Field Trials to Assess the Growth, Survival, and Stomatal Densities of Five Mexican Pine Species and Their Hybrids under Common Plantation Conditions
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Evaluation of Differences in the Development of Hybrid/Pure Individuals in the Field
2.3. Calculation of Stomatal Density in Needles
2.4. Statistical Analysis
3. Results
3.1. Growth Parameters and Survival
3.2. Stomatal Density and Growth in the Field
4. Discussion
4.1. Seedling Growth and Survival
4.2. Stomatal Density
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rieseberg, L.H. Hybrid origins of plant species. Annu. Rev. Ecol. Syst. 1997, 28, 359–389. [Google Scholar] [CrossRef]
- Mallet, J. Hybridization, ecological races and the nature of species: Empirical evidence for the ease of speciation. Philos. Trans. R. Soc. B Biol. Sci. 2008, 363, 2971–2986. [Google Scholar] [CrossRef]
- Wright, J.W. Hybridization between species and races. Unasylva 1964, 18, 30–39. [Google Scholar]
- Arnold, M.L.; Hodges, S.A. Are natural hybrids fit or unfit relative to their parents? Trends Ecol. Evol. 1995, 10, 67–71. [Google Scholar] [CrossRef]
- Rieseberg, L.H.; Carney, S.E. Plant hybridization. New Phytol. 1998, 140, 599–624. [Google Scholar] [CrossRef] [PubMed]
- Gernandt, D.S.; Aguirre Dugua, X.; Vázquez-Lobo, A.; Willyard, A.; Moreno Letelier, A.; Pérez de la Rosa, J.A.; Piñero, D.; Liston, A. Multi-locus phylogenetics, lineage sorting, and reticulation in Pinus subsection Australes. Am. J. Bot. 2018, 105, 711–725. [Google Scholar] [CrossRef]
- Hernández-Velasco, J.; Hernández-Díaz, J.C.; Vargas-Hernández, J.J.; Hipkins, V.; Prieto-Ruíz, J.Á.; Pérez-Luna, A.; Wehenkel, C. Natural hybridization in seed stands of seven Mexican Pinus species. New For. 2022, 53, 487–509. [Google Scholar] [CrossRef]
- Rzedowski, J. Vegetación de México; Comisión Nacional para el Conocimiento y Uso de la Biodiversidad: México, México, 2006. [Google Scholar]
- Rieseberg, L.H.; Raymond, O.; Rosenthal, D.M.; Lai, Z.; Livingstone, K.; Nakazato, T.; Durphy, J.L.; Schwarzbach, A.E.; Donovan, L.A.; Lexer, C. Major Ecological Transitions in Wild Sunflowers Facilitated by Hybridization. Science 2003, 301, 1211–1216. [Google Scholar] [CrossRef]
- Lindgren, D.; Ying, C.C. A model integrating seed source adaptation and seed use. New For. 2000, 20, 87–104. [Google Scholar] [CrossRef]
- Hereford, J. A Quantitative Survey of Local Adaptation and Fitness Trade-Offs. Am. Nat. 2009, 173, 579–588. [Google Scholar] [CrossRef]
- de Kort, H.; Mergeay, J.; vander Mijnsbrugge, K.; Decocq, G.; Maccherini, S.; Kehlet Bruun, H.H.; Honnay, O.; Vandepitte, K. An evaluation of seed zone delineation using phenotypic and population genomic data on black alder Alnus glutinosa. J. Appl. Ecol. 2014, 51, 1218–1227. [Google Scholar] [CrossRef]
- Poyatos, R.; Martínez-Vilalta, J.; Čermák, J.; Ceulemans, R.; Granier, A.; Irvine, J.; Köstner, B.; Lagergren, F.; Meiresonne, L.; Nadezhdina, N.; et al. Plasticity in hydraulic architecture of Scots pine across Eurasia. Oecologia 2007, 153, 245–259. [Google Scholar] [CrossRef] [PubMed]
- Trewavas, A. Aspects of Plant Intelligence. Ann. Bot. 2003, 92, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Hetherington, A.M.; Woodward, F.I. The role of stomata in sensing and driving environmental change. Nature 2003, 424, 901–908. [Google Scholar] [CrossRef]
- Kollist, H.; Nuhkat, M.; Roelfsema, M.R.G. Closing gaps: Linking elements that control stomatal movement. New Phytol. 2014, 203, 44–62. [Google Scholar] [CrossRef]
- Woodward, F.I. Ecophysiological studies on the shrub Vaccinium-Myrtillus L. taken from a wide altitudinal range. Oecologia 1986, 70, 580–586. [Google Scholar] [CrossRef]
- Chen, S.; Bai, Y.; Zhang, L.; Han, X. Comparing physiological responses of two dominant grass species to nitrogen addition in Xilin River Basin of China. Environ. Exp. Bot. 2005, 53, 65–75. [Google Scholar] [CrossRef]
- López Upton, J.; Velazco Fiscal, V.; Jasso Mata, J.; Ramírez Herrera, C.; Vargas Hernández, J. Hibridación natural entre Pinus oocarpa y P. pringlei. Acta Botánica Mex. 2001, 57, 51–66. [Google Scholar] [CrossRef]
- Livshits, G.; Kobyliansky, E. Lerner’s concept of developmental homeostasis and the problem of heterozygosity level in natural populations. Heredity 1985, 55, 341–353. [Google Scholar] [CrossRef]
- Gunn, D.; Hirnyck, R.; Shewmaker, G.; Takatori, S.; Ellis, L.T.; Controlando a las tuzas de Idaho. University of Idaho Extension CIS 1213-S. 2016. Available online: https://www.extension.uidaho.edu/publishing/pdf/CIS/CIS1213-S.pdf (accessed on 21 October 2021).
- McDonald, P.M.; Skinner, C.N.; Fiddler, G.O. Ponderosa pine needle length: An early indicator of release treatment effectiveness. Can. J. For. Res. 1992, 22, 761–764. [Google Scholar] [CrossRef]
- Squillance, A.E.; Silen, R.R. Racial variation in ponderosa pine. For. Sci. Monogr. 1962, 2, 27. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021; Available online: https://www.R-project.org/ (accessed on 24 October 2021).
- Gregorius, H.R.; Roberds, J.H. Measurement of genetical differentiation among subpopulations. Theor. Appl. Genet. 1986, 72, 826–834. [Google Scholar] [CrossRef] [PubMed]
- Simental-Rodriguez, S.L.; Pérez-Luna, A.; Hernández-Díaz, J.C.; Jaramillo-Correa, J.P.; López-Sánchez, C.A.; Flores-Rentería, L.; Carrillo-Parra, A.; Wehenkel, C. Modelling Shifts and Contraction of Seed Zones in Two Mexican Pine Species by Using Molecular Markers. Forests 2021, 12, 570. [Google Scholar] [CrossRef]
- Ávila-Flores, I.J.; Prieto-Ruíz, J.A.; Hernández-Díaz, J.C.; Wehenkel, C.A.; Corral-Rivas, J.J. Preconditioning Pinus engelmannii Carr. seedlings by irrigation deficit in nursery. Rev. Chapingo Ser. Cienc. For. Y Ambiente 2014, 20, 237–245. [Google Scholar] [CrossRef]
- Rosales Mata, S.; Prieto Ruíz, J.A.; García Rodríguez, J.L.; Madrid Aispuro, R.E.; Sigala Rodríguez, J.A. Preacondicionamiento de Pinus engelmannii Carr. bajo diferentes condiciones ambientales en vivero. Rev. Mex. Cienc. For. 2015, 6, 64–71. [Google Scholar] [CrossRef][Green Version]
- Ávila-Flores, I.J.; Hernández-Díaz, J.C.; Gonzáez-Elizondo, M.S.; Prieto-Ruíz, J.Á.; Wehenkel, C. Degree of hybridization in seed stands of Pinus engelmannii Carr. in the Sierra Madre Occidental, Durango, Mexico. PLoS ONE 2016, 11, e0152651. [Google Scholar] [CrossRef]
- Barton, A.M.; Teeri, J.A. The Ecology of elevational positions in plants: Drought resistance in five montane pine species in Southeastern Arizona. Am. J. Bot. 1993, 80, 15–25. [Google Scholar] [CrossRef]
- Friedrich, S.C.; Hernández-Díaz, J.C.; Leinemann, L.; Prieto-Ruíz, J.A.; Wehenkel, C. Spatial Genetic Structure in Seed Stands of Pinus arizonica Engelm. and Pinus cooperi Blanco in the State of Durango, Mexico. For. Sci. 2018, 64, 191–202. [Google Scholar] [CrossRef]
- Wehenkel, C.; Simental, L.; Silva-Flores, R.; Hernández-Díaz, J.C. Discrimination of 59 seed stands of various Mexican pine species based on 43 dendrometric, climatic, edaphic and genetic traits. Forstarchiv 2015, 86, 194–201. [Google Scholar]
- Chacón Sotelo, J.M.; Velázquez Martínez, A.; Musálem, M.A. Comportamiento de la repoblación natural de Pinus arizonica Engelm. bajo diferentes coberturas. Madera Y Bosques 1998, 4, 39–44. [Google Scholar] [CrossRef][Green Version]
- Perry, J. The Pines of Mexico and Central America; Timber Press: Portland, OR, USA, 1991. [Google Scholar]
- Wehenkel, C.; Samantha del Rocío, M.L.; Socorro, M.G.E.; Aguirre-Galindo, V.A.; Fladung, M.; López-Sánchez, C.A. Tall Pinus luzmariae trees with genes from P. Herrerae. PeerJ 2020, 8, e8648. [Google Scholar] [CrossRef] [PubMed]
- Denison, N.P.; Kietzka, J.E. The use and importance of hybrid intensive forestry in south africa. South Afr. J. 1993, 165, 55–60. [Google Scholar] [CrossRef]
- Hansson, B.; Westerberg, L. On the correlation between heterozygosity and fitness in natural populations. Mol. Ecol. 2002, 11, 2467–2474. [Google Scholar] [CrossRef] [PubMed]
- Lynch, M.; Walsh, B. Genetics and Analysis of Quantitative Traits; Sinauer Associates, Inc.: Sunderland, MA, USA, 1998. [Google Scholar]
- González-Varo, J.P.; Aparicio, A.; Lavergne, S.; Arroyo, J.; Albaladejo, R.G. Contrasting heterozygosity-fitness correlations between populations of a self-compatible shrub in a fragmented landscape. Genetica 2012, 140, 31–38. [Google Scholar] [CrossRef]
- Reed, D.H.; Fox, C.W.; Enders, L.S.; Kristensen, T.N. Inbreeding-stress interactions: Evolutionary and conservation consequences. Ann. N. Y. Acad. Sci. 2012, 1256, 33–48. [Google Scholar] [CrossRef]
- Abrahamsson, S.; Ahlinder, J.; Waldmann, P.; García-Gil, M.R. Maternal heterozygosity and progeny fitness association in an inbred Scots pine population. Genetica 2013, 141, 41–50. [Google Scholar] [CrossRef]
- Mejía, B.J.M.; García, R.J.L.; Muñoz, F.H.J.; Evaluación de plantaciones de cuatro especies forestales en el estado de Durango. Reaxion 2015, 2, 8–28. Available online: http://reaxion.utleon.edu.mx/Art_Evaluacion_plantaciones_cuatro_especies_forestales_Durango.html (accessed on 26 October 2021).
- Benítez, M.A.G. Supervivencia y Crecimiento de Plantaciones Forestales Comerciales en el Conjunto Predial “El Durangueño”, Canatlán, Durango. Tesis de Maestría, Facultad de Ciencias Forestales, Universidad Juárez del Estado de Durango, Durango, Dgo., México, México, 2016; 82p. [Google Scholar]
- Torres Rojo, J.M. Factores ambientales y físicos que afectan la supervivencia de siete especies forestales en el Estado de México. Revista mexicana de ciencias forestales. Rev. Mex. Cienc. For. 2021, 12, 66–91. [Google Scholar] [CrossRef]
- Prieto Ruíz, J.Á.; Duarte Santos, A.; Goche Télles, J.R.; González Orozco, M.M.; Pulgarín Gámiz, M.Á. Supervivencia y crecimiento de dos especies forestales, con base en la morfología inicial al plantarse. Rev. Mex. Cienc. For. 2018, 9, 151–168. [Google Scholar] [CrossRef][Green Version]
- Comisión Nacional del Agua (CONAGUA). Resúmenes Mensuales de Temperaturas y Lluvia. 2019. Available online: https://smn.conagua.gob.mx/es/climatologia/temperaturas-y-lluvias/resumenes-mensuales-de-temperaturas-y-lluvias (accessed on 25 April 2022).
- Shu, M. Association Genetics of Drought Tolerance in Ponderosa Pine (Pinus ponderosa); University of California: Merced, CA, USA, 2020. [Google Scholar]
- López, R.; Climent, J.; Gil, L. From desert to cloud forest: The non-trivial phenotypic variation of Canary Island pine needles. Trees 2008, 22, 843. [Google Scholar] [CrossRef]
- Dangasuk, O.G.; Panetsos, K.P. Altitudinal and longitudinal variations in Pinus brutia (Ten.) of Crete Island, Greece: Some needle, cone and seed traits under natural habitats. New For. 2004, 27, 269–284. [Google Scholar] [CrossRef]
- Wahid, N.; González-Martínez, S.C.; El Hadrami, I.; Boulli, A. Variation of morphological traits in natural populations of maritime pine (Pinus pinaster Ait.) in Morocco. Ann. For. Sci. 2006, 63, 83–92. [Google Scholar] [CrossRef][Green Version]
- Afas, N.A.; Marron, N.; Ceulemans, R. Variability in Populus leaf anatomy and morphology in relation to canopy position, biomass production, and varietal taxon. Ann. For. Sci. 2007, 64, 521–532. [Google Scholar] [CrossRef]
- Bulfe, N.; Fernández, M.E. Efecto del momento de ocurrencia del déficit hídrico sobre el crecimiento de plantines de Pinus taeda L. Rev. Fac. Agron. 2014, 113, 81–93. [Google Scholar]
- Poulson, M.E.; Boeger, M.R.T.; Donahue, R.A. Response of photosynthesis to high light and drought for Arabidopsis thaliana grown under a UV-B enhanced light regime. Photosynth. Res. 2007, 90, 79–90. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Ngwenyama, N.; Liu, Y.; Walker, J.C.; Zhang, S. Stomatal Development and Patterning Are Regulated by Environmentally Responsive Mitogen-Activated Protein Kinases in Arabidopsis. Plant Cell 2007, 19, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Gailing, O.; Langenfeld-Heyser, R.; Polle, A.; Finkeldey, R. Quantitative trait loci affecting stomatal density and growth in a Quercus robur progeny: Implications for the adaptation to changing environments. Glob. Chang. Biol. 2008, 14, 1934–1946. [Google Scholar] [CrossRef]
- Chebib, J.; Guillaume, F. Pleiotropy or linkage? Their relative contributions to the genetic correlation of quantitative traits and detection by multitrait GWA studies. Genetics 2021, 219, iyab159. [Google Scholar] [CrossRef]
- Al Afas, N.; Marron, N.; Ceulemans, R. Clonal variation in stomatal characteristics related to biomass production of 12 poplar (Populus) clones in a short rotation coppice culture. Environ. Exp. Bot. 2006, 58, 279–286. [Google Scholar] [CrossRef]
- Reich, P.B. Leaf stomatal density and diffusive conductance in three amphistomatous hybrid poplar cultivars. New Phytol. 1984, 98, 231–239. [Google Scholar] [CrossRef]
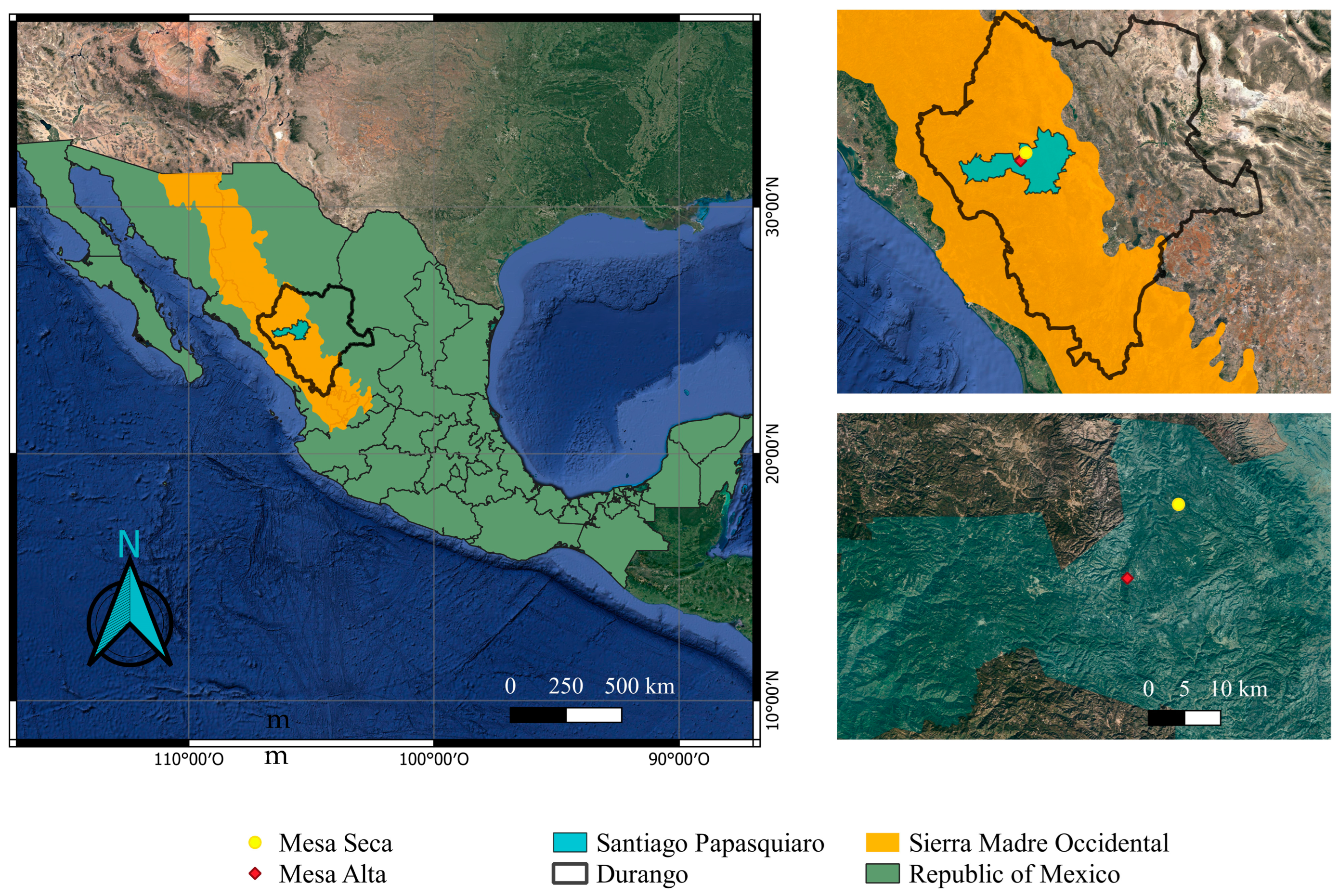
| Characteristic | Mesa Alta | Mesa Seca |
|---|---|---|
| Textural class | Sandy clay loam | Loam |
| Organic matter (OM, %) | 4.64 High | 1.94 Median |
| Nitrogen (N-NO3, kg/ha) | 12.32 | 7.39 |
| Phosphorus (ppm) | 9.66 | 7.39 |
| Potassium (ppm) | 220 | 116 |
| Magnesium (ppm) | 198 | 114 |
| Zinc (ppm) | 2.06 | 4.12 |
| pH 1:2 water | 5.88 | 5.25 |
| CaCO3 (ppm) | 1698 | 774 |
| CEC (meq/100 g) | 10.99 | 5.35 |
| Species | Mesa Alta | Mesa Seca | Both Trials Together | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Median | Mean | Sd | N | Median | Mean | Sd | N | Median | Mean | Sd | |
| PA-H | 5 | 13.1 ab | 13.1 | 0.8 | 5 | 21.5 ab | 21.5 | 5.2 | 10 | 13.6 ab | 15.9 | 4.9 |
| PA-P | 3 | 9.6 b | 9.6 | 0.6 | 4 | 11.0 b | 11.0 | 1.4 | 7 | 10.3 ab | 10.3 | 1.0 |
| PD-H | 18 | 10.6 b | 11.4 | 3.6 | 58 | 14.8 b | 15.1 | 5.5 | 76 | 13.2 b | 14.2 | 5.3 |
| PD-P | 14 | 11.6 b | 11.5 | 2.7 | 20 | 14.6 b | 15.3 | 5.2 | 34 | 12.6 ab | 13.7 | 4.7 |
| PE-H | 56 | 19.9 a | 19.4 | 9.0 | 211 | 24.4 a | 24.6 | 7.4 | 267 | 23.6 a | 23.5 | 8.0 |
| PE-P | 78 | 20.1 a | 20.2 | 7.8 | 218 | 23.3 a | 23.6 | 7.1 | 296 | 22.5 a | 22.6 | 7.4 |
| PL-H | 7 | 17.3 ab | 19.3 | 5.9 | 18 | 20.3 ab | 20.1 | 6.7 | 25 | 20.0 b | 19.9 | 6.4 |
| PL-P | 4 | 22.1 a | 22.5 | 5.8 | 13 | 14.6 b | 17.3 | 6.9 | 17 | 18.7 b | 18.5 | 6.9 |
| PT-H | 49 | 16.2 ab | 15.9 | 6.4 | 114 | 16.7 b | 16.9 | 6.3 | 163 | 16.6 b | 16.6 | 6.3 |
| PT-P | 24 | 15.3 ab | 15.9 | 5.4 | 77 | 15.3 b | 16.8 | 5.9 | 101 | 15.3 b | 16.6 | 5.7 |
| H | 135 | 15.4 a | 15.8 | 5.1 | 406 | 19.5 a | 19.6 | 6.2 | 541 | 17.4 a | 18.0 | 6.2 |
| P | 123 | 15.7 a | 15.9 | 4.5 | 332 | 15.8 a | 16.8 | 5.3 | 455 | 15.9 a | 16.3 | 5.2 |
| Species | Mesa Alta | Mesa Seca | Both Trials Together | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Median | Mean | Sd | N | Median | Mean | Sd | N | Median | Mean | Sd | |
| PA-H | 5 | 22.2 ab | 22.2 | 5.9 | 5 | 16.0 ab | 16.0 | 5.1 | 10 | 18.0 ab | 20.1 | 5.5 |
| PA-P | 3 | 30.0 a | 30.0 | 7.6 | 4 | 18.3 ab | 18.3 | 8.8 | 7 | 24.2 ab | 24.2 | 8.3 |
| PD-H | 18 | 33.2 a | 34.6 | 14.1 | 58 | 31.3 a | 32.7 | 12.2 | 76 | 31.5 a | 33.2 | 12.6 |
| PD-P | 14 | 31.3 a | 31.4 | 10.5 | 20 | 35.6 a | 36.9 | 14.7 | 34 | 32.8 a | 34.6 | 13.2 |
| PE-H | 56 | 14.2 b | 16.5 | 12.7 | 211 | 15.9 b | 16.6 | 6.7 | 267 | 15.2 b | 16.7 | 8.4 |
| PE-P | 78 | 14.2 b | 15.1 | 6.2 | 218 | 15.8 b | 17.6 | 8.3 | 296 | 15.3 b | 16.9 | 7.8 |
| PL-H | 7 | 30.0 a | 30.8 | 7.4 | 18 | 34.1 a | 33.3 | 9.1 | 25 | 31.2 a | 32.6 | 8.6 |
| PL-P | 4 | 43.3 a | 40.8 | 13.3 | 13 | 33.0 a | 34.4 | 8.2 | 17 | 37.0 a | 35.9 | 9.6 |
| PT-H | 49 | 33.7 a | 35.2 | 12.6 | 114 | 30.0 a | 31.3 | 10.9 | 163 | 32.0 a | 32.5 | 11.5 |
| PT-P | 24 | 34.5 a | 36.1 | 14.7 | 77 | 34.7 a | 36.0 | 17.2 | 101 | 34.5 a | 36.0 | 16.6 |
| H | 135 | 26.7 a | 27.9 | 10.5 | 406 | 25.5 a | 26.0 | 8.8 | 541 | 22.0 a | 24.6 | 12.9 |
| P | 123 | 30.7 b | 30.7 | 10.5 | 332 | 27.5 a | 28.6 | 11.4 | 455 | 19.5 b | 23.3 | 14.1 |
| Species | Mesa Alta | Mesa Seca | Overall, Both Trials | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Median | Mean | Sd | N | Median | Mean | Sd | N | Median | Mean | Sd | |
| PA-H | 5 | 31.4 b | 31.4 | 5.9 | 5 | 23.0 ab | 23.0 | 6.9 | 10 | 27.2 ab | 28.6 | 6.4 |
| PA-P | 3 | 31.6 b | 31.6 | 3.6 | 4 | 27.7 ab | 27.7 | 2.2 | 7 | 29.7 ab | 29.7 | 2.8 |
| PD-H | 18 | 44.5 a | 46.4 | 13.4 | 58 | 42.0 a | 43.9 | 12.9 | 76 | 42.0 a | 44.5 | 13.0 |
| PD-P | 14 | 42.1 a | 40.4 | 13.7 | 20 | 46.0 a | 47.7 | 14.2 | 34 | 42.9 a | 44.7 | 14.2 |
| PE-H | 56 | 32.8 b | 32.9 | 8.8 | 211 | 33.0 b | 34.3 | 8.5 | 267 | 33.0 b | 34.0 | 8.6 |
| PE-P | 78 | 32.0 b | 32.4 | 9.2 | 218 | 33.2 b | 34.6 | 9.6 | 296 | 33.0 b | 34.0 | 9.5 |
| PL-H | 7 | 34.0 a | 36.8 | 9.3 | 18 | 38.7 a | 39.3 | 10.3 | 25 | 38.4 ab | 38.6 | 9.9 |
| PL-P | 4 | 46.6 a | 44.7 | 16.1 | 13 | 42.0 a | 40.1 | 8.8 | 17 | 43.0 ab | 41.2 | 10.5 |
| PT-H | 49 | 40.2 a | 40.3 | 12.3 | 114 | 36.2 a | 37.4 | 10.4 | 163 | 36.0 a | 37.0 | 10.8 |
| PT-P | 24 | 39.6 a | 41.5 | 13.9 | 77 | 39.5 a | 42.1 | 16.7 | 101 | 35.1 a | 36.9 | 12.4 |
| H | 135 | 36.6 a | 37.5 | 9.9 | 406 | 34.6 a | 35.6 | 9.7 | 541 | 35.3 a | 36.5 | 9.7 |
| P | 123 | 38.4 a | 38.1 | 11.3 | 332 | 37.7 a | 38.4 | 10.4 | 455 | 36.7 a | 37.3 | 9.9 |
| Species | Mesa Alta | Mesa Seca | Mean Survival (%) | ||||
|---|---|---|---|---|---|---|---|
| N 2018 | N 2020 | Survival (%) | N 2018 | N 2020 | Survival (%) | 2020 | |
| PA-H | 11 | 5 | 45 a | 11 | 5 | 45 b | 45 ab |
| PA-P | 29 | 3 | 10 b | 29 | 4 | 14 d | 12 c |
| PD-H | 94 | 18 | 19 b | 94 | 58 | 61 ab | 40 a |
| PD-P | 66 | 14 | 21 b | 65 | 20 | 30 c | 26 b |
| PE-H | 294 | 56 | 19 b | 293 | 211 | 72 a | 46 a |
| PE-P | 354 | 78 | 22 b | 357 | 218 | 61 ab | 42 a |
| PL-H | 35 | 7 | 20 b | 34 | 18 | 52 b | 36 ab |
| PL-P | 27 | 4 | 15 b | 28 | 13 | 47 b | 31 ab |
| PT-H | 216 | 49 | 23 b | 215 | 114 | 52 b | 38 a |
| PT-P | 150 | 24 | 16 b | 150 | 77 | 51 b | 34 ab |
| H | 649 | 135 | 21 a | 649 | 406 | 63 a | 42 a |
| P | 628 | 123 | 20 a | 627 | 332 | 53 a | 36 a |
| Species | N Mesa Alta | N Mesa Seca | Total N | Median | Mean | Sd |
|---|---|---|---|---|---|---|
| PD-H | 12 | 16 | 28 | 94.0 b | 94.6 | 17.1 |
| PD-P | 10 | 6 | 16 | 96.5 b | 97.7 | 24.6 |
| PE-H | 27 | 29 | 56 | 144.3 a | 145.7 | 33.6 |
| PE-P | 30 | 35 | 65 | 142.0 a | 146.6 | 35.6 |
| PT-H | 23 | 24 | 47 | 109.5 b | 111.3 | 24.2 |
| PT-P | 15 | 18 | 33 | 108.0 b | 112.2 | 30.3 |
| H | 62 | 69 | 131 | 119.7 a | 122.4 | 34.6 |
| P | 55 | 59 | 114 | 127.0 a | 129.6 | 38.0 |
| Variable | rs | p-Value |
|---|---|---|
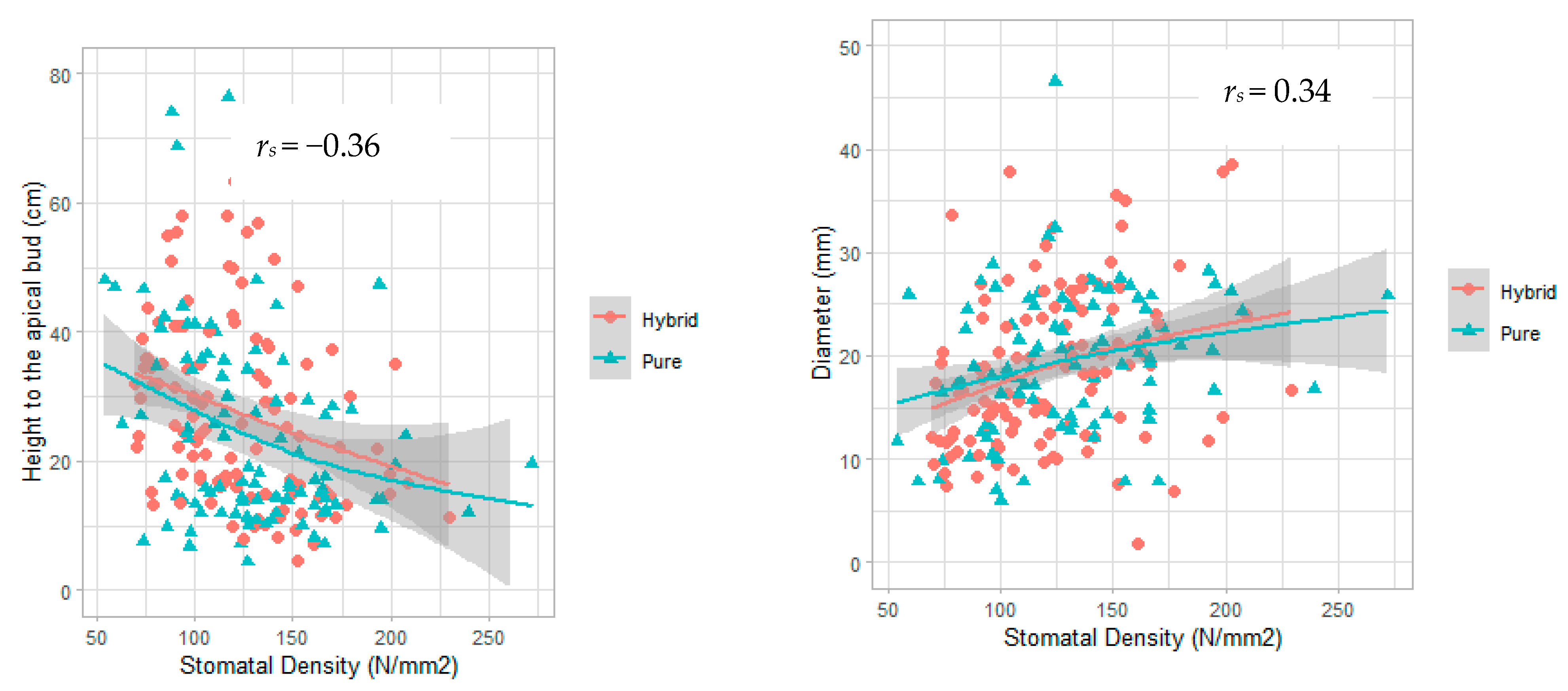
| Basal diameter | +0.34 | 3 × 10•7 |
| Height to apical bud | −0.36 | 5 × 10•8 |
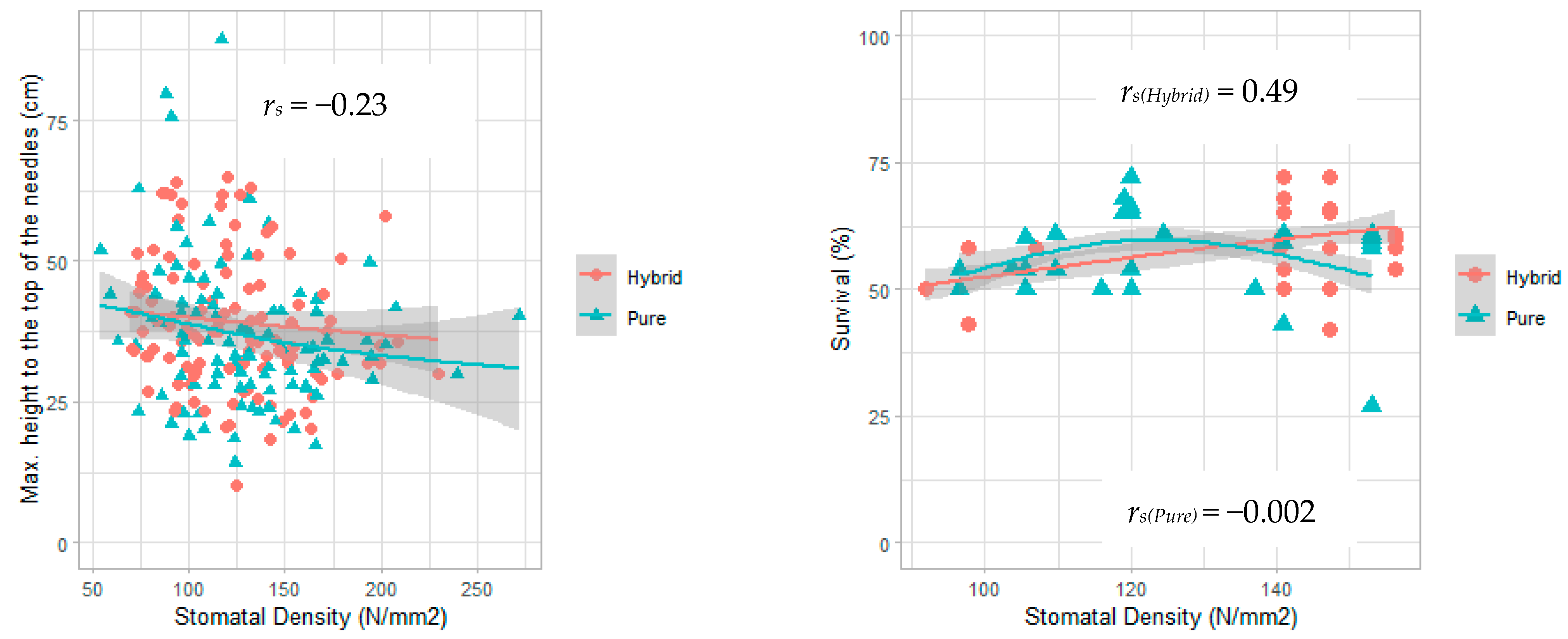
| Maximum height to the top of the needles | −0.23 | 0.0006 |
| Survival (hybrid seedlings) | +0.49 | 10•8 |
| Survival (pure seedlings) | −0.002 | 0.98 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sánchez-Hernández, R.S.; Quiñones-Pérez, C.Z.; Hernández-Díaz, J.C.; Prieto-Ruíz, J.Á.; Wehenkel, C. Field Trials to Assess the Growth, Survival, and Stomatal Densities of Five Mexican Pine Species and Their Hybrids under Common Plantation Conditions. Forests 2022, 13, 1791. https://doi.org/10.3390/f13111791
Sánchez-Hernández RS, Quiñones-Pérez CZ, Hernández-Díaz JC, Prieto-Ruíz JÁ, Wehenkel C. Field Trials to Assess the Growth, Survival, and Stomatal Densities of Five Mexican Pine Species and Their Hybrids under Common Plantation Conditions. Forests. 2022; 13(11):1791. https://doi.org/10.3390/f13111791
Chicago/Turabian StyleSánchez-Hernández, Ricardo Silas, Carmen Zulema Quiñones-Pérez, José Ciro Hernández-Díaz, José Ángel Prieto-Ruíz, and Christian Wehenkel. 2022. "Field Trials to Assess the Growth, Survival, and Stomatal Densities of Five Mexican Pine Species and Their Hybrids under Common Plantation Conditions" Forests 13, no. 11: 1791. https://doi.org/10.3390/f13111791
APA StyleSánchez-Hernández, R. S., Quiñones-Pérez, C. Z., Hernández-Díaz, J. C., Prieto-Ruíz, J. Á., & Wehenkel, C. (2022). Field Trials to Assess the Growth, Survival, and Stomatal Densities of Five Mexican Pine Species and Their Hybrids under Common Plantation Conditions. Forests, 13(11), 1791. https://doi.org/10.3390/f13111791






