Dehydration Treatment Improves Ulmus glabra Dormant Bud Regeneration from Cryostorage
Abstract
1. Introduction
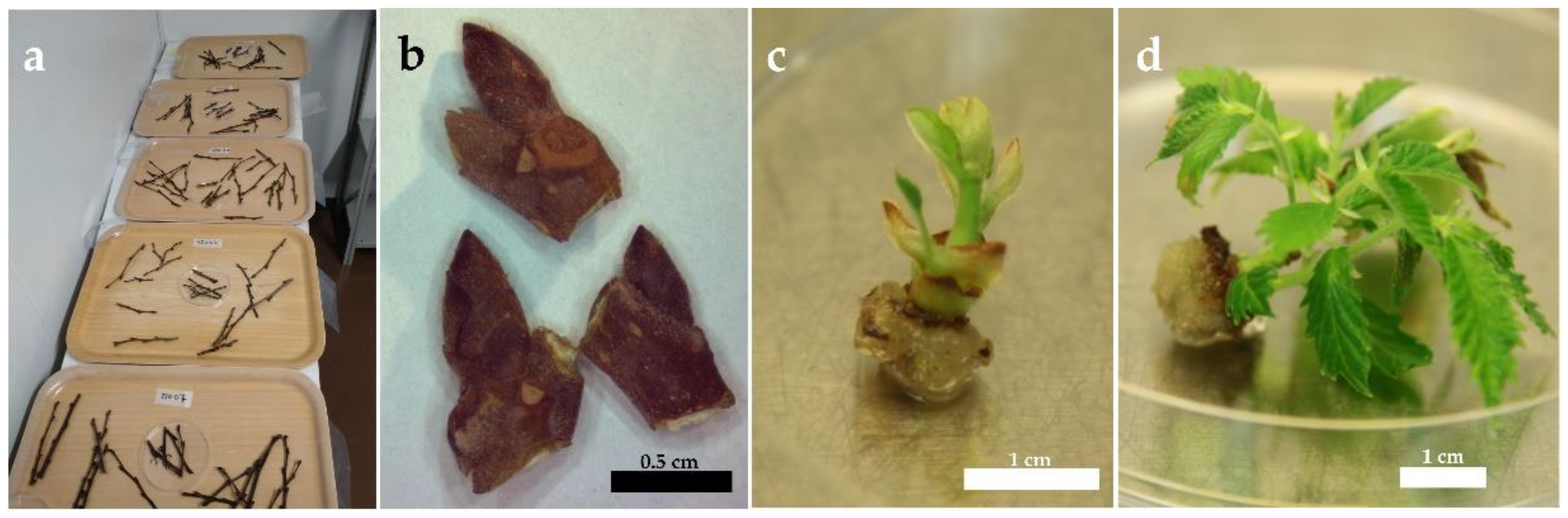
2. Materials and Methods
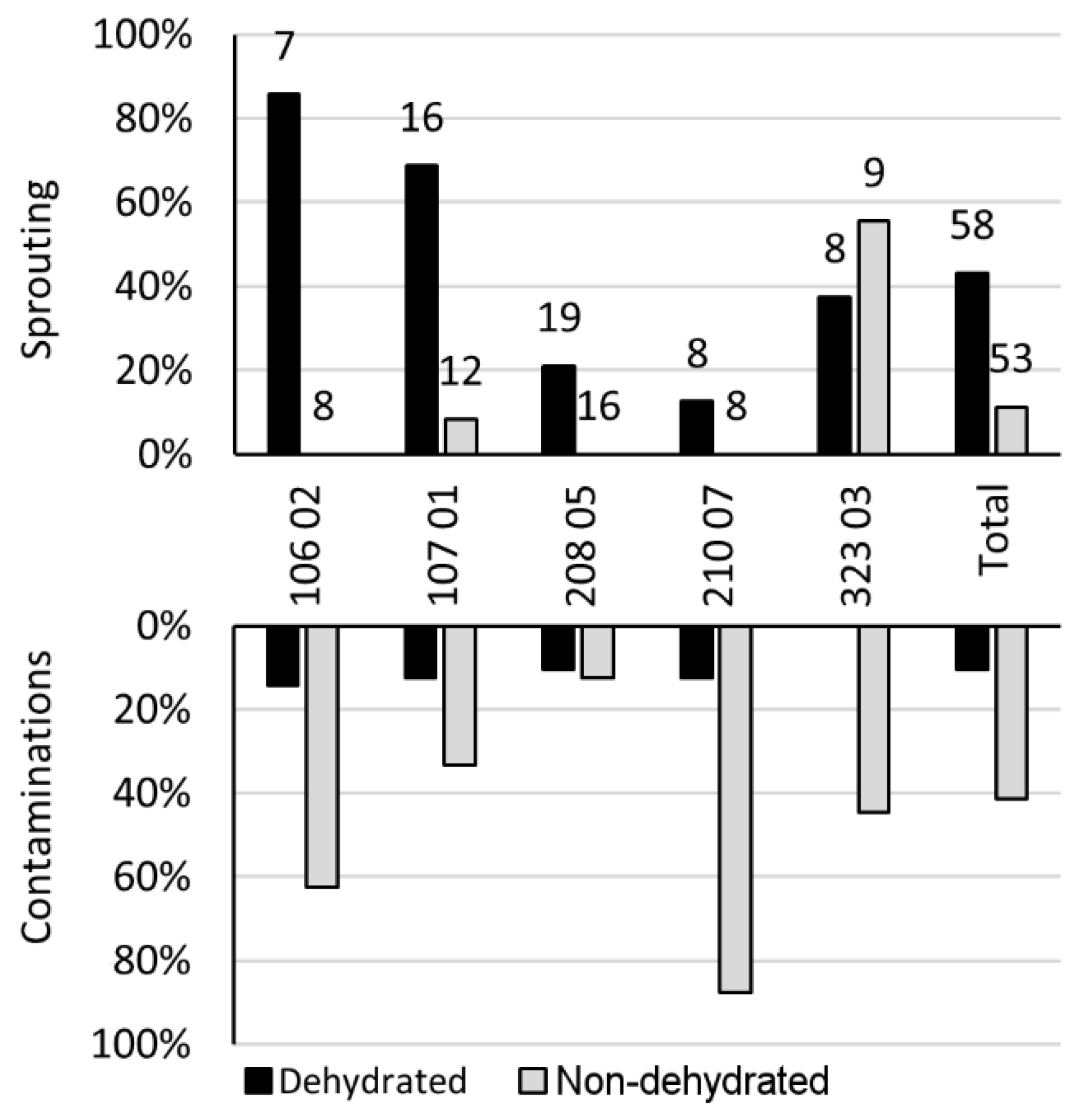
3. Results
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brasier, C.M. Ophiostoma Novo-Ulmi Sp. Nov., Causative Agent of Current Dutch Elm Disease Pandemics. Mycopathologia 1991, 115, 151–161. [Google Scholar] [CrossRef]
- Paques, M.; Poissonnier, M.; Dumas, E.; Monod, V. Cryopreservation of Dormant and Non Dormant Broad-Leaved Trees. Acta Hortic. 1997, 447, 491–498. [Google Scholar] [CrossRef]
- Harvengt, L.; Meier-Dinkel, A.; Dumas, E.; Collin, E. Establishment of a Cryopreserved Gene Bank of European Elms. Can. J. For. Res. 2004, 34, 43–55. [Google Scholar] [CrossRef]
- Uchendu, E.E.; Shukla, M.R.; Reed, B.M.; Saxena, P.K. Melatonin Enhances the Recovery of Cryopreserved Shoot Tips of A Merican Elm (Ulmus Americana L.). J. Pineal Res. 2013, 55, 435–442. [Google Scholar] [CrossRef]
- Collin, E.; Rondouin, M.; Joyeau, C.; Matz, S.; Raimbault, P.; Harvengt, L.; Bilger, I.; Guibert, M. Conservation and use of Elm Genetic Resources in France: Results and Perspectives. iForest 2020, 13, 41–47. [Google Scholar] [CrossRef]
- Välimäki, S.; Rusanen, M.; Pečínková, D.; Tikkinen, M.; Aronen, T. Cryopreservation and Micropropagation Methods for Conservation of Genetic Resources of Ulmus Laevis and Ulmus Glabra. Forests 2021, 12, 1121. [Google Scholar] [CrossRef]
- Towill, L.E.; Ellis, D.D. Cryopreservation of dormant buds. In Plant Cryopreservation: A Practical Guide; Reed, B.M., Ed.; Springer: New York, NY, USA, 2008; pp. 421–442. [Google Scholar]
- Seufferheld, M.J.; Stushnoff, C.; Forsline, P.L.; Gonzalez, G.H.T. Cryopreservation of Cold-Tender Apple Germplasm. J. Am. Soc. Hortic. Sci. 1999, 124, 612–618. [Google Scholar] [CrossRef]
- Aronen, T.; Ryynänen, L. Cryopreservation of Dormant in Vivo-Buds of Hybrid Aspen: Timing as Critical Factor. Cryo Lett. 2014, 35, 385–394. [Google Scholar]
- Reed, B.M.; Uchendu, E. Controlled rate cooling. In Plant Cryopreservation: A Practical Guide; Reed, B.M., Ed.; Springer: New York, NY, USA, 2008; pp. 77–92. [Google Scholar]
- Tyler, N.; Stushnoff, C. Dehydration of Dormant Apple Buds at Different Stages of Cold Acclimation to Induce Cryopreservability in Different Cultivars. Can. J. Plant Sci. 1988, 68, 1169–1176. [Google Scholar] [CrossRef]
- Zhumagulova, Z.B.; Kovalchuk, I.Y.; Reed, B.M.; Kampitova, G.A.; Turdiev, T.T. Effect of Pretreatment Methods of Dormant Pear Buds on Viability after Cryopreservation. World Appl. Sci. J. 2014, 30, 330–334. [Google Scholar] [CrossRef]
- Forsline, P.L.; Towill, L.E.; Waddell, J.W.; Stushnoff, C.; Lamboy, W.F.; McFerson, J.R. Recovery and Longevity of Cryopreserved Dormant Apple Buds. J. Am. Soc. Hortic. Sci. 1998, 123, 365–370. [Google Scholar] [CrossRef]
- Volk, G.M.; Bonnart, R.; Waddell, J.; Widrlechner, M.P. Cryopreservation of Dormant Buds from Diverse Fraxinus Species. Cryo Lett. 2009, 30, 262–267. [Google Scholar]
- Tanner, J.D.; Chen, K.Y.; Bonnart, R.M.; Minas, I.S.; Volk, G.M. Considerations for Large-Scale Implementation of Dormant Budwood Cryopreservation. Plant Cell Tissue Org. Cult. 2021, 144, 35–49. [Google Scholar] [CrossRef]
- Tanner, J.D.; Chen, K.Y.; Jenderek, M.M.; Wallner, S.J.; Minas, I.S. Determining the Effect of Pretreatments on Freeze Resistance and Survival of Cryopreserved Temperate Fruit Tree Dormant Buds. Cryobiology 2021, 101, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Bilavcik, A.; Faltus, M.; Zamecnik, J. The Survival of Pear Dormant Buds at Ultra-Low Temperatures. Plants 2021, 10, 2502. [Google Scholar] [CrossRef] [PubMed]
- Ryynänen, L. Survival and Regeneration of Dormant Silver Birch Buds Stored at Super-Low Temperatures. Can. J. For. Res. 1996, 26, 617–623. [Google Scholar] [CrossRef]
- Fenning, T.M.; Gartland, K.; Brasier, C.M. Micropropagation and Regeneration of English Elm, Ulmus Procera Salisbury. J. Exp. Bot. 1993, 44, 1211–1217. [Google Scholar] [CrossRef]
- Driver, J.A.; Kuniyuki, A.H. In Vitro Propagation of Paradox Walnut Rootstock. HortScience 1984, 19, 507–509. [Google Scholar] [CrossRef]
- Jenderek, M.M.; Tanner, J.D.; Chao, C.T.; Blackburn, H. How Applicable Are Dormant Buds in Cryopreservation of Horticultural Woody Plant Crops? The Malus Case. Acta Hortic. 2019, 1234, 317–322. [Google Scholar] [CrossRef]
- Volk, G.M.; Henk, A.D.; Jenderek, M.M.; Richards, C.M. Probabilistic Viability Calculations for Cryopreserving Vegetatively Propagated Collections in Genebanks. Genet. Resour. Crop Evol. 2017, 64, 1613–1622. [Google Scholar] [CrossRef]
| Model, log(p/1 − p) | Variable | p-Value | Odds Ratio (95% CI) | Donor Tree | % of Cases Predicted Correctly by Model |
|---|---|---|---|---|---|
| log(p/1 − p) = −1.539 + 2.181d − 0.113t1 − 2.099t2 − 2.722t3 + 0.370t4 | 80.2 | ||||
| dehydration status | <0.001 | 8.851 (2.879–27.209) | |||
| Tree | 0.004 | Reference | 106 02 | ||
| 0.88 | 0.893 (0.207–3.852) | 107 01 | |||
| 0.012 | 0.123 (0.024–0.629) | 208 05 | |||
| 0.027 | 0.066 (0.006–0.729) | 210 07 | |||
| 0.654 | 1.447 (0.288–7.285) | 323 03 | |||
| Model, log(p/1 − p) | Variable | p-Value | Odds Ratio (95% CI) | Donor Tree | % of Cases Predicted Correctly by Model |
|---|---|---|---|---|---|
| log(p/1 − p) = 0.400 − 1.966d − 0.846t1 − 1.738t2 + 0.583t3 − 0.909t4 | 82.0 | ||||
| dehydration status | <0.001 | 0.140 (0.047–0.414) | |||
| Tree | 0.045 | Reference | 106 02 | ||
| 0.270 | 0.429 (0.095–1.930) | 107 01 | |||
| 0.032 | 0.176 (0.036–0.857) | 208 05 | |||
| 0.475 | 1.791 (0.362–8.856) | 210 07 | |||
| 0.284 | 0.403 (0.076–2.125) | 323 03 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Välimäki, S.; Rusanen, M.; Aronen, T. Dehydration Treatment Improves Ulmus glabra Dormant Bud Regeneration from Cryostorage. Forests 2022, 13, 1923. https://doi.org/10.3390/f13111923
Välimäki S, Rusanen M, Aronen T. Dehydration Treatment Improves Ulmus glabra Dormant Bud Regeneration from Cryostorage. Forests. 2022; 13(11):1923. https://doi.org/10.3390/f13111923
Chicago/Turabian StyleVälimäki, Sakari, Mari Rusanen, and Tuija Aronen. 2022. "Dehydration Treatment Improves Ulmus glabra Dormant Bud Regeneration from Cryostorage" Forests 13, no. 11: 1923. https://doi.org/10.3390/f13111923
APA StyleVälimäki, S., Rusanen, M., & Aronen, T. (2022). Dehydration Treatment Improves Ulmus glabra Dormant Bud Regeneration from Cryostorage. Forests, 13(11), 1923. https://doi.org/10.3390/f13111923






