Abstract
The regeneration of natural Betula platyphylla Suk., a pioneer tree and predominant species in stand forest of the northern region, China faces a challenge, i.e., population decline. One of the bottlenecks relative to the successful seedling establishment is the effectiveness of seed germination. In this study, four different families (3-4, 3-22, 3-42, and 3-43) of B.platyphylla seeds were used as research objects to explore the effects of temperature and PEG simulated drought on seed germination. The result showed that seed germination ability from different families increased first and then decreased with the increase in temperature. The germination rate (GR), germination value coefficient (GVC), and germination index (GI) at 25 °C and 30 °C were significantly higher than those at lower temperatures (15 °C and 20 °C) and higher temperature (35 °C), and the time for germination percentage to reach 50% (T50) was faster. The germination ability decreased gradually with the increase in drought stress. Seeds are more sensitive to drought stress at low temperatures, their germination is severely inhibited, and the tolerance range of PEG concentration at 15 °C (0%–10%) is less than other temperatures (10%–20%), while they can germinate well at high temperatures and the tolerance to drought stress is stronger. Our results suggested that 3-43 had better germination ability at high temperature, while 3-22 had better germination ability at low temperature. Our findings highlight low temperatures and drought stress as key factors limiting seed germination, which may be one of the bottlenecks to population regeneration. This research provides a scientific reference for the natural regeneration and population restoration of B. platyphylla.
1. Introduction
The study of plant population renewal is essential to the understanding of biodiversity and ecosystems. The population renewal of the natural Betula forest faces a big challenge and the population quantity is gradually decreasing in recent years [1,2,3], which will probably lead to massive declines in the natural forest area. The damaging consequences of this include the loss of ecological services (such as biodiversity protection), the loss of many goods (such as timber and nontimber forest products), these losses have fallen particularly heavily to our normal life [4,5,6]. Thus, it is necessary to explore the bottleneck of B. platyphylla Suk. population regeneration [7,8]. Previous studies on B. platyphylla have focused on its intensive breeding [7], gene regulation [9,10,11], and provenance selection [12] due to its economic benefits (i.e., as a landscape tree species and could provide wood). However, the regeneration restriction of B. platyphylla, which is a pioneer species widely distributed in the north of China and plays an important role in ecological restoration, remains unclear. According to field observation, B. platyphylla has a large number of seeds, thus the number of seeds may not be the main factor restricting its population regeneration; but there were few regeneration seedlings under the forest [2,3], therefore we speculate that one of the bottlenecks of B. platyphylla population regeneration may be affected by the restriction from seed to the seedling stage [13,14].
Seed germination is the basis of seedling establishment and an important link affecting plant population regeneration, but it is easy to be affected by external environmental factors [15,16]. Seeds can germinate quickly and establish seedlings in time under suitable environmental conditions, which can increase the probability of successful regeneration of the population; while seeds cannot germinate effectively into seedlings and affect population regeneration under unsuitable environmental conditions [14,17,18]. Many studies support that temperature and water are key environmental factors for seed germination in the natural environment [17,19,20], and it is closely related to the germination of plant seeds and the establishment of seedlings [18,21,22].
Temperature can affect the germination of non-dormant seeds directly or indirectly by altering the dormancy of seeds, thus influencing seedling growth and population regeneration and its dynamics [17,23]. Generally, relatively high temperatures within a certain temperature range are conducive to seed germination, while excessively high and low temperatures outside a certain range are disadvantageous [24,25,26]. The appropriate temperature for seed germination is generally relatively narrow, and some seeds can only germinate at a particular temperature, but this varies considerably between species. For instance, a study demonstrated that the germination rate reached the maximum at 5 °C of Vicia amoena Fisch. ex DC., 1859 seeds in the Qinghai-Tibet Plateau [27]. However, some plants have a relatively high optimum temperature for seed germination, such as Picea asperata Mast., 1906 and Betula albo-sinensis Burk. (25 °C) [1,2]. Unexpectedly, the seeds of Sophora alopecuroides from the Alaska desert even maintained a high germination rate at 40 °C [27]. Drought stress is also an important environmental factor affecting seed germination [28]. Polyethylene glycol (PEG), a hydrophilic macromolecule, has been widely used for drought tolerance studies in plant seed germination because of its simplicity and short cycle time for simulating drought stress using PEG solutions [29,30,31]. Numerous studies have shown that as the concentration of PEG increases, the germination rate, germination index, and vigor index of plant seeds all tend to decrease [31,32,33]. while t it has been shown that low concentrations of PEG promote seed germination [34]. Studies have reported significant inhibition of germination of Swainsonia salsula Pall. seeds at PEG concentrations up to 10% [35]. However, seed germination of Quercus variabilis can tolerate a 30% PEG concentration [36]. In addition, PEG simulated the interaction between drought and temperature, which also had an important impact on the germination of seeds and was also affected by temperature [26].
In this experiment, four B. platyphylla seeds of different families were taken as the research object to study the effects of temperature, drought, and their combined effects on seed germination, to explore the environmental factors limiting the germination of B. platyphylla seeds. This study could provide a theoretical basis and scientific guidance for the difficulties of seedling regeneration of the B. platyphylla community.
2. Materials and Methods
2.1. Seed Materials
In mid-August 2017, the seedling seed orchard of half-sib families of B. platyphylla was collected in the B. platyphylla seed intensive garden of Northeast Forestry University (126.63 E, 45.72 °N) in Harbin, Heilongjiang Province, China. It belongs to the continental monsoon climate of the middle temperate zone. The winter is long, cold, and dry, with more northwest wind. The summer is warm and short, with concentrated rainfall. The early spring is easy to dry with less rain, and the autumn is fast in cooling. Frost damage often occurs. The early frost in the middle of September and the late frost in late May have an average annual temperature of 2.4 °C, the highest temperature of 34 °C, the lowest temperature of −40 °C, the annual accumulated temperature ≥10 °C about 2300 °C, and the annual average precipitation of 567 mm. In order to test whether different families have consistent responses and select seeds with stronger tolerance for environmental factors. We collected seeds from four families (3-4, 3-22, 3-42, and 3-43) with good phenotypic growth, and each family selected five trees and collected about 2000 seeds per tree. We use 1/10,000 electronic balance to weigh 1000 seeds and repeated three times. Then, the seed length (longitudinal axis length of the seed, n = 100) and width (transverse maximum width of seed plane perpendicular to the longitudinal axis, n = 100) were measured with a digital caliper and repeated eight times.
2.2. Germination Experiments
For this experiment, B. platyphylla seeds from different families were tested at five physical temperature constants (15, 20, 25, 30, and 35 °C) and chemical reagent PEG-6000 percentage concentration (0%, 5%, 10%, 15%, and 20%) respectively under each temperature [19]. Each treatment contained four replicates of forty seeds (16,000 seeds in total) in the artificial climate box (type: SPX-330-C) produced by Shanghai Langyi Experimental Equipment Corporation. Put the different families B. platyphylla seeds which are plump and the same size as the 110 mm diameter Petri dishes, and two layers of filter paper as germination substrate. Using a wash bottle, inject the PEG solution into the bottom of the Petri dish along the edge of the filter paper until it is completely saturated. After sowing the seeds, the dishes were placed in an artificial climate chamber at the above-mentioned different temperatures. The test was started by placing the petri dishes in an artificial climate chamber at the different temperatures mentioned above, with 14 h of light (light intensity of approximately 11,500lx) and 10 h of darkness. In order to eliminate the interference caused by the gradual increase in PEG concentration due to water evaporation, the filter paper was replaced once at every 2 days intervals and PEG solution with corresponding percentage concentration was added.
2.3. Calculation of Seed Germination Parameters
The standard of germination test is the radicle had emerged by at least 2 mm [26]. The germination number and radicle length of B. platyphylla seeds were observed and recorded at the same time every day. After recording, the germinated seeds were taken out of the Petri dish. The number of germinated seeds was counted each day until no seed germination in two consecutive weeks. The parameters for evaluating seed vigor we selected are germination rate (GR), Time for seeds to reach half germination rate (T50), germination value coefficient (GVC), Germination index (GI) in this experiment [8,26], and calculated according to the following formula.
where n and N are the numbers of germinating seeds and the total number of seeds used in the experiment.
MDG (mean daily germination) is the average number of seeds germinated every day, and PV (peak value) is the average maximum number of germinated seeds per day during the germination test [37].
Gt is the number of germinated seeds in t days, and Dt is the days the germination test.
2.4. Statistical Analysis
Data were square root transformed prior to analysis when necessary. All experimental data are summarized in Microsoft Excel 2007 and analyses were conducted using SPSS 21.0 software (IBM, New York, NY, USA) and the significant difference was set at p < 0.05. The effects of families, temperature, and drought stress on seed germination parameters of B. platyphylla were analyzed by multivariate variance analysis (Multi-way ANOVA). We also analyzed the effects of temperature and drought on seed germination of different B. platyphylla families. The significant differences in seed germination parameters in different temperatures, PEG concentrations and different families were analyzed by the least significant difference (LSD) method.
3. Results
3.1. Traits of Betula platyphylla Seeds from Different Families
The 1000 seed weight of families 3-4 and 3-22 is significantly lower than that of families 3-42 and 3-43, the seed lengths of the different families are not significantly different, and the width of the seeds of family 3-4 is significantly greater than that of families 3-22 and 3-43 (Table 1).

Table 1.
Seed traits of different Betula platyphylla families.
3.2. Effects of Temperature, Drought and Family on Seeds Germination of Betula platyphylla
The temperature had a significant effect on the GR, T50, GVC, GI B. platyphylla (p < 0.001) (Table 2). On the whole, with the increase in temperature, the GP, GVC, and GI of B. platyphylla seeds of different families increased first and then decreased; on the contrary, T50 decreased first and then increased. The seeds GR was significantly higher at 25 °C and 30 °C than at other temperatures of 15 °C, 20 °Cand 25 °C. (Figure 1, Figure 2, Figure 3 and Figure 4). Drought also had a significant effect on seed GR, T50, GVC, and GI (p < 0.001) (Table 2). Overall, seeds GR, GVC, and GI all decreased with increasing PEG concentration, but T50 increased with increasing PEG concentration. Compared with other concentrations of PEG, the seeds of 4 families had higher GR, GVC, and GI, and lower T50 at 0% PEG and 5% PEG. When PEG concentration reached 10%, the GR, GVC, and GV decreased significantly (p < 0.001) (Figure 1, Figure 2, Figure 3 and Figure 4). There are also significant differences in seed germination parameters among different families (p < 0.001) (Table 1). At 15 °C and 0% PEG, the GP and GVC of family 3-22 were higher than those of the other three families (Figure 1, Figure 2, Figure 3 and Figure 4). For 3-43 family, at 20 °C, the GR, GVC, and GI were significantly lower than those of the other three families. When the PEG concentration is 0%, 5%, 10%, 15% and the temperature is 35 °C, the GR, GVC, and GI of 3-43 family are higher than those of the other three families, while T50 is lower than those of the other three families (Figure 1, Figure 2, Figure 3 and Figure 4).

Table 2.
Three-way ANOVA on the effects of family (F), temperature (T), drought (D) and the interaction on seed germination. of Betula platyphyll.
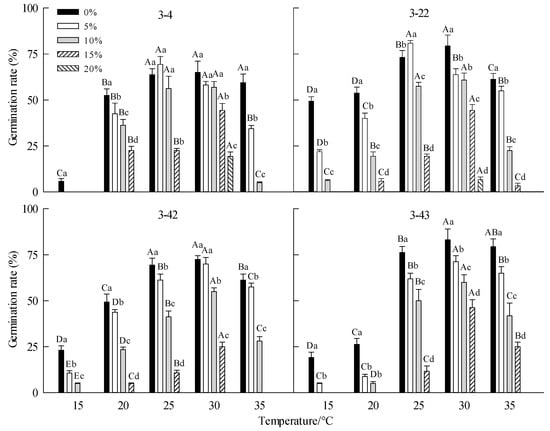
Figure 1.
Effect of drought stress on seed germination percentage of Betula platyphylla Suk. in different families under different temperatures. Different capital letters stand for significant differences between different temperatures of the same PEG concentration (p < 0.05), and different lowercase letters stand for significant differences between different PEG concentrations at the same temperature (p < 0.05); 0%, 5%, 10%, 15%, and 20% represent different concentrations of PEG; 3-4, 3-22, 3-42, 3-43 represent different families of Betula platyphylla seeds; Data are shown as mean ± Sd (n = 4).
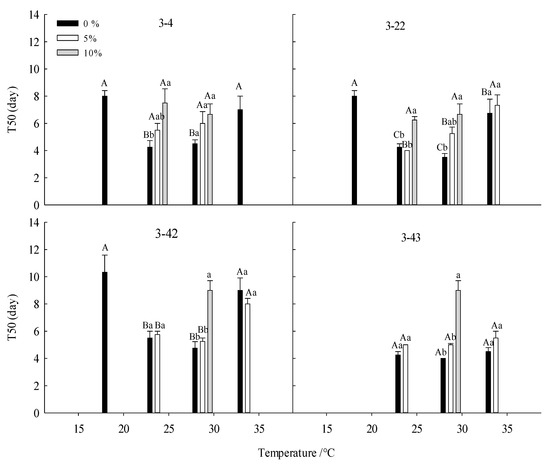
Figure 2.
Effect of drought stress on the time to 50% germination of seeds of Betula platyphylla in different families under different temperatures. Different capital letters stand for significant differences between different temperatures of the same PEG concentration (p < 0.05), and different lowercase letters stand for significant differences between different PEG concentrations at the same temperature (p < 0.05).
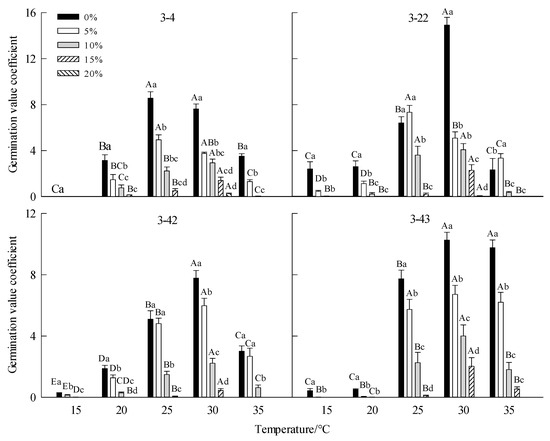
Figure 3.
Effect of drought stress on seed germination value of Betula platyphylla in different families under different temperatures. Different capital letters stand for significant differences between different temperatures of the same PEG concentration (p < 0.05), and different lowercase letters stand for significant differences between different PEG concentrations at the same temperature (p < 0.05).
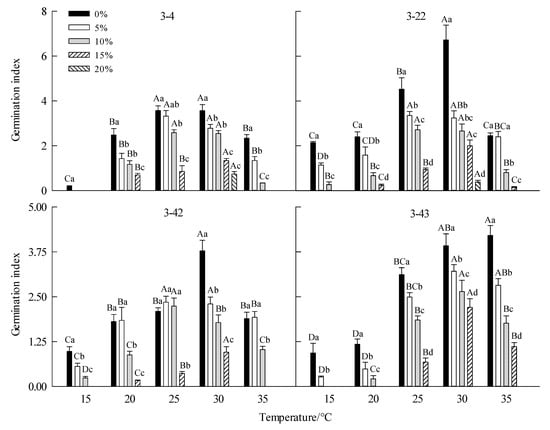
Figure 4.
Effect of drought stress on seed germination index of Betula platyphylla in different families under different temperatures. Different capital letters stand for significant differences between different temperatures of the same PEG concentration (p < 0.05), and different lowercase letters stand for significant differences between different PEG concentrations at the same temperature (p < 0.05).
3.3. Effect of Temperature on Seeds Germination of Betula platyphylla in Different Families
For 3-4 family, at 15 °C, the GR, GVC, and GI were the lowest. When the temperature was 25 °C and 30 °C, the GR, GVC, and GI were significantly higher than other temperatures, but T50 was lower than other temperatures (Table 3, Figure 1, Figure 2, Figure 3 and Figure 4). For family 3-22, at 25 °C and 30 °C, the seeds GR, GVI, and GI were significantly higher than those of low temperatures (15 °C and 20 °C) and high temperature (35 °C), while T50 was significantly lower than those of low temperature (15 °C and 20 °C) and high temperature (35 °C) (Table 3, Figure 1–4). For family 3-42, at 15 °C, the GR GVC and GI were the lowest. At 25 °C and 30 °C, the GR was significantly higher than that at low temperature (15 °C and 20 °C) and high temperature (35 °C), while T50 was significantly higher than that at 20 °C and 35 °C (Figure 1, Figure 2, Figure 3 and Figure 4). For family 3-43, at 15 °C and 20 °C, the GR did not reach 50%, at 25–35 °C, the seeds had high GR, GVC, and GI (Table 3, Figure 1, Figure 2, Figure 3 and Figure 4).

Table 3.
Two-way ANOVA on the effect of temperature and drought and their interaction on seed germination in different families of Betula platyphyll.
3.4. Effects of Drought on Seeds Germination of Betula platyphylla in Different Families
For family 3-4, with the increase in PEG concentrations, the GR, GVC, and GI decreased gradually, but T50 increased. There were differences under different temperatures. At 15 °C, no seeds germinated at other PEG concentrations except for 0% PEG. 15% PEG, some seeds germinated at 20 °C, 25 °C and 30 °C, and the drought tolerance was the highest at 30 °C (Table 3, Figure 1, Figure 2, Figure 3 and Figure 4). For family 3-22, with the increase in PEG concentrations, the GR, GVC, and GI decreased gradually, but T50 increased. The drought tolerance at 25 °C and 30 °C was higher than that at other temperatures, a few seeds germinated at 30 °C and 20% PEG (Table 3, Figure 1, Figure 2, Figure 3 and Figure 4). For family 3-42, with the increase in PEG concentration, the GR, GVI, and GI gradually decreased, but T50 increased. At 15 °C and 20 °C, the GR, GVC, and GI of different PEG treatments were relatively low. The drought resistance was the strongest at 30 °C, and the seeds GR of 10% PEG treatment was 55% (Table 3, Figure 1, Figure 2, Figure 3 and Figure 4). For family 3-43, with the increase in PEG concentration, the GR, GVC, and GI gradually decreased, but T50 increased. At 15 °Cand 20 °C, the GR, GVC, and GI of seeds treated with 10% PEG were significantly lower than that at other temperatures. The seeds had the strongest drought resistance at 30 °C, and the GR of seeds treated with 15% PEG at this temperature was 46.25% (Table 3, Figure 1, Figure 2, Figure 3 and Figure 4).
4. Discussion
4.1. Response of Seed Germination to Temperature
The process of seed germination is a series of material transformation and energy transfer processes of the seed embryo under the catalysis of various internal enzymes [25,26]. In this process, the temperature is closely related to the enzyme activity [34]. To maintain the maximum enzyme activity, an appropriate temperature threshold is required. Too high or too low a temperature will reduce the enzyme activity, which is reflected in the seed germination process [25,26,38,39]. Our results showed that the GR, GVC, and GI of B. platyphylla seeds of different families at 15 °C were lower than other temperatures. This was basically consistent with the reported by Kumar et al. [40], the germination rate of Andrographis paniculata (Burm.f.) Wall. ex Nees seeds is only 17.55% at the low temperature of 15 °C. Low temperature seriously affects the germination of seeds, which is due to the inhibition of enzyme activity in seeds, thus slowing down physiological metabolism activities and being unfavorable to seeds germination. In the area where we collect the seeds of B.platyphylla, the low temperature in spring makes few seeds germinate, thus reducing the establishment of B. platyphylla seedlings, which is consistent with our research results. With the temperature increased, the seeds GR, GVC and GI all tended to increase and then decrease, the time when the seeds GR reaches 50% decreased first and then increases, which is similar to the studies developed by Wu et al. [1] and Ren et al. [2]. The appropriate temperature can maintain the maximum enzyme activity, and excessive temperature also changes the membrane permeability and enzyme activity during germination, reducing metabolic activities [36]. Overall, relatively high GR, GVC, GI, and Lower T50 for the B.platyphylla families (3-4, 3-22, 3-42, and 3-43) at 25 °C and 30 °C. Therefore, we determined that the seeds of these four families of B.platyphylla were more suitable for germination in this temperature range. This is close to the results of a previous study on the optimum germination temperature of 25 °C for Betula albo-sinensis Burk. seeds [1]. In addition, we also found that different families have different responses to low temperature (15 °C) and high temperature (35 °C). Family 3-43 had larger germination ability at high temperature (35 °C), while the germination ability of family 3-22 was better at low temperature (15 °C). This may be attributed to the existence of regional temperature related to the survival of the original parents of different families.
4.2. Response of Seed Germination to Drought Stress
Water is one of the important environmental factors affecting the survival of plants in natural habitats, especially in the stage of plant regeneration [41,42]. In the process of germination, seeds can adapt to water stress by increasing the activity of protective enzymes and osmotic adjustment substances in vivo, thereby minimizing the damage caused by water stress to the germination process [41,42]. Many scholars explored the effect of drought stress on seed germination by using PEG and noticed that the germination parameters such as seed GR, GVC, and GI gradually decreased, and germination time prolonged with the increase in drought stress [31,32,33]. However, some studies have shown that light drought is conducive to seed germination [26,36]. Our results showed that with the increase in PEG concentration, the GR, GVC, and GI of B.platyphylla seeds from different families decreased gradually, but T50 increased with the increase in PEG concentration, which was basically consistent with the research result of Perala and Alm [40] that B. platyphylla seeds were extremely sensitive to drought stress. Gorai et al. [43] observed that Henophyton deserti Coss. & Durieu seed incubation is more tolerant to PEG drought stress at low temperature and even promoted incubation at low PEG concentrations. Similarly, Yan et al. [26] found that low PEG concentration under 20 °C promoted the germination of Caragana korshinskii seeds to a certain extent. At temperatures (25 °C and 30 °C), the GR, GVC, and GI decreased with the increase in PEG concentration, and the germination process slowed down. However, our study showed that the GR, GVC, and GI of different families of B.platyphylla seeds decreased with increasing PEG concentration at different temperatures, and that T50 increased with increasing PEG concentration at the temperature where the germination rate could reach 50%. This may be related to the small size of B.platyphylla seeds, where even low concentrations of PEG treatment can have a significant effect on their seed viability. Although the germination activity of seeds decreased with the increase in PEG concentration at different temperatures, we found that there were differences in the PEG tolerance of seeds at different temperatures. With the increase in temperature, the PEG tolerance concentration of B.platyphylla seeds increased first and then decreased. For example, when the PEG concentration exceeds 10% at 15 °C, the seeds of four families of B. platyphylla cannot germinate. However, at 25 °C and 30 °C, some seeds of four families of B.platyphylla still germinated at 15% EG concentration, which reflected that the drought tolerance of B.platyphylla seeds was stronger at relatively high temperatures. This is similar to the results reported by Li et al. [44], the rhubarb seeds germination is more sensitive to drought at low temperatures than at suitable temperatures. Maladaptive stresses accelerate the inhibition of seed germination by another stress, as the combined effects of temperature and drought create a physiological imbalance that in turn reduces germination rates, delays germination, and even causes seeds to lose viability [44]. This reflects that the germination ability of B.platyphylla seeds is affected by the interaction of temperature and PEG simulated drought. In addition, we found that there were differences in the activity and tolerance to PEG concentration of different B.platyphylla seeds. For example, (15 °C, 10% PEG), there are no germinating seeds in 3-4 and 3-43 families, while the seeds in 3-22 and 3%–425.6% and 3.7% germinate. (35 °C, 15% PEG), families 3-22 and 3-43 have 2.5% and 18.75% GP respectively. This may be related to the environmental difference of the original source of seed parents of B.platyphylla in different families.
5. Conclusions
Our study showed that the GR, GVC, GI, and germination process of four different families of B. platyphylla seeds increased first and then decreased with the increase in temperature. Low temperature (15 °C) had the greatest effect on seed germination. The temperature was 25 °C and 30 °C. The GR, GVC, and GI of seeds were higher than other temperatures, while T50 was lower than other temperatures. In addition, we found that the germination activity of seeds decreased with the increase in PEG concentration, and the drought tolerance was stronger at 25 °C and 30 °C. More importantly, there are differences in the response intensity of B.platyphylla seeds from different families to temperature and drought. The different germination parameters of families 3-22 at 15 °C were better than those of other families. Family 3-43, at 35 °C, PEG concentration is 0%, 5%, 10%, and 15%. Seed GR, GVC, and GI are higher than other families; on the contrary, T50 is smaller than other families. Our research suggests that low temperature may be the most important factor limiting the seed germination of four different families of B.platyphylla, and they are very sensitive to drought, but this drought sensitivity will decrease with the high temperature. In addition, we found that families 3-22 were more tolerant to low temperature, while families 3-43 were more tolerant to high temperature. Our results can provide a reference for population regeneration and artificial cultivation of B. platyphylla.
Author Contributions
Conceptualization, Y.L. and J.C. and J.Z. (Jinfeng Zhang); methodology, Y.L.; J.C. and X.Y.; validation, Y.L.; J.C.; X.Y. and J.Z. (Jinfeng Zhang); formal analysis, Y.L.; Cheng, J.-M; J.Z. (Jinfeng Zhang) and J.Z. (Jingbao Zhang); investigation, J.C.; resources, X.Y.; data curation, Y.L.; J.C.; J.Z. (Jinfeng Zhang) and J.Z. (Jingbao Zhang); writing—original draft preparation, Y.L.; J.C.; writing—review and editing, Y.L.; J.C.; X.Y.; J.Z. (Jinfeng Zhang) and Zhang, J.-B; visualization, Y.L.; J.C.; supervision, X.Y.; J.Z. (Jinfeng Zhang); project administration, X.Y.; funding acquisition, X.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (grant number: 31660195), the Key Research and Development Projects in Ningxia Hui Autonomous Region (grant number: 2018 BEG 02001).
Data Availability Statement
The data presented in this study are available in the forests data.xls.
Acknowledgments
Thanks to Jiang Jing, State Key Laboratory of Tree Genetics and Breeding, Northeast Forestry University, and Xu Wendi, Key Laboratory of Ecosystem Modeling and Applications of State Nationalities Affairs Commission, North Minzu University, for providing B. platyphylla seeds of different families (3-4, 3-22, 3-42 and 3-43). This research was funded by the National Natural Science Foundation of China (31660195), the Key Research and Development Projects in Ningxia Hui Autonomous Region (2018 BEG 02001).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Wu, Y.; Liu, Q.; He, H.; Lin, B.; Yin, H.J. Effects of light and temperature on seed germination of Picea asperata and Betula albo-sinensis. Chin. J. Appl. Ecol. 2004, 15, 2229–2232. [Google Scholar]
- Ren, Y.J.; Lin, Y.; Yue, M. Seed germination characteristics of Betula albo-sinensis at mountain Taibai, China. Chin. J. Plant Ecol. 2008, 32, 883–890. [Google Scholar]
- Chao, L.; Liu, Y.Y.; Wu, C.Z.; Hong, T.; Lin, Z.; Hong, W. Age structure and population dynamics of dominant species in a Betula platyphylla—Larix olgensis forest on swamp ecotone. Guihaia 2017, 37, 1406–1417. [Google Scholar]
- Lamb, D.; Erskine, P.D.; Parrotta, J.A. Restoration of degraded tropical forest landscapes. Science 2005, 310, 1628–1632. [Google Scholar] [CrossRef] [PubMed]
- Cardinale, B.J.; Duffy, J.E.; Gonzalez, A.; Hooper, D.U.; Perrings, C.; Venail, P.; Narwani, A.; Mace, G.M.; Tilman, D.; Wardle, D.A.; et al. Biodiversity loss and its impact on humanity. Nature 2012, 486, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Tittensor, D.P.; Walpole, M.; Hill, S.L.; Boyce, D.G.; Britten, G.L.; Burgess, N.D.; Butchart, S.H.; Leadley, P.W.; Regan, E.C.; Alkemade, R.; et al. A mid-term analysis of progress toward international biodiversity targets. Science 2014, 346, 241–244. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.P.; Liu, G.F.; Wei, Z.G.; Wu, Y.L.; Zhou, Y.M. Study on intensive breeding technique of accelerating Betula platyphylla flowering and seeding early. Scientia Silvae Sinicae 2004, 40, 14–17. [Google Scholar]
- Li, Z.X.; Pei, X.N.; Yin, S.P.; Lang, X.B.; Zhao, X.Y.; Qu, G.Z. Plant hormone treatments to alleviate the effects of salt stress on germination of Betula platyphylla seeds. J. For. Res. 2019, 30, 779–787. [Google Scholar] [CrossRef]
- Xu, W.; Han, R.; Xu, S.; Jiang, J.; Liu, G. Expression of BpIAA10 from Betula platyphylla (birch) is differentially regulated by different hormones and light intensities. Plant Cell Tissue Organ Cult. 2018, 132, 371–381. [Google Scholar] [CrossRef]
- Xu, W.; Chen, S.; Jiang, J.; Liu, G. Expression profiling of the BpIAA gene family and the determination of IAA levels in Betula platyphylla tetraploids. J. For. Res. 2019, 30, 855–867. [Google Scholar] [CrossRef]
- Gang, H.; Liu, G.; Chen, S.; Jiang, J. Physiological and transcriptome analysis of a yellow-green leaf mutant in birch (Betula platyphylla× B. Pendula). Forests 2019, 10, 120. [Google Scholar] [CrossRef]
- Liu, G.F.; Yang, C.P.; Liu, G.J.; Yang, R.H.; Kong, H.W. The configuration characters and germination percentages of various provenance seeds of Betula platyphylla. J. Northeast. For. Univ. 1999, 27, 1–4. [Google Scholar]
- Clark, J.S.; Macklin, E.; Wood, L. Stages and spatial scales of recruitment limitation in southern Appalachian forests. Ecol. Monogr. 1998, 68, 213–235. [Google Scholar] [CrossRef]
- Schupp, E.W.; Milleron, T.; Russo, S.E. Dissemination limitation and the origin and maintenance of species-rich tropical forests. In Seed Dispersal and Frugivory: Ecology, Evolution and Conservation; CABI: Wallingford, UK, 2002; pp. 19–33. [Google Scholar]
- Gutterman, Y. Strategies of seed dispersal and germination in plants inhabiting deserts. Bot. Rev. 1994, 60, 373–425. [Google Scholar] [CrossRef]
- Fennner, M.; Thompson, K. The Ecology of Seeds; Cambridge University Press: Cambridge, UK, 2005; pp. 23–26. [Google Scholar]
- Baskin, C.C.; Baskin, J.M. Germination ecophysiology of herbaceous plant species in a temperate region. Am. J. Bot. 1988, 75, 286–305. [Google Scholar] [CrossRef]
- Alvarado, V.; Bradford, K.J. A hydrothermal time model explains the cardinal temperatures for seed germination. Plant Cell Environ. 2002, 25, 1061–1069. [Google Scholar] [CrossRef]
- Baskin, J.M.; Baskin, C.C. A classification system for seed dormancy. Seed Sci. Res. 2004, 14, 1–16. [Google Scholar] [CrossRef]
- Hu, X.J.; Liu, C.; Li, A.R.; Yang, X.Y.; Baskin, C. Effect of temperature and moist conditions on seed dormancy cycling of two sympatric limestone species, Begonia guishanensis and Paraisometrum mileense, in southern China. Seed Sci. Res. 2020, 30, 29–36. [Google Scholar] [CrossRef]
- Whitmore, T.C. Secondary succession from seed in tropical rain forests. For. Abstr. 1983, 44, 767–779. [Google Scholar]
- Dürr, C.D.; Dickie, J.B.; Yang, X.Y.; Pritchard, H.W. Ranges of critical temperature and water potential values for the germination of species worldwide: Contribution to a seed trait database. Agric. For. Meteorol. 2015, 200, 222–232. [Google Scholar] [CrossRef]
- Gul, B.; Weber, D.J. Effect of salinity, light, and temperature on germination in Allenrolfea occidentalis. Can. J. Bot.-Rev. Can. Bot. 1999, 77, 240–246. [Google Scholar] [CrossRef]
- Gulzar, S.; Khan, M.A. Seed germination of halophytic grass Aeluropus lagopoides. Ann. Bot. 2001, 87, 319–324. [Google Scholar] [CrossRef]
- Pahlevani, A.H.; Rashed, M.H.; Ghorbani, R. Effects of environmental factors on germination and emergence of swallowwort. Weed Technol. 2008, 22, 303–308. [Google Scholar] [CrossRef]
- Yan, X.F.; Zhou, L.B.; Si, B.B.; Sun, Y.; Gao, Y.F.; Wang, R.X. Stress effects of simulated drought by polyethylene glycol on the germination of Caragana korshinskii Kom. seeds under different temperature conditions. Acta Ecol. Sin. 2016, 36, 1989–1996. [Google Scholar]
- Hu, X.W.; Fan, Y.; Baskin, C.C.; Baskin, J.M.; Wang, Y.R. Comparison of the effects of temperature and water potential on seed germination of Fabaceae species from desert and subalpine grassland. Am. J. Bot. 2015, 102, 649–660. [Google Scholar] [CrossRef]
- Bonjovani, M.R.; Barbedo, C.J. Induction of tolerance to desiccation and to subzero temperatures in embryos of recalcitrant seeds of inga. J. Seed Sci. 2014, 36, 419–426. [Google Scholar] [CrossRef]
- Inocente, M.C.; Barbedo, C.J. Germination of Eugenia brasiliensis, E. involucrata, E. pyriformis, and E. uniflora (Myrtaceae) under water-deficit conditions. J. Seed Sci. 2019, 41, 76–85. [Google Scholar] [CrossRef]
- Gholami, M.; Rahemi, M.; Kholdebarin, B. Effect of drought stress induced by polyethylene glycol on seed germination of four wild almond species. Aust. J. Basic Appl. Sci. 2010, 4, 785–791. [Google Scholar]
- Sevik, H.; Cetin, M. Effects of water stress on seed germination for select landscape plants. Pol. J. Environ. Stud. 2015, 24, 689–693. [Google Scholar] [CrossRef]
- Keller, M.; Kollmann, J. Effects of seed provenance on germination of herbs for agricultural compensation sites. Agric. Ecosyst. Environ. 1999, 72, 87–99. [Google Scholar] [CrossRef]
- Zeng, Y.J.; Wang, Y.R.; Baskin, C.C.; Baskin, J.M. Testing seed germination responses to water and salinity stresses to gain insight on suitable microhabitats for restoration of cold desert shrubs. J. Arid. Environ. 2014, 100, 89–92. [Google Scholar] [CrossRef]
- Vicente, M.J.; Martínez-Díaz, E.; Martínez-Sánchez, J.J.; Franco, J.A.; Bañón, S.; Conesa, E. Effect of light, temperature, and salinity and drought stresses on seed germination of Hypericum ericoides, a wild plant with ornamental potential. Sci. Hortic. 2020, 270, 109433. [Google Scholar] [CrossRef]
- Hui, Z.; Li, Z.; Liu, W.; Li, C.; Zhang, X.; Xu, Y.; Zhang, X.; Wang, D. Effects of fulvic acid soaking on seed germination and seedling growth of alfalfa under PEG-simulated drought stress. Chin. Northwest Bot. J. 2013, 33, 1621–1629. [Google Scholar]
- Li, Z.P.; Zhang, W.H.; Cui, Y.H. Effects of PEG-simulated drought stress on seed germination and growth physiology of Quercus cork. Chin. Northwest Bot. J. 2013, 33, 2043–2049. [Google Scholar]
- Czabator, F.J. Germination value: An index combining speed and completeness of pine seed germination. For. Sci. 1962, 8, 386–396. [Google Scholar]
- Boscagli, A.; Sette, B. Seed germination enhancement in Satureja montana L. ssp. montana. Seed Sci. Technol. 2001, 29, 347–355. [Google Scholar]
- Camacho, M.E.; Heitman, J.L.; Gannon, T.W.; Amoozegar, A.; Leon, R.G. Seed germination responses to soil hydraulic conductivity and polyethylene glycol (PEG) osmotic solutions. Plant Soil 2021, 462, 175–188. [Google Scholar] [CrossRef]
- Kumar, B.; Verma, S.K.; Singh, H.P. Effect of temperature on seed germination parameters in Kalmegh (Andrographis paniculata Wall. ex Nees.). Ind. Crops Prod. 2011, 34, 1241–1244. [Google Scholar] [CrossRef]
- Saeed, S.; Ullah, A.; Ullah, S.; Noor, J.; Ali, B.; Khan, M.N.; Hashem, M.; Mostafa, Y.S.; Alamri, S. Validating the impact of water potential and temperature on seed germination of wheat (Triticum aestivum L.) via hydrothermal time model. Life 2022, 12, 983. [Google Scholar] [CrossRef]
- Baskin, C.C.; Baskin, J.M. Seeds: Ecology, Biogeography, and Evolution of Dormancy and Germination; Academic Press: London, UK, 2014. [Google Scholar]
- Gorai, M.; El Aloui, W.; Yang, X.; Neffati, M. Toward understanding the ecological role of mucilage in seed germination of a desert shrub Henophyton deserti: Interactive effects of temperature, salinity and osmotic stress. Plant Soil 2014, 374, 727–738. [Google Scholar] [CrossRef]
- Borsai, O.; Al Hassan, M.; Boscaiu, M.; Sestras, R.E.; Vicente, O. Effects of temperature and drought stress on seed germination and seedling growth of three species of rhubarb. Chin. Herb. Med. 2022, 53, 2480–2489. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).