Balsam Fir (Abies balsamea (L.) Mill.) Wood Quality after Defoliation by Spruce Budworm (Choristoneura fumiferana Clem.) in the Boreal Forest of Quebec, Canada
Abstract
1. Introduction
2. Materials and Methods
2.1. Experiment Design and Sampling
2.2. Wood Quality Analyses
2.3. Moisture Content
2.4. Sapwood Proportion and Stem Decay and Coloration
2.5. Mechanical Properties
2.6. Tracheid Dimensions and Coarseness
2.7. Dendrochronology
2.8. Statistical Analyses
2.9. Discriminant Analysis
3. Results
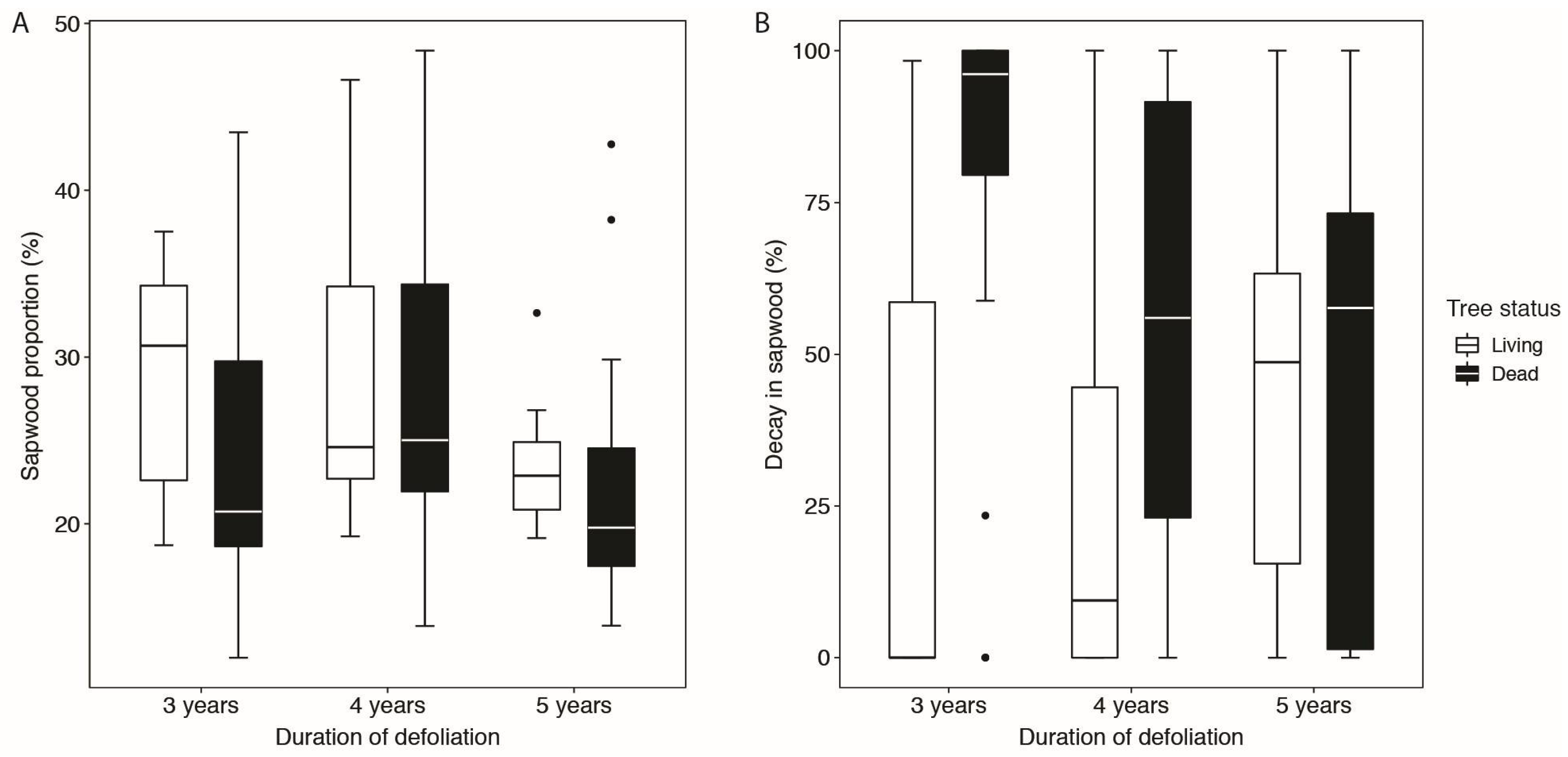
3.1. Proportion of Sapwood and Proportion of Decay and Coloration
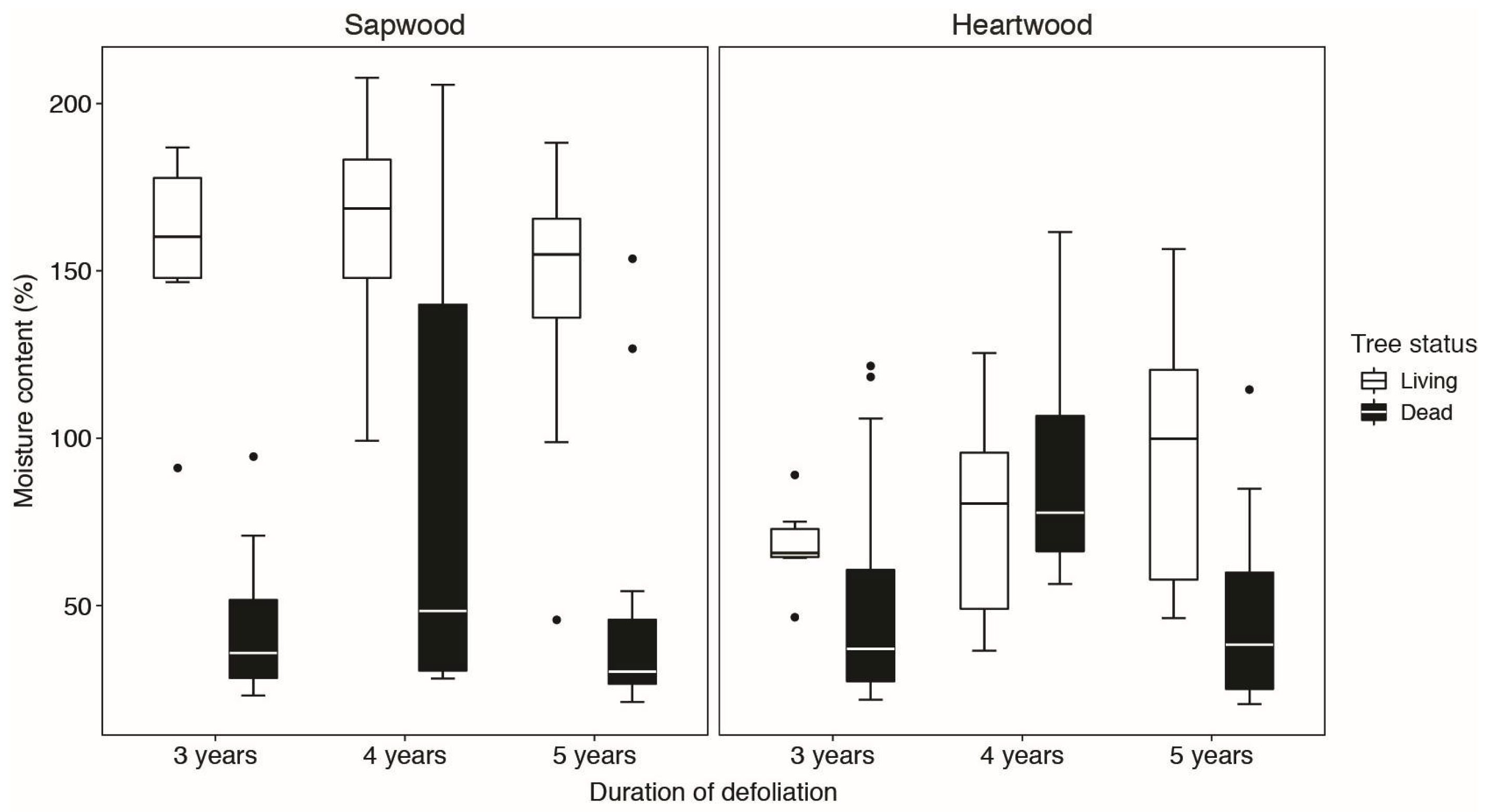
3.2. Moisture Content
3.3. Mechanical Properties
3.4. Tracheid Dimensions and Coarseness
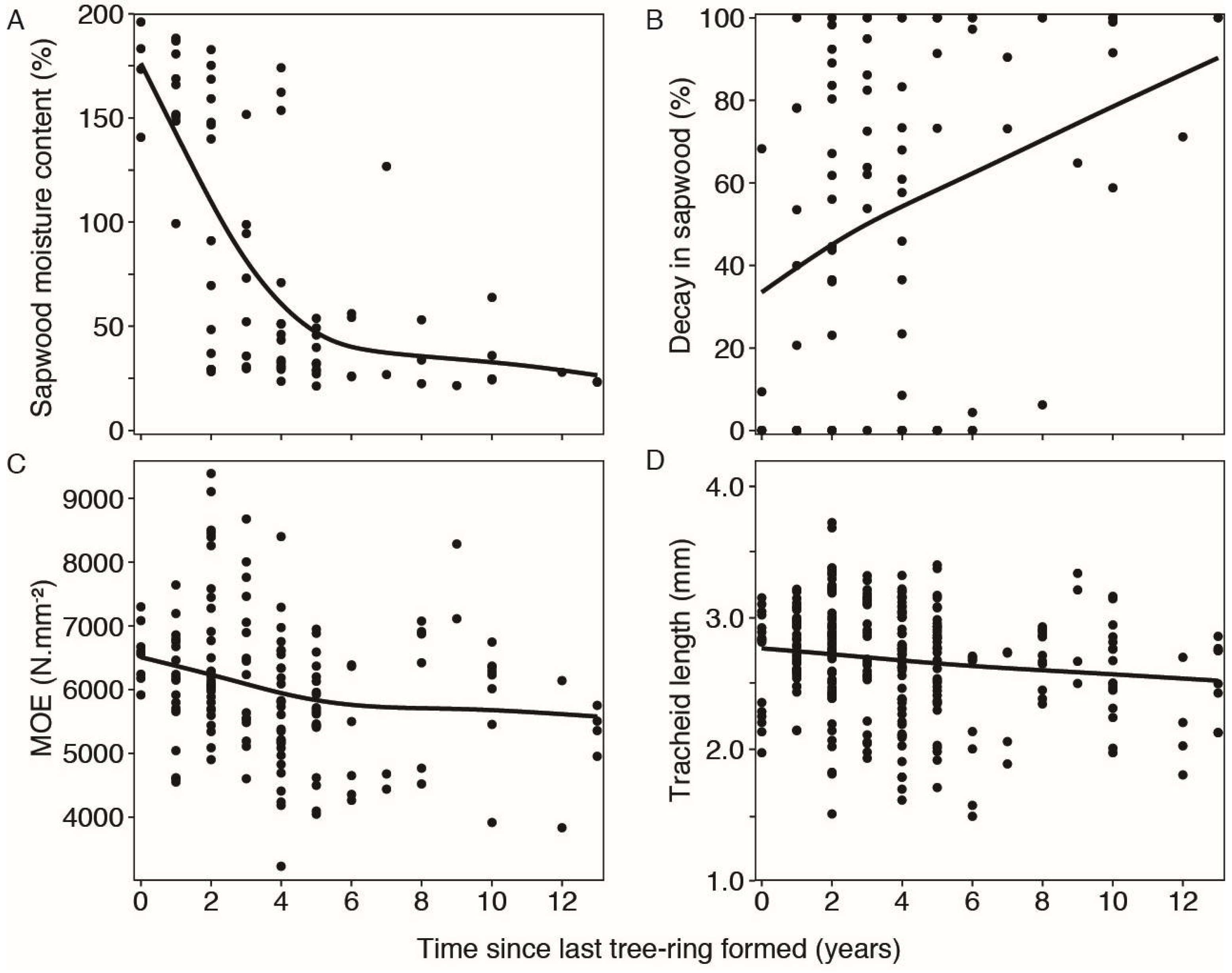
3.5. Wood Quality as a Function of Time
3.6. Discriminant Analysis
4. Discussion
4.1. Comparison between Balsam Firs Visually Classified as Living or Dead
4.2. Comparison between Durations of Defoliation
4.3. Comparison between SBW Epidemic and Endemic Periods
4.4. Variation of Wood Properties with Time since the Last Tree Ring Was Formed
4.5. Implications for Forest Managers and Industries
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Morin, H. Dynamics of balsam fir forests in relation to spruce budworm outbreaks in the Boreal Zone of Quebec. Can. J. For. Res. 1994, 24, 730–741. [Google Scholar] [CrossRef]
- Martineau, R. Insectes Nuisibles des Forêts de l’est du Canada (Insects Harmful to Forest Trees); Service Canadien des Forêts, Administration Centrale: Ottawa, ON, Canada, 1985. [Google Scholar]
- Morin, H. Importance et évolution des épidémies de la tordeuse des bourgeons de l’épinette dans l’est du Canada: L’apport de la dendrochronologie (Importance and evolution of spruce budworm outbreaks in Eastern Canada: The contribution of dendrochronology). Geogr. Phys. Quat. 1998, 52, 237–244. [Google Scholar] [CrossRef][Green Version]
- Navarro, L.; Harvey, A.-É.; Ali, A.; Bergeron, Y.; Morin, H. A Holocene landscape dynamic multiproxy reconstruction: How do interactions between fire and insect outbreaks shape an ecosystem over long time scales? PLoS ONE 2018, 13, e0204316. [Google Scholar] [CrossRef] [PubMed]
- Hennigar, C.R.; MacLean, D.A.; Quiring, D.T.; Kershaw, J.A., Jr. Differences in spruce budworm defoliation among balsam fir and white, red, and black spruce. For. Sci. 2008, 54, 158–166. [Google Scholar] [CrossRef]
- Nealis, V.G.; Régnière, J. Insect-host relationships influencing disturbance by the spruce budworm in a boreal mixedwood forest. Can. J. For. Res. 2004, 34, 1870–1882. [Google Scholar] [CrossRef]
- Boulanger, Y.; Arseneault, D. Spruce budworm outbreaks in eastern Quebec over the last 450 years. Can. J. For. Res. 2004, 34, 1035–1043. [Google Scholar] [CrossRef]
- Jardon, Y.; Morin, H.; Dutilleul, P. Périodicité et synchronisme des épidémies de la tordeuse des bourgeons de l’épinette au Québec (Periodicity and synchronism of spruce budworm outbreaks in Quebec). Can. J. For. Res. 2003, 33, 1947–1961. [Google Scholar] [CrossRef]
- Royama, T. Population dynamics of the spruce budworm Choristoneura fumiferana. Ecol. Monogr. 1984, 54, 429–462. [Google Scholar] [CrossRef]
- Pureswaran, D.S.; Johns, R.; Heard, S.B.; Quiring, D. Paradigms in eastern spruce budworm (Lepidoptera: Tortricidae) population ecology: A century of debate. Environ. Entomol. 2016, 45, 1333–1342. [Google Scholar] [CrossRef]
- Morris, R.F. The Dynamics of Epidemic Spruce Budworm Populations. Mem. Entomol. Soc. Can. 1963, 95, 1–12. [Google Scholar] [CrossRef]
- Blais, J.R. Trends in the frequency, extent, and severity of spruce budworm outbreaks in eastern Canada. Can. J. For. Res. 1983, 13, 539–547. [Google Scholar] [CrossRef]
- Blais, J.R. Impact of the Spruce Budworm on Balsam Fir and White Spruce in the Laurentian Reserve, Quebec: An Interim Report. Information Report LAU-X-68; Government of Canada, Canadian Forestry Service: Sainte-Foy, QC, Canada, 1985; 15p.
- MacLean, D.A. Vulnerability of fir-spruce stands during uncontrolled spruce budworm outbreaks: A review and discussion. For. Chron. 1980, 56, 213–221. [Google Scholar] [CrossRef]
- Bergeron, Y.; Leduc, A.; Morin, H.; Joyal, C. Balsam fir mortality following the last spruce budworm outbreak in northwestern Quebec. Can. J. For. Res. 1995, 25, 1375–1384. [Google Scholar] [CrossRef]
- Krause, C.; Luszczynski, B.; Morin, H.; Rossi, S.; Plourde, P.Y. Timing of growth reductions in black spruce stem and branches during the 1970s spruce budworm outbreak. Can. J. For. Res. 2012, 42, 1220–1227. [Google Scholar] [CrossRef]
- Krause, C.; Morin, H. Changes in radial increment in stems and roots of balsam fir [Abies balsamea (L.) Mill.] after defoliation by spruce budworm. For. Chron. 1995, 71, 747–754. [Google Scholar] [CrossRef]
- Paixao, C.; Krause, C.; Morin, H.; Achim, A. Wood quality of black spruce and balsam fir trees defoliated by spruce budworm: A case study in the boreal forest of Quebec, Canada. For. Ecol. Manag. 2019, 437, 201–210. [Google Scholar] [CrossRef]
- Deslauriers, A.; Caron, L.; Rossi, S. Carbon allocation during defoliation: Testing a defense-growth trade-off in balsam fir. Front. Plant Sci. 2015, 6, 338. [Google Scholar] [CrossRef] [PubMed]
- Krause, C.; Morin, H. Impact of spruce budworm defoliation on the number of latewood tracheids in balsam fir and black spruce. Can. J. For. Res. 1995, 25, 2029–2034. [Google Scholar] [CrossRef]
- Lewis, K.; Thompson, D. Degradation of wood in standing lodgepole pine killed by mountain pine beetle. Wood Fiber Sci. 2011, 43, 130–142. [Google Scholar]
- Barrette, J.; Pothier, D.; Duchesne, I. Lumber and wood chips properties of dead and sound black spruce trees grown in the boreal forest of Canada. Forestry 2014, 88, 108–120. [Google Scholar] [CrossRef]
- Basham, J.T. Biological factors influencing stem deterioration rates and salvage planning in balsam fir killed after defoliation by spruce budworm. Can. J. For. Res. 1986, 16, 1217–1229. [Google Scholar] [CrossRef]
- Hiratsuka, Y.; Chakravarty, P.; Bohning, R.A. A Field Guide to Decay and Stain in Conifers in Alberta; Natural Resources Canada, Canadian Forest Service: Edmonton, AB, Canada, 1996; 25p.
- Hiratsuka, Y.; Gibbard, D.A.; Bakowsky, O.; Maier, G.B. Classification and Measurement of Aspen Decay and Stain in Alberta; Forestry Canada, Northwest Region, Northern Forestry Centre: Edmonton, AB, Canada, 1990; 29p.
- Basham, J.T. Degradation and loss of wood fibre in spruce budworm-killed timber, and effects on utilization. For. Chron. 1984, 60, 10–14. [Google Scholar] [CrossRef]
- MFFP. Aires Infestées Par La Tordeuse des Bourgeons de L’épinette au Québec en 2021 (Areas Infested by the Spruce Budworm in Quebec in 2021); Ministère des Forêts, de la Faune et des Parcs: Québec, QC, Canada, 2021; 22p.
- Zobel, B.J.; Van Buijtenen, J.P. Wood Variation: Its Causes and Control; Springer: New York, NY, USA, 1989; 378p. [Google Scholar]
- Houndode, D.J.; Krause, C.; Morin, H. Predicting balsam fir mortality in boreal stands affected by spruce budworm. For. Ecol. Manag. 2021, 496, 119408. [Google Scholar] [CrossRef]
- ASTM. Standard Test Methods for Direct Moisture Content Measurment of Wood and Wood-Base Materials, ASTM D4442-07. In Annual Book of ASTM Standards, Vol. 04.10 on Wood; American Society for Testing and Materials; ASTM: West Conshohocken, PA, USA, 2007. [Google Scholar]
- Guay, R.; Gagnon, R.; Morin, H. A new automatic and interactive tree ring measurement system based on a line scan camera. For. Chron. 1992, 68, 138–141. [Google Scholar] [CrossRef]
- ASTM. Standard Test Methods for Small Clear Specimens of Timber, ASTM D143-09. In Annual Book of ASTM Standards, Vol. 04.10 on Wood; American Society for Testing and Materials; ASTM: West Conshohocken, PA, USA, 2010. [Google Scholar]
- Poncsák, S.; Kocaefe, D.; Bouazara, M.; Pichette, A. Effect of high temperature treatment on the mechanical properties of birch (Betula papyrifera). Wood Sci. Technol. 2006, 40, 647–663. [Google Scholar] [CrossRef]
- Franklin, G.L. Preparation of thin sections of synthetic resins and wood-resin composites, and a new macerating method for wood. Nature 1945, 155, 51. [Google Scholar] [CrossRef]
- Robertson, G.; Olson, J.; Allen, P.; Chan, B.; Seth, R. Measurement of fiber length, coarseness, and shape with the fiber quality analyzer. Tappi J. 1999, 82, 93–98. [Google Scholar]
- Herman, M.; Dutilleul, P.; Avella-Shaw, T. Intra-ring and inter-ring variations of tracheid length in fast-grown versus slow-grown Norway spruces (Picea abies). Iawa J. 1998, 19, 3–23. [Google Scholar] [CrossRef]
- Mäkinen, H.; Hynynen, J. Wood density and tracheid properties of Scots pine: Responses to repeated fertilization and timing of the first commercial thinning. Forestry 2014, 87, 437–447. [Google Scholar] [CrossRef]
- Mäkinen, H.; Hynynen, J.; Penttilä, T. Effect of thinning on wood density and tracheid properties of Scots pine on drained peatland stands. Forestry 2015, 88, 359–367. [Google Scholar] [CrossRef]
- SCIEM. PAST5 Personal Analysis System for Tree Ring Research. Version 5.0.576. Instruction Manual & Reference; Vienna, Austria. 2015. Available online: https://www.sciem.com/products/past/ (accessed on 19 September 2022).
- Holmes, R. Computer-assisted quality control in tree-ring dating and measurement. Tree-Ring Bull. 1983, 43, 69–78. [Google Scholar]
- Pinheiro, J.; Bates, D.; DebRoy, S.; Sarkar, D.; R Core Team. Package ‘nlme’: Linear and Nonlinear Mixed Effects Models; R Package Version 3.1-157. 2022. Available online: https://cran.r-project.org/web/packages/nlme/nlme.pdf (accessed on 19 September 2022).
- Quinn, G.P.; Keough, M.J. Experimental Design and Data Analysis for Biologists; Cambridge University Press: Cambridge, UK, 2002; 537p. [Google Scholar]
- Wood, S. Package ‘mgcv’: Mixed GAM Computation Vehicle with Automatic Smoothness Estimation; R Package Version 1.8-40. 2022. Available online: https://cran.r-project.org/web/packages/mgcv/mgcv.pdf (accessed on 19 September 2022).
- Friendly, M.; Fox, J. Package ‘candisc’: Visualizing Generalized Canonical Discriminant and Canonical Correlation Analysis; R Package Version 0.8-6. 2021. Available online: https://cran.r-project.org/web/packages/candisc/candisc.pdf (accessed on 19 September 2022).
- R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing; Vienna, Austria. 2022. Available online: https://www.yumpu.com/s/Fxit5xIdza5bKZeV (accessed on 19 September 2022).
- Barnes, D.P.; Sinclair, S.A. Time-related changes in specific gravity and moisture content of spruce budworm killed balsam fir. Can. J. For. Res. 1983, 13, 257–263. [Google Scholar] [CrossRef]
- Hazenberg, G.; Yang, K.C. Sapwood/heartwood width relationships with tree age in balsam fir. Iawa J. 1991, 12, 95–99. [Google Scholar] [CrossRef]
- Galván, J.D.; Camarero, J.J.; Sangüesa-Barreda, G.; Alla, A.Q.; Gutiérrez, E. Sapwood area drives growth in mountain conifer forests. J. Ecol. 2012, 100, 1233–1244. [Google Scholar] [CrossRef]
- Coyea, M.R.; Margolis, H.A. Factors affecting the relationship between sapwood area and leaf area of balsam fir. Can. J. For. Res. 1992, 22, 1684–1693. [Google Scholar] [CrossRef]
- Basham, J.T. Studies in forest pathology XX. Investigations of the pathological deterioration in killed balsam fir. Can. J. Bot. 1959, 37, 291–326. [Google Scholar] [CrossRef]
- Sinclair, S.A.; Barnes, D.P. Balsam Fir: Its Properties and Utilization, Agriculture Handbook No. 629; United States Department of Agriculture, Forest Service: Blacksburg, VA, USA, 1984; 69p.
- Lewis, K.J.; Hartley, I.D. Rate of deterioration, degrade, and fall of trees killed by mountain pine beetle. J. Ecosyst. Manag. 2006, 7, 11–19. [Google Scholar] [CrossRef]
- Sonderman, D.L. Balsam Fir (Abies balsamea); United States Department of Agriculture, Forest Service: Washington, DC, USA, 1970; 7p.
- Wood Degradation and Ligninolytic Fungi; Morel-Rouhier, M., Sormani, R., Eds.; Elsevier Science & Technology: London, UK, 2021; Volume 99, 252p. [Google Scholar]
- Barnes, D.P.; Sinclair, S.A.; Govett, R.L. Mechanical properties of small clear specimens and visually graded lumber from living and spruce budworm-killed balsam fir. Wood Fiber Sci. 1985, 17, 36–46. [Google Scholar]
- Basham, J.T. Reducing Stem Rot in Living Black Spruce in Ontario; Technical Note No. 83; Natural Resources Canada, Canadian Forest Service: Sault Ste. Marie, ON, Canada, 1974; 4p.
- Watanabe, Y.; Ohno, Y. Severe insect defoliation at different timing affects cell wall formation of tracheids in secondary xylem of Larix kaempferi. Trees 2020, 34, 931–941. [Google Scholar] [CrossRef]
- Krause, C.; Gionest, F.; Morin, H.; MacLean, D.A. Temporal relations between defoliation caused by spruce budworm (Choristoneura fumiferana Clem.) and growth of balsam fir (Abies balsamea (L.) Mill.). Dendrochronologia 2003, 21, 23–31. [Google Scholar] [CrossRef]
- Lindström, H. Fiber length, tracheid diameter, and latewood percentage in Norway spruce: Development from pith outwards. Wood Fiber Sci. 1997, 29, 21–34. [Google Scholar]
- Krauss, A.; Moliński, W.; Kúdela, J.; Čunderlík, I. Differences in the mechanical properties of early and latewood within individual annual rings in dominant pine tree (Pinus sylvestris L.). Wood Res. 2011, 56, 1–12. [Google Scholar]
- Büyüksarı, Ü.; Nusret, A.; Dündar, T. Mechanical properties of earlywood and latewood sections of scots pine wood. BioResources 2017, 12, 4004–4012. [Google Scholar] [CrossRef]
- Panshin, A.J.; De Zeeuw, C. Vol. Volume 1-Structure, identification, uses and properties of the commercial woods of the United States and Canada. In Textbook of Wood Technology, 3rd ed.; McGraw-Hill: New York, NY, USA, 1970; 705p. [Google Scholar]
- Fabisiak, E.; Fabisiak, B.; Krauss, A. Radial variation in tracheid lengths in dominant trees of selected coniferous species. BioResources 2020, 15, 7330–7341. [Google Scholar] [CrossRef]
- Dinwoodie, J.M. Tracheid and fibre length in timber: A review of literature. Forestry 1961, 34, 125–144. [Google Scholar] [CrossRef]
- Mäkinen, H.; Jyske, T.; Saranpää, P. Variation of tracheid length within annual rings of Scots pine and Norway spruce. Holzforschung 2008, 62, 123–128. [Google Scholar] [CrossRef]
- Pyörälä, J.; Piispanen, R.; Valkonen, S.; Lundqvist, S.-O. Tracheid dimensions of Norway spruce in uneven-aged stands. Can. J. For. Res. 2022, 52, 346–356. [Google Scholar] [CrossRef]
- Shmulsky, R.; Jones, P.D. Forest Products and Wood Science: An Introduction, 6th ed.; Wiley-Blackwell: West Sussex, UK, 2011; 477p. [Google Scholar]
- Trent, T.; Lawrence, V.; Woo, K. A wood and Fibre Quality-Deterioration Model for Mountain Pine Beetle-Killed Trees by Biogeoclimatic Subzone. Mountain Pine Beetle Initiative Working Paper 2006-10; Natural Resources Canada: Victoria, BC, Canada, 2006; 23p.
- Hudak, J.; Laflamme, G.; Meades, J.P. Deterioration of Balsam Fir Damaged by the Eastern Hemlock Looper in Newfoundland; Information Report N-X-157; Newfoundland Forest Research Centre: St-John’s, NL, Canada, 1978; 33p. [Google Scholar]
- Pearce, R.B. Tansley Review No. 87. Antimicrobial Defences in the Wood of Living Trees. New Phytol. 1996, 132, 203–233. [Google Scholar] [CrossRef]
- Yamada, T. Defense mechanisms in the sapwood of living trees against microbial infection. J. For. Res. 2001, 6, 127–137. [Google Scholar] [CrossRef]
- Basham, J.T.; Belyea, R.M. Death and deterioration of balsam fir weakened by spruce budworm defoliation in Ontario. Part III—The deterioration of dead trees. For. Sci. 1960, 6, 78–96. [Google Scholar] [CrossRef]
- Barrette, J.; Thiffault, E.; Saint-Pierre, F.; Wetzel, S.; Duchesne, I.; Krigstin, S. Dynamics of dead tree degradation and shelf-life following natural disturbances: Can salvaged trees from boreal forests ‘fuel’ the forestry and bioenergy sectors? Forestry 2015, 88, 275–290. [Google Scholar] [CrossRef]
- Byrne, A.; Stonestreet, C.; Peter, B. Current Knowledge of Characteristics and Utilization of Post-Mountain Pine Beetle Wood in Solid Wood Products; Working Paper 2005-8; Natural Resources Canada, Canadian Forest Service, Pacific Forestry Centre: Victoria, BC, Canada, 2005; 18p.
- Barrette, J.; Pothier, D.; Auty, D.; Achim, A.; Duchesne, I.; Gélinas, N. Lumber recovery and value of dead and sound black spruce trees grown in the North Shore region of Québec. Ann. For. Sci. 2012, 69, 603–615. [Google Scholar] [CrossRef]



| Stand Characteristics | Sample Trees | |||||
|---|---|---|---|---|---|---|
| Stand | GPS Coordinates | Duration of Defoliation (Years) | Intensity of Defoliation * | Stand Density (stems.ha−1) | Mean Age (Years) | Mean DBH (cm) |
| S1 | 49°31′8.9976″ N 72°18′43.9056″ W | 3 | Moderate | 979.3 | 60.0 | 14.3 |
| S2 | 49°37′32.4876″ N 72°11′19.2192″ W | 3 | Moderate | 858.3 | 55.0 | 13.7 |
| S3 | 49°27′28.9980″ N 72°25′47.1504″ W | 4 | Severe | 1099.2 | 60.3 | 14.2 |
| S4 | 49°42′56.9988″ N 72°15′53.8272″ W | 4 | Severe | 1357.9 | 76.1 | 14.3 |
| S5 | 49°36′1.6812″ N 72°9′47.1024″ W | 5 | Moderate | 871.9 | 54.2 | 13.2 |
| S6 | 49°28′32.8584″ N 72°21′14.9760″ W | 5 | Moderate | 1548.7 | 75.2 | 13.4 |
| Effect | df | F Value | Pr (>F) | F Value | Pr (>F) |
|---|---|---|---|---|---|
| Sapwood proportion | Total decay and coloration | ||||
| Tree status (living or dead) | 1 | 3.7856 | 0.0556 | 0.1019 | 0.7504 |
| Duration of defoliation | 2 | 0.7926 | 0.5292 | 0.7282 | 0.5523 |
| Tree status:Duration of defoliation | 2 | 0.1558 | 0.8560 | 2.6591 | 0.0772 |
| Residuals | 72 | ||||
| Decay in sapwood | Decay in heartwood | ||||
| Tree status | 1 | 5.4624 | 0.0223 | 2.5191 | 0.1171 |
| Duration of defoliation | 2 | 1.0347 | 0.4552 | 0.2427 | 0.7985 |
| Tree status:Duration of defoliation | 2 | 2.0455 | 0.1371 | 1.7078 | 0.1888 |
| Residuals | 72 | ||||
| Moisture content | |||||
| Wood type (sapwood/heartwood) | 2 | 571.9985 | <0.0001 | ||
| Tree status | 1 | 5.2559 | 0.0059 | ||
| Duration of defoliation | 2 | 151.4218 | <0.0001 | ||
| Wood type:Tree status | 2 | 10.7478 | 0.0428 | ||
| Wood type:Duration of defoliation | 4 | 33.1195 | <0.0001 | ||
| Tree status:Duration of defoliation | 2 | 0.0217 | 0.9991 | ||
| Wood type:Tree status:Duration of defoliation | 4 | 10.8929 | <0.0001 | ||
| Residuals | 216 | ||||
| MOE | MOR | ||||
| Tree status | 1 | 12.6899 | 0.0005 | 18.0281 | <0.0001 |
| Time period (during or outside SBW outbreak) | 1 | 4.8330 | 0.0296 | 6.7606 | 0.0103 |
| Duration of defoliation | 2 | 2.2843 | 0.2495 | 0.9020 | 0.4935 |
| Tree status:Time period | 1 | 0.0096 | 0.9220 | 0.0748 | 0.7848 |
| Tree status:Duration of defoliation | 2 | 0.1524 | 0.8588 | 0.0779 | 0.9251 |
| Time period:Duration of defoliation | 2 | 1.2650 | 0.2855 | 1.1723 | 0.3127 |
| Tree status:Time period:Duration of defoliation | 2 | 0.8222 | 0.4416 | 0.7366 | 0.4806 |
| Residuals | 142 | ||||
| Tracheid length | Tracheid width | ||||
| Tree status | 1 | 9.6030 | 0.0021 | 10.6350 | 0.0012 |
| Time period | 1 | 240.1850 | <0.0001 | 43.6780 | <0.0001 |
| Duration of defoliation | 2 | 5.2270 | 0.1053 | 1.3120 | 0.3896 |
| Tree status:Time period | 1 | 1.3190 | 0.2518 | 0.0580 | 0.8104 |
| Tree status:Duration of defoliation | 2 | 2.1560 | 0.1176 | 5.6380 | 0.0040 |
| Time period:Duration of defoliation | 2 | 0.7250 | 0.4851 | 2.3740 | 0.0950 |
| Tree status:Time period:Duration of defoliation | 2 | 0.0240 | 0.9762 | 0.5480 | 0.5788 |
| Residuals | 288 | ||||
| Coarseness | |||||
| Tree status | 1 | 1.4987 | 0.2220 | ||
| Time period | 1 | 5.5787 | 0.0189 | ||
| Duration of defoliation | 2 | 1.2281 | 0.4077 | ||
| Tree status:Time period | 1 | 0.6376 | 0.4253 | ||
| Tree status:Duration of defoliation | 2 | 2.0052 | 0.1367 | ||
| Time period:Duration of defoliation | 2 | 1.3322 | 0.2657 | ||
| Tree status:Time period:Duration of defoliation | 2 | 0.7919 | 0.4541 | ||
| Residuals | 260 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lemay, A.; Barrette, J.; Krause, C. Balsam Fir (Abies balsamea (L.) Mill.) Wood Quality after Defoliation by Spruce Budworm (Choristoneura fumiferana Clem.) in the Boreal Forest of Quebec, Canada. Forests 2022, 13, 1926. https://doi.org/10.3390/f13111926
Lemay A, Barrette J, Krause C. Balsam Fir (Abies balsamea (L.) Mill.) Wood Quality after Defoliation by Spruce Budworm (Choristoneura fumiferana Clem.) in the Boreal Forest of Quebec, Canada. Forests. 2022; 13(11):1926. https://doi.org/10.3390/f13111926
Chicago/Turabian StyleLemay, Audrey, Julie Barrette, and Cornelia Krause. 2022. "Balsam Fir (Abies balsamea (L.) Mill.) Wood Quality after Defoliation by Spruce Budworm (Choristoneura fumiferana Clem.) in the Boreal Forest of Quebec, Canada" Forests 13, no. 11: 1926. https://doi.org/10.3390/f13111926
APA StyleLemay, A., Barrette, J., & Krause, C. (2022). Balsam Fir (Abies balsamea (L.) Mill.) Wood Quality after Defoliation by Spruce Budworm (Choristoneura fumiferana Clem.) in the Boreal Forest of Quebec, Canada. Forests, 13(11), 1926. https://doi.org/10.3390/f13111926






