Abstract
The initial carbon (C) quality of plant litter is one of the major factors controlling the litter decomposition rate and regulating C sequestration, but a comprehensive understanding is still lacking. Here, we used proximate analysis and 13C nuclear magnetic resonance (NMR) with spectral editing techniques to quantify the variations in the initial C quality for four dominant species (fir: Abies faxoniana Rehd. et Wils.; spruce: Picea asperata Mast; willow: Salix paraplesia Schneid; and rosa: Rosa omeiensis Rolfe.), including the organic compositions and C-based chemical structures of newly shed foliar litter over eight months in an alpine forest on the eastern Tibetan Plateau. The results indicated that the fractions of acid-soluble extractives (ASE) and acid-unhydrolyzable residues (AUR) were the main fractions of organic components, and aliphatic C and O-alkyl C were the main functional C groups for all plant species. Under the effects of the plant species, higher levels of ASE (37.62%) and aliphatic C (35.44%) were detected in newly shed rosa foliar litter, while higher levels of AUR (fir: 37.05%; spruce: 41.45%; and willow: 40.04%) and O-alkyl C (fir: 32.03%; spruce: 35.02%; and willow: 32.34%) were detected in newly shed fir, spruce and willow foliar litter. Moreover, the A/O-A and HB/HI ratios in rosa litter were 0.88 and 1.15, respectively, which were higher than those in fir, spruce and willow litter. The C quality of newly shed foliar litter varied seasonally due to the litter quality and environmental conditions, especially nitrogen (N), dissolved organic carbon (DOC), manganese (Mn) and monthly air temperature. We also found that C loss during 4-year litter decomposition was highly related to the aromatic C and phenolic C contents in newly shed foliar litter, suggesting that litter decomposition was strongly controlled by the initial recalcitrant C fractions. We conclude that the C quality of newly shed foliar litter in rosa might be structurally stable and more resistant to degradation than that of fir, spruce and willow, which contain abundant labile C fractions, and the initial recalcitrant C fractions are closely related to C loss during litter decomposition, which might contribute to soil C sequestration in alpine forests.
1. Introduction
The initial litter quality, mainly referred to as the initial carbon (C) quality, which is commonly present in newly shed litter, is a critical factor controlling the processes of litter decomposition and soil C sequestration [1,2,3,4]. Generally, litter with abundant labile components has been widely documented to be more decomposable and to undergo rapid decomposition [5,6]. Simultaneously, as the main carrier of soil organic C, recalcitrant components mainly manage the accumulation and stabilization of soil organic matter [7,8]. The initial qualities of labile and recalcitrant components could be characterized by the organic C fractions [9,10,11], thus facilitating the evaluation and prediction of litter decomposition and subsequent soil C sequestration, but there remains a lack of comprehensive knowledge.
Traditionally, proximate C fraction analysis (PA) has been used to separate water-soluble extractives (WSE), organic-soluble extractives (OSE), and acid-soluble extractives (ASE) from acid-unhydrolyzable residues (AUR), which can indicate the C fractions of organic components in plant litter and soils [12,13]. This method provides a close approximation of the lignin content in materials such as wood containing a low tannin content [12,13]. Nevertheless, the insolubility and complicated chemical structures of AUR fractions make it difficult to be characterized via this method, especially the AUR in foliar litter which generally includes substantial contributions of tannins and aliphatic C (cutin and, surface waxes) [14]. Solid-state 13C cross polarization magic angle spinning magnetic resonance (CPMAS-NMR) spectroscopy has been proven to be an effective method that allows direct and nondestructive characterization of newly shed plant materials and litter and provides more specific information on the C quality, especially in terms of the AUR in foliar litter, which can be better defined [3,15,16]. Based on this method, previous studies demonstrated that O-alkyl C, which ranges from 60 to 95 ppm chemical shifts, is the most labile component originating from decomposable carbohydrates. Aliphatic C (0–45 ppm) is commonly referred to as a biologically stable structure, and it mainly contains long-chain aliphatic compounds, cutin and waxes [2,16,17,18]. Likewise, aromatic C (110–140 ppm) and phenolic C (140–165 ppm) have been determined as chemically recalcitrant fractions [2,18,19]. In addition, studies have found that the ratio of aliphatic C to O-alkyl C (A/O-A) or hydrophobic C to hydrophilic C (HB/HI) ((0–45 ppm + 110–165 ppm)/(45–110 ppm + 165–190 ppm)) could reflect the enhancement in chemical structures against degradation, and the higher these two ratios are, the more stable the chemical structures against degradation [20].
Seasonal climate change regulates the C quality in litter due to the plant phenology. The consumption of decomposable C fractions is accelerated by strong rainfall and transpiration, which results from the increased temperature and solar radiation as a direct consequence of the decline in the fractions of WSE, OSE fractions and carbohydrate O-alkyl C [21,22]. Similarly, the aforementioned climatic changes could lead to the accumulation of recalcitrant C fractions [23]. Moreover, as the main fractions determining the litter quality, structurally complex C fractions notably determine and vary with the various litter decomposition stages, particularly the early stage of decomposition, which is susceptible to climatic conditions, rather than the late stage of decomposition [24,25,26]. A large proportion of labile C input derived from newly shed litter rapidly decreases at the early stage of decomposition [7,22], whereas the limitation on litter decomposition at the late stage of decomposition is enhanced, and more recalcitrant C fractions are formed [27]. In addition, vegetation is often considered the key factor resulting in variation in the litter C quality [28]. It has been reported that ASE and AUR were the main C fractions in foliar litter, while the content of recalcitrant AUR was higher in coniferous litter than that in broadleaf litter [26,29]. The chemical structures and compositions of AUR vary with vegetation, which results in the differences in the decomposable capacity and C fraction contents [25,30]. It has been considered that a strong signal intensity is mostly found for O-alkyl C, which indicates that newly shed foliar litter comprises labile carbohydrates. Moreover, Ono et al. [19,31] found that more aliphatic C was detected in newly shed coniferous litter (cedar and cypress) than in broadleaf litter. Conversely, broadleaved litter (beech and oak) retained more recalcitrant aromatic C, which is more decomposable than aliphatic C. In addition, it was reported that phenolic C (lignin fractions) and methoxyl C in coniferous and broadleaf species were significantly related to the accumulation of AUR at the initial stage of decomposition, suggesting that the contribution of phenolic C and methoxyl C to the AUR fraction is higher and not limited by the species at this decomposition stage [26]. Therefore, we addressed the hypothesis that (1) the contents of C fractions would vary with the species, and more labile C fractions would be released from newly shed foliar litter, while more recalcitrant C fractions might accumulate during seasons with heavy rainfall and severe temperatures, while (2) the labile C fractions might be the major factor controlling C accumulation during litter decomposition.
To test the above hypotheses, we used PA analysis and 13C CPMAS NMR spectroscopy to detect the initial C quality of newly shed foliar litter for four dominant tree species, namely fir, spruce, willow and rosa, which were collected on a monthly basis from August 2015 to July 2016 in an alpine forest on the eastern Tibetan Plateau. In this study, we aimed to address three scientific questions: (1) How do the plant species and litter quality affect the C quality of newly shed foliar litter? (2) How does C quality of newly shed foliar litter vary seasonally? (3) How does initial C quality regulate decomposition processes? Therefore, our objective was to evaluate the capacity of the initial C input from plant litter and the initial potential of litter-derived C for C sequestration in the decomposition process in this study.
2. Materials and Methods
2.1. Site Description
This study was conducted at the Long-Term Research Station of the Alpine Forest Ecosystem in the Miyaluo Nature Reserve (31°14′ N, 102°53′ E, 2982–3020 m above sea level (masl)) which is located on the eastern Tibetan Plateau. This region is a typical and transitional winter-cold zone between the Tibetan Plateau and the Sichuan Basin. The annual mean temperature is 2.7 °C. The maximum and minimum temperatures are 23 °C (July) and −18 °C (January), respectively. The annual mean precipitation reaches approximately 850 mm. Winter normally extends from late October until the following April with snow accumulating, and the growing season with the most concentrated litterfall extends from April until November. The forest is dominated by coniferous fir (Abies faxoniana) and spruce (Picea asperata). The main understory shrubs include willow (Salix paraplesia), rosa (Rosa omeiensis) and azalea (Rhododendron lapponicum). The forest soil in this area is dark brown soil and brown soil, and it is considered to be poor and barren due to the frequent natural disasters and low temperature conditions [22,32,33].
2.2. Experimental Design and Sample Collection
Based on a previous field investigation, three replicate 50 × 50 m sampling sites exhibiting similar aspects and slopes were established in a spruce-fir forest (2982–3020 m above sea level (masl)), and the three sites were at least 50 m apart from each other. Twenty litterfall funnel-shaped trap collectors were placed randomly at each site and fixed approximately 1 m above the ground at the end of July 2015.
Newly shed foliar litter was collected on a monthly basis from August 2015 to April 2016. The collected litter samples were air-dried at room temperature for one week, and foliar litter was classified as fir, spruce, willow and rosa according to the litter species. All foliar litter materials were ground and passed through a 0.25-mm sieve as subsamples for chemical analysis.
2.3. Chemical Analysis
The samples were analyzed using a sequential extraction technique according to Hilli et al. [34] and Preston et al. [14]. The samples were sequentially treated with trichloromethane to extract organic-soluble extractives (OSE), followed by boiling water to extract water-soluble extractives (WSE) and finally 72% (v/v) sulfuric acid to extract acid-soluble extractives (ASE), and the remaining residues were considered acid-unhydrolyzable residues (AUR). We performed 3 laboratory replicates for each sample during the experiment.
During solid-state 13C NMR measurement, all samples were treated with hydrofluoric acid (HF) to remove paramagnetic materials such as iron oxide from samples as follows: 0.5 g subsamples were weighed into 50-mL falcon tubes, and the tubes were tightly capped and horizontally shaken after adding 10 mL of 46% HF. Then, 40 mL of deionized water was added to the tubes, and the tubes were centrifuged at 3000 rpm for 10 min. The supernatant was removed and discarded, and the residue was washed 3–5 times with deionized water to completely remove HF, and then dried at 60 °C in an oven. The HF-treated samples were used for solid-state 13C NMR analysis [19]. We did not perform laboratory replicate analyses of the samples. Generally, the measurements were better than the chemical extractions, and the replicates at each site were adequate to ensure the reliability of the data.
Solid-state 13C NMR spectra were obtained on a Bruker AVANCE III 400 spectrometer (Bruker BioSpin AG, Fällanden, Switzerland) at 100 MHz. The dried and finely powdered samples were packed in a zirconium dioxide (ZrO2) rotor topped with a Kel-F cap, which was spun at a 5 kHz rate. The contact time for the ramp sequence of the 1H ramp was 2 ms. A total of 2048 scans were recorded with a 1 s recycle delay for each sample, and the plotted spectral regions ranged from 0–220 ppm.
The contributions of various functional C groups to the total organic C (TOC) content were determined via the integration of their 13C signal intensity in the respective chemical shifts using MestreNova-9.0.1 (Mestrelab Research S.L., Santiago de Compostela, Spain). Functional C groups were assigned to aliphatic C (0–50 ppm), methoxyl C (50–60 ppm), O-alkyl C (60–95 ppm), di-O-alkyl C (95–110 ppm), aromatic C (110–140 ppm), phenolic C (140–165 ppm), and carboxyl C (165–220 ppm) [17,35]. Several hydrophobicity indices, including the ratio of aliphatic C to O-alkyl C (A/O-A) and the ratio of hydropholic-C to hydrophilic-C (HB/HI), i.e., (aliphatic C + functional C groups of the 110–165 ppm peak area)/(functional C groups of the 45–110 ppm peak area + functional C groups of the 165–190 ppm peak area), were used to reflect the stability of organic C. The higher these two ratios are, the higher the stability of organic C [20,36,37].
Total organic C, N and phosphorus (P) concentrations were determined via the dichromate oxidation, Kjeldahl determination (KDN, Top Ltd., Zhejiang, China) and phosphomolybdenum yellow spectrophotometry (TU-1901, Puxi Ltd., Beijing, China) methods, respectively [38]. Litter DOC was extracted by shaking 0.5 g of dried sample with 50 mL of deionized water for 30 min at room temperature, and the suspension was filtered through a 0.45-μm filter membrane. A total organic C analyzer (multi N/C 2100, Analytik Jena, Thüringen, Germany) was used to determine the litter DOC concentration [39]. The concentration of Mn via inductively coupled plasma-mass spectroscopy (ICP-MS, IRIS Advantage 1000, Thermo Elemental, Waltham, MA, USA) after litter materials (1.00 g) were digested in HNO3-HClO4 (5:1, v/v) at 160 °C for 5 h [40].
2.4. Statistical Analysis
A two-way ANOVA was performed to evaluate the effects of the collection time and plant species on the initial C quality and hydrophobicity indices. Multiple comparisons were used to assess the significance of the differences among the various plant species. The above analyses were performed in SPSS 22.0 (IBM SPSS Statistics Inc., Chicago, IL, USA). Linear analysis was used to determine the effects of methoxyl C, aromatic C and phenolic C in newly shed foliar litter on C loss during litter decomposition. Stepwise regression analysis was conducted to evaluate the effects of the environmental conditions and litter quality on the initial C quality. The above analyses were performed in Origin Pro 9.0 (OriginLab, Northampton, MA, USA). All graphics were generated in Origin Pro 9.0 (OriginLab, Northampton, MA, USA). C loss data for fir and willow litter in the 4-year decomposition process were collected from Ni et al. [41], which were used to detect the effects of the C quality of newly shed fir and willow foliar litter on C loss during litter decomposition.
3. Results
3.1. Proportions of Organic Components in Newly Shed Foliar Litter
The fractions of ASE and AUR were the main components among all plant species. In rosa litter, the content of ASE (37.62%) was higher than that of other C fractions, while in fir, spruce and willow litter, the content of AUR was higher than that of other C fractions (fir: 37.05%; spruce: 41.45%; willow: 40.04%) (Table 1). ASE and AUR were significantly affected by the collection time (ASE: p = 0.015; AUR: p = 0.003) and plant species (ASE: p = 0.020; AUR: p < 0.001) (Table 2). Regarding WSE, similar trends were observed between the fir and spruce litter materials, with peaks occurring in April (Figure 1). Regarding AUR, the content in fir, willow and rosa litter generally decreased from August to November (Figure 1).

Table 1.
Properties of the C quality of newly shed foliar litter for each plant species. Different lowercase letters indicate significant (p < 0.05) differences among the various plant species for each C quality aspect (plant species: fir, spruce, willow and rosa; functional C groups: aliphatic C, methoxyl C, O-alkyl C, di-O-alkyl C, aromatic C, phenolic C and carboxyl C; organic components: WSE, water-soluble extractives, OSE, organic-soluble extractives, ASE, acid-soluble extractives, AUR, acid-unhydrolyzable residues; HB/HI, (0–45 ppm + 110–165 ppm)/(45–110 ppm + 165–190 ppm), and A/O-A, aliphatic C/O-alkyl C)).

Table 2.
Results of two-way ANOVA testing for the effects of the collection time (from August 2015 to July 2016) and plant species on the C quality of newly shed foliar litter (plant species: fir, spruce, willow and rosa; functional C groups: aliphatic C, methoxyl C, O-alkyl C, di-O-alkyl C, aromatic C, phenolic C and carboxyl C; organic components: WSE, water-soluble extractives, OSE, organic-soluble extractives, ASE, acid-soluble extractives, AUR, acid-unhydrolyzable residues; HB/HI, (0–45 ppm + 110–165 ppm)/(45–110 ppm + 165–190 ppm), and A/O-A, aliphatic C/O-alkyl C)).
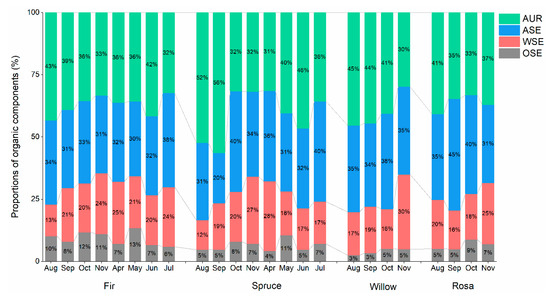
Figure 1.
Monthly dynamics of the proportions of organic components (%) (WSE: water-soluble extractives; OSE: organic-soluble extractives; ASE: acid-soluble extractives; AUR: acid-unhydrolyzable residues) in newly shed foliar litter determined via proximate C fraction analysis.
3.2. Proportions of Functional C Groups in Newly Shed Foliar Litter
Aliphatic C and O-alkyl C were the main functional C groups, while methoxyl C and carboxyl C were less abundant than the other functional groups (Table 1). In rosa litter, the content of aliphatic C (35.44%) was higher than that of the other functional C groups, while fir, spruce and willow litter contained more O-alkyl C (fir: 32.03%; spruce: 35.02%; willow: 32.34%). The plant species (p < 0.001) and collection time (p < 0.01) remarkably affected the functional C groups (Table 2). Smaller proportions of O-alkyl C in the fir and spruce litter samples were observed in April (Figure 2). The aromatic C and phenolic C contents in the fir and spruce litter samples remained stable with low proportions (Figure 2). The aliphatic C content in willow litter continuously decreased from August to November, while the aliphatic C content in rosa litter increased from August to October (Figure 2).
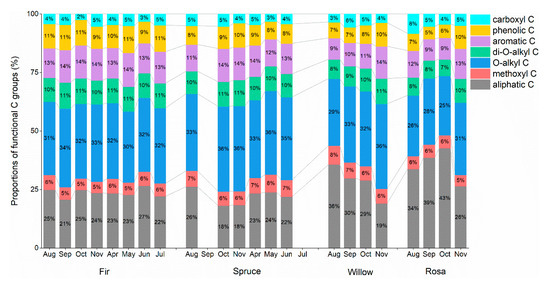
Figure 2.
Monthly dynamics of the proportions of functional C fractions (%) (aliphatic C, methoxyl C, O-alkyl C, di-O-alkyl C, aromatic C, phenolic C, and carboxyl C) in newly shed foliar litter obtained from the integration of different chemical shifts determined via CP/MAS 13C NMR spectroscopy.
3.3. A/O-A and HB/HI Ratios of Newly Shed Foliar Litter
The ratios of A/O-A (0.88) and HB/HI (1.15) in rosa litter were higher than those in the litter of the other species, while those in spruce litter were the lowest (A/O-A: 0.43; HB/HI: 0.78) (Table 1). The plant species (p < 0.001) and collection time (p < 0.001) significantly influenced A/O-A and HB/HI (Table 2). The ratios of A/O-A in the fir and spruce litter samples were significantly lower than those in the willow and rosa litter samples (Figure 3a). The ratios of HB/HI in the fir and spruce litter samples were more stable than those in the willow and rosa litter samples, respectively (Figure 3b).
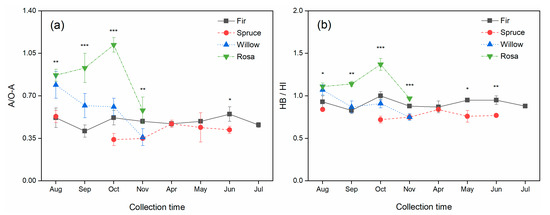
Figure 3.
Monthly dynamics of the ratio of (a) aliphatic C to O-alkyl C (A/O-A) and (b) the index of hydrophobicity (HB/HI, (0–45 ppm + 110–165 ppm)/(45–110 ppm + 165–190 ppm)) in newly shed foliar litter determined via CP/MAS 13C NMR spectroscopy. The * indicates significant (p < 0.05) differences among the various plant species. * p < 0.05, ** p < 0.01, and *** p < 0.001.
3.4. Relationships between the Initial Functional C Groups and C Loss during Litter Decomposition
Among all the functional C groups, methoxyl C, aromatic C and phenolic C significantly (p < 0.05) affected C loss during litter decomposition. Methoxyl C (coefficient: 0.49) and aromatic C (coefficient: −0.54) markedly controlled C loss in the third and first years of litter decomposition, respectively, and phenolic C significantly controlled C loss in both the second (coefficient: −0.51) and fourth (coefficient: −0.47) years of litter decomposition (Figure 4).
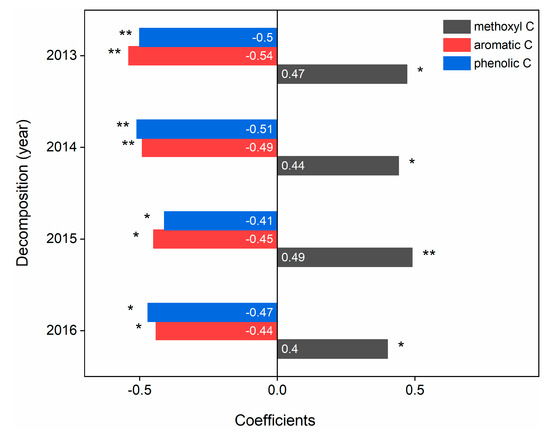
Figure 4.
Results of linear regression analysis between functional C groups and C loss in litter decomposition processes. The * indicates the significance (p < 0.05) between functional C groups and C loss at different decomposition stages in the regression model. * p < 0.05, ** p < 0.01, and Pearson’s r values from linear regression are shown in each panel.
4. Discussion
In this study, ASE and AUR were the main organic C fractions for all species, which is consistent with the study results of Yang et al. [29]. Consistent with our hypothesis, a large amount of labile O-alkyl C was detected in the newly shed foliar litter, so that it could be sufficiently consumed by a large microbial population in the subsequent decomposition process, which has been commonly reported [3,11]. Moreover, the functional C group contents in newly shed foliar litter varied with the plant species, and labile O-alkyl C was mostly detected in the fir, spruce and willow litter materials, whereas rosa litter contained abundant recalcitrant aliphatic C. Therefore, this result suggested that the initial C quality might be species-dependent, which is consistent with our hypothesis. This might occur because rosa litter could highly protect the leaf surface from leaching as a result of the abundant recalcitrant aliphatic C and higher hydrophobicity indices (Table 1) [11,18], while the initial C fractions in fir, spruce and willow litter might potentially contribute to C turnover in alpine forests. Moreover, in accordance with the results of other studies on litter decomposition, the initial C quality in foliar litter was controlled by DOC, N and Mn (Figure 5) [1,4,6], which illustrated the key role of the litter quality in the formation and preservation of litter-derived C in soils. Methoxyl C, aromatic C and phenolic C in newly shed foliar litter significantly regulated C loss during litter decomposition, which indicated that the variation in the C quality of newly shed litter was considered a critical factor influencing litter decomposition and greatly contributed to soil C sequestration in alpine forests.
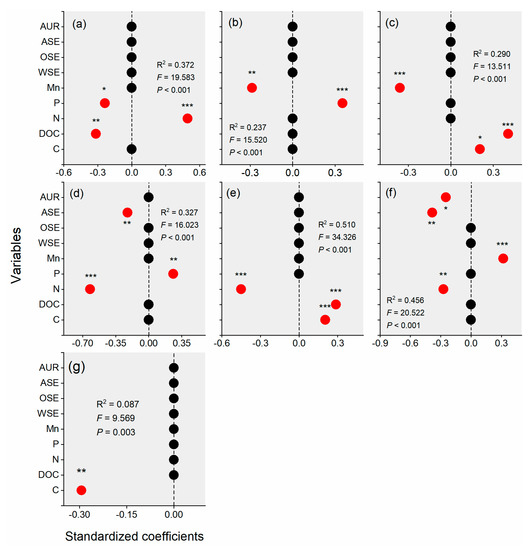
Figure 5.
Results of stepwise regression analysis of the functional C groups ((a): aliphatic C, (b): methoxyl C, (c): O-alkyl C, (d): di-O-alkyl C, (e): aromatic C, (f): phenolic C, and (g): carboxyl (C)) using the litter quality (C: carbon; DOC: dissolved organic carbon; N: nitrogen; P: phosphorus; Mn: manganese; WSE: water-soluble extractives; OSE: organic-soluble extractives; ASE: acid-soluble extractives; AUR: acid-unhydrolyzable residues). The dominant variables are emphasized in red in the regression model. The values represent standardized coefficients, and the * indicates significant effects of the variables on the functional C fractions in a given regression model * p < 0.05, ** p < 0.01, and *** p < 0.001. For example, aliphatic C was significantly impacted by P (p < 0.05), DOC (p < 0.01) and N (p < 0.001) in the stepwise regression testing the effects of litter chemical quality on aliphatic C (a).
The plant species significantly regulated the initial C quality of newly shed foliar litter. In our study, the initial C quality of newly shed fir, spruce and willow foliar litter was dominated by labile carbohydrates because strong signals were detected in the O-alkyl C regions [35,42]. Furthermore, previous studies found that the rapid decrease in O-alkyl C attributed to polysaccharide materials at the initial decomposition stage generally occurred due to the preferential degradation of labile cellulosic compounds [2,43]. Hence, the result whereby O-alkyl C release in rosa litter was higher than that in fir, spruce and willow litter in this study might mostly occur because carbohydrates in O-alkyl C in rosa litter might be easier to degrade than those in fir, spruce and willow litter. Moreover, xylose is considered the dominant hemicellulose monosaccharide in broad-leaved tissues, and its degradation occurs at least one year after broad-leaved litter decomposition. Nevertheless, arabinose and galactose, the main dominant hemicellulose monosaccharides in coniferous tissues, immediately degrade upon litter formation [27,44]. Therefore, the ASE content in rosa and willow litter was higher than that in fir and spruce litter. Our study also found that the aliphatic C content and A/O-A and HB/HI ratios in rosa litter were higher than those in the litter of the other species, suggesting that the C structure in rosa litter was mainly assigned to aliphatic C and was more complex than that in the litter of the other species [20,45]. The results are consistent with the studies of Hishinuma et al. [2] and De Marco et al. [26] but inconsistent with the studies of Ono et al. [31] and Ono et al. [25], which could be explained by the variations in biosynthetic lipid processes among plant species. In addition, the less complex chemical structure determined by the indices of A/O-A and HB/HI in coniferous species versus broadleaved species might also be attributable to the plant species [45,46]. Thus, rosa litter might be more resistant to decomposition. In addition, the abundance of guaiacyl lignins and stable tannins in coniferous fir and spruce and broadleaf willow and rosa might lead to differences in the proportions of aromatic C and phenolic C among the various litter types [25,45]. In general, the contents of ASE and aliphatic C might be the key C quality factors controlling the chemical structure of newly shed foliar litter, particularly that of broad-leaved rosa in this study.
The litter quality also dramatically determined the C quality of newly shed foliar litter, and this might lead to seasonal variations in the C quality together with environmental conditions. Our analysis results showed that the N content also dominated the recalcitrant C quality of newly shed foliar litter (Figure 5), which is consistent with the decomposition process. This might be due to foliar litter releasing a large amount of nutrients, such as N, at the early stage of decomposition under the notable influences of the litter quality and environmental conditions and then further stimulating microbial activity that might easily accelerate the degradation of recalcitrant substances [47,48,49]. This might also be the reason that stable aromatic C and phenolic C and low ratios of A/O-A and HB/HI in willow and rosa litter were detected in November. The insulating effect of snow cover at our study site during this period might promote microbial activity, thus resulting in fast decomposition [41,50]. Similarly, soluble nutrients, such as DOC, were rapidly released at the early stage of decomposition. In our study, DOC was the most significant factor influencing WSE and O-alkyl C and strongly responded to environmental conditions (Figure 5); therefore, a large amount of easily available compounds may be formed as a result of the thick litter layer and could be easily leached from foliar litter by light snowfall in October and November of this region, thus leading to more labile WSE and O-alkyl C released from foliar litter [51]. Moreover, our analysis results showed that DOC significantly affected the recalcitrant AUR (Figure S1), aliphatic C and aromatic C, which might have resulted from DOC derived from the oxidative degradation of lignin. Water-soluble components primarily controlled the formation and persistence of recalcitrant substances in forest soils by significantly affecting microbial activity [52,53]. This might be the main reason why the temporal characteristics of the aliphatic C and AUR fraction were opposite to those of WSE and O-alkyl C during the periods of October and November. In addition, the initial Mn generally functioned as a cofactor of lignin-degrading enzymes to regulate litter decomposition and humus stability, revealing a high contribution to the control of C sequestration in the organic layer [4,53]. The significant correlations between Mn and C quality, especially O-alkyl C and O-alkyl C, in newly shed foliar litter in our study might also indicate that the initial Mn might potentially contribute to C sequestration in soils.
In accordance with our hypothesis (2), the initial C quality notably controlled litter decomposition processes and could further affect C sequestration in soils. In combination with the litter decomposition experiment of Ni et al. [41], we found that the C loss in the 4-year decomposition process was highly related to the initial aromatic C and phenolic C, which are commonly referred to as structurally complex guaiacyl- and syringyl-lignin, respectively. This result notably illustrated that the initial recalcitrant C quality mainly determined C sequestration in soils in the decomposition process, which is contrary to the results of recent studies whereby labile components were the main factor influencing the decomposition process [18,20]. Nevertheless, the initial methoxyl C, which is also commonly reported as the aromatic rings of guaiacyl and syringyl units in lignin and could be structurally stable [11,36], was positively related to the C loss of decomposing litter in our study. This might be due to the region of initial methoxyl C also arising from other sources including proteins and amino acids [14].
5. Conclusions
Our results indicated that the initial C quality of newly shed foliar litter significantly varied with the plant species over time. Newly shed foliar litter of fir, spruce and willow comprising more labile C might contribute to the magnitude of accumulation of the soil C pool in alpine forests. Rosa litter, obtaining more structurally complex recalcitrant C fractions, might qualitatively contribute to the stability and sequestration of soil organic C. Moreover, other litter quality-related factors, such as N, DOC, and Mn, strongly controlled the initial C quality of newly shed foliar litter. In addition, the initial methoxyl C, aromatic C and phenolic C were the best predictors of C loss during litter decomposition in the alpine forests. However, our study did not consider the C quality in soils, and we could speculate that labile and recalcitrant C fractions could exhibit different characteristics in soils. Therefore, future efforts should be devoted to thoroughly studying the C quality based on the continuous process of “newly shed litter-decomposing litter-soils” to obtain the contributions of litter-derived C to soil C sequestration in alpine forests.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/f13111886/s1, Figure S1: Results of stepwise regression analyses of initial organic components ((a): WSE, water-soluble extractives; (b): OSE, organic-soluble extractives; (c): ASE, acid-soluble extractives; (d): AUR, acid-unhydrolyzable residues) using litter quality and environmental conditions (C: carbon; DOC: dissolved organic carbon; N: nitrogen; P: phosphorus; Mn: manganese; MAT: monthly average temperature; MAP: monthly average precipitation). The dominant variables are emphasized by red color in the regression model, Values represent the standardized coefficients, The * indicates significant effects of variables on C fractions in a given regression model * p < 0.05, ** p < 0.01, and *** p < 0.001. For example, OSE was significantly impacted by MAT (p < 0.05), Mn (p < 0.001), P (p < 0.001), and DOC (p < 0.001) in the stepwise regression testing the effects of litter chemical quality and environmental conditions on OSE (a).
Author Contributions
Conceptualization, methodology, validation, resources, supervision and writing—review and editing, J.M. and Q.W.; Project administration and funding acquisition, Q.W.; Conceptualization, data curation, investigation, methodology and writing—original draft, J.Y.; Investigation, Q.D., Y.Y., Y.Z. and C.F. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (31800521, 32022056, 31800373, 31922052 and 32071747), the Fok Ying-Tong Education Foundation for Young Teachers (161101), the National Natural Science Foundation of Sichuan Province (2022NSFSC0087), the Key R&D Program of Sichuan (18ZDYF0307), the Research Fund of Mianyang Normal University (QD2020A18), and the Open Fund of Ecological Security and Protection Key Laboratory of Sichuan Province, Mianyang Normal University (ESP1807).
Institutional Review Board Statement
Not applicable.
Acknowledgments
We wish to thank Zhuang Wang, Fan Yang, Xinyu Wei, Ling Mou, Qun Liu, Fujia Wu, Ji Yuan, and Long Jiang for the sample collecting.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Li, Q.; Zhang, M.; Geng, Q.; Jin, C.; Zhu, J.; Ruan, H.; Xu, X. The roles of initial litter traits in regulating litter decomposition: A “common plot” experiment in a subtropical evergreen broadleaf forest. Plant Soil 2020, 452, 207–216. [Google Scholar] [CrossRef]
- Hishinuma, T.; Osono, T.; Fukasawa, Y.; Azuma, J.-i.; Takeda, H. Application of 13C NMR spectroscopy to characterize organic chemical components of decomposing coarse woody debris from different climatic regions. Ann. For. Res. 2015, 58, 3–13. [Google Scholar] [CrossRef]
- Sarker, T.C.; Maisto, G.; De Marco, A.; Esposito, F.; Panico, S.C.; Alam, M.F.; Mazzoleni, S.; Bonanomi, G. Explaining trajectories of chemical changes during decomposition of tropical litter by 13C-CPMAS NMR, proximate and nutrients analysis. Plant Soil 2019, 436, 13–28. [Google Scholar] [CrossRef]
- De Marco, A.; Fioretto, A.; Giordano, M.; Innangi, M.; Menta, C.; Papa, S.; Virzo De Santo, A. C stocks in forest floor and mineral soil of two mediterranean beech forests. Forests 2016, 7, 181. [Google Scholar] [CrossRef]
- Mastný, J.; Kaštovská, E.; Bárta, J.; Chroňáková, A.; Borovec, J.; Šantrůčková, H.; Urbanová, Z.; Edwards, K.R.; Picek, T. Quality of DOC produced during litter decomposition of peatland plant dominants. Soil Biol. Biochem. 2018, 121, 221–230. [Google Scholar] [CrossRef]
- Ni, X.Y.; Yang, W.Q.; Liao, S.; Li, H.; Tan, B.; Yue, K.; Xu, Z.F.; Zhang, L.; Wu, F.Z. Rapid release of labile components limits the accumulation of humic substances in decomposing litter in an alpine forest. Ecosphere 2018, 9, e02434. [Google Scholar] [CrossRef]
- Kögel-Knabner, I. The macromolecular organic composition of plant and microbial residues as inputs to soil organic matter: Fourteen years on. Soil Biol. Biochem. 2017, 105, A3–A8. [Google Scholar] [CrossRef]
- Pisani, O.; Lin, L.H.; Lun, O.O.Y.; Lajtha, K.; Nadelhoffer, K.J.; Simpson, A.J.; Simpson, M.J. Long-term doubling of litter inputs accelerates soil organic matter degradation and reduces soil carbon stocks. Biogeochemistry 2016, 127, 1–14. [Google Scholar] [CrossRef]
- Grossman, J.J.; Cavender-Bares, J.; Hobbie, S.E. Functional diversity of leaf litter mixtures slows decomposition of labile but not recalcitrant carbon over two years. Ecol. Monogr. 2020, 90, e01407. [Google Scholar] [CrossRef]
- Wang, Y.; Zheng, J.; Boyd, S.E.; Xu, Z.; Zhou, Q. Effects of litter quality and quantity on chemical changes during eucalyptus litter decomposition in subtropical Australia. Plant Soil 2019, 442, 65–78. [Google Scholar] [CrossRef]
- De Marco, A.; Spaccini, R.; Virzo De Santo, A. Differences in nutrients, organic components and decomposition pattern of Phillyrea angustifolia leaf litter across a low maquis. Plant Soil 2021, 464, 559–578. [Google Scholar] [CrossRef]
- Preston, C.M.; Nault, J.R.; Trofymow, J.A.; Smyth, C. Chemical changes during 6 years of decomposition of 11 litters in some Canadian forest sites. Part 1. elemental composition, tannins, phenolics, and proximate fractions. Ecosystems 2009, 12, 1053–1077. [Google Scholar] [CrossRef]
- Hilli, S.; Stark, S.; Willför, S.; Smeds, A.; Reunanen, M.; Hautajärvi, R. What is the composition of AIR? Pyrolysis-GC–MS characterization of acid-insoluble residue from fresh litter and organic horizons under boreal forests in southern Finland. Geoderma 2012, 179–180, 63–72. [Google Scholar] [CrossRef]
- Preston, C.M.; Nault, J.R.; Trofymow, J.A. Chemical changes during 6 years of decomposition of 11 litters in some Canadian forest sites. Part 2. 13C abundance, solid-state 13C NMR spectroscopy and the meaning of “lignin”. Ecosystems 2009, 12, 1078–1102. [Google Scholar] [CrossRef]
- Assuncao, S.A.; Pereira, M.G.; Rosset, J.S.; Berbara, R.L.L.; Garcia, A.C. Carbon input and the structural quality of soil organic matter as a function of agricultural management in a tropical climate region of Brazil. Sci. Total Environ. 2019, 658, 901–911. [Google Scholar] [CrossRef]
- Yeasmin, S.; Singh, B.; Smernik, R.J.; Johnston, C.T. Effect of land use on organic matter composition in density fractions of contrasting soils: A comparative study using 13C NMR and DRIFT spectroscopy. Sci. Total Environ. 2020, 726, 138395. [Google Scholar] [CrossRef]
- Mathers, N.J.; Jalota, R.K.; Dalal, R.C.; Boyd, S.E. 13C-NMR analysis of decomposing litter and fine roots in the semi-arid Mulga Lands of southern Queensland. Soil Biol. Biochem. 2007, 39, 993–1006. [Google Scholar] [CrossRef]
- Lorenz, K.; Lal, R.; Preston, C.M.; Nierop, K.G.J. Strengthening the soil organic carbon pool by increasing contributions from recalcitrant aliphatic bio(macro)molecules. Geoderma 2007, 142, 1–10. [Google Scholar] [CrossRef]
- Ono, K.; Hirai, K.; Morita, S.; Ohse, K.; Hiradate, S. Organic carbon accumulation processes on a forest floor during an early humification stage in a temperate deciduous forest in Japan: Evaluations of chemical compositional changes by 13C NMR and their decomposition rates from litterbag experiment. Geoderma 2009, 151, 351–356. [Google Scholar] [CrossRef]
- Ji, H.; Han, J.; Xue, J.; Hatten, J.A.; Wang, M.; Guo, Y.; Li, P. Soil organic carbon pool and chemical composition under different types of land use in wetland: Implication for carbon sequestration in wetlands. Sci. Total Environ. 2020, 716, 136996. [Google Scholar] [CrossRef]
- de Wit, H.A.; Ledesma, J.L.J.; Futter, M.N. Aquatic DOC export from subarctic Atlantic blanket bog in Norway is controlled by seasalt deposition, temperature and precipitation. Biogeochemistry 2016, 127, 305–321. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, J.P.; Yang, W.Q.; Tan, B.; Fu, C.K.; Wu, F.Z. Climate, plant organs and species control dissolved nitrogen and phosphorus in fresh litter in a subalpine forest on the eastern Tibetan Plateau. Ann. Sci. 2018, 75, 51. [Google Scholar] [CrossRef]
- Ni, X.Y.; Yang, W.Q.; Tan, B.; He, J.; Xu, L.Y.; Li, H.; Wu, F.Z. Accelerated foliar litter humification in forest gaps: Dual feedbacks of carbon sequestration during winter and the growing season in an alpine forest. Geoderma 2015, 241–242, 136–144. [Google Scholar] [CrossRef]
- Prescott, C.E.; Maynard, D.G.; Laiho, R. Humus in northern forests: Friend or foe? Ecol. Manag. 2000, 133, 23–36. [Google Scholar] [CrossRef]
- Ono, K.; Hiradate, S.; Morita, S.; Hirai, K. Fate of organic carbon during decomposition of different litter types in Japan. Biogeochemistry 2013, 112, 7–21. [Google Scholar] [CrossRef]
- De Marco, A.; Spaccini, R.; Vittozzi, P.; Esposito, F.; Berg, B.; Virzo De Santo, A. Decomposition of black locust and black pine leaf litter in two coeval forest stands on Mount Vesuvius and dynamics of organic components assessed through proximate analysis and NMR spectroscopy. Soil Biol. Biochem. 2012, 51, 1–15. [Google Scholar] [CrossRef]
- Berg, B. Decomposition patterns for foliar litter—A theory for influencing factors. Soil Biol. Biochem. 2014, 78, 222–232. [Google Scholar] [CrossRef]
- Chapin, F.S. Effects of plant traits on ecosystem and regional processes: A conceptual framework for predicting the consequences of global change. Ann. Bot. 2003, 91, 455–463. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, Y.; Fu, C.; Liang, Z.; Yue, K.; Xu, Z.; Ni, X.; Wu, F. Seasonal dynamics of organic components in fresh foliar litters at different gap positions in an alpine forest on the eastern Tibetan Plateau. J. Soils Sediments 2021, 21, 810–820. [Google Scholar] [CrossRef]
- Khan, M.U.; Ahring, B.K. Lignin degradation under anaerobic digestion: Influence of lignin modifications—A review. Biomass Bioenergy 2019, 128, 105325. [Google Scholar] [CrossRef]
- Ono, K.; Hiradate, S.; Morita, S.; Ohse, K.; Hirai, K. Humification processes of needle litters on forest floors in Japanese cedar (Cryptomeria japonica) and Hinoki cypress (Chamaecyparis obtusa) plantations in Japan. Plant Soil 2010, 338, 171–181. [Google Scholar] [CrossRef]
- Wu, F.Z.; Yang, W.Q.; Zhang, J.; Deng, R.J. Litter decomposition in two subalpine forests during the freeze–thaw season. Acta Oecol. 2010, 36, 135–140. [Google Scholar] [CrossRef]
- IUSS Working Group WRB. World Reference Base for Soil Resources 2014, update 2015. In International Soil Classification System for Naming Soils and Creating Legends for Soil Maps; Schad, P., van Huyssteen, C., Micheli, E., Eds.; World Soil Resources Reports No. 106; FAO: Rome, Italy, 2015; 192p. [Google Scholar]
- Hilli, S.; Stark, S.; Derome, J. Carbon quality and stocks in organic horizons in boreal forest soils. Ecosystems 2008, 11, 270–282. [Google Scholar] [CrossRef]
- Carrasco, B.; Cabaneiro, A.; Fernandez, I. Exploring potential pine litter biodegradability as a natural tool for low-carbon forestry. Ecol. Manag. 2017, 401, 166–176. [Google Scholar] [CrossRef]
- Bonanomi, G.; Incerti, G.; Giannino, F.; Mingo, A.; Lanzotti, V.; Mazzoleni, S. Litter quality assessed by solid state 13C NMR spectroscopy predicts decay rate better than C/N and Lignin/N ratios. Soil Biol. Biochem. 2013, 56, 40–48. [Google Scholar] [CrossRef]
- He, Y.; Chen, C.; Xu, Z.; Williams, D.; Xu, J. Assessing management impacts on soil organic matter quality in subtropical Australian forests using physical and chemical fractionation as well as 13C NMR spectroscopy. Soil Biol. Biochem. 2009, 41, 640–650. [Google Scholar] [CrossRef]
- Zhu, J.X.; He, X.H.; Wu, F.Z.; Yang, W.Q.; Tan, B. Decomposition of abies faxoniana litter varies with freeze–thaw stages and altitudes in subalpine/alpine forests of southwest China. Scand. J. For. Res. 2012, 27, 586–596. [Google Scholar] [CrossRef]
- Wang, D.; Abdullah, K.M.; Xu, Z.; Wang, W. Water extractable organic C and total N: The most sensitive indicator of soil labile C and N pools in response to the prescribed burning in a suburban natural forest of subtropical Australia. Geoderma 2020, 377, 114586. [Google Scholar] [CrossRef]
- Peng, Y.; Yang, W.Q.; Yue, K.; Tan, B.; Huang, C.P.; Xu, Z.F.; Ni, X.Y.; Zhang, L.; Wu, F.Z. Temporal dynamics of phosphorus during aquatic and terrestrial litter decomposition in an alpine forest. Sci. Total Environ. 2018, 642, 832–841. [Google Scholar] [CrossRef]
- Ni, X.Y.; Berg, B.; Yang, W.Q.; Li, H.; Liao, S.; Tan, B.; Yue, K.; Xu, Z.F.; Zhang, L.; Wu, F.Z. Formation of forest gaps accelerates C, N and P release from foliar litter during 4 years of decomposition in an alpine forest. Biogeochemistry 2018, 139, 321–335. [Google Scholar] [CrossRef]
- Baldock, J.A.; Masiello, C.A.; Gélinas, Y.; Hedges, J.I. Cycling and composition of organic matter in terrestrial and marine ecosystems. Mar. Chem. 2004, 92, 39–64. [Google Scholar] [CrossRef]
- McKee, G.A.; Soong, J.L.; Caldéron, F.; Borch, T.; Cotrufo, M.F. An integrated spectroscopic and wet chemical approach to investigate grass litter decomposition chemistry. Biogeochemistry 2016, 128, 107–123. [Google Scholar] [CrossRef]
- Schädel, C.; Blöchl, A.; Richter, A.; Hoch, G. Quantification and monosaccharide composition of hemicelluloses from different plant functional types. Plant Physiol. Biochem. 2010, 48, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Liu, S.; Wang, J.; Shi, Z.; Lu, L.; Guo, W.; Jia, H.; Cai, D. Dynamics and speciation of organic carbon during decomposition of leaf litter and fine roots in four subtropical plantations of China. Ecol. Manag. 2013, 300, 43–52. [Google Scholar] [CrossRef]
- Ussiri, D.A.N.; Johnson, C.E. Characterization of organic matter in a northern hardwood forest soil by 13C NMR spectroscopy and chemical methods. Geoderma 2003, 111, 123–149. [Google Scholar] [CrossRef]
- Yue, K.; Peng, C.; Yang, W.; Peng, Y.; Zhang, C.; Huang, C.; Wu, F. Degradation of lignin and cellulose during foliar litter decomposition in an alpine forest river. Ecosphere 2016, 7, e01523. [Google Scholar] [CrossRef]
- Maisto, G.; De Marco, A.; Meola, A.; Sessa, L.; Virzo De Santo, A. Nutrient dynamics in litter mixtures of four Mediterranean maquis species decomposing in situ. Soil Biol. Biochem. 2011, 43, 520–530. [Google Scholar] [CrossRef]
- Hopkins, D.W.; Sparrow, A.D.; Elberling, B.; Gregorich, E.G.; Novis, P.M.; Greenfield, L.G.; Tilston, E.L. Carbon, nitrogen and temperature controls on microbial activity in soils from an Antarctic dry valley. Soil Biol. Biochem. 2006, 38, 3130–3140. [Google Scholar] [CrossRef]
- Saccone, P.; Morin, S.; Baptist, F.; Bonneville, J.-M.; Colace, M.-P.; Domine, F.; Faure, M.; Geremia, R.; Lochet, J.; Poly, F.; et al. The effects of snowpack properties and plant strategies on litter decomposition during winter in subalpine meadows. Plant Soil 2013, 363, 215–229. [Google Scholar] [CrossRef]
- Yuan, Z.Y.; Chen, H.Y.H. Global trends in senesced-leaf nitrogen and phosphorus. Glob. Ecol. Biogeogr. 2009, 18, 532–542. [Google Scholar] [CrossRef]
- Klotzbücher, T.; Kaiser, K.; Guggenberger, G.; Gatzek, C.; Kalbitz, K. A new conceptual model for the fate of lignin in decomposing plant litter. Ecology 2011, 92, 1052–1062. [Google Scholar] [CrossRef] [PubMed]
- Trum, F.; Titeux, H.; Ponette, Q.; Berg, B. Influence of manganese on decomposition of common beech (Fagus sylvatica L.) leaf litter during field incubation. Biogeochemistry 2015, 125, 349–358. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).