Abstract
The availability of soil-stored seed determines initial plant functional types in post-fire landscapes. We evaluated the post-fire regeneration of Nothofagus pumilio forests, in Patagonia, Argentina, analyzing the soil seed bank (SSB) and the above-ground vegetation (AV). At three sites: La Colisión, Río Turbio and Monte Zeballos, burned in 2008, 1980 and 1941, respectively, we sampled the SSB and AV in two transects from the edge of the remaining forest, up to 90 m within the burned area, and recorded the emergence (198 soil samples) and presence of vascular species. To determine the effect of the distance to the remnant forest on the germinable seed bank, we performed simple linear regression analysis through the use of linear mixed-effect models, and we analyzed the similarity between the composition of SSB and AV with PERMANOVA. The emergence of plant growth forms had different patterns in relation to the distance from the forest in the three sites, which might be associated with the time of fire occurrence, and specific characteristics of each site. The emergence of N. pumilio was registered at more than one distance at the recent burning site. Herbs constituted the main source of cover with 69% of the composition, and native/endemic species represented 71%. This study contributes to the understanding of the relationship between the seed bank and standing vegetation and a better understanding of the resilience of post-fire N. pumilio forests. Our findings suggest that from 15–20 m from the edge, the SSB would be insufficient to ensure the spontaneous recovery of the forest, making active restoration necessary in order to tend to a recovery of the structure and functionality of the original community.
1. Introduction
The seed bank present in the soil is a relevant factor in the dynamics and structure of plant communities in the recovery process after a disturbance [1]. Fire represents one of the main recurrent disturbances that affect temperate forests depending on severity and frequency, as well as the adaptations of the affected species [2,3,4]. The term fire severity provides a description of how fire intensity affects ecosystems, particularly after wildfires with variable responses depending on the type of ecosystem [5]. The fire had a severe but differential impact on the soil seed bank: species with transient seed reserves accumulated on the soil surface are usually eliminated from the site after a fire event; whereas, species with persistent buried seed reserves tended to remain in the soil [6]. If the fire severity is moderate, the ecosystem maintains part of its ecological integrity, resilience and the ability to recover through natural regeneration. On the other hand, if the severity is high, several ecological processes are affected and the recovery of the ecosystem is delayed or disrupted [7]. Additionally, climatic conditions and the presence of other disturbances, such as herbivory, can negatively interact with the ecological conditions of the ecosystem [8].
In Patagonia Argentina, Nothofagus pumilio (Poepp. & Endl.) Krasser (lenga) is the most important tree species in terms of distribution area (1.2 million ha of pure forests) and timber production from 350,000 ha corresponding to productive forests [9]. Its distribution ranges from 35°35’ S in the province of Neuquén to 55°03’ S in the province of Tierra del Fuego. It occupies the altitude limit of vegetation (treeline) from 2000 m a.s.l. in the northern areas and the sea level in the extreme south of the Island of Tierra del Fuego [2,3,10]. In the western sector of the provinces of Chubut and Santa Cruz, there are extensive areas of N. pumilio forest degraded by fire and grazing [11,12]. Regeneration of N. pumilio occurs exclusively by seeds [13], which form a transient seed bank [14,15,16]. Seed production is very irregular between years, with high seed production events (masting year) and viability [10,17]. Seed viability decreases significantly from seed dispersal time in late summer and early fall, until germination in mid-spring [15,16,18]. Moreover, if the environmental conditions are not favorable after germination, a large number of seedlings die [18].
Although fire is one of the most important disturbance factors in N. pumilio forests [3], which normally occurs during extreme drought years affecting hundreds of hectares, the species does not present adaptive mechanisms for rapid recovery after the occurrence of fires. The seedling establishment after wildfires relies exclusively on their ability to colonize burned areas from unburned areas depending on seed production, seed dispersal distance, seed germination rate in the post-fire environment and distance from the seed source [19].
Fire affects, directly and indirectly, the regeneration processes of this species, where the resulting system after disturbance may differ substantially from the original forest structure and composition [20,21]. In general, burned forests of N. pumilio in northwestern Patagonia show slow and low post-fire regeneration, characterized by a successional process dominated in its early stages by opportunistic exotic herbaceous species [22]. In areas with grazing, the “empastado” process usually occurs, which consists of the formation of a layer of herbaceous vegetation with a dense superficial root system, that avoids the establishment of regeneration of N. pumilio [15]. Thus, after intense fires and depending on the type of forest considered, transformations from forest to grassland (steppe), or open forest can be expected [20,23]. To improve the knowledge about the effects of fire on the ecosystem components and its resilience is essential to restore forests after this type of disturbance [24,25].
The objectives of this work were to determine the regeneration capacity of N. pumilio forests after the occurrence of fire events of different ages in the center–south region of its continental distribution in Argentina, and to define the relationship between post-fire establishments with distance to the seed source. The germinable seed bank of N. pumilio and associated species were analyzed across a spatial gradient from the edge of the remaining unburned forest to the burned area, and the composition of the soil seed bank and the above-ground vegetation present in the burned area. We postulated as a hypothesis that, in burned forests of N. pumilio, the regeneration potential of the dominant tree species is insufficient to ensure forest re-establishment.
2. Materials and Methods
2.1. Study Area
We carried out the study in three N. pumilio sites distributed in a latitudinal gradient of 8° in Patagonia, Argentina. Each site consisted of a burned N. pumilio forest and the adjacent unburned remnant forest (Figure 1). From north to south, the first site La Colisión is located near Esquel (LC: 42°56’ S; 71°30’ W) Chubut province, the second site Monte Zeballos in the Los Antiguos (MZ: 46°49’ S; 71°54’ W) province of Santa Cruz and the third site Río Turbio (RT: 51°29′ S; 72°19′ W) located also in Santa Cruz. In LC the fire occurred in 2008, in MZ in 1941 and in RT in the early 1980′s. Regarding the fire severity, in LC, we determined a very high severity, category 5 in the classification of Mutch and Swetman (1995) [26], characterized by most understorey and >50% of canopy trees killed. In MZ and RT fire severity was not determined with precision due to the time elapsed since fire events. However, considering the state of the burned forest adjacent to the remaining forest, both sites are likely to have suffered high-severity events.

Figure 1.
Images of the remnant forest and burned area in (a) La Colisión, (b) Monte Zeballos and (c) Río Turbio, burned in 2008, 1941 and 1980, respectively. The white circles indicate the sampling zones.
In addition to the difference in time elapsed since the fire event, sites presented differences in other parameters such as altitude, rainfall, soils and livestock pressure. La Colisión site is located at 1050 m a.s.l. and has an average annual rainfall (AAR) of ~1000 mm, MZ is located at 880 m asl and it has an AAR ~300 m while RT is located at 575 m a.s.l. and the AAR is ~650 mm [27]. The average annual temperature is 6.8 °C in LC, 4.8 °C in MZ and 3.8 °C in RT, while the average temperatures of the warmest month (January) are 17.0, 14.3 and 11.1 °C, respectively [27]. All sites are located at the eastern limit of N. pumilio distribution, representing dry environmental conditions [28]. The soil physical–chemical properties in LC and MZ are characteristic of deposits of volcanic ash, allophane and imogolite, respectively, while RT presents halloysite. The fertility of the remaining forests is high in LC, intermediate in RT and low in MZ. In LC, more than one year after the occurrence of fire, soil conductivity, carbon, nitrogen, phosphorus, calcium and potassium were lower compared to the unburned forest, while the pH, magnesium and sulfur were higher in the burned sector. In RT and MZ, carbon and nitrogen in the burned sectors were similar to that of the unburned forest [25]. After the fires, in the LC site livestock was removed, while animal grazing occurred in MZ for sheep farming and in RT for cattle rearing. At the edge of the unburned forest in RT, a belt of regeneration (40 m wide) of N. pumilio of two m high was established after the fire.
2.2. Seed Viability
Given the inter-annual variability in the production and quality of N. pumilio seeds, in the fall of 2009, we collected seeds in unburned forest areas in each sampling site. We placed nets under the tree canopy during the period of massive seed dispersal [18].
2.3. Experimental Design and Sampling
At each site we established two sampling transects 50 m apart from each other, from the edge of the remnant unburned forest towards the burned area following the direction of the prevailing winds. In each transect, we established circular plots of 1 m2, every 15 m from the forest edge up to 90 m (0, 15, 30, 45, 60, 75 and 90 m) (Figure 2). In each plot, we sampled both fractions: above-ground vegetation (AV) and soil seed bank (SSB).
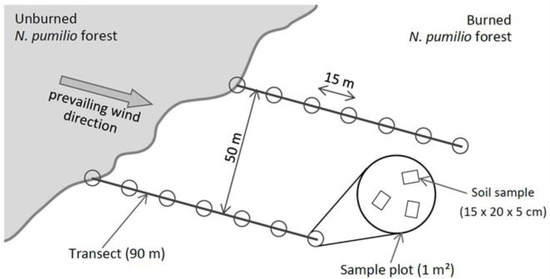
Figure 2.
Diagram of sample design: two transects 50 m apart from each other, from the edge of the unburned forest towards the burned area and in the direction of prevailing winds, each with seven plots of 1 m2, every 15 m from the edge of the forest up to 90 m.
To evaluate above-ground vegetation, we sampled the total vascular plant species composition in each plot in March 2009. Species were herborized and identified in the laboratory according to Correa (1969–1988) [29] and Zuloaga et al. (2013) [30]. We classified species by growth-form (grass, herbs, dwarf shrub-shrub and trees), and by native/endemic and adventive/exotic according to Zuloaga et al. (2013) [30].
To evaluate the soil seed bank, we sampled in each sample plot three rectangular soil samples of 15 × 20 cm at 5 cm depth in autumn after seed dispersal (April 2009) and in spring after winter seed stratification (September 2009). In the autumn sampling in RT, samples were collected up to a distance of 60 m from the remaining forest, and MZ sampling could not be carried out due to site inaccessibility. In total, 198 soil samples were collected. The set of autumn samples (n = 72) was stored in the refrigerator at 4 °C for 4 months and the set collected in spring was stored for 1 month to homogenize the stratification period from the three sites.
2.4. Seed Bank
We used the incubation method [31] to evaluate the seed bank. For this, we placed soil samples in trays in a growth chamber, sprinkled them with water and covered them with polyethylene bags to maintain humidity according to ISTA (1999) [32]. The trays were kept for seven weeks in the chamber at a temperature of 20 °C with 16 h of light and 8 h of darkness. The response variable was the emergence of seedlings of N. pumilio and other species (N° of emerged seedlings sensu Harper (1977) [33] during the study period). We classified the identified species by growth-form as grass, herbs, subshrubs-shrubs and trees, and by native/endemic and adventive/exotic [30].
2.5. Data Analysis
2.5.1. Nothofagus pumilio Germination Capacity
To determine the germination capacity of the N. pumilio seeds, they were subjected to a pre-germinative treatment of cold-humid stratification for 60 days. For this treatment, the seeds were hydrated for 48 h, drained and placed in a refrigerator (3–5 °C). Subsequently, 400 seeds from LC, 400 from RT and 200 from MZ were sown. The seeds of LC and RT were distributed in 4 plastic trays in groups of 100 seeds each, while those of MZ were distributed in groups of 50 seeds [32]. The trays were prepared with 250 g of sterilized volcanic sand, previously moistened with 150 mL of distilled water. All trays were kept in a growth chamber at 20 °C with 16 h of light and 8 h of darkness for 7 weeks. The number of germinated seedlings was recorded. To evaluate the non-germinated seeds, at the end of the experiment, we longitudinally sectioned each one with a sharp blade, examining the state of their interior using a Wild M3Z Heerbrugg Switzerland loupe. We classified seeds as empty, fresh and dead [32]. The results were expressed as the percentage ± error standard of germination capacity (germinated plus fresh seeds), empty and dead seeds.
2.5.2. Soil Seed Bank Response
To determine the effect of the distance to the remnant forest on the germinable seed bank, we performed simple linear regressions through the use of linear mixed-effect models (function lmer, package lme4) [34]. We analyzed data independently for each site because of the differences in the time since fire disturbance events. The distance to the limit of the remnant forest (0, 15, 30, 45, 60, 75, 90 m) was treated as a fixed effect. The transect (with two levels) and the soil sample plots (with three levels) nested in the transect, were treated as random effects. The response variables analyzed were the number of emerged seedlings of N. pumilio, dwarf shrub-shrub, herbaceous and grass species. Two values of R2 were obtained [35]: the marginal R2, which refers to the amount of variability explained by the fixed effects, and the conditional R2, which refers to the amount of variability explained by the complete model, including the random effects (r.squaredGLMM function, MuMIn package) [36]. We transformed the response variables to logarithmic or square root, to meet normality and homoscedasticity assumptions, which were evaluated through the Shapiro–Wilk test (shapiro.test function, stats package) [37] and through residuals analysis, respectively. For those variables with few emerged seedlings, we did not adjust a model and only present the observed values. To carry out these analyses we used R software [37].
2.5.3. Comparison between Above-Ground Vegetation and Soil Seed Bank Composition
To compare the vegetation composition and seed bank fractions in the three sites, we performed a permutational multivariate analysis of variance (PERMANOVA) using species presence data at plot level with the binary form of Bray’s distance, which is equal to Sorensen’s (function adonis, package Vegan) [38]. For the seed bank, we only considered the identified the emerged species. Then, we displayed plant community data graphically in ordination space using a non-metric multidimensional scaling (NMDS) based on species presence with Sorensen’s distance (function metaMDS, package Vegan). These analyses were performed using the software R [37].
3. Results
3.1. Nothofagus pumilio Germination Capacity
The germination capacity of N. pumilio seeds varied between 3.5 ± 0.3% in LC, 10.0 ± 1.7% in MZ and 15.8 ± 1.2% in RT. La Colisión showed a higher percentage of empty seeds (87.5 ± 1.0%) than MZ and RT (46.0 ± 2.2% y 42.8 ± 1.3%, respectively). Contrary, MZ and RT showed a higher percentage of dead seeds (44.0 ± 1.2% and 41.5 ± 2.1%) than LC (9.0 ± 1.1%).
3.2. Emergence from the Soil Seed Bank
3.2.1. Emergence of Tree Species
Nothofagus pumilio seeds emerged in the autumn and spring samples in LC, in the spring samples in MZ, and only in the autumn samples in RT (Figure 3). In no site or season, the emergence of N. pumilio was associated with the distance to the remaining forest, and the emergence was almost zero from 30 m distances in all sites. In LC, 50 seedlings/m2 on average of N. pumilio emerged at the edge of forests in autumn. From a distance of 30 m and 45 m, around 10 seedlings/m2 on average were recorded in the same site in autumn and spring. In MZ and RT, emergence was recorded only in one soil sample (Figure 3).
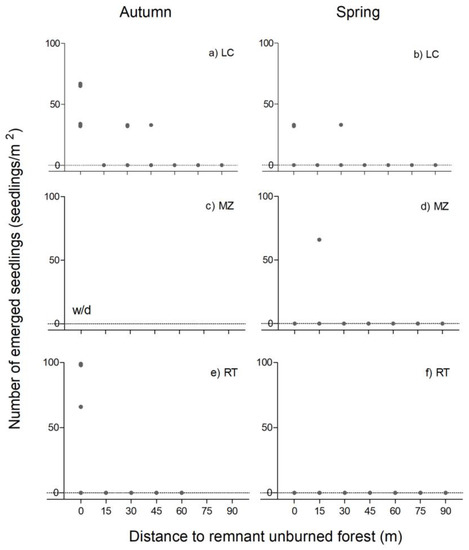
Figure 3.
Emergence of N. pumilio seeds in La Colisión (LC, (a,b)), Monte Zeballos (MZ, (c,d)), and Río Turbio (RT, (e,f)), as a function of the distance from the edge of the remnant forest to the burned area, in autumn (left) and spring (right). Dots represent the observed emergence in each sample soil. w/d: without data.
3.2.2. Emergence of Dwarf Shrub-Shrub Species
The emergence of dwarf shrub-shrub species presented different patterns at the three sites independently of the distance to the remnant forest. In LC, there was a tendency for greater emergence in the proximity of the remnant forest in autumn (Figure 4a). In spring, the seed bank was represented by 40 emerged seedlings/m2 from the edge of the forest to 90 m (Figure 4b). In MZ, in spring, there was a trend towards a greater abundance of dwarf shrub-shrub species at greater distances from the remaining forest, which, in some cases, exceeded the 100 seedlings/m2 (Figure 4d). In RT, there was less emergence than in LC and MZ (Figure 4e,f).
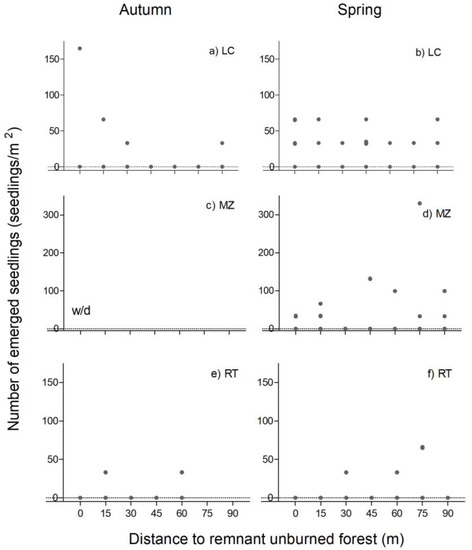
Figure 4.
Emergence of dwarf shrub-shrub species in La Colisión (LC, (a,b)), Monte Zeballos (MZ, (c,d)) and Río Turbio (RT, (e,f)), as a function of the distance from the edge of the remnant forest to the area burned in autumn (left) and spring (right). Dot represents the observed emergence in each sample soil. w/d: without data.
3.2.3. Emergence of Herbaceous and Grass Species
The emergence of the herbaceous species also showed a different pattern in the three sites, and differences associated with distance were observed. At LC, a marked decrease in emergence was found in the first 15 m, with a similar pattern in autumn and spring, although only in autumn was the adjustment significant and the distance to the edge explains 67% of the variability (Figure 5a). The seed bank varied for more than 2000 emerged seeds/m2 in autumn and 20,000 in spring (Figure 5a,b) at the edge of the burned forest, to values close to zero at 30 m. MZ-only samples from the edge of the remnant forest did not present the emergence of herbaceous species. From 15 m of the remaining forest, a bimodal pattern was observed in spring with two peaks at 30 and 90 m from the edge, with an average of 5000 and 3800 seedlings/m2 (Figure 5d). In RT, on the other hand, the pattern observed was contrary to that of LC and in both seasons emergence was associated with distance and this factor explains 66% of the variability in autumn and 52% in spring (Figure 5e,f). No emergence was recorded at the edge of the remaining forest; however, in both autumn and spring, emergence increased with distance, reaching a maximum of 45–60 m. In both seasons this value achieves 2000 seedlings/m2.
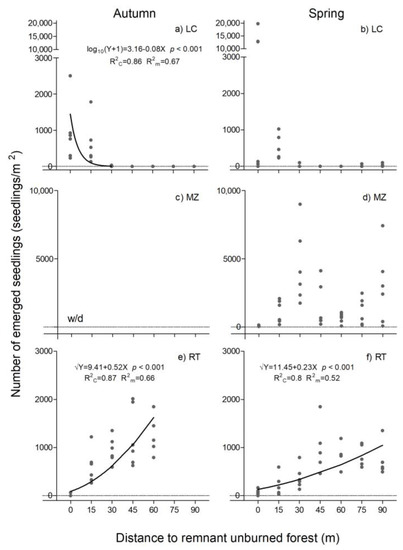
Figure 5.
Emergence of herbs species in La Colisión (LC, (a,b)), Monte Zeballos (MZ, (c,d)) and Río Turbio (RT, (e,f)), as a function of the distance from the edge of the remnant forest to the burned area, in autumn (left) and spring (right). Dots represent the observed emergence in each sample soil and lines indicate the simple linear regression for the evaluated range. w/d: without data.
Related to the grasses, the emergence in LC is very low and null in RT in any of the seasons analyzed (Figure 6). MZ is higher and did not show a variation associated with the distance from the remnant forest. In MZ the emergence is on average 80 seedlings/m2 from the edge to 45 m, and then decreases a bit (Figure 6b).
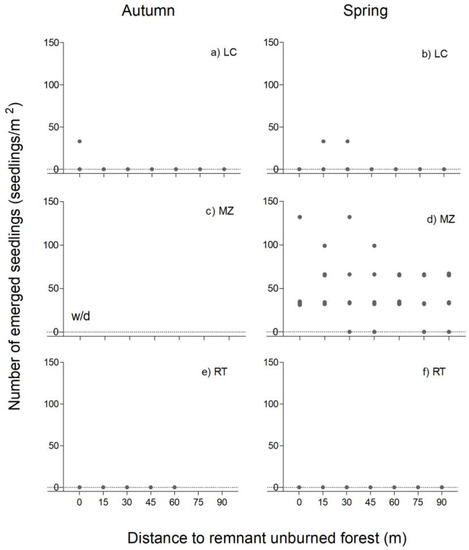
Figure 6.
Grass emergence in La Colisión (LC, (a,b)) and Monte Zeballos (MZ, (c,d)), and Río Turbio (RT, (e,f)), as a function of the distance from the edge of the remnant forest to the burned area, in autumn (left) and spring (right). Dots represent the observed emergence in each sample soil. w/d: without data.
3.3. Above-Ground Vegetation and Soil Seed Bank Composition
We identified 48 species in both fractions, above-ground vegetation and soil seed bank (Table 1). In the seed bank, the majority (29) were identified to the species level, while seven plants were identified to the genus level and 12 could not be determined. Regarding the habit, 70.8% corresponds to herbaceous species, 20.8% to dwarf shrub-shrubs and 8.3% to trees and grasses. Regarding status, 77.7% of the determined species are native/endemic, and 22.8% are adventive/exotic (Table 1).

Table 1.
Vascular plant species present in both fractions: soil seed bank (SSB) and above-ground vegetation (AV) at each study site La Colisión: LC, Monte Zeballos: MZ and Río Turbio: RT. Habit and Status according to Zuloaga et al. (2013) H = herbaceous, S = dwarf shrub-shrub and T = trees; N/E = native or endemic and A/Ex = adventive or exotic.
Species composition was different between sites (LC, MZ and RT) and fractions (AV and SSB) (p < 0.001). The species composition was mainly explained by the site (50%) and the fraction (5%) (Figure 7). In addition, the site*fraction interaction was recorded, which explains 17% of the variability in composition. In LC all the species present in the vegetation (n = 14) were found in the seed bank (n = 24). In this site, the herbaceous species represented 64.3% in both fractions, while the tree species were only found in the seed bank (7.1%). Dwarf shrub-shrub species represented 28.6% of the bank and 35.7% of the vegetation. Regarding status, 79% of the species were native/endemic. The remaining 21% corresponds to adventitious/exotic species, mainly herbaceous such as Cerastium sp., Cirsium vulgare and Taraxacum officinale (Table 1). In contrast, in MZ, although the number of species found in each fraction was similar (16 in SSB and 15 in VA), seven species in AV were not found in SSB and three in the SSB (Ribes cucullatum, Baccharis sp. and Cerastium sp.) were not found in the vegetation (Table 1). This was confirmed in the PERMANOVA and NMDS analyses (Figure 6). In this site, the herbaceous species also presented the highest proportion (68.7% in SSB and 73.3% in AV) followed by dwarf shrub-shrub (25% in SSB and 13% in AV) and a low percentage of trees and grasses. Regarding status, 81% were native/endemic, and the rest were adventitious/exotic mainly herbaceous such as Draba verna, Rumex acetosella and Taraxacum officinale. In RT, the SSB was more diverse than the AV with 15 species in total (four not determined) as evidenced also in the NMDS (Figure 6). The four main species of the AV, B. magellanica, Hieracium glaucifolium, N. pumilio and Senecio trifurcatus, were present in the SSB. In this site, herbaceous species represented 73.3% of SSB and 50% of AV, while dwarf shrub-shrub species represented 6.6% of SSB and 25% of AV. The trees were 13.3% of SSB and 25% of AV, and the grasses were 6.7% of SSB. Regarding the habit, 82% were native/endemic, the remaining percentage corresponding to the adventitious herbaceous plants R. acetosella and T. officinale (Table 1).
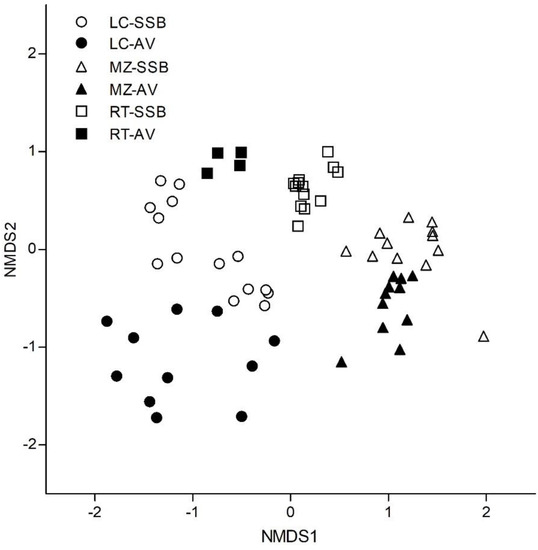
Figure 7.
Two-dimensional non-metric multidimensional scaling (NMDS, stress = 0.0946) ordination of above-ground vegetation (AV) and soil seed bank (SSB) for sites (LC, MZ and RT). Ordination is based on species presence, using Sorensen’s distance.
4. Discussion
In this work, we studied the soil seed bank and the above-ground vegetation established in the proximity of the remnant unburned N. pumilio forests at different times since the fire events. The study sites had differences in topography, soil properties, water regime, vascular plant composition, and livestock pressure that influence the results. Associated with this we found a high variability between and within sites, consistent with previous studies [39,40]. In the seed bank, the emergence of N. pumilio occurred in all sites but at LC and RT, the number of emerged seeds was the maximum at the edge of the remaining forest. In RT and MZ the emergence was almost zero from 15 m. This pattern is consistent with the wind-mediated dispersal mechanism that, in many species, occurs at short distances [41]. Seeds dispersal for N. pumilio reaches a distance of 25–60 m from the forest line [28,42].
Without disturbances, the regeneration of N. pumilio varied between 50 and 300 seedlings/m2 ensuring natural regeneration [18,43,44,45]. In LC, the density of 50 seedlings/m2 in autumn and 10 seedlings/m2 in spring in the first 10 to 20 m from the edge of the remnant forest could allow the gradual recovery of the forest without the herbivory pressure from domestic cattle or hares. However, it is probable that once a regeneration belt has been established, its advance towards the burned area will be strongly limited by different factors, as has been observed in N. pumilio burned sites over 25 years old [46]. In MZ, the very low emergence of N. pumilio from the seed bank is the main limitation to recovering the forest cover. This could be due to the decrease in the quality of the transitory seed bank that normally occurs from autumn to spring [41], as well as to the scarcity of seed-producing trees. Likewise, collected seeds in all sites showed a low germination capacity, normal in years of low production. N. pumilio presents abundant seed cycles called masting, with periods that can vary from 4 to 8 years, and with also a higher quality of seeds.
La Colisión is the most recently burned forest, and this determined the low similarity of the species composition compared to other sites (30 and 70 years since burned). The greater emergence of N. pumilio in LC could be associated with the absence of soil erosion and grazing. In the case of MZ, although the fire occurred more than 70 years ago, the intense livestock use has caused significant soil degradation and erosion affecting seed germination and seedlings establishment. In ecosystems where the spatial configuration of the community is of patches immersed in a matrix of bare soil, the shrubs and the vegetation present function as structures for retaining seeds [47]. It is also possible that in the burned area, the micro-environmental conditions during the winter are more extreme (e. g. low temperatures, high radiation, desiccation) and cause irreversible mechanical damage to the few fall seeds without the protection of litter leaves. This condition regarding the seeds and the death of seed-producing trees associated with high-severity fires are critical factors that influence the recovery of the N. pumilio forest after a disturbance [43].
A large part of the shrub species that emerged from the seed bank at the three sites was found in the vegetation and have also been identified in other burned areas [25]. These species have the ability to sprout from vegetative parts that have been buried and provide seeds to the soil seed bank, regardless of the distance to the edge of the remnant’s forest. The larger size of the germinable seed bank of shrubs on the edge of the remaining forest in LC could be associated with the recent fire event and better microsite conditions (e.g., litter, shade levels) and seeds provided by the forest, similar to those determined in others studies [48]. The low density of germinable seeds distant the edge of the remaining forest could delay the recovery of the species due to spontaneous recolonization of the burned area from propagules, as occurs in different species [49]. Instead, the size of the germinable bank of shrub species was small, in MZ from a distance of 45 m native/endemic species Azorella prolifera, Bacchariss sp., Berberis microphylla and Ribes cucullatum germinated, and in RT from 60 m occurred with B. magellanica and B. microphylla. In this case, both sites have livestock grazing impact on plant reproduction depending on the palatability of each plant species. Significant changes in the flowering span in one palatable species (R. magellanicum), with flowers opening first in fenced plots, were found in a postfire N. pumilio forest [50]. In contrast, flowering periods of the non-palatable species, B. microphylla and B. darwinii, and the exotic forb, C. vulgare, were extended by cattle browsing effects.
In part, the regenerating understory response after fires was due to the interaction between the spatial legacy of disturbance and the ability of different species to recolonize. The spatial and temporal pattern for regeneration depends upon the presence of the seed and vegetative banks as well as dispersal from surviving aboveground seed sources. The former provides in situ sources of regeneration, while the latter provides opportunities for species not previously on-site to invade a community [49]. In our study herbaceous species represented, on average, 69% of the composition of the bank and constituted the main source of soil cover, which could represent a mechanism of temporary protection, considering the important losses of soil fertility registered in recently burned forests [24], especially in areas with high rainfall and steep slopes [22]. The different patterns regarding the distance to the edge of the remnant forest in the three studied sites showed a significant trend of change between 15 and 30 m probably associated with the fire age and the presence of the seeds from anemophilous dispersal species that colonize burned areas. Moreover, it could be that the sampling design with only two transects for the site has been insufficient to cover the environmental heterogeneity within each site. In the first years, the remaining forest would be the main source of seeds that allows their dispersion towards the burned area, as was observed in LC with more than 10,000 seedlings/m2. However, since the time of the fire event and the herbaceous species colonizing the site, the main source of dispersal will be the same herbaceous species established in the burned area. In this site, three herbaceous species were very abundant (Galium aparine, Osmorhiza berteroi and Veronica serpyllifolia) and it is possible that, in the first stages of succession, these species play an essential role for the ecosystem by facilitating the installation of other species, as proposed by White and Pickett [51]. These results agree with those obtained from the analysis of the post-fire seed bank of the largest fire recorded in the last 100 years in Patagonia [52]. Thus, where a low abundance of herbaceous species was recorded in the seed bank in relation to shrubs and trees, most native/endemic species and a significant effect of fire severity on the emergence and abundance of species were detected [53]. In other forests, deep burial in seed banks is characteristic of early successional species and those with low seed weights [54]. The organic layer loss after intense burns should release this plant group. For early succession specialists, the presence of a persistent seed bank and a short period of favorable conditions immediately after wildfires are critical to the maintenance of viable populations. Seed banks may renovate during this short window of recruitment after lying dormant for years between wildfire cycles [49]. For example, the estimated interval between burns in the boreal forest ranges from 50 to 300 years [55].
However, the variation in the amount of seeds differed in the oldest burned sites, since RT displayed certain stability from 30 m from the forest edge, where the vegetation has a homogeneous distribution, while MZ showed great variation, surely associated with the heterogeneous distribution of the vegetation. In the case of RT, although the species present in the vegetation were found in the seed bank, the great number of species in the bank explains the different composition between fractions. This low presence may be related to the high Hieracium glaucifolium cover, a perennial invasive herb that covers the ground with values greater than 40% in nearby sites [25]. This can hinder seed germination due to the effect called empastado, as has been observed for N. pumilio in burned [15] and unburned [23] forests.
Integrating the results of this work and identifying a possible succession trajectory for each site, it would be expected that in LC with livestock excluded, a natural regeneration belt with a width of between 10 and 30 m would be established, but towards the inside of the burnt area, a replacement of N. pumilio seedlings by a stratum composed of endemic species, such as Berberis, Ribes, Schinus and Maytenus, may occur. For MZ and RT, it is expected that the ecotone grassland will remain. This is due to the fact that the intense and severe fires left low cover and an insufficient number of seed-producing trees, causing a decrease in the regeneration of N. pumilio and favoring the invasion of shrubby and herbaceous species. It is possible that due to the more marginal environmental conditions and the intense grazing use in MZ and RT, the system trespasses a threshold that limits the natural recovery succession, determining the need to carry out the ecological restoration.
5. Conclusions
Comparable to other studies in forest ecosystems, standing vegetation and its associated seed bank were not similar [56]. Knowing the relationships between seed banks and standing vegetation may help managers and conservationists to manage against exotic species, plan for community responses to disturbances, restore diversity, and better understand the resilience of an ecosystem [56].
In N. pumilio forests disturbed by fire, the size of the germinable seed bank of the dominant tree species would be, in the short term, insufficient to ensure spontaneous recovery through natural regeneration beyond 15–30 m from the forest limit remainder. In recently burned sites, the forest would produce a facilitating effect in the first 30 m, while in older sites where a regeneration belt has been established, the effect would be the opposite. The effect of these interactions is relevant to study in future works on succession in forests affected by the fire. It is also necessary to expand the knowledge about the composition of the seed bank in the different stages of post-fire succession in the forests of N. pumilio in the south-central of its distribution, and what is the ecological function of the species present in them since, unlike other works in N. pumilio forests affected by fire, most of the species determined in the study sites are native/endemic.
Regardless of the distance to the remaining forest, the presence of livestock could alter native species regeneration and possibly change successional trajectories following the burning of N. pumilio forests. Furthermore, animal browsing effects on reproduction phenology showed strong contrasting patterns between palatable and non-palatable species that could modify regeneration in the northern of N. pumilio distribution. Further research is needed to determine the patterns of postfire browsing effects at different sites in the south-central forests of N. pumilio. In this type of fire-sensitive forests, with traditional livestock use, with very low or no resilience capacity, the need to carry out restoration tasks that include the planting of native tree and shrub species is highlighted, in order to tend to a recovery of the structure and functionality of the original community.
Author Contributions
Conceptualization, M.F.U., V.A., L.M. and P.L.P.; methodology, M.F.U., V.A., I.O., P.L.B., L.M. and P.L.P.; formal analysis, V.A., P.L.B., M.M.R. and M.F.U.; investigation, M.F.U., V.A., I.O., P.L.B., L.M. and P.L.P.; writing—original draft preparation, V.A., M.F.U., P.L.B., and I.O.; writing—review and editing, M.F.U., I.O., M.M.R. and P.L.P.; supervision, M.F.U.; project administration, M.F.U.; funding acquisition, M.F.U., L.M. and P.L.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by FONCYT Argentina PICTO 36663 Project “Ecological restoration in degraded forests of the Andean Patagonia of Chubut and Santa Cruz: basic aspects and development of technologies”.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Acknowledgments
The authors wish to acknowledge M.F. Oyharçabal, J. Monges, and H. Arriola for their sampling collaboration, P. Quinteros for her assisting in the identification of vascular species and the owners where the studies were carried. We also thank P. Masera for his collaboration with Chelsa-climatic data.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Pickett, S.; McDonnell, M. Seed bank dynamics in temperate deciduous forest. In Ecology of Soil Seed Banks; Leck, M., Parker, V., Simpson, R., Eds.; Academic Press Inc.: San Diego, CA, USA, 1989; pp. 123–147. [Google Scholar]
- Donoso Zegers, C. Bosques Templados de Chile y Argentina. Variación, Estructura y Dinámica, 1st ed.; Editorial Universitaria SA: Valdivia, Chile, 1993; p. 484. [Google Scholar]
- Veblen, T.T.; Donoso Zegers, C.; Kitzberger, T.; Rebertus, A.J. Ecology of southern Chilean and southern Argentinean Nothofagus forests. In The Ecology and Biogeography of Nothofagus Forests; Veblen, T., Hill, R.S., Read, J., Eds.; Yale University Press: New Haven, CT, USA; London, UK, 1996; pp. 293–353. [Google Scholar]
- Little, S. Effects of fire on temperate forests: Northeastern United States. In Fire and Ecosystems; Kozlowski, T., Ahlgren, C., Eds.; Academic Press: New York, NY, USA, 1974; pp. 225–250. [Google Scholar]
- Keeley, J.E. Fire intensity, fire severity and burn severity: A brief review and suggested usage. Int. J. Wildland Fire 2009, 18, 116–126. [Google Scholar] [CrossRef]
- Ferrandis, P.; Herranz, J.; Martínez-Sánchez, J. The role of soil seed bank in the early stages of plant recovery after fire in a Pinus pinaster forest in SE Spain. Int. J. Wildland Fire 1996, 6, 31–35. [Google Scholar] [CrossRef]
- Falk, D.A.; van Mantgem, P.J.; Keeley, J.E.; Gregg, R.M.; Guiterman, C.H.; Tepley, A.J.; Young, D.J.; Marshall, L.A. Mechanisms of forest resilience. Forest Ecol. Manag. 2022, 512, 120–129. [Google Scholar] [CrossRef]
- Veblen, T.T.; Kitzberger, T.; Raffaele, E.; Mermoz, M.; González, M.E.; Sibold, J.S.; Holz, A. The historical range of variability of fires in the Andean–Patagonian Nothofagus forest region. Int. J. Wildland Fire 2008, 17, 724–741. [Google Scholar] [CrossRef]
- CIEFAP; SAyDS. Monitoreo de la Superficie de Bosque Nativo de la República Argentina. Región Forestal Bosque Andino Patagónico; Centro de Investigación y Extensión Forestal Andino Patagónico-Secretaría de Ambiente y Desarrollo Sustentable: Buenos Aires, Argentina, 2019; p. 86. Available online: https://www.argentina.gob.ar/sites/default/files/monitoreo_de_la_superficie_de_bosque_nativo_bap_2_de_octubre_2019.pdf (accessed on 10 June 2022).
- Donoso Zegers, C. Variación natural en especies de Nothofagus en Chile. Bosque 1987, 8, 85–97. [Google Scholar] [CrossRef]
- Bava, J.O.; Lencinas, J.D.; Haag, A. Determinación De La Materia Prima Disponible Para Proyectos de Inversión Forestales En Cuencas De La Provincia Del Chubut; Consejo Federal de Inversiones, Provincia del Chubut: Esquel, Argentina, 2006; p. 151. [Google Scholar]
- Mohr Bell, D. Superficies Afectadas por Incendios en la Región Bosque Andino Patagónico Durante los Veranos de 2013–2014 y 2014–2015—Report (Unpublished work) Nodo Regional Bosque Andino Patagónico (SAyDS–CIEFAP). Unpublished Work. 2015; 12. [Google Scholar]
- Dimitri, M. La Región de los Bosques Andino-Patagónicos; Ediciones INTA: Buenos Aires, Argentina, 1983; p. 381. [Google Scholar]
- Thompson, K.; Grime, J.P. Seasonal variation in the seed banks of herbaceous species in ten contrasting habitats. J. Ecol. 1979, 67, 893–921. [Google Scholar] [CrossRef]
- Rusch, V. Estudio sobre la regeneración de la lenga (Nothofagus pumilio) en la cuenca del Río Manso Superior, Río Negro. In Informe Final Beca de Iniciación; CONICET: Buenos Aires, Argentina, 1987; p. 113. [Google Scholar]
- Cuevas, J.G.; Arroyo, M.T. Ausencia de banco de semillas persistente en Nothofagus pumilio (Fagaceae) en Tierra del Fuego, Chile. Rev. Chil. Hist. Nat. 1999, 72, 73–82. [Google Scholar]
- Urretavizcaya, M.F.; Contardi, L.; Oyharçabal, M.F.; Pasquini, M. Calidad de semillas de especies nativas del bosque andino patagónico de la provincia de Chubut y su importancia para la producción de plantines. Rev. Fac. Agron. Univ. Nac. La Plata 2016, 115, 9–18. [Google Scholar]
- González, M.; Donoso Zegers, C.; Ovalle, P.; Martínez-Pastur, G. Nothofagus pumilio (Poep. et Endl.) Krasser. Lenga, roble blanco, leñar, roble de Tierra del Fuego. In Las Especies Arbóreas de los Bosques Templados de Chile y Argentina; Donoso Zegers, C., Ed.; Marisa Cuneo Ediciones: Valdivia, Chile, 2006; pp. 486–500. [Google Scholar]
- Rodrigo, A.; Arnan, X.; Retana, J. Relevance of soil seed bank and seed rain to immediate seed supply after a large wildfire. Int. J. Wildland Fire 2012, 21, 449–458. [Google Scholar] [CrossRef]
- Rusch, V. Determinación de las transiciones de estado en bosques de lenga (Nothofagus pumilio). In Informe Final Beca de Avance; CONICET: Buenos Aires, Argentina, 1989; p. 76. [Google Scholar]
- Kitzberger, T.; Raffaele, E.; Heinemann, K.; Mazzarino, M.J. Effects of fire severity in a north Patagonian subalpine forest. J. Veg. Sci. 2005, 16, 5–12. [Google Scholar] [CrossRef]
- Varela, S.A.; Gobbi, M.E.; Laos, F. Banco de semillas de un bosque quemado de Nothofagus pumilio: Efecto de la aplicación de compost de biosólidos. Ecol. Austral 2006, 16, 63–78. [Google Scholar]
- Quinteros, C.P.; Bava, J.O.; López Bernal, P.M.; Gobbi, M.E.; Defossé, G.E. Competition effects of grazing-modified herbaceous vegetation on growth, survival and water relations of lenga (Nothofagus pumilio) seedlings in a temperate forest of Patagonia, Argentina. Agroforest Syst. 2017, 91, 597–611. [Google Scholar] [CrossRef]
- Urretavizcaya, M.F. Propiedades del suelo en bosques quemados de Austrocedrus chilensis en Patagonia, Argentina. Bosque 2010, 31, 140–149. [Google Scholar] [CrossRef]
- Urretavizcaya, M.F.; Peri, P.L.; Monelos, L.; Arriola, H.; Oyharçabal, M.F.; Contardi, L.T.; Muñoz, M.; Sepúlveda, E.; Defossé, G.E. Condiciones de suelo y vegetación en tres bosques quemados de Nothofagus pumilio en Argentina y experiencias para su restauración activa. Ecol. Austral 2018, 28, 383–399. [Google Scholar] [CrossRef]
- Mutch, L.S.; Swetnam, T.W. Effects of fire severity and climate on ring-width growth of giant sequoia after fire. In Proceedings of the Symposium on Fire in Wilderness and Park Management: Past Lessons and Future Opportunities; Brown, J.K., Mutch, R.W., Spoon, C.W., Wakimoto, R.H., Eds.; Gen. Tech. Rep. INT-GTR-320; US Department of Agriculture, Forest Service: Ogden, UT, USA, 1995. [Google Scholar]
- Karger, D.N.; Zimmermann, N.E. Climatologies at High Resolution for the Earth Land Surface Areas CHELSA V1. 2: Technical Specification; Swiss Federal Research Institute WSL: Birmensdorf, Switzerland, 2019.
- López Bernal, P.M.; Urretavizcaya, M.F.; Defossé, G.E. Seedling Dynamics in an Environmental Gradient of Andean Patagonia, Argentina. In From Seed Germination to Young Plants: Ecology, Growth and Environmental Influences; Busso, C., Ed.; Nova Science Publishers, Inc.: New York, NY, USA, 2012; pp. 189–210. [Google Scholar]
- Correa, M. Flora Patagónica I-VIII; Instituto Nacional de Tecnología Agropecuaria: Buenos Aires, Argentina, 1969–1988. [Google Scholar]
- Zuloaga, F.; Morrone, O.; Belgrano, M. Flora deldono Sur. Catálogo de las Plantas Vasculares. 2013. Available online: http://www.darwin.edu.ar/Proyectos/FloraArgentina/fa.htm (accessed on 1 February 2022).
- van der Valk, A.; Pederson, R. Seed banks and the management and restoration of natural vegetation. In Ecology of soil seed banks; Leck, M., Parker, V., Simpson, R., Eds.; Academic Press: San Diego, CA, USA, 1989; pp. 329–346. [Google Scholar]
- ISTA. International Rules for Seed Testing; International Seed Testing Association: Zürich, Switzerland, 1999. [Google Scholar]
- Harper, J.L. Population Biology of Plants; Academic Press: London, UK, 1977. [Google Scholar]
- Bates, D.; Maechler, M.; Bolker, B.; Walker, S. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Nakagawa, S.; Schielzeth, H. A general and simple method for obtaining R2 from generalized linear mixed-effects models. Methods Ecol. Evol. 2013, 4, 133–145. [Google Scholar] [CrossRef]
- Barton, K. MuMIn: Multi-Model Inference. R package version 1.43.17. Available online: https://CRAN.R-project.org/package=MuMIn (accessed on 10 June 2022).
- Team, R.C. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. Available online: https://www.r-project.org/ (accessed on 10 June 2022).
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D. Community Ecology Package. R package version 2.5-7. 2020. Available online: https://CRAN.R-project.org/package=vegan (accessed on 10 June 2022).
- Cavallero, L. Heterogeneidad Ambiental y Dispersión de Semillas en Comunidades de Distinta Edad Post-Fuego del Noroeste de Patagonia; Universidad Nacional del Comahue: Bariloche, Argentina, 2012. [Google Scholar]
- Pickett, S.; Cadenasso, M.; Jones, C. Generation of heterogeneity by organisms: Creation, maintenance and transformation. In The Ecological Consequences of Environmental Heterogeneity; Hutchings, M., John, E., Stewart, J., Eds.; Blacwell Science: London, UK, 2000; pp. 33–52. [Google Scholar]
- Bakker, J.; Poschlod, P.; Strykstra, R.; Bekker, R.; Thompson, K. Seed banks and seed dispersal: Important topics in restoration ecology. Acta Bot. Neerl. 1996, 45, 461–490. [Google Scholar] [CrossRef]
- Cellini, J.M. Estructura y Regeneración Bajo Distintas Propuestas de Manejo de Bosques de Nothofagus Pumilio (Poepp. Et. Endl.) Krasser en Tierra del Fuego, Argentina; Universidad Nacional de La Plata: La Plata, Argentina, 2010. [Google Scholar]
- Rusch, V. Principales limitantes para la regeneración de la lenga en la zona NE de su área de distribución. In Proceedings of the Seminario de manejo forestal de la lenga y aspectos ecológicos relacionados, Esquel, Argentina, 23–24 June 1992; pp. 61–73. [Google Scholar]
- Bava, J.O. Aportes Ecológicos y Silviculturales a la Transformación de Bosques Virgenes de Lenga (Nothofagus Pumilio (Poepp. et Endl.) Krasser en Bosques Manejados en Sector Argentino de Tierra del Fuego; CIEFAP, Centro de Investigación y Extensión Forestal Andino Patagónico: Esquel, Argentina, 1999; p. 138. [Google Scholar]
- Martínez Pastur, G.; Peri, P.; Fernández, C.; Staffieri, G.; Rodríguez, D. Desarrollo de la regeneración a lo largo del ciclo del manejo forestal de un bosque de Nothofagus pumilio: 1. Incidencia de la cobertura y el aprovechamiento o cosecha. Bosque 1999, 20, 39–46. [Google Scholar] [CrossRef]
- Bertolin, M.L.; Urretavizcaya, M.; Defossé, G. Fire emissions and car-bon uptake in severely burned lenga beech (Nothofagus pumilio) forests of Patagonia, Argentina. Fire Ecol. 2015, 11, 32–54. [Google Scholar] [CrossRef]
- Walker, L.R.; del Moral, R. Lessons from primary succession for restoration of severely damaged habitats. Appl. Veg. Sci. 2009, 12, 55–67. [Google Scholar] [CrossRef]
- Cubiña, A.; Aide, T.M. The effect of distance from forest edge on seed rain and soil seed bank in a tropical pasture 1. Biotropica 2001, 33, 260–267. [Google Scholar] [CrossRef]
- Lee, P. The impact of burn intensity from wildfires on seed and vegetative banks, and emergent understory in aspen-dominated boreal forests. Can. J. Bot. 2004, 82, 1468–1480. [Google Scholar] [CrossRef]
- de Paz, M.; Raffaele, E. Cattle change plant reproductive phenology, promoting community changes in a post-fire Nothofagus forest in northern Patagonia, Argentina. J. Plant Ecol. 2013, 6, 459–467. [Google Scholar] [CrossRef][Green Version]
- White, P.S.; Pickett, S. Natural disturbance and patch dynamics: An introduction. In Natural Disturbance and Patch Dynamics; Pickett, S., White, P.S., Eds.; Academic Press: San Diego, CA, USA, 1985; pp. 3–13. [Google Scholar]
- SSB; CIEFAP; INTA. Programa Integral de Manejo y Restauración de las Grandes Áreas Afectadas por los Incendios Forestales de la Temporada 2014–2015 en la Provincia de Chubut Subsecretaría de Bosques de Chubut; Centro de Investigación y Extensión Forestal Andino Patagónico, Instituto Nacional de Tecnologia Agropecuearia: Esquel, Argentina, 2015; p. 144. [Google Scholar]
- Williams, A.; Orellana, I.; Bertoldi, G.; von Müller, A.; Roveta, R.; Urretavizcaya, M. Análisis del banco de semillas post-incendio en “Las Horquetas”, Cholila, Chubut. In Proceedings of the IV Jornadas Patagónicas de Biología, III Jornadas Patagónicas de Ciencias Ambientales y VI Jornadas Estudiantiles de Ciencias Biológicas, Trelew, Argentina, 19–21 September 2018. [Google Scholar]
- Grandin, U.; Rydin, H. Attributes of the seed bank after a century of primary succession on islands in Lake Hjälmaren, Sweden. J. Ecol. 1998, 86, 293–303. [Google Scholar] [CrossRef]
- Armstrong, G.W. A stochastic characterisation of the natural disturbance regime of the boreal mixedwood forest with implications for sustainable forest management. Can. J. For. Res. 1999, 29, 424–433. [Google Scholar] [CrossRef]
- Hopfensperger, K.N. A review of similarity between seed bank and standing vegetation across ecosystems. Oikos 2007, 116, 1438–1448. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).