New Symptoms in Castanea sativa Stands in Italy: Chestnut Mosaic Virus and Nutrient Deficiency
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Field Investigations
2.2.1. Phytosanitary Visual Assessment in the Plots
2.2.2. SPAD Measurements
2.2.3. Sample Collection
2.3. Laboratory Tests
2.3.1. Molecular Detection of Phytoplasma and Chestnut Mosaic Virus in Chestnut Samples
2.3.2. Quantification of Nutrients in Leaf Samples and Chemical Analysis of Soils
2.4. Statistical Analysis
3. Results
3.1. Field Investigations
3.1.1. Tree Visual Assessment in the Plots
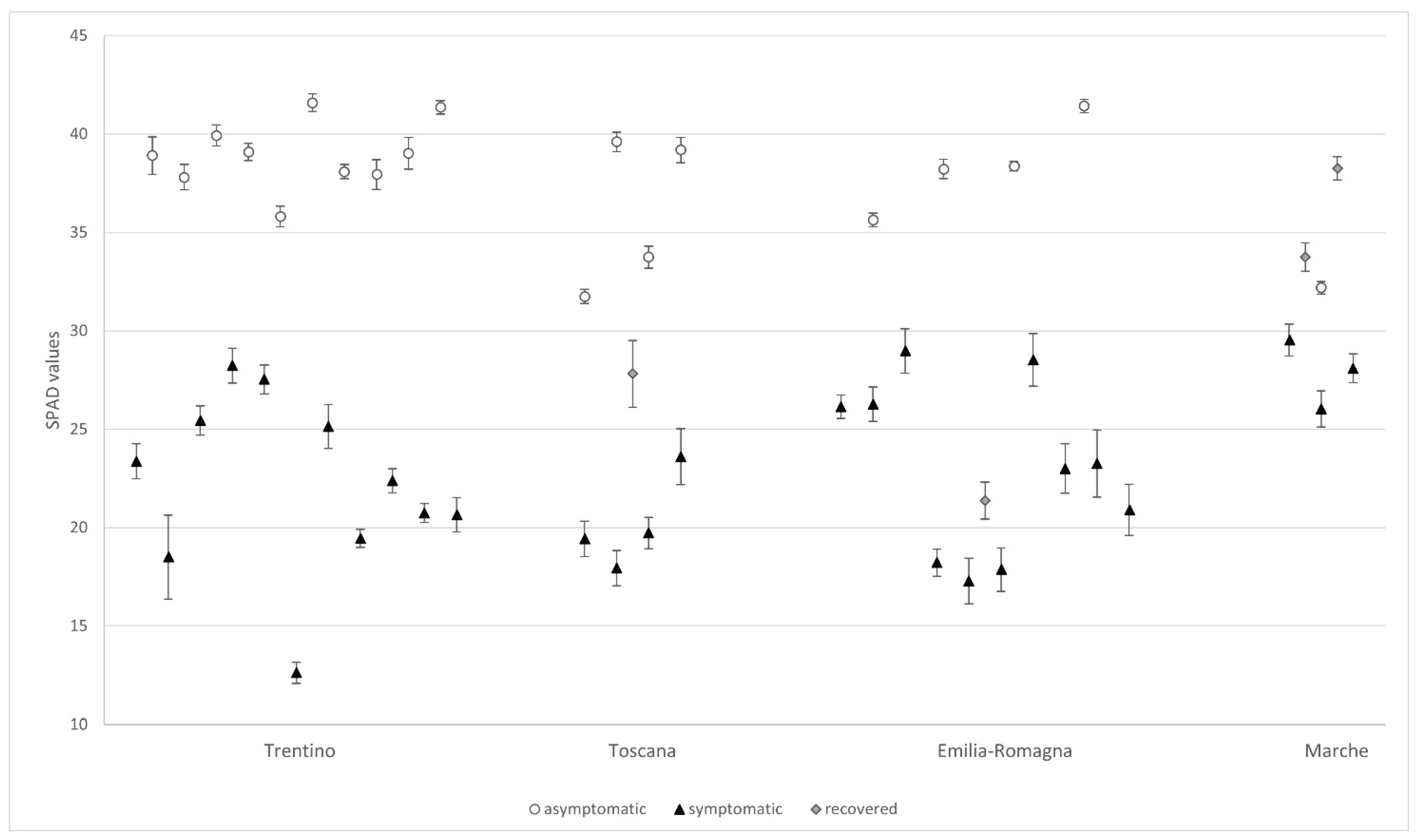
3.1.2. SPAD Measurements
3.2. Laboratory Tests
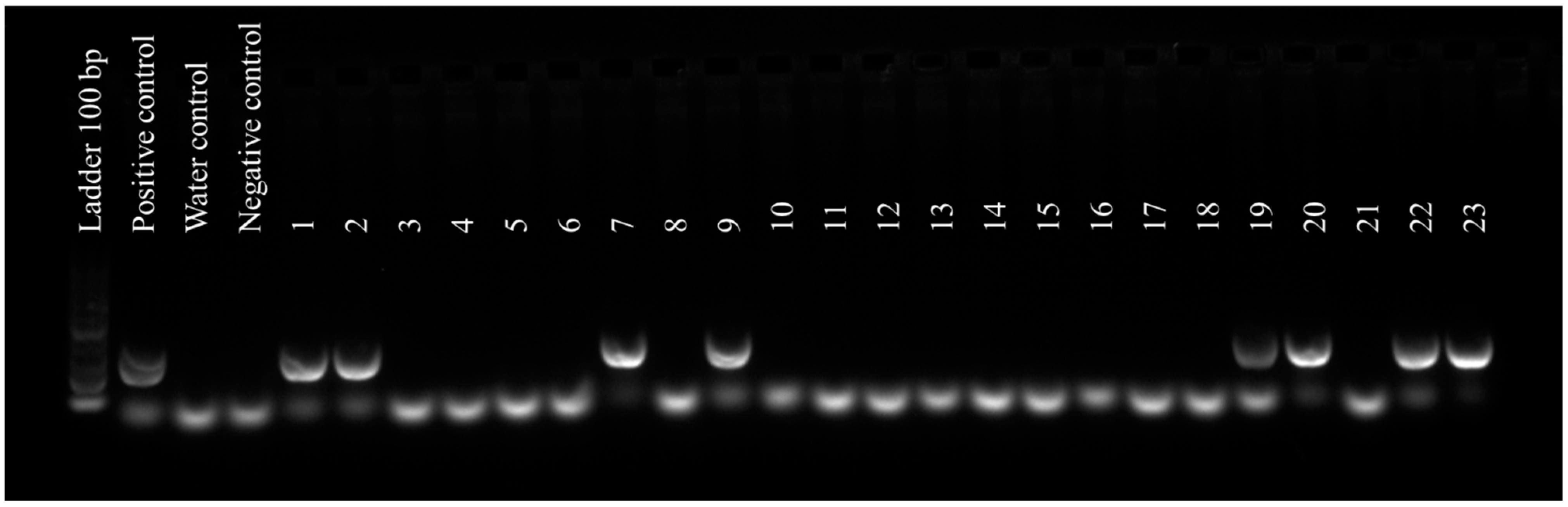
3.2.1. Molecular Detection of Phytoplasma and Chestnut Mosaic Virus in Chestnut Samples
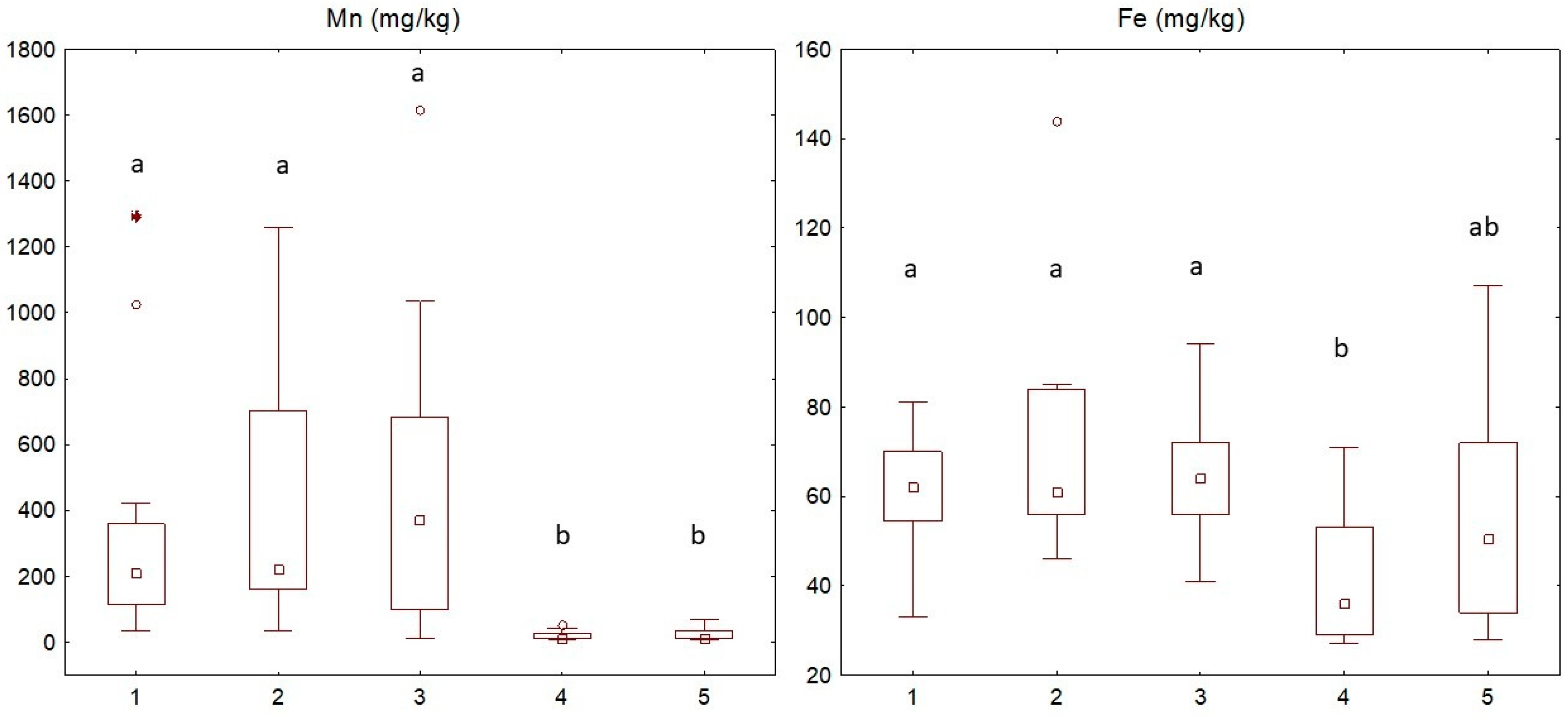
3.2.2. Nutrient Elements in Leaf Samples and Chemical Analysis of Soils
3.2.3. Trends of Mineral Element Contents in Leaves during the Season
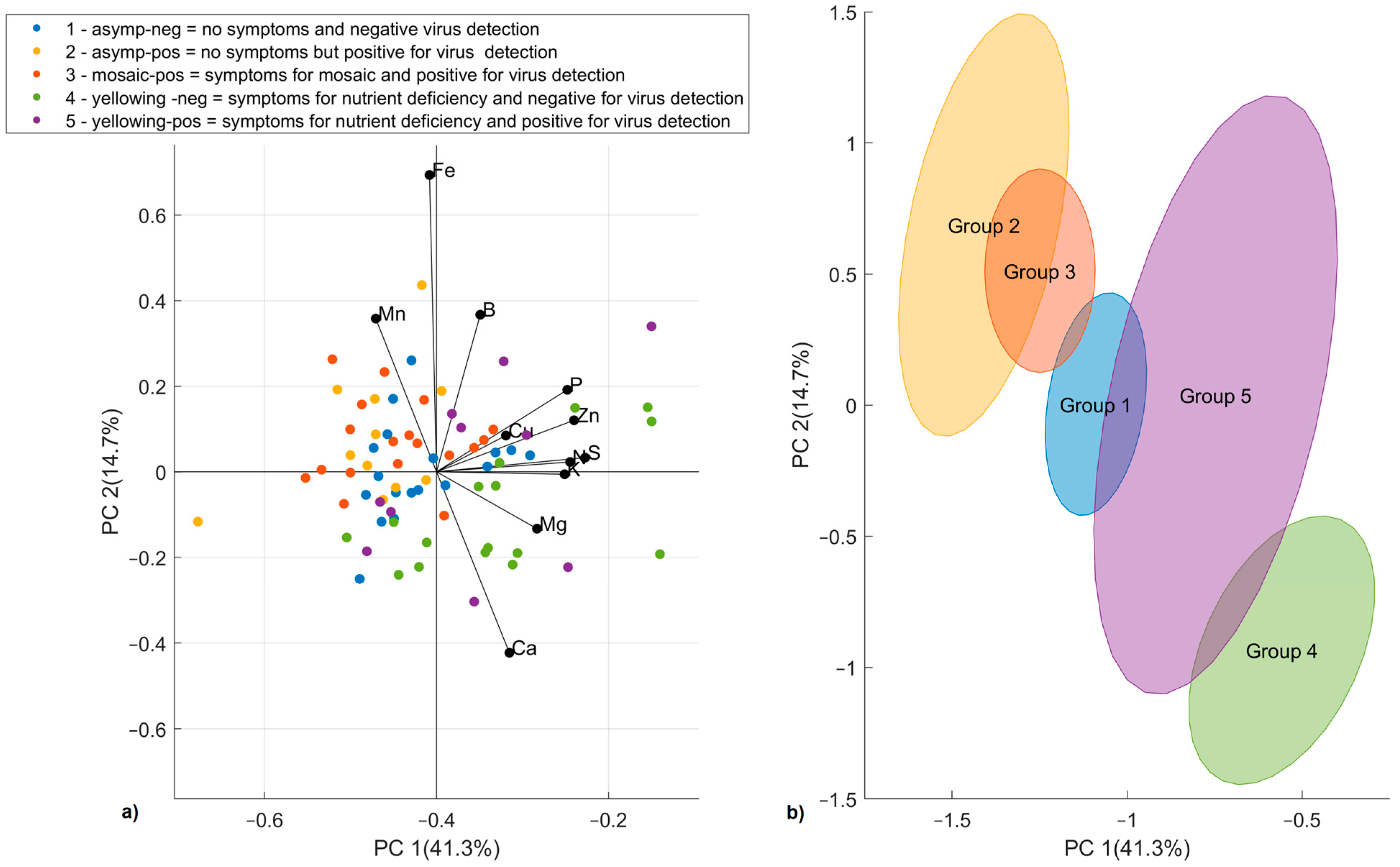
3.2.4. PCA Elaboration
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Conedera, M.; Krebs, P.; Tinner, W.; Pradella, M.; Torriani, D. The Cultivation of Castanea Sativa (Mill.) in Europe, from Its Origin to Its Diffusion on a Continental Scale. Veg. Hist. Archaeobot. 2004, 13, 161–179. [Google Scholar] [CrossRef]
- Gabrielli, A. La Civiltà Del Castagno. Monti Boschi 1994, 65, 3. [Google Scholar]
- Pezzi, G.; Gambini, S.; Buldrini, F.; Ferretti, F.; Muzzi, E.; Maresi, G.; Nascimbene, J. Contrasting Patterns of Tree Features, Lichen, and Plant Diversity in Managed and Abandoned Old-Growth Chestnut Orchards of the Northern Apennines (Italy). For. Ecol. Manag. 2020, 470–471, 118207. [Google Scholar] [CrossRef]
- Pezzi, G.; Ferretti, F.; Maltoni, A.; Krebs, P.; Conedera, M.; Maresi, G. The Chestnut Orchards in the Bolognese Apennines: A Vanishing Socio-Ecological Habitat. In Historical Ecology: Learning from the Past to Understand the Present and Forecast the Future of Ecosystems; Wiley: London, UK; ISTE: Hoboken, NJ, USA, 2022; pp. 195–205. ISBN 9781789450903. [Google Scholar]
- Maresi, G.; Battisti, A.; Maltoni, A.; Turchetti, T. Chestnut Wood Management in Relation to Phytosanitary Problems. In Proceedings of the Atti del Secondo Congresso Internazionale di Selvicoltura (the Second International Congress of Silviculture), Florence, Italy, 26–29 November 2014; Accademia Italiana di Scienze Forestali: Firenze, Italy, 2015; pp. 148–154. [Google Scholar]
- Puccinelli, M. Giornale Di Agricoltura; Camera del Commercio e dell’Agricoltura: Lucca, Italy, 1859. [Google Scholar]
- Vettraino, A.M.; Natili, G.; Anselmi, N.; Vannini, A. Recovery and Pathogenicity of Phytophthora Species Associated with a Resurgence of Ink Disease in Castanea Sativa in Italy. Plant Pathol. 2001, 50, 90–96. [Google Scholar] [CrossRef]
- Biraghi, A. Il Cancro Del Castagno Causato Da Endothia Parasitica. L’Italia Agric. 1946, 7, 406. [Google Scholar]
- Quacchia, A.; Moriya, S.; Bosio, G.; Scapin, I.; Alma, A. Rearing, Release and Settlement Prospect in Italy of Torymus Sinensis, the Biological Control Agent of the Chestnut Gall Wasp Dryocosmus Kuriphilus. BioControl 2008, 53, 829–839. [Google Scholar] [CrossRef]
- Turchetti, T.; Ferretti, F.; Maresi, G. Natural Spread of Cryphonectria Parasitica and Persistence of Hypovirulence in Three Italian Coppiced Chestnut Stands. For. Pathol. 2008, 38, 227–243. [Google Scholar] [CrossRef]
- Murolo, S.; Angelini, R.M.d.M.; Faretra, F.; Romanazzi, G. Phenotypic and Molecular Investigations on Hypovirulent Cryphonectria Parasitica in Italy. Plant Dis. 2018, 102, 540–545. [Google Scholar] [CrossRef]
- Maresi, G.; Oliveira Longa, C.M.; Turchetti, T. Brown Rot on Nuts of Castanea Sativa Mill: An Emerging Disease and Its Causal Agent. iForest—Biogeosci. For. 2013, 6, 294. [Google Scholar] [CrossRef]
- Lione, G.; Danti, R.; Fernandez-Conradi, P.; Ferreira-Cardoso, J.V.; Lefort, F.; Marques, G.; Meyer, J.B.; Prospero, S.; Radócz, L.; Robin, C.; et al. The Emerging Pathogen of Chestnut Gnomoniopsis Castaneae: The Challenge Posed by a Versatile Fungus. Eur. J. Plant Pathol. 2019, 153, 671–685. [Google Scholar] [CrossRef]
- Vettraino, A.M.; Vannini, A.; Flamini, L.; Lagnese, R.; Pizzichini, L.; Talevi, S.; Fulbright, D.W. A New Transmissible Symptomology on Sweet Chestnut in Italy. Acta Hortic. 2005, 693, 547–550. [Google Scholar] [CrossRef]
- Mittempergher, L.; Sfalanga, A. Chestnut Yellows: A New Disease for Europe. Phytopathol. Mediterr. 1998, 37, 143–145. [Google Scholar]
- Jung, H.-Y.; Sawayanagi, T.; Kakizawa, S.; Nishigawa, H.; Miyata, S.; Oshima, K.; Ugaki, M.; Lee, J.-T.; Hibi, T.; Namba, S. “Candidatus Phytoplasma Castaneae”, a Novel Phytoplasma Taxon Associated with Chestnut Witches’ Broom Disease. Int. J. Syst. Evol. Microbiol. 2002, 52, 1543–1549. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lin, C.L.; Li, H.F.; Zhang, G.Z.; Wei, W.; Zhu, X.Q.; Li, Z.P.; Wang, H.; Xu, Q.C.; Zhou, T.; Tian, G.Z. Molecular Identification and Characterization of a New Phytoplasma Strain Associated with Chinese Chestnut Yellow Crinkle Disease in China. For. Camera del Commer. Pathol. 2011, 41, 233–236. [Google Scholar] [CrossRef]
- Bertoldi, D.; Miorelli, P.; Pedrazzoli, F.; Delugan, S.; Deromedi, M.; Maresi, G. Investigations on Yellowing of Chestnut Crowns in Trentino (Alps, Northern Italy). iForest—Biogeosci. For. 2020, 13, 466–472. [Google Scholar] [CrossRef]
- Marais, A.; Murolo, S.; Faure, C.; Brans, Y.; Larue, C.; Maclot, F.; Massart, S.; Chiumenti, M.; Minafra, A.; Romanazzi, G.; et al. Sixty Years from the First Disease Description, a Novel Badnavirus Associated with Chestnut Mosaic Disease. Phytopathology 2021, 111, 1051–1058. [Google Scholar] [CrossRef]
- Gualaccini, G. Una Virosi Nuova Del Castagno. Boll. Stn. Patol. Veg. Roma 1958, 16, 67–75. [Google Scholar]
- Desvignes, J.C. Sweet Chestnut Incompatibility and Mosaics Caused by the Chestnut Mosaic Virus (ChMV). Acta Hortic. 1999, 494, 451–458. [Google Scholar] [CrossRef]
- Rumbou, A.; Vainio, E.J.; Büttner, C. Towards the Forest Virome: High-Throughput Sequencing Drastically Expands Our Understanding on Virosphere in Temperate Forest Ecosystems. Microorganisms 2021, 9, 1730. [Google Scholar] [CrossRef]
- Hislop, M.; Twery, M.; Vihemäki, H. Involving People in Forestry: A Toolbox for Public Involvement in Forest and Woodland Planning; Forestry Commission: Edinburgh, UK, 2004. [Google Scholar]
- Tongco, M.D.C. Purposive Sampling as a Tool for Informant Selection. Ethnobot. Res. Appl. 2007, 5, 147–158. [Google Scholar] [CrossRef]
- Marquard, R.D.; Tipton, J.L. Relationship between Extractable Chlorophyll and an in Situ Method to Estimate Leaf Greenness. Hortscience 1987, 22, 1327. [Google Scholar] [CrossRef]
- Madeira, A.C.; Ferreira, A.; De Varennes, A.; Vieira, M.I. SPAD Meter versus Tristimulus Colorimeter to Estimate Chlorophyll Content and Leaf Color in Sweet Pepper. Commun. Soil Sci. Plant Anal. 2003, 34, 2461–2470. [Google Scholar] [CrossRef]
- Sim, C.C.; Zaharah, A.R.; Tan, M.S.; Goh, K.J. Rapid Determination of Leaf Chlorophyll Concentration, Photosynthetic Activity and NK Concentration of Elaies Guineensis via Correlated SPAD-502 Chlorophyll Index. Asian J. Agric. Res. 2015, 9, 132–138. [Google Scholar] [CrossRef]
- Jiang, C.; Johkan, M.; Hohjo, M.; Tsukagoshi, S.; Maruo, T. A Correlation Analysis on Chlorophyll Content and SPAD Value in Tomato Leaves. HortResearch 2017, 71, 37–42. [Google Scholar] [CrossRef]
- Doyle, J.J.; Doyle, J.L. Isolation of Plant DNA from Fresh Tissue. Focus 1990, 12, 13–15. [Google Scholar]
- Deng, S.; Hiruki, C. Amplification of 16S RRNA Genes from Culturable and Nonculturable Mollicutes. J. Microbiol. Methods 1991, 14, 53–61. [Google Scholar] [CrossRef]
- Schneider, B.; Seemueller, E.; Smart, C.D.; Kirkpatrick, B.C. E6 -Phylogenetic Classification of Plant Pathogenic Mycoplasma-like Organisms or Phytoplasmas. In Molecular and Diagnostic Procedures in Mycoplasmology; Razin, S., Tully, J.G., Eds.; Academic Press: San Diego, CA, USA, 1995; pp. 369–380. ISBN 978-0-12-583805-4. [Google Scholar]
- Lee, I.-M.; Hammond, R.W.; Davis, R.E.; Gundersen, D.E. Universal Amplification and Analysis of Pathogen 16S RDNA for Classification and Identification of Mycoplasmalike Organisms. Phytopathology 1993, 83, 834. [Google Scholar] [CrossRef]
- Gundersen, D.E.; Lee, I.-M. Ultrasensitive Detection of Phytoplasmas by Nested-PCR Assays Using Two Universal Primer Pairs. Phytopathol. Mediterr. 1996, 35, 144–151. [Google Scholar]
- Standard-Diagnostics, E.P.O.O. PM 7/133 (1) Generic Detection of Phytoplasmas. EPPO Bull. 2018, 48, 414–424. [Google Scholar] [CrossRef]
- Shimada, S. Chestnut Yellows. Pl. Prot. Tokyo 1962, 16, 253–254. [Google Scholar]
- Okuda, S.; Doi, Y.; Yora, K. Mycoplasmalike Bodies Associated with Chestnut Yellows. Ann. Phytopathol. Soc. Japan 1974, 40, 464–468. [Google Scholar] [CrossRef]
- Horvath, J.; Ecke, I.; Gal, T.; Dezcery, M. Demonstration of Virus-like Particles in Sweet Chestnut and Oak with Leaf Deformations in Hungary. Z. Pflkrankh. Pfsdrntz. 1975, 82, 498–502. [Google Scholar]
- Han, S.; Kim, Y.H.; So, I.Y.; Chai, J.K. Association of Phytoplasma with Chestnut (Castanea Crenata Sieb. et Zucc.) Little Leaf Disease in Korea. Korean J. Mycoplasmol. 1997, 8, 48–54. [Google Scholar]
- Ferrari, D.; Contaldo, N.; Bertaccini, A. Association of Phytoplasmas with a Chestnut Yellows in Italy. Phytopathogenic Mollicutes 2018, 8, 69. [Google Scholar] [CrossRef]
- Ragozzino, A.; Lahoz, E. Una Malattia Virus-Simile Del Castagno in Provincia Di Avellino. In Giornate di Studio sul Castagno; Soc. Orti. Italiana: Firenze, Italy, 1986; pp. 307–311. [Google Scholar]
- Antonaroli, R.; Perna, M.R. Una Fitopatia Ad Eziologia Ancora Incerta: Il Giallume Del Castagno in Emilia-Romagna e Nelle Marche. Sherwood 2000, 57, 43–46. [Google Scholar]
- Desvignes, J.C. Characterization of the Chestnut Mosaic. In Proceedings of the Acta Horticulturae, International Society for Horticultural Science (ISHS), Leuven, Belgium, 1 May 1992; pp. 353–358. [Google Scholar]
- Desvignes, J.C. Mosaïque Du Chataignier. In Maladies à Virus des Arbres Fruitiers; Centre Technique Interprofessionnel des Fruits et Légumes: Paris, France, 1999; pp. 179–181. [Google Scholar]
- Desvignes, J.C.; Lecocq, G. New Knowledges on the Chestnut Mosaic Virus. Acta Hortic. 1995, 578–584. [Google Scholar] [CrossRef]
- Desvignes, J.C.; Cornaggia, D. Mosaïque Du Chataignier: Transmission Par Le Puceron Myzocallis Castanicola. Phytoma 1996, 481, 39–41. [Google Scholar]
- Desvignes, J.-C.; Boye, R.; Cornaggia, D.; Grasseau, N. Virus Diseases of Fruit Trees; Centre Technique Interprofessionnel des Fruits et Légumes: Paris, France, 1999; ISBN 2879111439. [Google Scholar]
- Zhao, J.; Zhang, X.; Hong, Y.; Liu, Y. Chloroplast in Plant-Virus Interaction. Front. Microbiol. 2016, 7, 1565. [Google Scholar] [CrossRef]
- Vettraino, A.M.; Morel, O.; Perlerou, C.; Robin, C.; Diamandis, S.; Vannini, A. Occurrence and Distribution of Phytophthora Species in European Chestnut Stands, and Their Association with Ink Disease and Crown Decline. Eur. J. Plant Pathol. 2005, 111, 169–180. [Google Scholar] [CrossRef]
- Trebicki, P. Climate Change and Plant Virus Epidemiology. Virus Res. 2020, 286, 198059. [Google Scholar] [CrossRef]

| Site ID | Location | Province/Region | Geographical Coordinates | Elevation m a.s.l. | Aspect | Substrate | Stand Type | Symptomatic Trees | Average Tree Age (y) |
|---|---|---|---|---|---|---|---|---|---|
| M1 | Venamartello | AP–Marche | 42,7882883; 13,4108881 | 657 | southeast | calcareous | orchard | grafted, sprouts | 40–50 |
| M2 | Acquasanta Terme | AP–Marche | 42.729.622; 13.403.105 | 918 | north west | calcareous | orchard | grafted, sprouts | 20–100 |
| ER1 | Selva la Maddalena | BO–Emilia Romagna | 44.240.029; 11.496.345 | 393 | north | marly siliceous | orchard | grafted | 100–200 |
| ER2 | Loiano | BO–Emilia Romagna | 44.253.954; 11.333.899 | 789 | west | siliceous | orchard | grafted | 20–50 |
| ER3 | Ca’ di Balloni | BO–Emilia Romagna | 44.265.234; 11.339.537 | 653 | east | siliceous | orchard | grafted | 100 |
| ER4 | Gragnano | BO–Emilia Romagna | 44.245.631; 11.345.321 | 719 | northwest | siliceous | orchard | grafted, sprouts | 20 |
| ER5 | Malalbergo 1 | MO–Emilia Romagna | 44.223.022; 10.929.023 | 923 | northwest | siliceous | orchard | grafted | 10–200 |
| ER6 | Malalbergo 2 | MO–Emilia Romagna | 44.223.022; 10.929.023 | 923 | northwest | siliceous | orchard | grafted | 200 |
| ER7 | Alberelli | MO–Emilia Romagna | 44.231.896; 10.926.836 | 783 | west | siliceous | orchard | grafted | 100–200 |
| ER8 | Monteombraro Lavezze | MO–Emilia Romagna | 44.384.805; 10.994.774 | 628 | west | marly siliceous | orchard | grafted, sprouts | 20–30 |
| ER9 | Monteombraro Caseificio | MO–Emilia Romagna | 44.382.960; 11.007.369 | 664 | north | siliceous | new plantation | grafted | 5 |
| ER10 | Montese | MO–Emilia Romagna | 44.2471.51; 10.930.374 | 740 | west | siliceous | mixed wood | sprout | |
| TO1 | Castagneto | FI–Toscana | 43.937.377; 11.615.097 | 520 | southwest | siliceous | orchard, mixed wood | grafted, sprouts | 20–150 |
| TO2 | Gaggiolo | GR–Toscana | 42.889.658; 11.562.061 | 823 | northwest | siliceous | orchard | sprout (nonsymptomatic) | 20–150 |
| TN1 | Mezzolombarbo | TN–Trentino Alto Adige | 46.226.333; 11.072.695 | 328 | east | calcareous | orchard | grafted (nonsymptomatic) | 100 |
| TN2 | Drena | TN–Trentino Alto Adige | 45.962.994; 10.949.407 | 520 | west | calcareous | orchard | grafted | 100 |
| TN3 | Pranzo 1 | TN–Trentino Alto Adige | 45.919.926; 10.821.334 | 457 | east | calcareous | orchard, mixed wood | grafted, sprouts | 10–100 |
| TN4 | Pranzo 2 | TN–Trentino Alto Adige | 45.925.029; 10.815.196 | 537 | east | calcareous | orchard | grafted, sprouts | 10–100 |
| TN5 | Pranzo3 | TN–Trentino Alto Adige | 45.927.132; 10.814.403 | 590 | west | calcareous | orchard, mixed wood | grafted, sprouts | 10–100 |
| TN6 | Campi | TN–Trentino Alto Adige | 45.911.697; 10.817.839 | 670 | northwest | calcareous | orchard, mixed wood | grafted, sprouts | 10–100 |
| TN7 | Valterigo | TN–Trentino Alto Adige | 46.160.358; 11.163.589 | 711 | northwest | marly siliceous | new plantation | grafted | 5 |
| TN8 | Nursery San Michele | TN–Trentino Alto Adige | 46.203.289; 11.139.945 | 204 | west | calcareous | nursery | seedling | 1 |
| AD1 | Nursery Piccolongo | BZ–Trentino Alto Adige | 46.358.521; 11.284.808 | 231 | east | calcareous | nursery | seedling | 1 |
| Site ID | Location | Main Phytosanitary Problems | |||||
|---|---|---|---|---|---|---|---|
| Mosaic | Yellowing | Blight | Ink Disease | ACGW | Other | ||
| M1 | Venamartello | 1 | 1 | 4 | 0 | 2 | 0 |
| M2 | Acquasanta Terme–Umito | 4 | 0 | 4 | 4 | 2 | 0 |
| ER1 | Selva la Maddalena | 0 | 2 | 4 | 1 | 2 | 0 |
| ER2 | Loiano | 1 | 1 | 4 | 0 | 2 | 0 |
| ER3 | Ca’ di Balloni | 1 | 0 | 4 | 1 | 2 | 0 |
| ER4 | Gragnano | 2 | 0 | 4 | 0 | 2 | 0 |
| ER5 | Malarbergo 1 | 1 | 0 | 4 | 0 | 2 | 0 |
| ER6 | Malalbergo 2 | 1 | 0 | 4 | 0 | 2 | 0 |
| ER7 | Alberelli | 1 | 0 | 4 | 0 | 2 | 0 |
| ER8 | Monteombraro Lavezze | 1 | 1 | 4 | 0 | 2 | 0 |
| ER9 | Monteombraro Caseificio | 1 | 0 | 4 | 0 | 2 | 0 |
| ER10 | Montese | 0 | 1 | 4 | 0 | 2 | 0 |
| TO1 | Castagneto | 2 | 2 | 4 | 0 | 2 | 0 |
| TO2 | Gaggiolo | 1 | 0 | 4 | 0 | 2 | 0 |
| TN1 | Mezzolombarbdo | 0 | 0 | 4 | 0 | 2 | 0 |
| TN2 | Drena | 0 | 1 | 4 | 0 | 2 | 0 |
| TN3 | Pranzo 1 | 1 | 1 | 4 | 0 | 2 | 0 |
| TN4 | Pranzo 2 | 0 | 1 | 4 | 0 | 2 | 0 |
| TN5 | Pranzo 3 | 0 | 1 | 4 | 0 | 2 | 0 |
| TN6 | Campi | 0 | 1 | 4 | 0 | 2 | 0 |
| TN7 | Valternigo | 2 | 2 | 0 | 0 | 2 | 0 |
| TN8 | Nursery San Michele | 1 | 0 | 0 | 0 | 0 | 0 |
| AD1 | Nursery Piccolongo | 0 | 1 | 0 | 0 | 0 | 0 |
| Symptoms | No. Samples | Molecular Analysis | |
|---|---|---|---|
| ChMV (%) | Phytoplasma (%) | ||
| Mosaic | 23 | 20 (91.3) | 0 |
| Nutrient deficiency | 38 | 12 (31.6) | 0 |
| No symptom | 40 | 12 (30.00) | 0 |
| Total | 101 | 45 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Murolo, S.; Bertoldi, D.; Pedrazzoli, F.; Mancini, M.; Romanazzi, G.; Maresi, G. New Symptoms in Castanea sativa Stands in Italy: Chestnut Mosaic Virus and Nutrient Deficiency. Forests 2022, 13, 1894. https://doi.org/10.3390/f13111894
Murolo S, Bertoldi D, Pedrazzoli F, Mancini M, Romanazzi G, Maresi G. New Symptoms in Castanea sativa Stands in Italy: Chestnut Mosaic Virus and Nutrient Deficiency. Forests. 2022; 13(11):1894. https://doi.org/10.3390/f13111894
Chicago/Turabian StyleMurolo, Sergio, Daniela Bertoldi, Federico Pedrazzoli, Manuela Mancini, Gianfranco Romanazzi, and Giorgio Maresi. 2022. "New Symptoms in Castanea sativa Stands in Italy: Chestnut Mosaic Virus and Nutrient Deficiency" Forests 13, no. 11: 1894. https://doi.org/10.3390/f13111894
APA StyleMurolo, S., Bertoldi, D., Pedrazzoli, F., Mancini, M., Romanazzi, G., & Maresi, G. (2022). New Symptoms in Castanea sativa Stands in Italy: Chestnut Mosaic Virus and Nutrient Deficiency. Forests, 13(11), 1894. https://doi.org/10.3390/f13111894







