Soil Degradation Due to Conversion from Natural to Plantation Forests in Indonesia
Abstract
1. Introduction
2. Methods
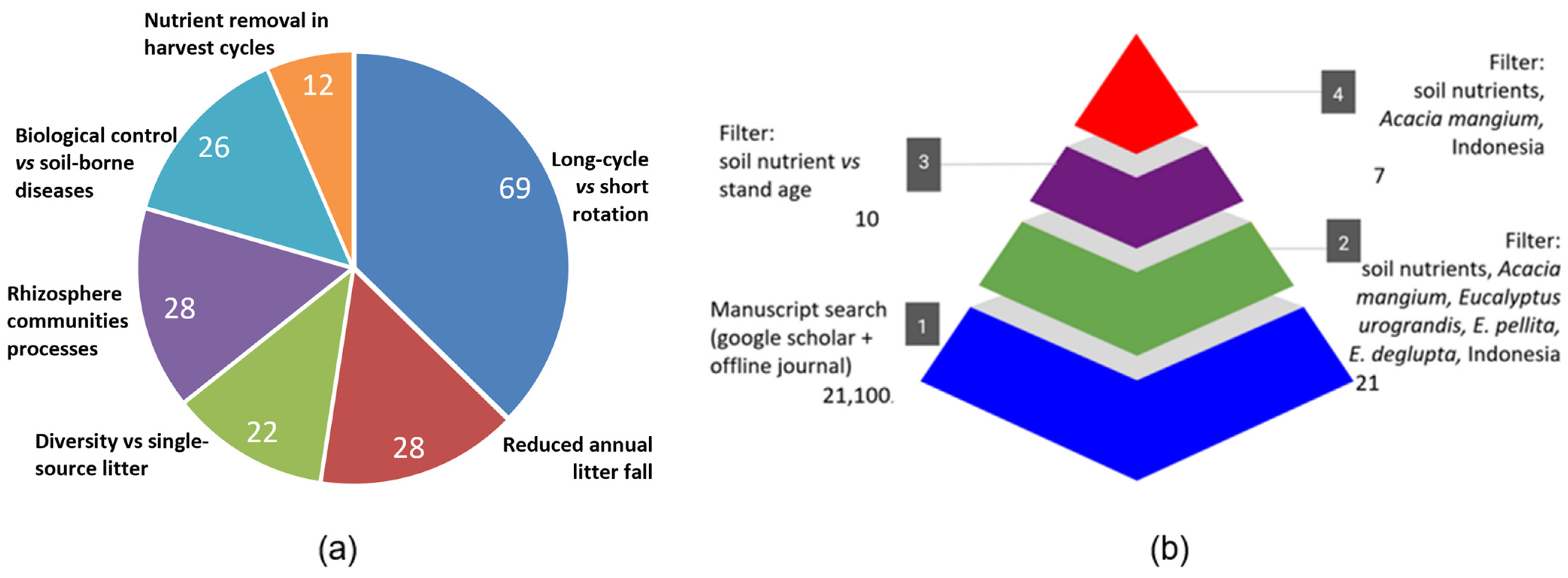
2.1. PRISMA Systematic Review and Meta-Analysis
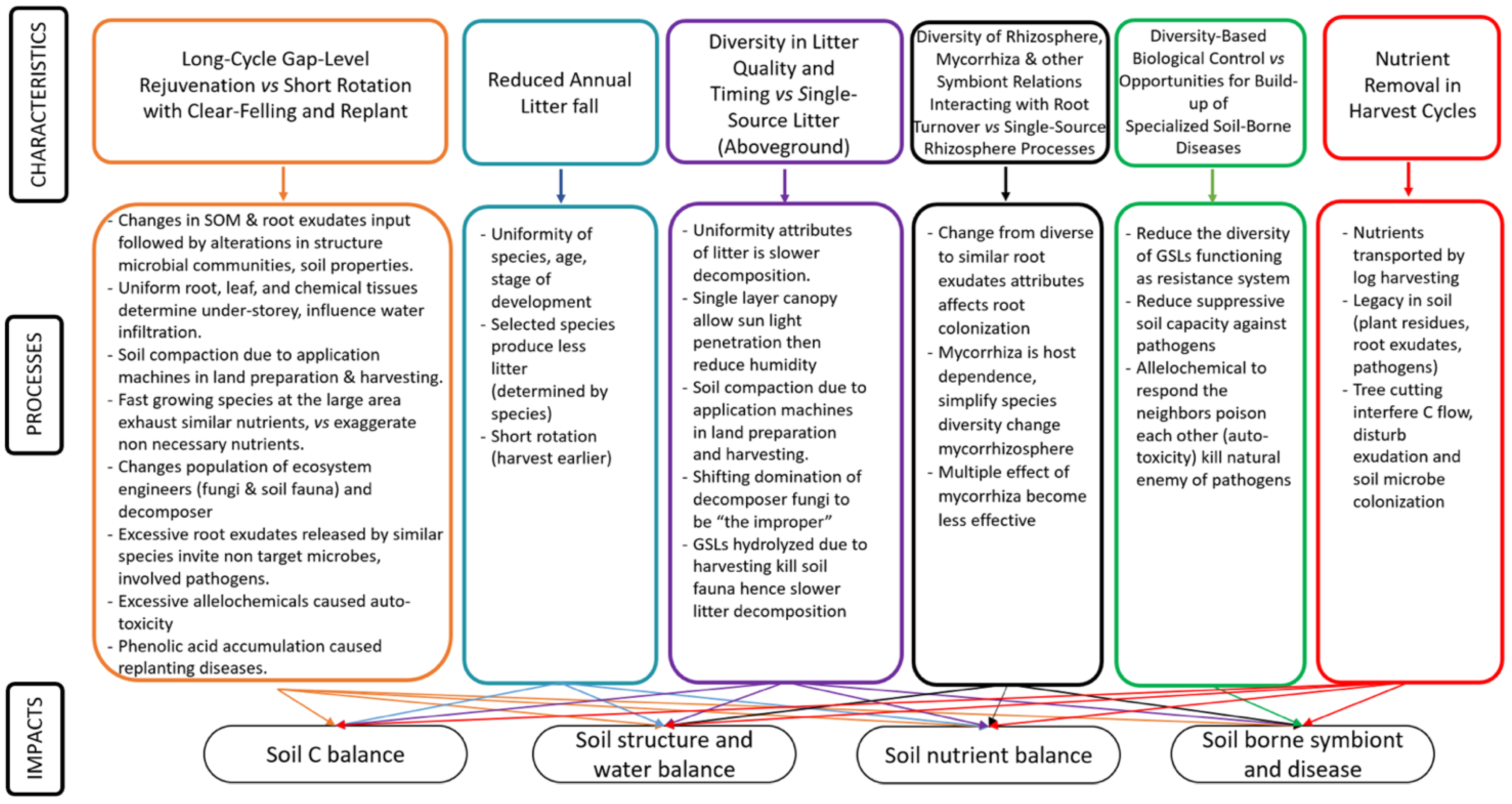
2.2. Scheme Linking Characteristics, Processes and Impacts
3. Long-Cycle Gap-Level Rejuvenation vs. Short Rotation with Clear-Felling and Replant
3.1. Impact on Soil C Balance
3.2. Impact on Soil Structure and Water Balance
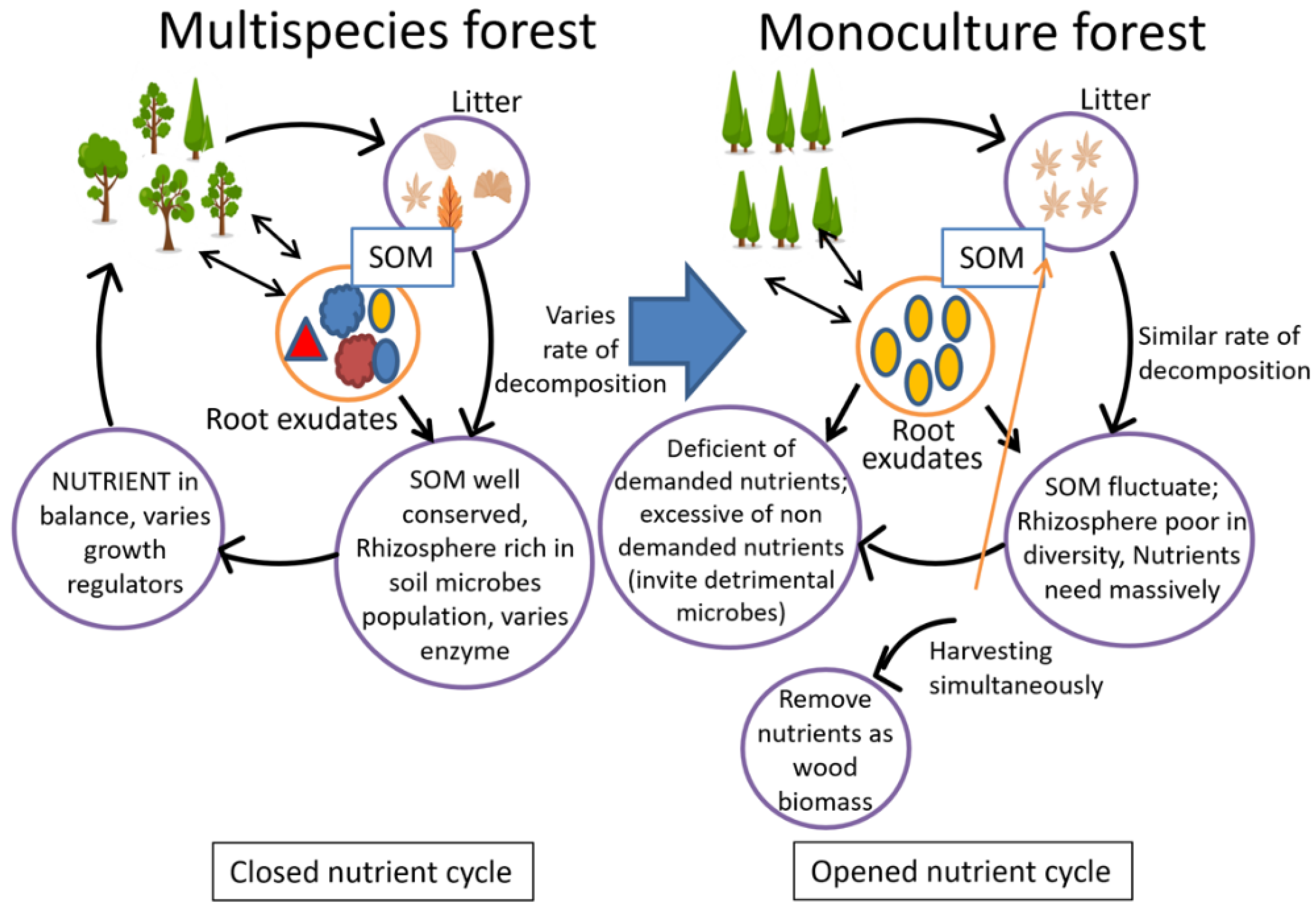
3.3. Impact on Soil Nutrient Balance
3.4. Impact on Soil-Borne Symbionts and Disease
4. Reduced Annual Litterfall
5. Diversity in Litter Quality and Timing vs. Single-Source Litter (Aboveground)
6. Diversity of Rhizosphere, Mycorrhiza and Other Symbiont Relations Interacting with Root Turnover vs. Single-Source Rhizosphere Processes
7. Diversity-Based Biological Control vs. Opportunities for Build-Up of Specialized Soil-Borne Diseases
8. Nutrient Removal in Harvest Cycles
9. Some Limitations of Existing Literature
10. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A
| Year of Publication | Number of Data | Source | |||
|---|---|---|---|---|---|
| A. mangium | E. pellita | E. urograndis | Age (Year) | ||
| 1997 | 9 | 5–8 | [79] | ||
| 1998 | 16 | 2, 5, 8, 11 | [80] | ||
| 2000 | 10 | 9 | [81] | ||
| 1 | n.a | [82] | |||
| 2004 | 4 | 1–4 | [83] | ||
| 8 | 5–8 | [84] | |||
| 5 | 5 | 4, 6 | [85] | ||
| 2005 | 6 | 2–6 | [86] | ||
| 2006 | 8 | 1–3 | [87] | ||
| 15 | 1–5 | [88] | |||
| 2008 | 7 | 17 | [89] | ||
| 3 | 5–8 | [90] | |||
| 10 | 8 | [91] | |||
| 2011 | 15 | 1–5 | [32] | ||
| 2019 | 15 | 2, 4, 6 | [92] | ||
References
- Watson, R.; Baste, I.; Larigauderie, A.; Leadley, P.; Pascual, U.; Baptiste, B.; Demissew, S.; Dziba, L.; Erpul, G.; Fazel, A.; et al. Summary for Policymakers of the Global Assessment Report on Biodiversity and Ecosystem Services of the Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services; IPBES Secretariat: Bonn, Germany, 2019; pp. 22–47. [Google Scholar] [CrossRef]
- Creed, I.F.; van Noordwijk, M. Forest and Water on a Changing Planet: Vulnerability, Adaptation and Governance Opportunities. A Global Assessment Report; IUFRO World Series, 38; IUFRO: Vienna, Austria, 2018. [Google Scholar]
- Ellison, D.; Morris, C.E.; Locatelli, B.; Sheil, D.; Cohen, J.; Murdiyarso, D.; Gutierrez, V.; van Noordwijk, M.; Creed, I.F.; Pokorny, J.; et al. Trees, forests and water: Cool insights for a hot world. Glob. Environ. Chang. 2017, 43, 51–61. [Google Scholar] [CrossRef]
- Binkley, D.; Fisher, R.F. Ecology and Management of Forest Soils; John Wiley & Sons: Chichester, UK, 2019. [Google Scholar]
- Basuki, T.M. Peningkatan Peran Hutan Melalui Sinergi Fungsi Hutan sebagai Pengatur Tata Air Daerah Aliran Sungai dan Penyerap CO2; BLI KLHK: Jakarta, Indonesia, 2020; pp. 5–21. [Google Scholar]
- Pramono, I.B. Peningkatan Peran Hutan dalam Mengendalikan Hasil Air untuk Mitigasi Banjir dan Kekeringan; BLI KLHK: Jakarta, Indonesia, 2019; pp. 11–20. [Google Scholar]
- Tsujino, R.; Yumoto, T.; Kitamura, S.; Djamaluddin, I.; Darnaedi, D. History of forest loss and degradation in Indonesia. Land Use Policy 2016, 57, 335–347. [Google Scholar] [CrossRef]
- Williams, M. Deforesting the Earth: From Prehistory to Global Crisis; University of Chicago Press: Chicago, IL, USA, 2003. [Google Scholar]
- MoEF (Ministry of Environment and Forestry). The State of Indonesia’s Forest 2020; MoEF: Jakarta, Indonesia, 2020. [Google Scholar]
- Scholes, R.J.; Montanarella, L.; Brainich, E.; Barger, N.; Ten Brink, B.; Cantele, M.; Erasmus, B.; Fisher, J.; Gardner, T.; Holland, T.G.; et al. Summary for Policymakers of the Assessment Report on Land Degradation and Restoration of the Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services; IPBES Secretariat: Bonn, Germany, 2018. [Google Scholar]
- van Noordwijk, M.; Suyamto, D.A.; Lusiana, B.; Ekadinata, A.; Hairiah, K. Facilitating agroforestation of landscapes for sustainable benefits: Tradeoffs between carbon stocks and local development benefits in Indonesia according to the FALLOW model. Agric Ecosyst. Environ. 2008, 126, 98–112. [Google Scholar] [CrossRef]
- Romijn, E.; Ainembabazi, J.H.; Wijaya, A.; Herold, M.; Angelsen, A.; Verchot, L.; Murdiyarso, D. Exploring different forest definitions and their impact on developing REDD+ reference emission levels: A case study for Indonesia. Environ. Sci. Policy 2013, 33, 246–259. [Google Scholar] [CrossRef]
- van Noordwijk, M.; Agus, F.; Dewi, S.; Purnomo, H. Reducing emissions from land use in Indonesia: Motivation, policy instruments and expected funding streams. Mitig. Adapt. Strat. Glob. Chang. 2004, 19, 677–692. [Google Scholar] [CrossRef]
- Ekawati, S.; Budiningsih, K.; Sari, G.K.; Muttaqin, M.Z. Policies affecting the implementation of REDD+ in Indonesia (cases in Papua, Riau and Central Kalimantan). For. Policy Econ. 2019, 108, 101939. [Google Scholar] [CrossRef]
- Gaveau, D.L.A.; Santos, L.; Locatelli, B.; Salim, M.A.; Husnayaen, H.; Meijaard, E.; Heatubun, C.; Sheil, D. Forest loss in Indonesia New Guinea (2001–2019): Trends, drivers, and outlook. Biol. Conserv. 2021, 261, 109225. [Google Scholar] [CrossRef]
- KLHK. Analisa Data Luas Areal Kebakaran Hutan dan Lahan tahun 2019; Direktorat Inventarisasi dan Pemantauan Sumberdaya Hutan, KLHK: Jakarta, Indonesia, 2019; pp. 2–3. [Google Scholar]
- KLHK (Kementerian Lingkungan Hidup dan Kehutanan). Deforestasi Indonesia 2015–2016; Direktorat Inventarisasi dan Sumber Daya Hutan: Jakarta, Indonesia; Direktorat Jenderal Planologi Kehutanan dan Tata Lingkungan: Jakarta, Indonesia; Kementerian Lingkungan Hidup dan Kehutanan (KLHK): Jakarta, Indonesia, 2017; pp. 9–28. [Google Scholar]
- KLHK (Kementerian Lingkungan Hidup dan Kehutanan). Statistik 2019 Kementerian Lingkungan Hidup dan Kehutanan; Pusat Data dan Informasi KLHK: Jakarta, Indonesia, 2021; pp. 48–51. [Google Scholar]
- Purwanto, E.; Santoso, H.; Jelsma, I.; Widayati, A.; Nugroho, H.Y.; van Noordwijk, M. Agroforestry as policy option for forest-zone oil palm production in Indonesia. Land 2020, 9, 531. [Google Scholar] [CrossRef]
- Saatchi, S.; Longo, M.; Xu, L.; Yang, Y.; Abe, H.; Andre, M.; Aukema, J.E.; Carvalhais, N.; Cadillo-Quiroz, H.; Cerbu, G.A.; et al. Detecting vulnerability of humid tropical forests to multiple stressors. One Earth 2021, 4, 988–1003. [Google Scholar] [CrossRef]
- Khasanah, N.; van Noordwijk, M.; Ningsih, H.; Rahayu, S. Carbon neutral? No change in mineral soil carbon stock under oil palm plantations derived from forest or non-forest in Indonesia. Agric. Ecosyst. Environ. 2015, 211, 195–206. [Google Scholar] [CrossRef]
- Dislich, C.; Keyel, A.C.; Salecker, J.; Kisel, Y.; Meyer, K.M.; Auliya, M.; Barnes, A.D.; Corre, M.D.; Darras, K.; Faust, H.; et al. A review of the ecosystem functions in oil palm plantations, using forests as a reference system. Biol. Rev. 2016, 92, 1539–1569. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, B.M.; Edwards, D.P.; Mendes, L.W.; Kim, M.; Dong, K.; Kim, H.; Adams, J.M. The impact of tropical forest logging and oil palm agriculture on the soil microbiome. Mol. Ecol. 2016, 25, 2244–2257. [Google Scholar] [CrossRef] [PubMed]
- Pirard, R.; Petit, H.; Baral, H.; Achdiawan, R. Impacts of Industrial Timber Plantations in Indonesia: An Analysis of Perceptions by Rural Populations in Sumatra, Java and Borneo CIFOR Occasional Paper 149; Center for International Forestry Research: Bogor, Indonesia, 2016. [Google Scholar]
- KLHK (Kementerian Lingkungan Hidup dan Kehutanan). Keputusan Menteri Lingkungan Hidup dan Kehutanan Nomor P.62/MENLHK/SETJEN/KUM.1/10/2019 Tentang Pembangunan Hutan Tanaman Industri; KLHK: Jakarta, Indonesia, 2019; p. 34. [Google Scholar]
- Bouillet, J.P.; Bernhard-Reservat, F. General objectives and sites. In Effect of Exotic Tree Plantations on Plant Diversity and Biological Soil Fertility in the Congo Savanna: With Special Reference to Eucalyptus; Bernhard-Reservat, F., Ed.; Center for International Forestry Research: Bogor, Indonesia, 2001; pp. 1–7. [Google Scholar] [CrossRef]
- Marais, A.; Hardy, M.; Booyse; Botha, A. Effects of monoculture, crop rotation and soil moisture content on selected soil physicochemical and microbial parameters in wheat field. Appl. Environ. Soil Sci. 2012, 2012, 593623. [Google Scholar] [CrossRef]
- Zubek, S.; Blaszkowski, J.; Seidler-Lozykowska, K.; Baba, W.; Mleczko, P. Arbuscular mycorrhizal fungi abundance, species ricness and composition under monoculturs of five medical lants. Acta Sci. Pol. Hortorum Cultus 2013, 12, 127–141. [Google Scholar]
- Mensah, A.K. Effects of Eucalyptus Plantation on Soil Physico-Chemical Properties in Thiririka Sub-Catchment, Kiambu County, Kenya. Master’s Thesis, Kenyata University, Nairobi, Kenya, 2016. Available online: https://www.researchgate.net/publication/301567610 (accessed on 11 February 2022).
- Widyati, E. Biologi Tanah. Membedah Kerapuhan Sistem Budidaya Monokultur; Deepublish: Yogyakarta, Indonesia, 2018; pp. 12–63. [Google Scholar]
- Liu, C.L.C.; Kuchma, O.; Krutovsky, K.V. Mixed-species versus monoculture in plantation forestry: Development, benefits, ecosystem services and perspecitves for the future. Glob. Ecol. Conserv. 2018, 15, e00419. [Google Scholar] [CrossRef]
- Mindawati, N. Kajian Kualitas Tapak Eucalyptus urograndis Sebagai Bahan Baku Industri Pulp Dalam Pengelolaan Hutan Lestari. Ph.D. Dissertation, Institut Pertanian Bogor, Bogor, Indonesia, 2011. [Google Scholar]
- Widyati, E.; Wahyudi, A. Dari Hutan Kembali ke Hutan. Sluge Industry Kertas Memperbaiki Produktivitas Tanah Pertanian, Kehutanan dan Pertambangan; Pusat Litbang Peningkatan Produktivitas Hutan: Bogor, Indonesia, 2011; p. 58. [Google Scholar]
- Moher, D.; Shamseer, L.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: Elaboration and explanation. BMJ 2014, 349, g7647. [Google Scholar] [CrossRef]
- Liang, M.Q.; Zhang, C.F.; Peng, C.L.; Lai, Z.L.; Chen, D.F.; Chen, Z.H. Plant growth, community structure, and nutrient removal in monoculture and mixed constructed wetlands. Ecol. Eng. 2011, 37, 309–316. [Google Scholar] [CrossRef]
- Guo, X.; Meng, M.; Zhang, J.; Chen, H.Y.H. Vegetation impacts on soil organic carbon chemical composition in subtropical forests. Sci. Rep. 2016, 6, 29607. [Google Scholar] [CrossRef]
- Hennings, N.; Becker, J.N.; Guillaume, T.; Damris, M.; Dippold, M.A.; Kuzyakov, Y. Riparian wetland properties counter the effect of land-use change on soil carbon stocks after rainforest conversion to plantations. Catena 2021, 196, 104941. [Google Scholar] [CrossRef]
- Mackensen, J. Penelitian Hutan Tropis. Kajian Suplai Hara Lestari Pada Hutan Tanaman Cepat Tumbuh. Implikasi Ekologi dan Ekonomi di Kalimantan Timur; Badan kerjasama teknis Jerman-Deutsche Gesellschaft fur Technische Zusammenarbeit (GTZ) GmbH, Jerman: Jakarta, Indonesia, 2000; pp. 34–36. [Google Scholar]
- Wu, J.; Su, Y.; Chen, X.; Liu, L.; Yang, X.; Gong, F.; Zhang, H.; Xiong, X.; Zhang, D. Leaf shedding of Pan-Asian tropical evergreen forests depends on the synchrony of seasonal variations of rainfall and incoming solar radiation. Agric. For. Meteorol. 2021, 311, 108691. [Google Scholar] [CrossRef]
- Feichtinger, F.; Erhart, E.; Hartl, W. Net N-mineralisation related to soil organic matter pools. Plant Soil Environ. 2004, 50, 273–276. [Google Scholar] [CrossRef]
- Wright, S.J.; Van Schaik, C.P. Light and the phenology of tropical trees. Am. Nat. 1994, 143, 192–199. [Google Scholar] [CrossRef]
- Hairiah, K.; Widianto, W.; Suprayogo, D.; van Noordwijk, M. Tree roots anchoring and binding soil: Reducing landslide risk in Indonesian agroforestry. Land 2020, 9, 25. [Google Scholar] [CrossRef]
- Wiersum, K.F. Effects of various vegetation layers in an Acacia auriculiformis forest plantation on surface erosion in Java, Indonesia. In Soil Erosion and Conservation; El-Swaify, S.A., Moldenhauer, W.C., Lo, A., Eds.; Soil Conservation Society of America: Ankeny, IA, USA, 1985; pp. 79–89. [Google Scholar]
- Bruijnzeel, L.A. Hydrological functions of tropical forests: Not seeing the soil for the trees? Agric. Ecosyst. Environ. 2004, 104, 185–228. [Google Scholar] [CrossRef]
- Myers, R.J.K.; van Noordwijk, M.; Vityakon, P. Synchrony of nutrient release and plant demand: Plant litter quality, soil environment and farmer management options. In Driven by Nature: Plant Litter Quality and Decomposition; Cadisch, K.G., Ed.; CAB International: Wallingford, UK, 1997. [Google Scholar]
- Quideau, S.A.; Chadwick, O.A.; Benesi, A.; Graham, R.C.; Anderson, M.A. A direct link between forest vegetation type and soil organic matter composition. Geoderma 2001, 104, 1–60. [Google Scholar] [CrossRef]
- Gannes, V.; Bekele, I.; Dipschansingh, D.; Wuddivira, M.N.; de Cairies, S.; Boman, M.; Hickey, W.J. Microbial community structure and function of soil following ecosystem conversion from native forests to teak plantation forests. Front. Microbiol. 2016, 7, 1976. [Google Scholar] [CrossRef]
- Bennet, J.A.; Klironomos, J. Mechanisms of plant-soil feedback: Interactions among biotic and abiotic drivers. New Phytol. 2019, 222, 91–96. [Google Scholar] [CrossRef]
- Hodge, A. Root decisions. Plant Cell Environ. 2009, 32, 628–640. [Google Scholar] [CrossRef]
- Gruntman, M.; Gros, D.; Majekova, M.; Tielborger, K. Decision-making in plants under competition. Nat. Commun. 2017, 8, 2235. [Google Scholar] [CrossRef]
- Fan, X.; Zhou, X.; Chen, H.; Tang, M.; Xie, X. Cross-talk between macro- and micronutrient uptake and signaling in plants. Front. Plant Sci. 2021, 12, 663477. [Google Scholar] [CrossRef]
- Rubio, G.; Walk, T.; Ge, Z.; Yan, X.; Liao, H.; Lynch, J. Root gravitropism and below-ground competition among neighbouring plants: A modelling approach. Ann. Bot. 2001, 88, 924–940. [Google Scholar] [CrossRef]
- van Noordwijk, M.; Lawson, G.; Hairiah, K.; Wilson, J. Root distribution of trees and crops: Competition and/or complementarity. In Tree-Crop Interactions: Agroforestry in a Changing Climate; CAB International: Wallingford, UK, 2015; pp. 221–257. [Google Scholar]
- Wang, Y.; Kim, J.H.; Mao, Z.; Ramel, M.; Pailler, F.; Perez, F.; Rey, H.; Tron, S.; Jourdan, C.; Stokes, A. Tree root dynamics in montane and sub-alpine mixed forest patches. Ann. Bot. 2018, 122, 861–872. [Google Scholar] [CrossRef]
- Foxx, A.J.; Fort, F. Root and shoot competition lead to contrasting competitiveoutcomes under water stress: A systematic review and meta-analysis. PLoS ONE 2019, 14, e0220674. [Google Scholar] [CrossRef] [PubMed]
- Ehrenfeld, J.G. Effects of exotic plant invasions on soil nutrient cycling processes. Ecosystems 2003, 6, 503–523. [Google Scholar] [CrossRef]
- Widyati, E.; Sutiyono; Darwo; Mindawati, N.; Yulianti, M.; Prameswari, D.; Abdulah, L.; Yuniarti, K.; Baral, H. Optimum plant density and harvest age for maximizing productivity and minimizing competition in a Calliandra short-rotation-coppice plantation in West Java, Indonesia. For. Sci. Tech. 2022, 18, 26–35. [Google Scholar] [CrossRef]
- Guillaume, T.; Damris, M.; Kuzyakov, Y. Losses of soil carbon by converting tropical forest to plantations: Erosion and decomposition estimated by δ(13) C. Glob. Chang. Biol. 2015, 21, 3548–3560. [Google Scholar] [CrossRef] [PubMed]
- Guillaume, T.; Maranguit, D.; Murtilaksono, K.; Kuzyakov, Y. Sensitivity and resistance of soil fertility indicators to land-use changes: New concept and examples from conversion of Indonesian rainforest to plantations. Ecol. Indic. 2016, 67, 49–57. [Google Scholar] [CrossRef]
- Krashevska, V.; Klarner, B.; Widyastuti, R.; Maraun, M.; Scheu, S. Impact of tropical lowland rainforest conversion into rubber and oil palm plantations on soil microbial communities. Biol. Fertil. Soils 2015, 51, 697–705. [Google Scholar] [CrossRef]
- Monkai, J.; Goldberg, S.D.; Hyde, K.D.; Harrison, R.D.; Mortimer, P.E.; Xu, J. Natural forests maintain a greater soil microbial diversity than that in rubber plantations in Southwest China. Agric. Ecosyst. Environ. 2018, 265, 190–197. [Google Scholar] [CrossRef]
- Guo, J.; Feng, H.; Roberge, G.; Feng, L.; Pan, C.; McNie, P.; Yu, Y. The negative effect of Chinese fir (Cunninghamia lanceolata) monoculture plantations on soil physicochemical properties, microbial biomass, fungal communities, and enzymatic activities. For. Ecol. Manag. 2022, 519, 120297. [Google Scholar] [CrossRef]
- Gao, C.; Zhang, Y.u.; Shi, N.N.; Zheng, Y.; Chen, L.; Wubet, T.; Bruelheide, H.; Both, S.; Buscot, F.; Ding, Q.; et al. Community assembly of ectomycorrhizal fungi along a subtropical secondary forest succession. New Phytol. 2015, 205, 771–785. [Google Scholar] [CrossRef]
- Cai, X.; Lin, Z.; Penttinen, P.; Li, Y.; Li, Y.; Luo, Y.; Yue, T.; Jiang, P.; Fu, W. Effects of conversion from a natural evergreen broadleaf forest to a Moso bamboo plantation on the soil nutrient pools, microbial biomass and enzyme activities in a subtropical area. For. Ecol. Manag. 2018, 422, 161–171. [Google Scholar] [CrossRef]
- He, F.; Yang, B.; Wang, H.; Yan, Q.; Cao, Y.; He, X. Changes in composition and diversity of fungal communities along Quercus mongolica forests developments in Northeast China. Appl. Soil Ecol. 2016, 100, 162–171. [Google Scholar] [CrossRef]
- Shen, F.; Wu, J.; Fan, H.; Liu, W.; Guo, X.; Duan, H.; Hu, L.; Lei, X.; Wei, X. Soil N/P and C/P ratio regulate the responses of soil microbial community composition and enzyme activities in a long-term nitrogen loaded Chinese fir forest. Plant Soil 2019, 436, 91–107. [Google Scholar] [CrossRef]
- Gibbons, S.M.; Lekberg, Y.; Mummey, D.L.; Sangwan, N.; Ramsey, P.W.; Gilbert, J.A. Invasive plants rapidly reshape soil properties in a grassland ecosystem. mSystems 2017, 2, e00178-16. [Google Scholar] [CrossRef]
- Lone, P.A.; Dar, J.A.; Subashree, K.; Raha, D.; Pandey, P.K.; Ray, T.; Khare, P.K.; Khan, M.L. Impact of plant invasion on physical, chemical and biological aspects of ecosystems: A review. Trop. Plant Res. 2019, 6, 528–544. [Google Scholar] [CrossRef]
- Levine, J.M.; Vila, M.; Antonio, C.M.D.; Dukes, J.S.; Grigulis, K.; Lavorel, S. Mechanisms underlying the impacts of exotic plant invasions. Proc. R. Soc. Lond. B Biol. Sci. 2003, 270, 775–781. [Google Scholar] [CrossRef] [PubMed]
- Si, C.; Liu, X.; Wang, C.; Wang, L.; Dai, Z.; Qi, S.; Du, D. Different degrees of plant invasion significantly affect the richness of the soil fungal community. PLoS ONE 2013, 8, e85490. [Google Scholar] [CrossRef]
- Evan, J. Sustainability of Productivity in Successive Rotations. Available online: https://www.fao.org/3/AC781E/AC781E11.htm (accessed on 3 August 2022).
- Suprayogo, D.; van Noordwijk, M.; Hairiah, K.; Meilasari, N.; Rabbani, A.L.; Ishaq, R.M.; Widianto, W. Infiltration-friendly agroforestry land uses on volcanic slopes in the Rejoso Watershed, East Java, Indonesia. Land 2020, 9, 240. [Google Scholar] [CrossRef]
- Liu, W.; Wu, J.; Fan, H.; Duan, H.; Li, Q.; Yuan, Y.; Zhang, H. Estimations of evapotranspiration in an age sequence of Eucalyptus plantations in subtropical China. PLoS ONE 2017, 12, e0174208. [Google Scholar] [CrossRef]
- Wardle, D.A.; Bardgett, R.D.; Klironomos, J.N.; Setälä, H.; van der Putten, W.H.; Wall, D.H. Ecological linkages between aboveground and belowground biota. Science 2004, 304, 1629–1633. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.Z.; Luo, Y.Q.; Fang, C.M.; Chen, J.K.; Li, B. The effects of plantation practice on soil properties based on the comparison between natural and planted forests: A meta-analysis. Glob. Ecol. Biogeogr. 2012, 21, 318–327. [Google Scholar] [CrossRef]
- Fierer, N.; Schimel, J.P.; Holden, P.A. Variations in microbial community composi- tion through two soil depth profiles. Soil Biol. Biochem. 2003, 35, 167–176. [Google Scholar] [CrossRef]
- Acosta-Martinez, V.; Zobeck, T.M.; Allen, V. Soil microbial, chemical and physical properties in continuous cotton and integrated crop-livestock systems. Soil Sci. Soc. Am. J. 2004, 68, 1875–1884. [Google Scholar] [CrossRef]
- Fang, S.; Liu, D.; Tian, Y.; Deng, S.; Shang, X. Tree species composition influences enzyme activities and microbial biomass in the rhizosphere: A rhizobox approach. PLoS ONE 2013, 8, e61461. [Google Scholar] [CrossRef]
- Mindawati, N.; Rostiwati, T. Ketersediaan unsur hara makro pada beberapa umur tegakan Acacia mangium Wild. di PT. Wirakarya Sakti, Jambi. Bul. Penelit. Hutan 1997, 609, 43–49. [Google Scholar]
- Siregar, S.T.H.; Hardiyanto, E.B.; Gales, K. Acacia mangium Plantations in PT Musi Hutan Persada, South Sumatera, Indonesia. In Proceedings of the Site Management and Productivity in Tropical Plantation Forests, Workshop Proceedings, Pietermaritzburg, South Africa, 16–20 February 1998; Nambiar, E.K.S., Cossalter, C., Tiarks, A., Eds.; CIFOR: Pietermaritzburg, South Africa, 1998. [Google Scholar]
- Hardiyanto, E.B.; Ryantoko, A.; Anshori, S. Effects of Site Management in Acacia mangium Plantations at PT. Musi Hutan Persada, South Sumatra, Indonesia. In Site Management and Productivity in Tropical Plantation Forests: A Progress Report; Nambiar, E.K.S., Toarks, A., Cossalter, C., Ranger, J., Eds.; Center for International Forestry Research: Bogor, Indonesia, 2000; pp. 41–49. [Google Scholar]
- Mok, C.K.; Cheah, L.C.; Chan, Y.K. Site Management and Productivity of Acacia mangium in Humid Tropical Sumatra, Indonesia. In Site Management and Productivity in Tropical Plantation Forests: A Progress Report; Nambiar, E.K.S., Toarks, A., Cossalter, C., Ranger, J., Eds.; Center for International Forestry Research: Bogor, Indonesia, 2000; pp. 87–94. [Google Scholar]
- Astuti, Y. Kandungan Unsur Hara Kalium pada Tanah dan Tanaman Acacia mangium Willd. Bachelor’s Thesis, IPB University, Bogor, Indonesia, 2004. [Google Scholar]
- Mindawati, N.; Pratiwi. Kondisi unsur hara di berbagai umur tegakan Acacia mangium di Riau. Bul. Penelit. Hutan 2004, 645, 75–83. [Google Scholar]
- Prameswari, D.; Mindawati, N. Ketersediaan unsur hara makro pada tegakan Eucalyptus pellita umur 4 dan 6 tahun di PT. Wirakarya Sakti, Jambi. In Ekspose Penerapan Hasil Litbang Hutan dan Konservasi Alam; Palembang, Indonesia, 15 December 2004; Gadas, S.R., Dewodaru, R., Daryono, H., Pratiwi, Murniati, Anwar, C., Haryono, Soekardi, A., Eds.; Pusat Litbang Hutan dan Konservasi Alam: Bogor, Indonesia, 2004. [Google Scholar]
- Latifah, S.; Setiadi, Y.; Kusmana, C.; Suhendang, E. Perbandingan pertumbuhan tegakan Acacia Mangium dan sifat kimia tanah pada lahan revegetasi dengan lahan hutan tanaman industri. Peronema For. Sci. J. 2005, 1, 55–60. [Google Scholar]
- Anhar, S. Kandungan Magnesium pada Biomassa Tanaman Acacia mangium Willd dan pada Podsolik Merah Kuning di HPHTI PT Musi Hutan Persada, Sumatera Selatan. Bachelor’s Thesis, IPB University, Bogor, Indonesia, 2006. [Google Scholar]
- Wasis, B. Perbandingan Kualitas Tempat Tumbuh Antara Daur Pertama dengan Daur Kedua pada Hutan Tanaman Acacia mangium Willd. Ph.D. Dissertation, IPB University, Bogor, Indonesia, 2006. [Google Scholar]
- Hartati, W. Evaluasi distribusi hara tanah dan tegakan mangium, sengon dan leda pada akhir daur untuk kelestarian produksi hutan tanaman di Umr Gowa PT Inhutani I Unit III Makassar. J. Hutan dan Masyarakat 2008, 3, 8195. [Google Scholar]
- Mindawati, N.; Pratiwi. Kajian penetapan daur optimal hutan tanaman Acacia mangium ditinjau dari kesuburan tanah. J. Penelit. Hutan Tanam. 2008, 5, 109–118. [Google Scholar] [CrossRef]
- Yamashita, N.; Ohta, S.; Hardjono, A. Soil changes induced by Acacia mangium plantation establishment: Comparison with secondary forest and Imperata cylindrica grassland soils in South Sumatra, Indonesia. For. Ecol. Manag. 2008, 254, 362–370. [Google Scholar] [CrossRef]
- Gunawan, G.; Wijayanto, N.; Budi, S.W. Karakteristik sifat kimia tanah dan status kesuburan tanah pada agroforestri tanaman sayuran berbasis Eucalyptus sp. J. Trop. Silvic. 2019, 10, 63–69. [Google Scholar] [CrossRef]
- Sanon, A.; Andrianjaka, Z.N.; Prin, Y.; Bally, R.; Thioulouse, J.; Comte, G.; Duponnois, R. Rhizosphere microbiota interfers with plant- plant interactions. Plant Soil 2009, 321, 259–278. [Google Scholar] [CrossRef]
- Coats, V.C.; Rumpho, M.E. The rhizosphere microbiota of plant invaders: An overview of recent advances in the microbiomics of new-excotic plants. Front. Microbiol. 2014, 5, 368. [Google Scholar] [CrossRef] [PubMed]
- Mendes, R.; Garbeva, P.; Raaijmakers, J.M. The rhizosphere microbiome: Significance of plant beneficial, plant pathogenic, and human pathogenic microorganisms. FEMS Microbiol. Rev. 2013, 37, 634–663. [Google Scholar] [CrossRef]
- Lelana, N.E.; Utami, S.; Darmawan, U.W.; Nuroniah, H.S.; Darwo; Asmaliyah; Haneda, N.F.; Arinana; Darwiati, W.; Anggraeni, I. Bagworms in Indonesian plantation forests: Species composition, pest status, and factors that contribute to outbreaks. Diversity 2022, 14, 471. [Google Scholar] [CrossRef]
- Zhou, X.; Liu, J.; Wu, F. Soil microbial communities in cucumber monoculture and rotation systems and their feedback effects on cucumber seedling growth. Plant Soil 2017, 415, 507–520. [Google Scholar] [CrossRef]
- Pollock, J.A.; Kogan, L.A.; Thorpe, A.S.; Holben, W.E. (±)-Catechin, a root exudate of the invasive Centaurea stoebe Lam. (Spotted Knapweed) exhibits bacteriostatic activity against multiple soil bacterial populations. J. Chem. Ecol. 2011, 37, 1044–1053. [Google Scholar] [CrossRef]
- Liu, X.B.; Herbert, S.J.; Hashemi, A.M.; Zhang, X.; Ding, G. Effects of agricultural management on soil organic matter and carbon transformation—A review. Plant Soil Environ. 2006, 52, 531–543. [Google Scholar] [CrossRef]
- Bhatti, A.A.; Haq, S.; Bhat, R.A. Actinomycetes benefaction role in soil and plant health. Microb. Pathog. 2017, 111, 458–467. [Google Scholar] [CrossRef]
- Wu, L.; Wang, J.; Huang, W.; Wu, H.; Chen, J.; Yang, Y.; Zhang, Z.; Lin, W. Plant-microbe rhizosphere interactions mediated by Rehmannia glutinosa root exudates under consecutive monoculture. Sci. Rep. 2015, 5, 15871. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.O.; Pang, L.; Dong, N.; Yang, S. LC-ESI-MS/MS analysis and pharmacokinetics of heterophyllin B, a cyclic octapeptide from Pseudostellaria heterophylla in rat plasma. Biomed. Chromatogr. 2015, 29, 1693–1699. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Jiang, X.; Singh, A.K.; Zeng, H.; Chen, C.; Lu, E.; Liu, W. Reduced litterfall and decomposition alters nutrient cycling following conversion of tropical natural forests to rubber plantations. Ecol. Indic. 2022, 138, 108819. [Google Scholar] [CrossRef]
- Kitayama, K.; Ushio, M.; Aiba, S.I.; Zotz, G. Temperature is a dominant driver of distinct annual seasonality of leaf litter production of equatorial tropical rain forests. J. Ecol. 2021, 109, 727–736. [Google Scholar] [CrossRef]
- Liu, C.; Westman, C.J.; Berg, B.; Kutsch, W.L.; Wang, G.Z.; Man, R.; Ilvesniemi, H. Variation in litterfall-climate relationships between coniferous and broadleaf forests in Eurasia. Glob. Ecol. Biogeogr. 2004, 13, 105–114. [Google Scholar] [CrossRef]
- Zhu, X.; Zou, X.; Lu, E.; Deng, Y.; Luo, Y.; Chen, H.; Liu, W. Litterfall biomass and nutrient cycling in karst and nearby non-karst forests in tropical China: A 10-year comparison. Sci. Total Environ. 2021, 758, 143619. [Google Scholar] [CrossRef] [PubMed]
- Kurniasari, S. Produktivitas Serasah dan Laju Dekomposisi di Kebun Campur Senjoyo Semarang Jawa Tengah serta Uji Laboratorium Anakan Mahoni (Swietenia macrophylla King) pada Beragam Dosis Kompos Yang Dicampur EM4. Master’s Thesis, IPB University, Bogor, Indonesia, 2009. [Google Scholar]
- Kotowska, M.M.; Leuschner, C.; Triadiati, T.; Hertel, D. Conversion of tropical lowland forest reduces nutrient return through litterfall, and alters nutrient use efficiency and seasonality of net primary production. Oecologia 2016, 180, 601–618. [Google Scholar] [CrossRef]
- Vigulu, V.; Blumfield, T.J.; Reverchon, F.; Bai, S.H.; Xu, Z. Nitrogen and carbon cycling associated with litterfall production in monoculture teak and mixed species teak and flueggea stands. J. Soils Sediments 2019, 19, 1672–1684. [Google Scholar] [CrossRef]
- Sari, R.R.; Rozendaal, D.; Saputra, D.D.; Hairiah, K.; Roshetko, J.M.; van Noordwijk, M. Balancing litterfall, and decomposition in cacao agroforestry systems. Plant Soil 2022, 473, 251–271. [Google Scholar] [CrossRef]
- Chairul; Muchktar, E.; Mansyurdin; Tesri, M.; Indra, G. Struktur kerapatan vegetasi dan estimasi kandungan karbon pada beberapa kondisi hutan di Pulau Siberut Sumatera Barat. Jurnal Metomorfosa 2016, 3, 15–22. [Google Scholar]
- Mekuo, C.S.; Ahmad, S.W.; Muhsin. Kandungan karbon serasah daun kuma (Palaquium luzoniense) di kawasan hutan lindung Nanga-Nanga Papalia Sulawesi Tenggara. Biowallaceae 2018, 5, 796–802. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, S.; Huang, Y. Comparisons of litterfall, litter decomposition and nutrient return in a monoculture Cunninghamia lanceolata and a mixed stand in southern China. For. Ecol. Manag. 2008, 255, 1210–1218. [Google Scholar] [CrossRef]
- Hermansah; Masunaga, T.; Wakatsuki, T.; Aflizar. Dynamic of litter production and its quality in relation to climate factors in a super wet tropical rain forest, West Sumatera Indonesia. Tropics 2002, 12, 115–130. [Google Scholar]
- Santos, F.M.; Balieiro; Fabiano, F.d.C.; Fontes, M.A.; Chaer, G.M. Understanding the enhanced litter decomposition of mixed-species plantations of Eucalyptus and Acacia mangium. Plant Soil 2018, 423, 141–155. [Google Scholar] [CrossRef]
- Saharjo, B.H.; Watanabe, H. Estimation of litter fall and seed production of Acacia mangium in a forest plantation in South Sumatra, Indonesia. For. Ecol. Manag. 2000, 130, 265–268. [Google Scholar] [CrossRef]
- Zhao, L.; Hou, R.; Fang, Q. Differences in interception storage capacities of undecomposed broad-leaf and needle-leaf litter under simulated rainfall conditions. For. Ecol. Manag. 2019, 446, 135–142. [Google Scholar] [CrossRef]
- Hairiah, K.; Sulistyani, H.; Suprayogo, D.; Purnomosidhi, P.; Widodo, R.H.; van Noordwijk, M. Litter layer residence time in forest and coffee agroforestry systems in Sumberjaya, West Lampung. For. Ecol. Manag. 2006, 224, 45–57. [Google Scholar] [CrossRef]
- Li, X.; Niu, J.; Xie, B. Study on hydrological functions of litter layers in North China. PLoS ONE 2013, 8, e70328. [Google Scholar] [CrossRef]
- Bulcock, H.H.; Jewitt, G.P.W. Field data collection and analysis of canopy and litter interception in commercial forest plantations in the KwaZulu-Natal Midlands, South Africa. Hydrol. Earth Syst. Sci. 2012, 16, 3717–3728. [Google Scholar] [CrossRef]
- Magliano, P.N.; Gimenez, R.; Houspanossian, J.; Paez, R.A.; Nosetto, M.D.; Fernandez, R.J.; Jobbagy, E.G. Litter is more effective than forest canopy reducing soil evaporation in Dry Chaco rangelands. Ecohydrology 2017, 10, e1879. [Google Scholar] [CrossRef]
- Sidle, R.C.; Gomi, T.; Usuga, J.C.L.; Jarihani, B. Hydrogeomorphic processes and scaling issues in the continuum from soil pedons to catchments. Earth-Sci. Rev. 2017, 175, 75–96. [Google Scholar] [CrossRef]
- Zhu, X.; Chen, C.; Wu, J.; Yang, J.; Zhang, W.; Zou, X.; Liu, W.; Jiang, X. Can intercrops improve soil water infiltrability and preferential flow in rubber-based agroforestry system? Soil Tillage Res. 2019, 191, 327–339. [Google Scholar] [CrossRef]
- Zhu, X.; Zhang, W.; Jiang, X.; Zakari, S.; Lu, E.; Singh, A.K.; Yang, B.; Liu, W. Conversion of primary tropical rainforest into rubber plantation degrades the hydrological functions of forest litter: Insights from experimental study. Catena 2021, 200, 105172. [Google Scholar] [CrossRef]
- Tesfay, F.; Tsehai, K.K.; Gebrekirstos, A.; Hadgu, K. Litterfall production and associated carbon and nitrogen flux along exclosure chronosequence at Kewet district, central lowland of Ethiopia. Environ. Syst. Res. 2020, 9, 11. [Google Scholar] [CrossRef]
- Singh, D.; Slik, J.; Jeon, Y.S.; Tomlinson, K.W.; Yang, X.; Wang, J.; Kerfahi, D.; Porazinska, D.L.; Adams, J.M. Tropical forest conversion to rubber plantation affects soil micro- and mesofaunal community & diversity. Sci. Rep. 2019, 9, 5893. [Google Scholar] [CrossRef]
- Zhou, J.; Lang, X.; Du, B.; Zhang, H.; Liu, H.; Zhang, Y.; Shang, L. Litterfall and nutrient return in moist evergreen broad-leaved primary forest and mixed subtropical secondary deciduous broad-leaved forest in China. Eur. J. Forest Res. 2016, 135, 77–86. [Google Scholar] [CrossRef]
- Zhang, Y.; Liao, X.; Wang, Z.; Wei, X.; Jia, X.; Shao, M. Synchronous sequestration of organic carbon and nitrogen in mineral soils after conversion agricultural land to forest. Agric. Ecosyst. Environ. 2020, 295, 106866. [Google Scholar] [CrossRef]
- Wang, Y.P.; Li, X.G.; Fu, T.; Wang, L.; Turner, N.C.; Siddique, K.H.M.; Li, F.M. Multi-site assessment of the effects of plastic-film mulch on the soil organic carbon balance in semiarid areas of China. Agric. For. Meteorol. 2016, 228–229, 42–51. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, S.; Zhong, M. Ecosystem carbon storage and soil organic carbon stability in pure and mixed stands of Cunninghamia lanceolata and Michelia macclurei. Plant Soil 2013, 370, 295–304. [Google Scholar] [CrossRef]
- Rożek, K.; Rola, K.; Błaszkowski, J.; Leski, T.; Zubek, S. How do monocultures of fourteen forest tree species affect arbuscular mycorrhizal fungi abundance and species richness and composition in soil? For. Ecol. Manag. 2020, 465, 118091. [Google Scholar] [CrossRef]
- Berg, B.; McClaugherty, C.A. Plant Litter Decomposition, 3rd ed.; Springer: Berlin, Germany, 2014. [Google Scholar] [CrossRef]
- Penner, J.F.; Frank, D.A. Litter decomposition in Yellowstone grasslands: The roles of large herbivores, litter quality, and climate. Ecosystems 2019, 22, 929–937. [Google Scholar] [CrossRef]
- Prescott, C. Decomposition and mineralization of nutrients from litter and humus. In Nutrient Acquisition by Plants; BassiriRad, H., Ed.; Springer: Berlin, Germany, 2005; Volume 181, pp. 15–41. [Google Scholar]
- Weil, R.C.; Brady, N.C. The Nature and Properties of Soils, 15th ed.; Pearson: Harlow, UK, 2017; pp. 550–552. [Google Scholar]
- Andrianto, F.; Bintoro, A.; Yuwono, S.B. Produksi dan laju dekomposisi seresah mangrove (Rhizophora so.) di desa Durian dan Batu Menyan Kecamatan padang Cermin Kabupaten Pesawaran. J. Sylva Lestari 2015, 3, 9–20. [Google Scholar] [CrossRef]
- Inagaki, M.; Kamo, K.; Miyamoto, K.; Titin, J.; Jamalung, L.; Lapongan, J.; Miura, S. Nitrogen and phosphorus retranslocation and N:P ratios of litterfall in three tropical plantations: Luxurious N and efficient P use by Acacia mangium. Plant Soil 2011, 341, 295–307. [Google Scholar] [CrossRef]
- Jex, C.N.; Pate, G.H.; Blyth, A.J.; Spencer, R.G.; Hernes, P.J.; Khan, S.J.; Baker, A. Lignin biogeochemistry: From modern processes to Quaternary archives. Quat. Sci. Rev. 2014, 87, 46–59. [Google Scholar] [CrossRef]
- Santos, F.M.; Chaer, G.M.; Diniz, A.R.; Balieiro, F.d.C. Nutrient cycling over five years of mixed-species plantations of Eucalyptus and Acacia on a sandy tropical soil. For. Ecol. Manag. 2017, 384, 110–121. [Google Scholar] [CrossRef]
- Berg, B.; Ekbohm, G. Litter mass-loss rates and decomposition patterns in some needle and leaf litter types. Long-term decomposition in a Scots pine forest. VII. Can. J. Bot. 1991, 69, 1449–1456. [Google Scholar] [CrossRef]
- Carreiro, M.M.; Sinsabaugh, R.L.; Repert, D.A.; Parkhurst, D.F. Microbial enzyme shifts explain litter decay responses to simulated nitrogen deposition. Ecology 2000, 81, 2359–2365. [Google Scholar] [CrossRef]
- Bachega, L.R.; Bouillet, J.P.; Piccolo, M.d.C.; Saint-Andre, L.; Bouvet, J.M.; Nouvellon, Y.; Gonçalves, J.L.d.M.; Robin, A.; Laclau, J.P. Decomposition of Eucalyptus grandis and Acacia mangium leaves and fine roots in tropical conditions did not meet the Home Field Advantage hypothesis. For. Ecol. Manag. 2016, 359, 33–43. [Google Scholar] [CrossRef]
- Rachid, C.T.; Balieiro, F.C.; Fonseca, E.S.; Peixoto, R.S.; Chaer, G.M.; Tiedje, J.M.; Rosado, A.S. Intercropped silviculture systems, a key to achieving soil fungal community management in eucalyptus plantations. PLoS ONE 2015, 10, e0118515. [Google Scholar] [CrossRef]
- Junaedi, A.; Mindawati, N.; Pribadi, A.; Hardiwinoto, S. Leaf litter decomposition and nutrient release of three native tree species in a drained tropical peatland in Riau, Indonesia. Hayati J. Biosci. 2022, 29, 182–191. [Google Scholar] [CrossRef]
- Aprianis, Y. Produksi dan laju dekomposisi serasah Acacia crassicarpa A.Cunn. di PT. Arara Abadi. Tekno Hutan Tanaman 2011, 4, 41–47. [Google Scholar]
- Silvianingsih, Y.A.; van Noordwijk, M.; Suprayogo, D.; Hairiah, K. Litter decomposition in wet rubber and fruit agroforests: Below the threshold for tropical peat formation. Soil Syst. 2022, 6, 19. [Google Scholar] [CrossRef]
- Bones, A.M.; Rossiter, J.T. The enzymic and chemically induced decomposition of glucosinolates. Phytochemistry 2006, 67, 1053–1067. [Google Scholar] [CrossRef] [PubMed]
- Clay, N.K.; Adio, A.M.; Denoux, C.; Jander, G.; Ausubel, F.M. Glucosinolate metabolites required for an Arabidopsis innate immune response. Science 2009, 323, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Gilliam, F.S. The ecological significance of the herbaceous layer in temperate forest ecosystems. Bioscience 2007, 57, 845–858. [Google Scholar] [CrossRef]
- Semchenko, M.; Saar, S.; Lepik, A. Plant root exudates mediate neighbour recognition and trigger complex behavioural changes. New Phytol. 2014, 204, 631–637. [Google Scholar] [CrossRef]
- Balser, T.C.; Firestone, M.K. Linking microbial community composition and soil processes in a California annual grassland and mixed-conifer forest. Biogeochemistry 2005, 73, 395–415. [Google Scholar] [CrossRef]
- Ushio, M.; Kitayama, K.; Balser, T.C. Tree species effects on soil enzyme activities through effects on soil physicochemical and microbial properties in a tropical montane forest on Mt. Kinabalu, Borneo. Pedobiologia 2010, 53, 227–233. [Google Scholar] [CrossRef]
- Gajda, A.; Martyniuk, S. Microbial biomass C and N and activity of enzymes in soil under winter wheat grown in different crop management systems. Pol. J. Environ. Stud. 2005, 14, 159–163. [Google Scholar]
- Oostra, S.; Majdi, H.; Olsson, M. Impact of tree species on soil carbon stocks and soil acidity in southern Sweden. Scand. For. Res. 2006, 21, 364–371. [Google Scholar] [CrossRef]
- Hobbie, S.E.; Ogdahl, M.; Chorover, J.; Chadwick, O.A.; Oleksyn, J.; Zytkowiak, R.; Reich, P.B. Tree species effects on soil organic matter dynamics: The role of soil cation composition. Ecosystems 2007, 10, 999–1018. [Google Scholar] [CrossRef]
- Rożek, K.; Rola, K.; Błaszkowski, J.; Zubek, S. Associations of root-inhabiting fungi with herbaceous plant species of temperate forests in relation to soil chemical properties. Sci. Total Environ. 2019, 649, 1573–1579. [Google Scholar] [CrossRef]
- Williams, A.; Langridge, H.; Straathof, A.L.; Muhamadali, H.; Hollywood, K.; Goodacre, R.; de Vries, F.T. Root functional traits explain root exudation rate and composition across a range of grassland species. J. Ecol. 2022, 110, 21–33. [Google Scholar] [CrossRef]
- Wilson, G.W.T.; Rice, C.W.; Rillig, M.C.; Springer, A.; Hartnett, D.C. Soil aggregation and carbon sequestrationare tightly correlated with the abundance of arbuscular mycorrhizal fungi: Results from long-term fieldexperiments. Ecol. Lett. 2009, 12, 452–461. [Google Scholar] [CrossRef]
- Soka, G.; Ritchie, M.E. Arbuscular mycorrhizal symbiosis, ecosystem processes and environmental changes in tropical soils. Appl. Ecol. Environ. Res. 2015, 13, 229–245. [Google Scholar] [CrossRef]
- Augé, R.M. Arbuscular mycorrhizae and soil/plant water relations. Can. J. Soil Sci. 2004, 84, 373–381. [Google Scholar] [CrossRef]
- Saxton, K.E.; Rawls, W.J. Soil water characteristic estimates by texture and organic matter for hydrologic solutions. Soil Sci. Soc. Am. J. 2006, 70, 1569–1578. [Google Scholar] [CrossRef]
- Policelli, N.; Horton, T.R.; Hudon, A.T.; Patterson, T.; Bhatnagar, J.M. Back to roots: The role of ectomycorrhizal fungi in boreal and temperate forest restoration. Front. For. Glob. Chang. 2020, 3, 97. [Google Scholar] [CrossRef]
- Smith, S.E.; Jakobsen, I.; Grønlund, M.; Smith, F.A. Roles of arbuscular mycorrhizas in plant phosphorus nutrition: Interactions between pathways of phosphorus uptake in arbuscular mycorrhizal roots have important implications for understanding and manipulating plant phosphorus acquisition. Plant Physiol. 2011, 156, 1050–1057. [Google Scholar] [CrossRef]
- Lin, C.; Wang, Y.; Liu, M.; Li, Q.; Xiao, W.; Song, X. Effects of nitrogen deposition and phosphorus addition on arbuscular mycorrhizal fungi of Chinese fir (Cunninghamia lanceolata). Sci. Rep. 2020, 10, 12260. [Google Scholar] [CrossRef]
- Hawkins, B.J.; Jones, M.D.; Kranabetter, J.M. Ectomycorrhizae and tree seedling nitrogen nutrition in forest restoration. New For. 2015, 46, 747–771. [Google Scholar] [CrossRef]
- McGuire, K.L.; Treseder, K.K. Microbial communities and their relevance for ecosystem models: Decomposition as a case study. Soil Biol. Biochem. 2010, 42, 529–535. [Google Scholar] [CrossRef]
- Burke, D.J.; Weintraub, M.N.; Hewins, C.R.; Kalisz, S. Relationship between soil enzyme activities, nutrient cycling and soil fungal communities in a northern hardwood forest. Soil Biol. Biochem. 2011, 43, 795–803. [Google Scholar] [CrossRef]
- Chavarría, D.N.; Verdenelli, R.A.; Serri, D.L.; Restovich, S.B.; Andriulo, A.E.; Meriles, J.M.; Vargas-Gil, S. Effect of cover crops on microbial community structure and related enzyme activities and macronutrient availability. Eur. J. Soil Biol. 2016, 76, 74–82. [Google Scholar] [CrossRef]
- Baker, K.; Cook, R.J. Biological Control of Plant Pathogens; WH Freeman and Company: San Fransisco, CA, USA, 1974. [Google Scholar]
- Chandrashekara, C.; Bhatt, J.C.; Kumar, R.; Chandrashekara, K.N. Supressive Soils in Plant Disease Management. In Eco-Friendly Innovative Approaches in Plant Disease Management; Singh, V.K., Singh, Y., Singh, A., Eds.; International Book Distributors: Uttarakhand, India, 2012; pp. 241–256. [Google Scholar]
- Sullivan, P.; Sustainable Management of Soil-Borne Plant Diseases. ATTRA National Sustainable Agriculture Information Service 2004. Available online: https://www.attra.ncat.org (accessed on 18 August 2022).
- Weller, D.M.; Raaijmakers, J.M.; Gardener, B.B.M.; Thomashow, L.S. Microbial populations responsible for specific soil suppressiveness to plant Pathogens. Ann. Rev. Phytopathol. 2002, 40, 309–348. [Google Scholar] [CrossRef] [PubMed]
- Garbeva, P.; Postma, J.; van Veen, J.A.; van Elsas, J.D. Effect of above-ground plant species on soil microbial community structure and its impact on soil microbial community structure and its impact on suppression of Rhizoctonia solani AG3. Environ. Microbiol. 2006, 8, 233–246. [Google Scholar] [CrossRef]
- Pervaiz, Z.H.; Iqbal, J.; Zhang, Q.; Chen, D.; Wei, H.; Saleem, M. Continuous cropping alters multiple biotic and abiotic indicators of soil health. Soil Syst. 2020, 4, 59. [Google Scholar] [CrossRef]
- Barry, K.M.; Irianto, R.S.B.; Santoso, E.; Turjaman, M.; Widyati, E.; Sitepu, I.; Mohammed, C.L. Incidence of heartrot in harvest-age Acacia mangium in Indonesia, using a rapid survey method. For. Ecol. Manag. 2004, 190, 273–280. [Google Scholar] [CrossRef][Green Version]
- Francis, A.; Beadle, C.; Puspitasari, D.; Irianto, R.; Agustini, L.; Rimbawanto, A.; Gafur, A.; Hardiyanto, E.; Hidyati, N.; Tjahjono, B.; et al. Disease progression in plantations of Acacia mangium affected by red root rot (Ganoderma philippii). For. Pathol. 2014, 44, 447–459. [Google Scholar] [CrossRef]
- Irianto, R.S.B.; Barry, K.; Hidayati, N.; Ito, S.; Fiani, A.; Rimbawanto, A.; Mohammed, C. Incidence and spatial analysis of root rot of Acacia mangium in Indonesia. J. Trop. For. Sci. 2006, 18, 157–165. [Google Scholar]
- Eyles, A.; Beadle, C.; Barry, K.; Francis, A.; Glen, M.; Mohammed, C. Management of fungal root-rot pathogens in tropical Acacia mangium plantations. For. Pathol. 2008, 38, 332–355. [Google Scholar] [CrossRef]
- Tarigan, M.; Roux, J.; Van Wyx, M.; Tjahjono, B.; Wingfield, M.J. A new wilt and die-back disease of Acacia mangium associated with Ceratocystis manginecans and C. acaciivora sp. nov. in Indonesia. S. Afr. J. Bot. 2011, 7, 292–304. [Google Scholar] [CrossRef]
- Old, K.M.; Wingfield, M.J.; Yuan, Z.Q. A Manual of Diseases of Eucalypts in South-East Asia; Center for International Forestry Research: Jakarta, Indonesia, 2003; pp. 60–66. [Google Scholar]
- Siregar, B.A.; Giyanto; Hidayat, S.H.; Siregar, I.Z.; Tjahjono, B. Diversity of Ralstonia pseudosolanacearum, the causal agent of bacterial wilt on Eucalyptus pellita in Indonesia. Biodiversitas 2021, 22, 2538–2546. [Google Scholar] [CrossRef]
- Nambiar, E.K.S.; Harwood, C.E.; Mendham, D.S. Paths to sustainable wood supply to the pulp and paper industry in Indonesia after diseases have forced a change of species from acacia to eucalypts. Aust. For. 2018, 81, 148–161. [Google Scholar] [CrossRef]
- Wu, H.; Xu, J.; Wang, J.; Qin, X.; Wu, L.; Li, Z.; Lin, S.; Lin, W.; Zhu, Q.; Khan, M.U.; et al. Insights into the mechanism of proliferation on the special microbes mediated by phenolic acids in the Radix pseudostellariae rhizosphere under continuous monoculture regimes. Front Plant Sci. 2017, 8, 659. [Google Scholar] [CrossRef] [PubMed]
- Phitiviraj, B.; Paschke, M.W.; Vivanco, J.M. Root Communication: The role of root exudates. In Encyclopedia of Plant and Crop Science; Goodman, R.M., Ed.; Marcel Dekker, Inc.: New York, NY, USA, 2006; pp. 1–5. [Google Scholar] [CrossRef]
- Li, X.G.; Zhang, T.L.; Wang, X.X.; Hua, K.; Zhao, L.; Han, Z.M. The composition of root exudates from two different resistant peanut cultivars and their effects on the growth of soil-borne pathogen. Int. J. Biol. Sci. 2013, 9, 164–173. [Google Scholar] [CrossRef] [PubMed]
- Sontowski, R.; Gorringe, N.J.; Pencs, S.; Schedl, A.; Touw, A.J.; van Dam, N.M. Same Difference? Low and high glucosinolate Brassica rapa varieties show similar responses upon feeding by two specialist root herbivores. Front Plant Sci. 2019, 10, 1451. [Google Scholar] [CrossRef]
- Fahey, J.W.; Zalcmann, A.T.; Talalay, P. The chemical diversity and distribution of glucosinolates and isothiocyanates among plants. Phytochemistry 2001, 56, 5–51. [Google Scholar] [CrossRef]
- Agerbirk, N.; De Vos, M.; Kim, J.H.; Jander, G. Indole glucosinolate breakdown and its biological effects. Phytochem. Rev. 2009, 8, 101. [Google Scholar] [CrossRef]
- Redovnikovic, I.R.; Glivetic, T.; Delonga, K.; Furac, J.V. Glucosinolates and their potential role in plant. Period. Biol. 2008, 110, 297–309. [Google Scholar]
- Jurgensen, M.; Harvey, A.; Graham, R.; Page-Dumroese, D.S. Review article: Impacts of timber harvesting on soil organic matter, nitrogen, productivity, and health of inland norwest forests. For. Sci. 1997, 43, 121–144. [Google Scholar]
- Grigal, D.F. Effects of extensive forest management on soil productivity. For. Ecol. Manag. 2000, 138, 167–185. [Google Scholar] [CrossRef]
- Turner, J.; Lambert, M.J. Effects of forest harvesting nutrient removals on soil nutrient reserves. Oecologia 1986, 70, 140–148. [Google Scholar] [CrossRef] [PubMed]
- Ballard, T.M. Impacts of forest management on northern forest soils. For. Ecol. Manag. 2000, 133, 37–42. [Google Scholar] [CrossRef]
- Weetman, G.F.; Webber, B.D. The influence of wood harvesting on the nutrient status of two spruce stands. Can. J. For. Res. 1972, 2, 351–369. [Google Scholar] [CrossRef]
- Foster, N.W.; Morrison, I.K. Distribution and cycling of nutrients in natural Pinus banksiana ecosystem. Ecology 1976, 57, 110–120. [Google Scholar] [CrossRef]
- Hernesmaa, A.; Björkölf, K.; Kiikkilä, O.; Fritze, H.; Haahtela, K.; Romantschuk, M. Structure and function of microbial communities in the rhizosphere of Scot pine after tree-felling. Soil Biol. Biochem. 2005, 37, 777–785. [Google Scholar] [CrossRef]
- Hannula, S.E.; Heinen, R.; Huberty, M.; Steinauer, K.; De Long, J.R.; Jongen, R.; Bezemer, T.M. Persistence of plant-mediated microbial soil legacy effects in soil and inside roots. Nat. Commun. 2021, 12, 5686. [Google Scholar] [CrossRef]
- Anderson, M.J.; Crist, T.O.; Chase, J.M.; Velled, M.; Inouye, B.D.; Freestone, A.L.; Sanders, N.J.; Cornell, H.V.; Comita, L.Z.; Davies, K.F.; et al. Navigating the multiple meaning of Beta diversity: A roadmap for the practising ecologist. Ecol. Lett. 2011, 14, 19–28. [Google Scholar] [CrossRef]
- Fox, A.; Lüscher, A.; Widmer, F. Plant species identity drives soil microbial community structures that persist under a following crop. Ecol Evol. 2020, 10, 8652–8668. [Google Scholar] [CrossRef]
- Hairiah, K.; Fiantis, D.; Utami, S.R.; Nurbaity, A.; Utami, S.N.H.; Ginting, F.I.; Ariyanto, D.P.; Nurcholis, M.; Minasny, B.; van Noordwijk, M. Hundred fifty years of soil security research in Indonesia: Shifting topics, modes of research and gender balance. Soil Secur. 2022, 6, 100049. [Google Scholar] [CrossRef]
- van Noordwijk, M.; Cerri, C.; Woomer, P.L.; Nugroho, K.; Bernoux, M. Soil carbon dynamics in the humid tropical forest zone. Geoderma 1997, 79, 187–225. [Google Scholar] [CrossRef]
- Hairiah, K.; van Noordwijk, M.; Sari, R.R.; Saputra, D.D.; Suprayogo, D.; Kurniawan, S.; Prayogo, C.; Gusli, S. Soil carbon stocks in Indonesian (agro) forest transitions: Compaction conceals lower carbon concentrations in standard accounting. Agric. Ecosyst. Environ. 2020, 294, 106879. [Google Scholar] [CrossRef]
| Host | Disease | Pathogen | Reference |
|---|---|---|---|
| Acacia mangium | Root rot, heart rot | Ganoderma philippii | [175,176,177] |
| Brown root rot | Phellinus spp. | [178] | |
| Wilt disease | Ceratosystis spp. | [179] | |
| Eucalyptus pellita | Stem and branch cancer | Botryosphaeria spp. | [180] |
| Wilt disease | Ralstonia pseudosolanacearum | [181] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Widyati, E.; Nuroniah, H.S.; Tata, H.L.; Mindawati, N.; Lisnawati, Y.; Darwo; Abdulah, L.; Lelana, N.E.; Mawazin; Octavia, D.; et al. Soil Degradation Due to Conversion from Natural to Plantation Forests in Indonesia. Forests 2022, 13, 1913. https://doi.org/10.3390/f13111913
Widyati E, Nuroniah HS, Tata HL, Mindawati N, Lisnawati Y, Darwo, Abdulah L, Lelana NE, Mawazin, Octavia D, et al. Soil Degradation Due to Conversion from Natural to Plantation Forests in Indonesia. Forests. 2022; 13(11):1913. https://doi.org/10.3390/f13111913
Chicago/Turabian StyleWidyati, Enny, Hani Sitti Nuroniah, Hesti Lestari Tata, Nina Mindawati, Yunita Lisnawati, Darwo, Lutfy Abdulah, Neo Endra Lelana, Mawazin, Dona Octavia, and et al. 2022. "Soil Degradation Due to Conversion from Natural to Plantation Forests in Indonesia" Forests 13, no. 11: 1913. https://doi.org/10.3390/f13111913
APA StyleWidyati, E., Nuroniah, H. S., Tata, H. L., Mindawati, N., Lisnawati, Y., Darwo, Abdulah, L., Lelana, N. E., Mawazin, Octavia, D., Prameswari, D., Rachmat, H. H., Sutiyono, Darwiati, W., Wardani, M., Kalima, T., Yulianti, & van Noordwijk, M. (2022). Soil Degradation Due to Conversion from Natural to Plantation Forests in Indonesia. Forests, 13(11), 1913. https://doi.org/10.3390/f13111913









