.P.; Grabner, M.; Karanitsch-Ackerl, S.; Mayer, K.; Weißenbacher, L.; Schueler, S. Genetic Variation, Phenotypic Stability, and Repeatability of Drought Response in European Larch throughout 50 Years in a Common Garden Experiment. Tree Physiol. 2017, 37, 33–46. [Google Scholar] [CrossRef]
- Zas, R.; Sampedro, L.; Solla, A.; Vivas, M.; Lombardero, M.J.; Alía, R.; Rozas, V. Dendroecology in Common Gardens: Population Differentiation and Plasticity in Resistance, Recovery and Resilience to Extreme Drought Events in Pinus pinaster. Agric. For. Meteorol. 2020, 291, 108060. [Google Scholar] [CrossRef]
- Fritts, H.C. Tree Rings, a Record of Seasonal Variations in Past Climate. Naturwissenschaften 1978, 65, 48–56. [Google Scholar] [CrossRef]
- Lloret, F.; Keeling, E.G.; Sala, A. Components of Tree Resilience: Effects of Successive Low-Growth Episodes in Old Ponderosa Pine Forests. Oikos 2011, 120, 1909–1920. [Google Scholar] [CrossRef]
- San-Miguel-Ayanz, J.; de Rig, D.; Caudullo, G.; Houston Durrant Achille, T. European Atlas of Forest Tree Species; Publication Office of the European Union: Luxembourg, 2016; Volume 11, ISBN 9789279367403. [Google Scholar]
- Eilmann, B.; de Vries, S.M.G.; den Ouden, J.; Mohren, G.M.J.; Sauren, P.; Sass-Klaassen, U. Origin Matters! Difference in Drought Tolerance and Productivity of Coastal Douglas-Fir (Pseudotsuga Menziesii (Mirb.)) Provenances. For. Ecol. Manag. 2013, 302, 133–143. [Google Scholar] [CrossRef]
- Bogino, S.M.; Bravo, F. Growth Response of Pinus pinaster Ait. To Climatic Variables in Central Spanish Forests. Ann. For. Sci. 2008, 65, 506. [Google Scholar] [CrossRef]
- Mazza, G.; Cutini, A.; Manetti, M.C. Influence of Tree Density on Climate-Growth Relationships in a Pinus pinaster Ait. Forest in the Northern Mountains of Sardinia (Italy). IForest 2015, 8, 456–463. [Google Scholar] [CrossRef]
- Corcuera, L.; Cochard, H.; Gil-Pelegrin, E.; Notivol, E. Phenotypic Plasticity in Mesic Populations of Pinus pinaster Improves Resistance to Xylem Embolism (P50) under Severe Drought. Trees-Struct. Funct. 2011, 25, 1033–1042. [Google Scholar] [CrossRef]
- Corcuera, L.; Gil-Pelegrín, E.; Notivol, E. Differences in Hydraulic Architecture between Mesic and Xeric Pinus pinaster Populations at the Seedling Stage. Tree Physiol. 2012, 32, 1442–1457. [Google Scholar] [CrossRef]
- De La Mata, R.; Merlo, E.; Zas, R. Among-Population Variation and Plasticity to Drought of Atlantic, Mediterranean, and Interprovenance Hybrid Populations of Maritime Pine. Tree Genet. Genomes 2014, 10, 1191–1203. [Google Scholar] [CrossRef]
- Tognetti, R.; Michelozzi, M.; Lauteri, M.; Brugnoli, E.; Giannini, R. Geographic Variation in Growth, Carbon Isotope, Discrimination, and Monoterpene Composition in Pinus pinaster Ait. Provenances. Can. J. For. Res. 2000, 30, 1682–1690. [Google Scholar] [CrossRef]
- Giannini, R.; Palmas, G.; Tani, A. Prove di Provenienza di Pinus pinaster Ait. in Sardegna. Monti Boschi 1992, 1, 55–60. [Google Scholar]
- Giannini, R.; Calamini, G.; Lavra, S.; Michelozzi, M.; Paffetti, D.; Rossi, F.; Tani, A. Risultati di Prove di Provenienza di Pino Marittimo in Sardegna. IFM 2002, 3, 293–301. [Google Scholar]
- Tabacchi, G.; Cosmo, L.; Gasparini, P. Stima del Volume e della Fitomassa delle Principali Specie Forestali Italiane. Equazioni di Previsione, Tavole del Volume e Tavole della Fitomassa Arborea Epigea; CRA: Trento, Italia, 2011; ISBN 9788897081111. [Google Scholar]
- Lombardi, F.; Cocozza, C.; Lasserre, B. Dendrochronological Assessment of the Time since Death of Dead Wood in an Old Growth Magellan’s Beech Forest, Navarino Island (Chile). Austral Ecol. 2011, 36, 329–340. [Google Scholar] [CrossRef]
- Eckstein, D.; Bauch, J. Beitrag Zur Rationalisierung Eines Dendrochronologischen Verfahrens Und Zur Analyse Seiner Aussagesicherheit. Forstwissenschaftliches Centralblatt 2007, 88, 230–250. [Google Scholar] [CrossRef]
- Holmes, R. Computer-Assisted Quality Control in Tree-Ring Dating and Measurement. Tree-Ring Bull. 1983, 43, 51–67. [Google Scholar]
- Speer, B.J.H. Fundamentals of Tree-Ring Research; Indiana State University: Terre Haute, IN, USA, 2009. [Google Scholar]
- Bunn, A.; Korpela, M.; Biondi, F.; Campelo, F.; Mérian, P.; Qeadan, F.; Zang, C.; Buras, A.; Cecile, A.; Mudelsee, M.; et al. DplR: Dendrochronology Program Library in R 2022. Available online: https://CRAN.R-project.org/package=dplR (accessed on 8 September 2022).
- Biondi, F.; Qeadan, F. A Theory-Driven Approach to Tree-Ring Standardization: Defining the Biological Trend from Expected Basal Area Increment. Tree Ring Res. 2008, 64, 81–96. [Google Scholar] [CrossRef]
- Thornthwaite, C.W. An Approach toward a Rational Classification of Climate. Geogr. Rev. 1948, 38, 55–94. [Google Scholar] [CrossRef]
- Beguería, S.; Vicente-Serrano, S.M. SPEI: Calculation of the Standardised Precipitation-Evapotranspiration Index, R package version 1.7; 2017. Available online: https://CRAN.R-project.org/package=SPEI (accessed on 8 September 2022).
- Mckee, T.B.; Doesken, N.J.; Kleist, J. The Relationship of Drought Frequency and Duration to Time Scales. In Proceedings of the 8th Conference on Applied Climatology, Anaheim, CA, USA, 17–22 January 1993; pp. 17–22. [Google Scholar]
- Sohn, J.A.; Hartig, F.; Kohler, M.; Huss, J.; Bauhus, J. Heavy and Frequent Thinning Promotes Drought Adaptation in Pinus Sylvestris Forests. Ecological Applications 2016, 26, 2190–2205. [Google Scholar] [CrossRef]
- Fuchs, S.; Schuldt, B.; Leuschner, C. Forest Ecology and Management Identification of Drought-Tolerant Tree Species through Climate Sensitivity Analysis of Radial Growth in Central European Mixed Broadleaf Forests. For. Ecol. Manag. 2021, 494, 119287. [Google Scholar] [CrossRef]
- De Martonne, E.L. Indice d Aridité. La Meteorol. 1926, 2, 3–5. [Google Scholar]
- Emadodin, I.; Reinsch, T.; Taube, F. Drought and Desertification in Iran. Hydrology 2019, 6, 66. [Google Scholar] [CrossRef]
- Signorell, A.; Aho, K.; Alfons, A.; Anderegg, N.; Aragon, T.; Arachchige, C.; Arppe, A.; Baddeley, A.; Barton, K.; Bolker, B.; et al. DescTools: Tools for Descriptive Statistics 2022. R package version 0.99.45. Available online: https://cran.r-project.org/package=DescTools (accessed on 8 September 2022).
- Naimi, B.; Hamm, N.; Groen, T.A.; Skidmore, A.K.; Toxopeus, A.G. Where Is Positional Uncertainty a Problem for Species Distribution Modelling. Ecography 2014, 37, 191–203. [Google Scholar] [CrossRef]
- Zhang, D. rsq: R-Squared and Related Measures; R package version 2.5. 2022. Available online: https://CRAN.R-project.org/package=rsq (accessed on 8 September 2022).
- Versace, S.; Antonucci, S.; Santopuoli, G.; Marchetti, M.; Tognetti, R. Local Environment Prevails over Population Variations in Growth-Climate Relationships of Pinus pinaster Provenances. Dendrochronologia 2022, 75, 125983. [Google Scholar] [CrossRef]
- Moreno-Fernández, D.; Cañellas, I.; Calama, R.; Gordo, J.; Sánchez-González, M. Thinning Increases Cone Production of Stone Pine (Pinus Pinea L.) Stands in the Northern Plateau (Spain). Ann. For. Sci. 2013, 70, 761–768. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Tai, D.T.; Zhang, P.; Razaq, M.; Shen, H.L. Effect of Thinning Intensity on Tree Growth and Temporal Variation of Seed and Cone Production in a Pinus Koraiensis Plantation. J. For. Res. 2019, 30, 835–845. [Google Scholar] [CrossRef]
- Aussenac, G.; Granier, A. Effects of Thinning on Water Stress and Growth in Douglas-Fir. Can. J. For. Res. 1988, 18, 100–105. [Google Scholar] [CrossRef]
- Camarero, J.; Linares, J.; Sangüesa-Barreda, G.; Sanchez-Salguero, R.; Gazol, A.; Navarro Cerrillo, R.; Carreira, J. The Multiple Causes of Forest Decline in Spain: Drought, Historical Logging, Competition and Biotic Stressors; Dendroecology; Springer: Cham, Switzerland, 2017; Volume 231, pp. 307–323. [Google Scholar] [CrossRef]
- Manrique-alba, À.; Beguer, S.; Tomas-burguera, M. Increased Post-Drought Growth after Thinning in Pinus Nigra Plantations. Forests 2021, 12, 985. [Google Scholar] [CrossRef]
- Sohn, J.A.; Saha, S.; Bauhus, J. Potential of Forest Thinning to Mitigate Drought Stress: A Meta-Analysis. For. Ecol. Manag. 2016, 380, 261–273. [Google Scholar] [CrossRef]
- Sohn, J.A.; Gebhardt, T.; Ammer, C.; Bauhus, J.; Häberle, K.H.; Matyssek, R.; Grams, T.E.E. Mitigation of Drought by Thinning: Short-Term and Long-Term Effects on Growth and Physiological Performance of Norway Spruce (Picea abies). For. Ecol. Manag. 2013, 308, 188–197. [Google Scholar] [CrossRef]
- Matejka, F.; Janouš, D.; Hurtalová, T.; Rožnovský, J. Effects of Thinning on Microclimate of a Young Spruce Forest. Ekol. Bratisl. 2004, 23, 30–38. [Google Scholar]
- Hale, S.E. The Effect of Thinning Intensity on the Below-Canopy Light Environment in a Sitka Spruce Plantation. For. Ecol. Manag. 2003, 179, 341–349. [Google Scholar] [CrossRef]
- Di Matteo, G.; Voltas, J. Multienvironment Evaluation of Pinus pinaster Provenances: Evidence of Genetic Trade-Offs between Adaptation to Optimal Conditions and Resistance to the Maritime Pine Bast Scale (Matsucoccus feytaudi). For. Sci. 2016, 62, 553–563. [Google Scholar] [CrossRef]
- Moreno-Gutiérrez, C.; Battipaglia, G.; Cherubini, P.; Saurer, M.; Nicolás, E.; Contreras, S.; Querejeta, J.I. Stand Structure Modulates the Long-Term Vulnerability of Pinus halepensis to Climatic Drought in a Semiarid Mediterranean Ecosystem. Plant Cell Environ. 2012, 35, 1026–1039. [Google Scholar] [CrossRef]
- Mazza, G.; Amorini, E.; Cutini, A.; Manetti, M.C. The Influence of Thinning on Rainfall Interception by Pinus pinea L. in Mediterranean Coastal Stands (Castel Fusano-Rome). Ann. For. Sci. 2011, 68, 1323–1332. [Google Scholar] [CrossRef]
- Cocozza, C.; Palombo, C.; Tognetti, R.; la Porta, N.; Anichini, M.; Giovannelli, A.; Emiliani, G. Monitoring Intra-Annual Dynamics of Wood Formation with Microcores and Dendrometers in Picea abies at Two Different Altitudes. Tree Physiol. 2016, 36, 832–846. [Google Scholar] [CrossRef] [PubMed]
- Chambel, M.R.; Climent, J.; Alía, R. Divergence among Species and Populations of Mediterranean Pines in Biomass Allocation of Seedlings Grown under Two Watering Regimes. Ann. For. Sci. 2007, 64, 87–97. [Google Scholar] [CrossRef]
- Kuusk, V.; Niinemets, Ü.; Valladares, F. A Major Trade-off between Structural and Photosynthetic Investments Operative across Plant and Needle Ages in Three Mediterranean Pines. Tree Physiol. 2018, 38, 543–547. [Google Scholar] [CrossRef]
- Sánchez-Salguero, R.; Camarero, J.J.; Rozas, V.; Génova, M.; Olano, J.M.; Arzac, A.; Gazol, A.; Caminero, L.; Tejedor, E.; de Luis, M.; et al. Resist, Recover or Both? Growth Plasticity in Response to Drought Is Geographically Structured and Linked to Intraspecific Variability in Pinus pinaster. J. Biogeogr. 2018, 45, 1126–1139. [Google Scholar] [CrossRef]
- Greenwood, S.; Ruiz-Benito, P.; Martínez-Vilalta, J.; Lloret, F.; Kitzberger, T.; Allen, C.D.; Fensham, R.; Laughlin, D.C.; Kattge, J.; Bönisch, G.; et al. Tree Mortality across Biomes Is Promoted by Drought Intensity, Lower Wood Density and Higher Specific Leaf Area. Ecol. Lett. 2017, 20, 539–553. [Google Scholar] [CrossRef]
- Serra-Maluquer, X.; Mencuccini, M.; Martínez-Vilalta, J. Changes in Tree Resistance, Recovery and Resilience across Three Successive Extreme Droughts in the Northeast Iberian Peninsula. Oecologia 2018, 187, 343–354. [Google Scholar] [CrossRef]
- Mazza, G.; Manetti, M.C. Growth Rate and Climate Responses of Pinus pinea L. in Italian Coastal Stands over the Last Century. Clim. Change 2013, 121, 713–725. [Google Scholar] [CrossRef]
- Schwarz, J.; Skiadaresis, G.; Kohler, M.; Kunz, J.; Schnabel, F.; Vitali, V.; Bauhus, J. Quantifying Growth Responses of Trees to Drought—A Critique of Commonly Used Resilience Indices and Recommendations for Future Studies. Curr. For. Rep. 2020, 6, 185–200. [Google Scholar] [CrossRef]
- Kuusk, V.; Niinemets, Ü.; Valladares, F. Structural Controls on Photosynthetic Capacity through Juvenile-to-Adult Transition and Needle Ageing in Mediterranean Pines. Funct. Ecol. 2018, 32, 1479–1491. [Google Scholar] [CrossRef]
- Sarris, D.; Christodoulakis, D.; Körner, C. Recent Decline in Precipitation and Tree Growth in the Eastern Mediterranean. Glob. Chang. Biol. 2007, 13, 1187–1200. [Google Scholar] [CrossRef]
- Ryan, M.G.; Yoder, B.J. Hydraulic Limits to Tree Height and Tree Growth. Bioscience 1997, 47, 235–242. [Google Scholar] [CrossRef]
- Forrester, D.I.; Limousin, J.-M.; Pfautsch, S. The Relationship between Tree Size and Tree Water Use: Is Competition for Water Size-Symmetric or Size-Asymmetric? Tree Physiol. 2022, 42, 1916–1927. [Google Scholar] [CrossRef] [PubMed]
- Schwalm, C.R.; Anderegg, W.R.L.; Michalak, A.M.; Fisher, J.B.; Biondi, F.; Koch, G.; Litvak, M.; Ogle, K.; Shaw, J.D.; Wolf, A.; et al. Global Patterns of Drought Recovery. Nature 2017, 548, 202–205. [Google Scholar] [CrossRef] [PubMed]
- Rebetez, M.; Mayer, H.; Dupont, O.; Schinlder, D.; Gartner, K. Heat and Drought 2003 in Europe: A Climate Synthesis. Ann. For. Sci. 2006, 63, 569–577. [Google Scholar] [CrossRef]
- Hodgson, D.; McDonald, J.L.; Hosken, D.J. What Do You Mean, “Resilient”? Trends Ecol. Evol. 2015, 30, 503–506. [Google Scholar] [CrossRef]
- Tognetti, R.; Michelozzi, M.; Giovannelli, A. Geographical Variation in Water Relations, Hydraulic Architecture and Terpene Composition of Aleppo Pine Seedlings from Italian Provenances. Tree Physiol. 1997, 17, 241–250. [Google Scholar] [CrossRef]
- Pretzsch, H. The Social Drift of Trees. Consequence for Growth Trend Detection, Stand Dynamics, and Silviculture. Eur. J. For. Res. 2021, 140, 703–719. [Google Scholar] [CrossRef]
- Pretzsch, H. The Emergent Past: Past Natural and Human Disturbances of Trees Can Reduce Their Present Resistance to Drought Stress. Eur. J. For. Res. 2021, 141, 87–104. [Google Scholar] [CrossRef]
- Yang, B.; He, M.; Shishov, V.; Tychkov, I.; Vaganov, E.; Rossi, S.; Ljungqvist, F.C.; Bräuning, A.; Grießinger, J. New Perspective on Spring Vegetation Phenology and Global Climate Change Based on Tibetan Plateau Tree-Ring Data. Proc. Natl. Acad. Sci. USA 2017, 114, 6966–6971. [Google Scholar] [CrossRef]
- Grossiord, C.; Sevanto, S.; Limousin, J.M.; Meir, P.; Mencuccini, M.; Pangle, R.E.; Pockman, W.T.; Salmon, Y.; Zweifel, R.; McDowell, N.G. Manipulative Experiments Demonstrate How Long-Term Soil Moisture Changes Alter Controls of Plant Water Use. Environ. Exp. Bot. 2018, 152, 19–27. [Google Scholar] [CrossRef]
- Peltier, D.M.P.; Ogle, K. Legacies of More Frequent Drought in Ponderosa Pine across the Western United States. Glob. Chang. Biol. 2019, 25, 3803–3816. [Google Scholar] [CrossRef] [PubMed]
- Caminero, L.; Génova, M.; Camarero, J.J.; Sánchez-Salguero, R. Growth Responses to Climate and Drought at the Southernmost European Limit of Mediterranean Pinus pinaster Forests. Dendrochronologia 2018, 48, 20–29. [Google Scholar] [CrossRef]
- Alakärppä, E.; Salo, H.M.; Valledor, L.; Cañal, M.J.; Häggman, H.; Vuosku, J. Natural Variation of DNA Methylation and Gene Expression May Determine Local Adaptations of Scots Pine Populations. J. Exp. Bot. 2018, 69, 5293–5305. [Google Scholar] [CrossRef] [PubMed]
- Correia, I.; Alía, R.; Yan, W.; David, T.; Aguiar, A.; Correia, I.; Alía, R.; Yan, W.; David, T.; Aguiar, A.; et al. Genotype x Environment Interactions in Pinus pinaster at Age 10 in a Multi-Environment Trial in Portugal: A Maximum Likelihood Approach. Ann. For. Sci. 2010, 67, 612. [Google Scholar] [CrossRef]
- Rodriguez-Vallejo, C.; Navarro-Cerrillo, R.M.; Manzanedo, R.D.; Palacios Rodriguez, G.; Gazol, A.; Julio Camarero, J. High Resilience, but Low Viability, of Pine Plantations in the Face of a Shift towards a Drier Climate. For. Ecol. Manag. 2021, 479, 118537. [Google Scholar] [CrossRef]
- Müller, L.M.; Bahn, M. Drought Legacies and Ecosystem Responses to Subsequent Drought. Glob. Chang. Biol. 2022, 28, 5086–5103. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).