An Integrated Similarity Analysis of Anatomical and Physical Wood Properties of Tropical Species from India, Mozambique, and East Timor
Abstract
1. Introduction
2. Materials and Methods
2.1. Collections
2.2. Wood Characterization
2.2.1. Anatomical Characterization
2.2.2. Physical Properties Determination
2.2.3. Color Measurements
2.3. Character Selection
2.4. Data Analysis
3. Results
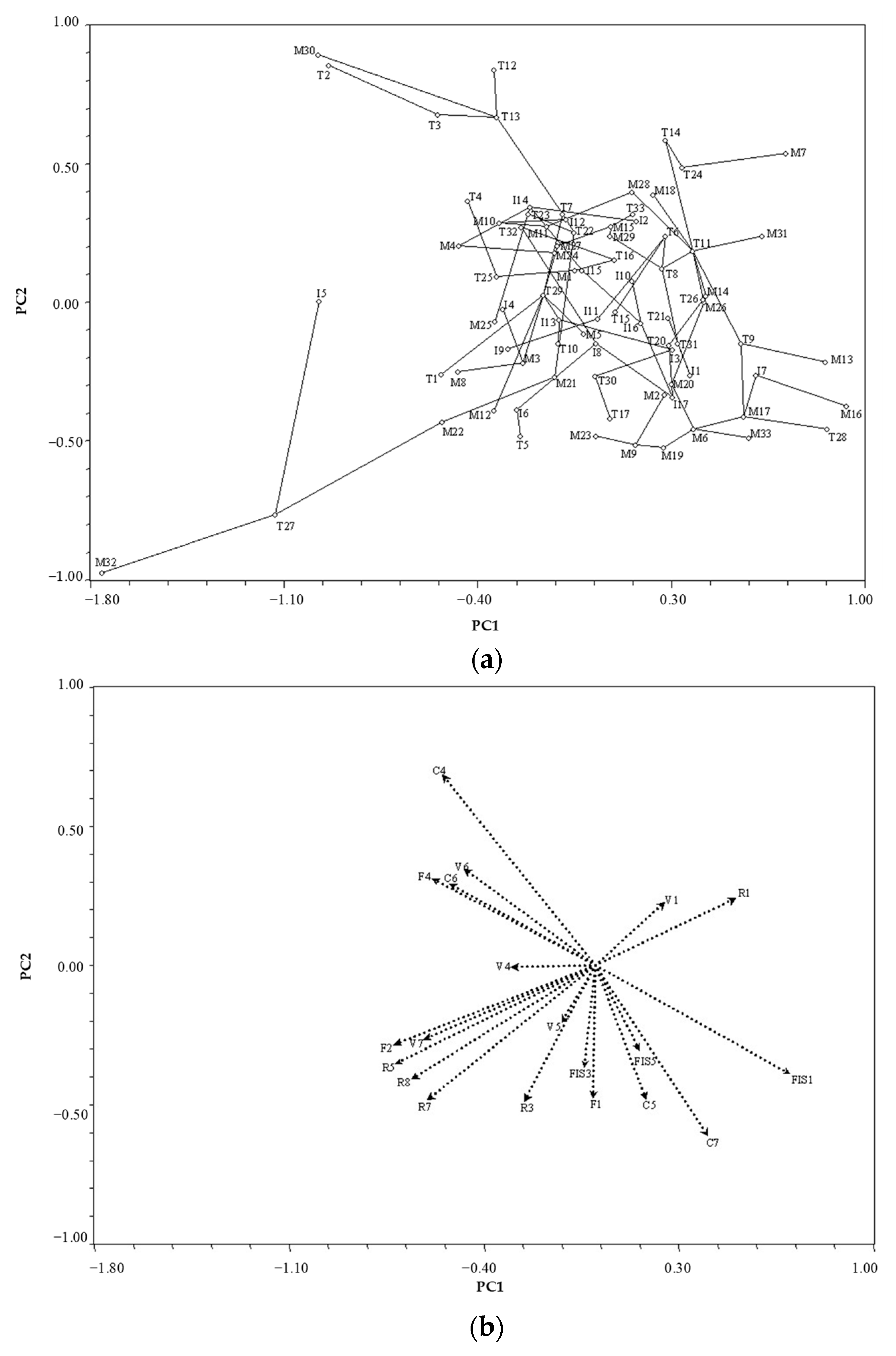
3.1. Species Variability Based on Anatomical and Physical Wood Characteristics
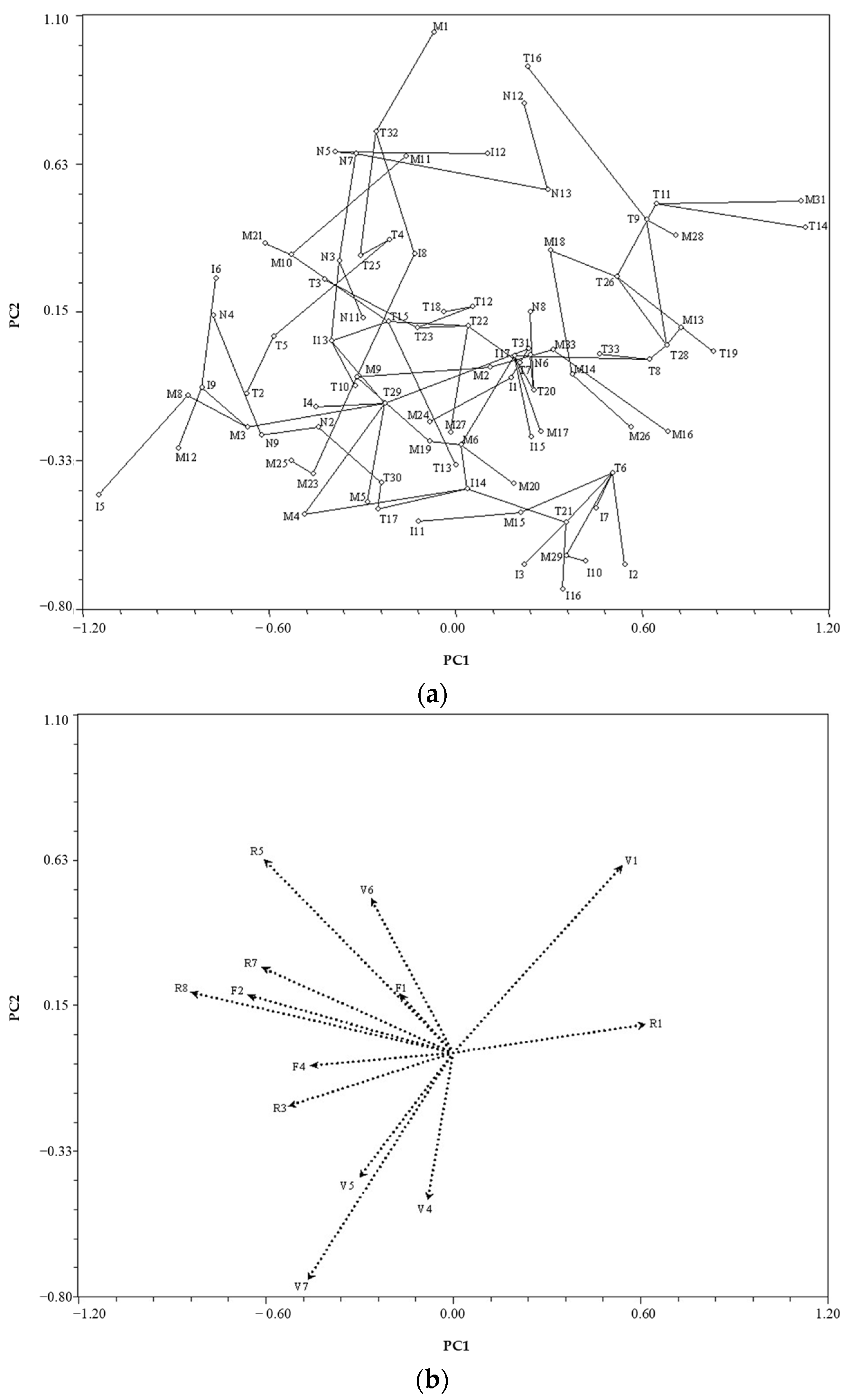
3.2. Species Variability Based on Anatomical Characteristics
3.3. Species Variability Based on Physical Characteristics
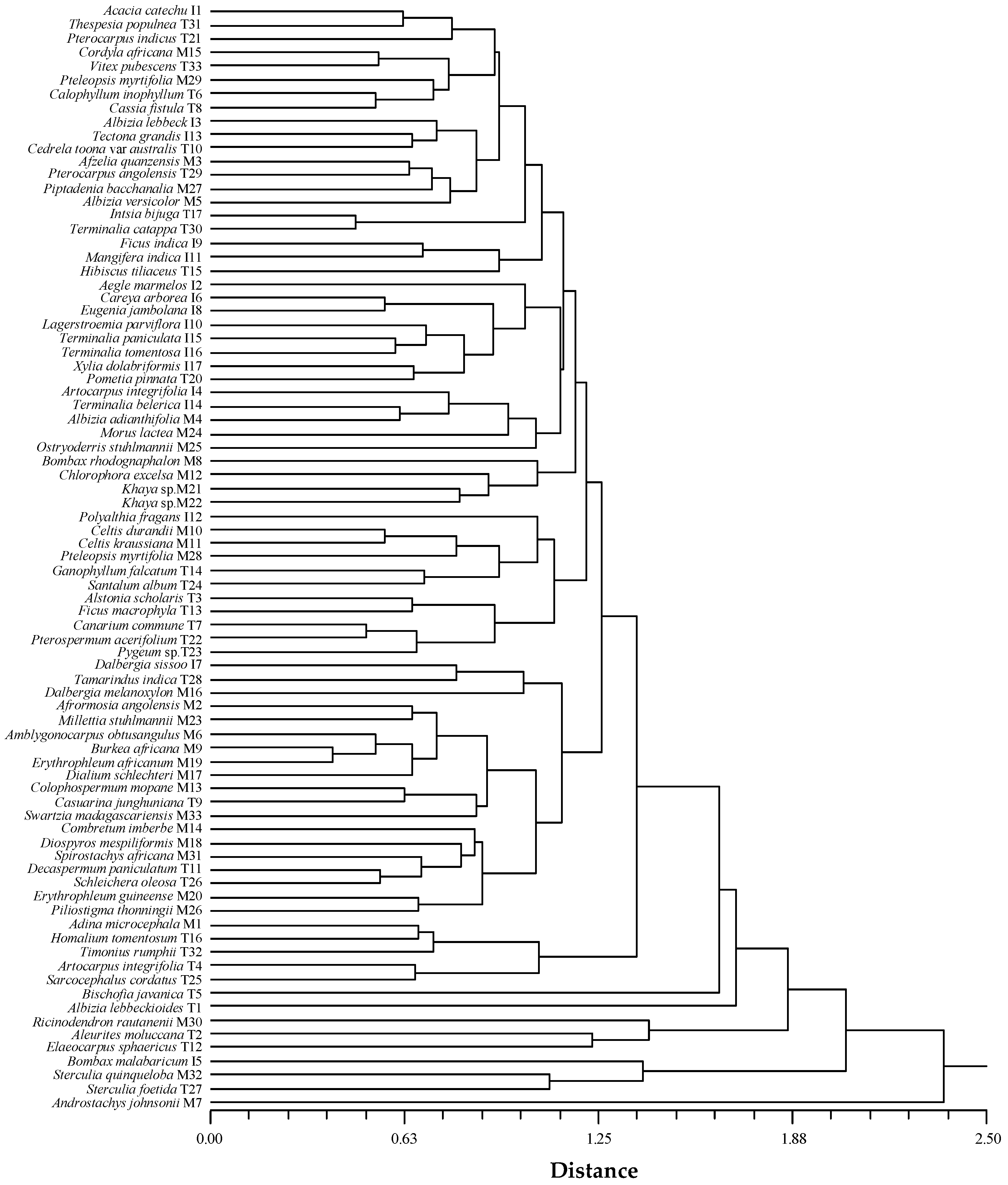
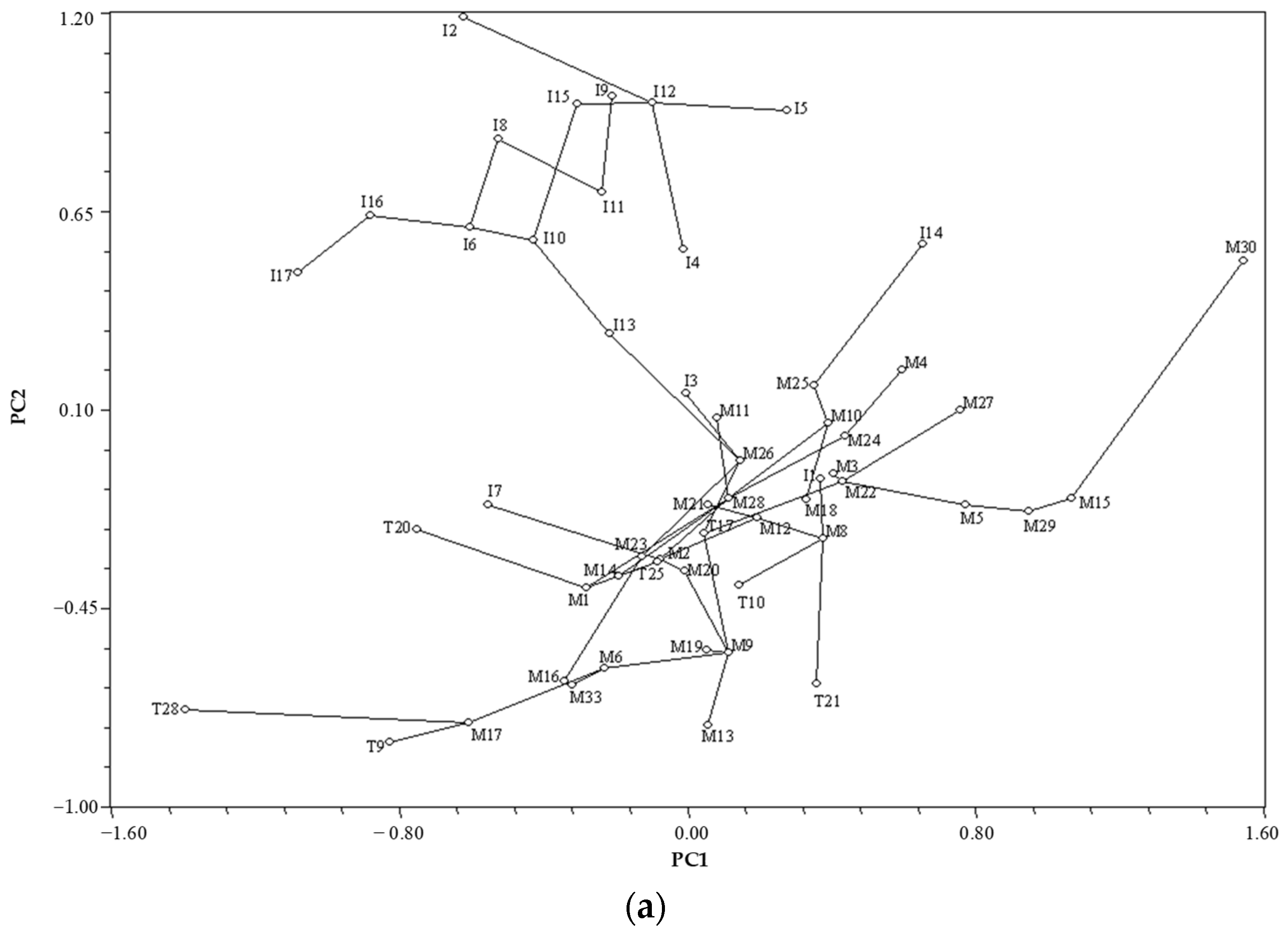
3.4. Similarity within Species and Genus
3.4.1. Similarity Based on Both Anatomical and Physical Wood Characteristics
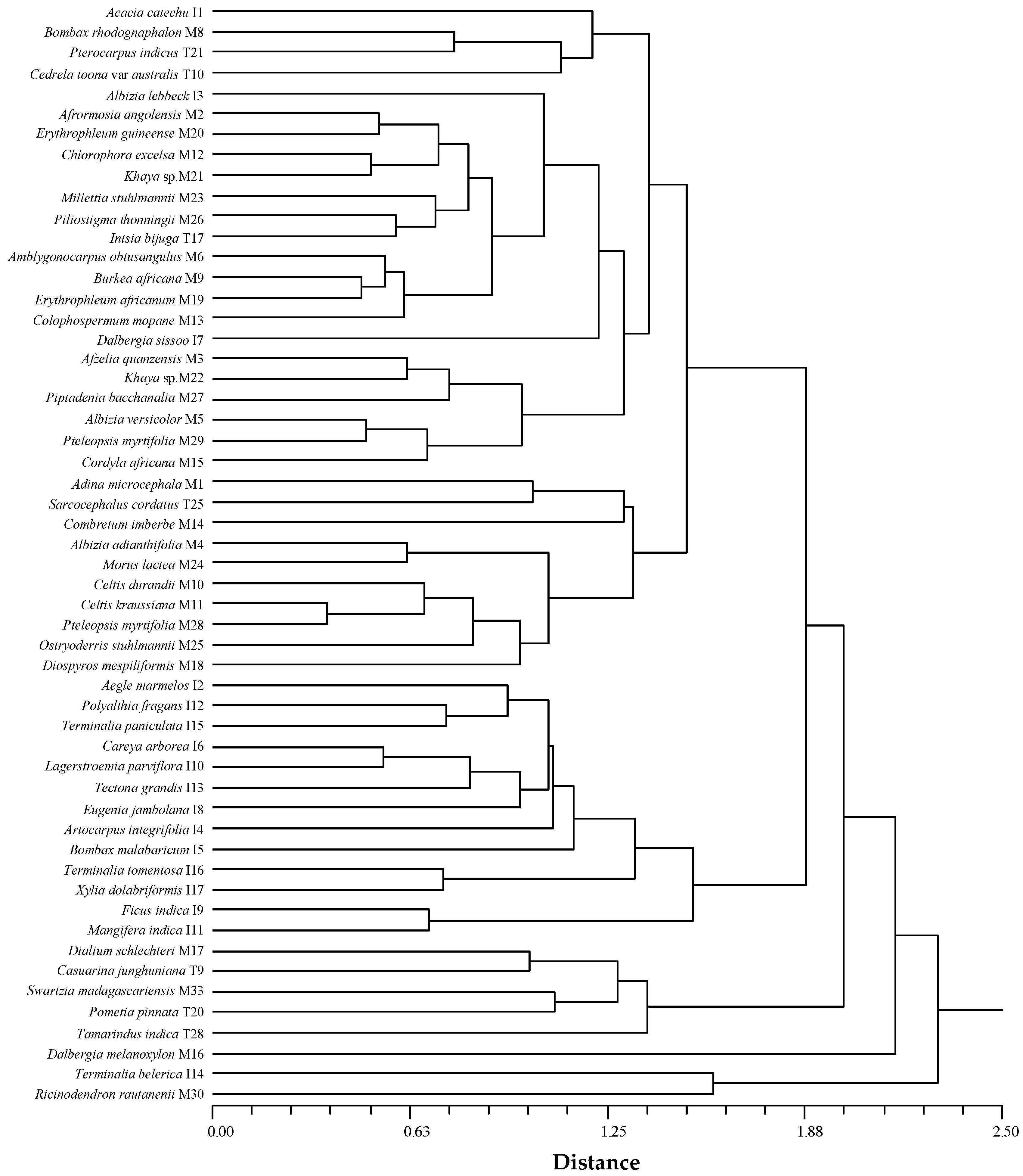
3.4.2. Similarity Based on Anatomical Characteristics
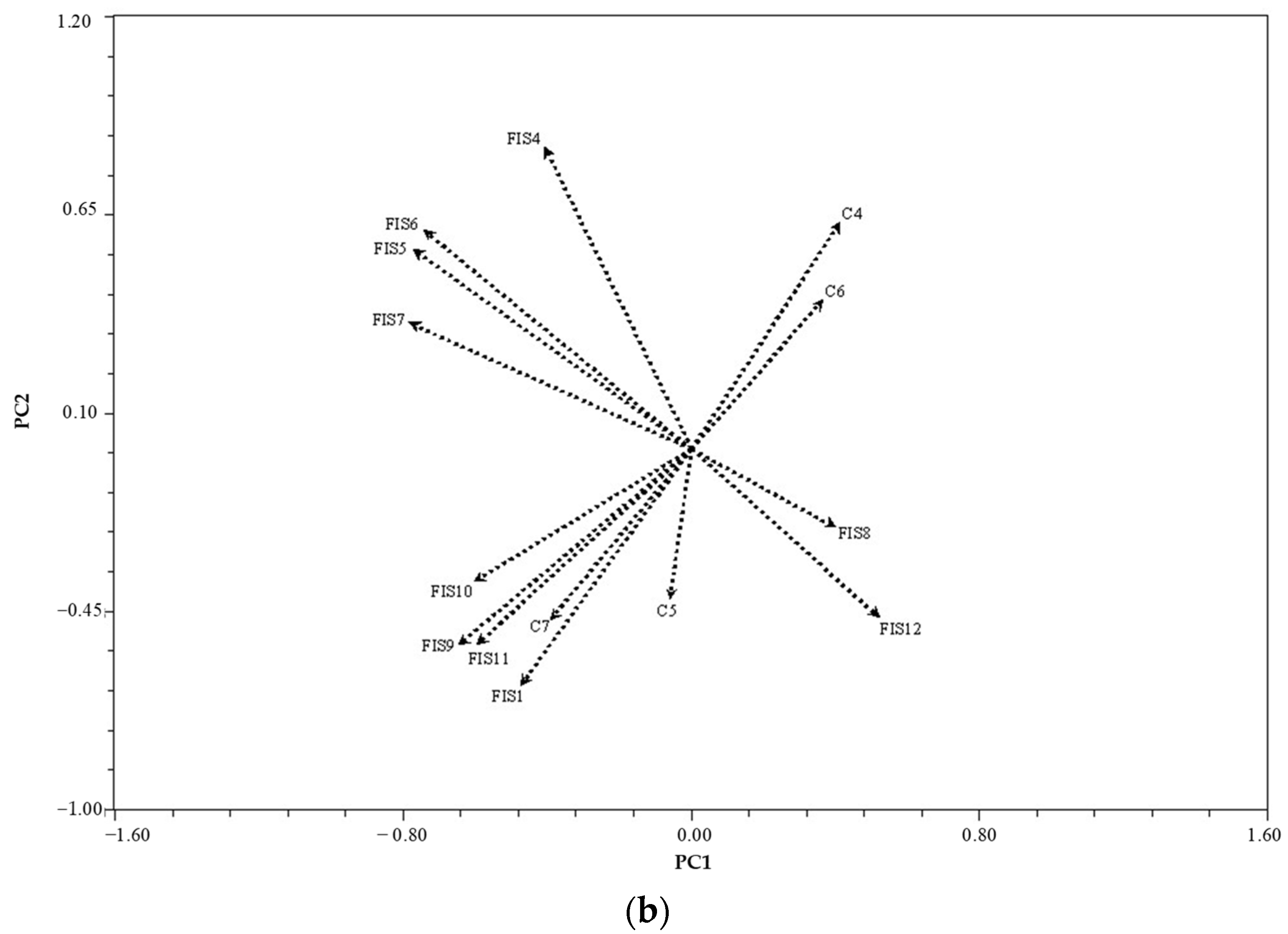
3.4.3. Similarity Based on Physical Characteristics
4. Discussion
4.1. Species Clustering
4.2. Variability and Interaction of Wood Properties
4.2.1. Both Anatomical and Physical Properties
4.2.2. Quantitative Wood Anatomy
4.2.3. Physical Properties
4.3. Similarity within Species and Genus
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gasson, P.; Baas, P.; Wheeler, E. Wood Anatomy of Cites-Listed Tree Species. IAWA J. 2011, 32, 155–198. [Google Scholar] [CrossRef]
- Perdigão, C.R.V.; Júnior, M.M.B.; Gonçalves, T.A.P.; de Araujo, C.S.; Mori, F.A.; Barbosa, A.C.M.C.; Souza, F.I.B.d.; Motta, J.P.; Melo, L.E.d.L. Forestry Control in the Brazilian Amazon I: Wood and Charcoal Anatomy of Three Endangered Species. IAWA J. 2020, 41, 490–509. [Google Scholar] [CrossRef]
- Koch, G.; Haag, V. Control of Internationally Traded Timber—The Role of Macroscopic and Microscopic Wood Identification against Illegal Logging. J. Forensic Res. 2015, 6, 317. [Google Scholar] [CrossRef]
- Gasson, P.E.; Lancaster, C.A.; Young, R.; Redstone, S.; Miles-Bunch, I.A.; Rees, G.; Guillery, R.P.; Parker-Forney, M.; Lebow, E.T. WorldForestID: Addressing the Need for Standardized Wood Reference Collections to Support Authentication Analysis Technologies; a Way Forward for Checking the Origin and Identity of Traded Timber. Plants People Planet 2021, 3, 130–141. [Google Scholar] [CrossRef]
- Dulbecco, P.; Luco, D. L’ Essentiel Sur Le Bois; Centre Technique du Bois et de L’ameublement: Paris, France, 2001; ISBN 978-2-85684-040-5. [Google Scholar]
- CITAB. Guide Pour Le Choix Des Bois En Menuiserie; FCBA, CIRAD: Montpellier, France, 2002; ISBN 978-2-85689-025-7. [Google Scholar]
- ATIBT. Tropical Timber Atlas—Volume One—Africa; ATIBT: Nogent-sur-Marne, France, 1986. [Google Scholar]
- Alves, E.S.; Angyalossy-Alfonso, V. Ecological trends in the wood anatomy of some brazilian species. 2. Axial parenchyma, rays and fibres. IAWA J. 2002, 23, 391–418. [Google Scholar] [CrossRef]
- Jansen, S.; Robbrecht, E.; Beeckman, H.; Smets, E. A Survey of the Systematic Wood Anatomy of the Rubiaceae. IAWA J. 2002, 23, 1–67. [Google Scholar] [CrossRef]
- Wheeler, E.; Baas, P.; Rodgers, S. Variations In Dieot Wood Anatomy: A Global Analysis Based on the Insidewood Database. IAWA J. 2007, 28, 229–258. [Google Scholar] [CrossRef]
- Gasson, P.; Miller, R.; Stekel, D.J.; Whinder, F.; Zieminska, K. Wood Identification of Dalbergia Nigra (CITES Appendix I) Using Quantitative Wood Anatomy, Principal Components Analysis and Naive Bayes Classification. Ann. Bot. 2010, 105, 45–56. [Google Scholar] [CrossRef]
- Fichtler, E.; Worbes, M. Wood Anatomical Variables in Tropical Trees and Their Relation to Site Conditions and Individual Tree Morphology. IAWA J. 2012, 33, 119–140. [Google Scholar] [CrossRef]
- Tarelkin, Y.; Delvaux, C.; Ridder, M.D.; Berkani, T.E.; Cannière, C.D.; Beeckman, H. Growth-ring distinctness and boundary anatomy variability in tropical trees. IAWA J. 2016, 37, 275–294. [Google Scholar] [CrossRef]
- De Palacios, P.; Esteban, L.G.; Gasson, P.; García-Fernández, F.; de Marco, A.; García-Iruela, A.; García-Esteban, L.; González-de-Vega, D. Using Lenses Attached to a Smartphone as a Macroscopic Early Warning Tool in the Illegal Timber Trade, in Particular for CITES-Listed Species. Forests 2020, 11, 1147. [Google Scholar] [CrossRef]
- Bodin, S.C.; Scheel-Ybert, R.; Beauchêne, J.; Molino, J.-F.; Bremond, L. CharKey: An Electronic Identification Key for Wood Charcoals of French Guiana. IAWA J. 2019, 40, 75-S20. [Google Scholar] [CrossRef]
- Kitin, P.; Espinoza, E.; Beeckman, H.; Abe, H.; McClure, P.J. Direct Analysis in Real-Time (DART) Time-of-Flight Mass Spectrometry (TOFMS) of Wood Reveals Distinct Chemical Signatures of Two Species of Afzelia. Ann. For. Sci. 2021, 78, 31. [Google Scholar] [CrossRef]
- Scholz, G.; Windeisen, E.; Liebner, F.; Bäucker, E.; Bues, C.-T. Wood Anatomical Features and Chemical Composition of Prosopis Kuntzei from the Paraguayan Chaco. IAWA J. 2010, 31, 39–52. [Google Scholar] [CrossRef]
- Beeckman, H.; Blanc-Jolivet, C.; Boeschoten, L.; Braga, J.; Cabezas, J.A.; Chaix, G.; Crameri, S.; Degen, B.; Deklerck, V.; Dormontt, E.; et al. Overview of Current Practices in Data Analysis for Wood Identification. A Guide for the Different Timber Tracking Methods; HAL Open Science: Lyon, France, 2020. [Google Scholar]
- Zobel, B.J.; Van Buijtenen, J. Wood Variation. Its Causes and Control; Springer: Berlin/Heidelberg, Germany, 1989. [Google Scholar]
- Chave, J.; Muller-Landau, H.C.; Baker, T.R.; Easdale, T.A.; ter Steege, H.; Webb, C.O. Regional and Phylogenetic Variation of Wood Density across 2456 Neotropical Tree Species. Ecol. Appl. 2006, 16, 2356–2367. [Google Scholar] [CrossRef]
- Miranda, I.; Sousa, V.; Pereira, H. Wood Properties of Teak (Tectona grandis) from a Mature Unmanaged Stand in East Timor. J. Wood Sci. 2011, 57, 171–178. [Google Scholar] [CrossRef]
- Weber, J.C.; Sotelo Montes, C. Geographic Variation in Tree Growth and Wood Density of Guazuma crinita Mart. in the Peruvian Amazon. New For. 2008, 36, 29. [Google Scholar] [CrossRef]
- Nocetti, M.; Rozenberg, P.; Chaix, G.; Macchioni, N. Provenance Effect on the Ring Structure of Teak (Tectona grandis L.f.) Wood by X-Ray Microdensitometry. Ann. For. Sci. 2011, 68, 1375–1383. [Google Scholar] [CrossRef]
- Nazari, N.; Bahmani, M.; Kahyani, S.; Humar, M.; Koch, G. Geographic Variations of the Wood Density and Fiber Dimensions of the Persian Oak Wood. Forests 2020, 11, 1003. [Google Scholar] [CrossRef]
- Sousa, V.; Silva, M.E.; Louzada, J.L.; Pereira, H. Wood Density and Ring Width in Quercus rotundifolia Trees in Southern Portugal. Forests 2021, 12, 1499. [Google Scholar] [CrossRef]
- Moore, J.; Cown, D. Wood Quality Variability—What Is It, What Are the Consequences and What Can We Do about It? N. Z. J. For. 2015, 59, 3–9. [Google Scholar]
- Preston, K.; Cornwell, W.; Denoyer, J. Wood Density and Vessel Traits as Distinct Correlates of Ecological Strategy in 51 California Coast Range Angiosperms. New Phytol. 2006, 170, 807–818. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Cabrera, H.I.; Jones, C.S.; Espino, S.; Schenk, H.J. Wood Anatomy and Wood Density in Shrubs: Responses to Varying Aridity along Transcontinental Transects. Am. J. Bot. 2009, 96, 1388–1398. [Google Scholar] [CrossRef]
- Ziemińska, K.; Butler, D.W.; Gleason, S.M.; Wright, I.J.; Westoby, M. Fibre Wall and Lumen Fractions Drive Wood Density Variation across 24 Australian Angiosperms. AoB Plants 2013, 5, plt046. [Google Scholar] [CrossRef]
- Nabais, C.; Hansen, J.K.; David-Schwartz, R.; Klisz, M.; López, R.; Rozenberg, P. The Effect of Climate on Wood Density: What Provenance Trials Tell Us? For. Ecol. Manag. 2018, 408, 148–156. [Google Scholar] [CrossRef]
- Sedjo, R.A. Wood Use and Trade. In ternational Trade in Wood Products. In Encyclopedia of Forest Sciences; Burley, J., Ed.; Elsevier: Oxford, UK, 2004; pp. 1857–1863. ISBN 978-0-12-145160-8. [Google Scholar]
- Ali, A.C.; Uetimane, E.; Lhate, I.A.; Terziev, N. Anatomical Characteristics, Properties and Use of Traditionally Used and Lesser-Known Wood Species from Mozambique: A Literature Review. Wood Sci. Technol. 2008, 42, 453–472. [Google Scholar] [CrossRef]
- Junior, E.U.; Terziev, N.; Daniel, G. Wood Anatomy of Three Lesser Known Species from Mozambique. IAWA J. 2009, 30, 277–291. [Google Scholar] [CrossRef]
- Ferreirinha, M.P. Catálogo da Madeiras de Moçambique—Parte I; Memórias, Série Botânica II; Ministério do Ultramar, Junta de Investigações do Ultramar: Lisbon, Portugal, 1955. [Google Scholar]
- Freitas, M.C. Estudo das Madeiras de Timor—I Contribuição; Memórias, Série Botânica III; Ministério do Ultramar, Junta de Investigações do Ultramar: Lisbon, Portugal, 1955. [Google Scholar]
- Freitas, M.C. Estudo das Madeiras de Timor. II Contribuição; Memórias da Junta de Investigações do Ultramar; Ministério do Ultramar: Lisbon, Portugal, 1958. [Google Scholar]
- Freitas, M.C. Madeiras da Índia Portuguesa; Memórias da Junta de Investigações do Ultramar; Ministério do Ultramar: Lisbon, Portugal, 1963; Volume 47. [Google Scholar]
- Freitas, M.C. Madeiras de Moçambique. Características Anatómicas Físicas e Mecânicas; Instituto de Investigação Científica Tropical: Lisbon, Portugal, 1986. [Google Scholar]
- IAWA Committee. IAWA List of Microscopic Features for Hardwood Identification. IAWA Bull. 1989, 10, 219–232. [Google Scholar]
- Sneath, P.H.A.; Sokal, R.R. Numerical Taxonomy: The Principles and Practice of Numerical Classification; A Series of Books in Biology; W. H. Freeman: San Francisco, CA, USA, 1973; ISBN 978-0-7167-0697-7. [Google Scholar]
- Sokal, R.R.; Rohlf, F.J. The Comparison of Dendrograms by Objective Methods. Taxon 11: 33–40. Taxon 1962, 11, 33–40. [Google Scholar] [CrossRef]
- Martínez-Cabrera, H.I.; Cevallos-Ferriz, S.R.S. New Species of Tapirira (Anacardiaceae) from Early Miocene Sediments of the El Cien Formation, Baja California Sur, Mexico. IAWA J. 2004, 25, 103–117. [Google Scholar] [CrossRef]
- Barros, C.F.; Marcon-Ferreira, M.L.; Callado, C.H.; Lima, H.R.P.; da Cunha, M.; Marquete, O.; Costa, C.G. Tendências Ecológicas Na Anatomia Da Madeira de Espécies Da Comunidade Arbórea Da Reserva Biológica de Poço Das Antas, Rio de Janeiro, Brasil. Rodriguésia 2006, 57, 443–460. [Google Scholar] [CrossRef][Green Version]
- Wickremasinghe, B.; Herat, T. A Comparative Wood Anatomical Study of the Genus diospyros L. (Ebenaceae) in Sri Lanka. Ceylon J. Sci. 2006, 35, 115–136. [Google Scholar]
- Pande, P.K.; Negi, K.; Singh, M. Wood Anatomy of Shorea of White Meranti (Meranti Pa’ang) Group of the Malay Peninsula. Curr. Sci. 2007, 92, 1748–1754. [Google Scholar]
- MacLachlan, I.R.; Gasson, P. PCA of Cites Listed Pterocarpus santalinus (Leguminosae) Wood. IAWA J. 2010, 31, 121–138. [Google Scholar] [CrossRef]
- Trugilho, P.; Mori, F.; Lima, J. Correlação Canônica Das Características Químicas e Físicas Da Madeira de Clones de Eucalyptus grandis e Eucalyptus saligna. CERNE 2003, 9, 66–80. [Google Scholar]
- Ziemińska, K.; Westoby, M.; Wright, I.J. Broad Anatomical Variation within a Narrow Wood Density Range—A Study of Twig Wood across 69 Australian Angiosperms. PLoS ONE 2015, 10, e0124892. [Google Scholar] [CrossRef]
- Zimmermann, M. Xylem Structure and the Ascent of Sap; Springer: Berlin/Heidelberg, Germany, 1983. [Google Scholar]
- Noshiro, S.; Baas, P. Systematic Wood Anatomy of Cornaceae and Allies. IAWA J. 1998, 19, 43–97. [Google Scholar] [CrossRef]
- Noshiro, S.; Baas, P. Latitudinal Trends in Wood Anatomy within Species and Genera: Case Study in Cornus S.L. (Cornaceae). Am. J. Bot. 2000, 87, 1495–1506. [Google Scholar] [CrossRef]
- Carlquist, S. Comparative Wood Anatomy Systematic, Ecological, and Evolutionary Aspects of Dicotyledon Wood, 2nd ed.; Springer: Berlin/Heidelberg, Germany, 2001. [Google Scholar]
- Baas, P.; Ewers, F.; Davis, S.; Wheeler, E. Evolution of Xylem Physiology. In The Evolution of Plant Physiology, Linnean Society symposium series Number 21; Academic Press: Oxford, UK, 2004; pp. 273–295. ISBN 978-0-12-339552-8. [Google Scholar]
- Vieira, H.; Rios, A.; Quilhó, T.; Cunha, A.; Brand, M.; Danielli, D.; Florez, J.; Stange, R.; Buss, R.; Higuchi, P. Agrupamento e Caracterização Anatômica Da Madeira de Espécies Nativas Da Floresta Ombrófila Mista. Rodriguésia 2019, 70, 1–16. [Google Scholar] [CrossRef]
- Hacke, U.G.; Sperry, J.S.; Pockman, W.T.; Davis, S.D.; McCulloh, K.A. Trends in Wood Density and Structure Are Linked to Prevention of Xylem Implosion by Negative Pressure. Oecologia 2001, 126, 457–461. [Google Scholar] [CrossRef]
- Hacke, U.G.; Sperry, J.S.; Wheeler, J.K.; Castro, L. Scaling of Angiosperm Xylem Structure with Safety and Efficiency. Tree Physiol 2006, 26, 689–701. [Google Scholar] [CrossRef]
- Ewers, F.W. Xylem’ Structure and Water Conduction in Conifer Trees, Dicot Trees, and Llanas. IAWA J. 1985, 6, 309–317. [Google Scholar] [CrossRef]
- Bessa, F. Caracterização anatómica, física, química e acústica de madeiras de várias espécies para a construção de instrumentos musicais—uma aplicação à viola dedilhada. Master’s Thesis, Instituto Superior de Agronomia, Universidade Técnica de Lisboa, Lisboa, Portugal, 2000. [Google Scholar]
- Aguilar-Rodríguez, S.; Terrazas, T.; López-Mata, L. Anatomical Wood Variation of Buddleja Cordata (Buddlejaceae) along Its Natural Range in Mexico. Trees 2005, 20, 253. [Google Scholar] [CrossRef]
- Miranda, I.; Almeida, M.H.; Pereira, H. Provenance and Site Variation of Wood Density in Eucalyptus globulus Labill. at Harvest Age and Its Relation to a Non-Destructive Early Assessment. For. Ecol. Manag. 2001, 149, 235–240. [Google Scholar] [CrossRef]
- Reyes, G.; Brown, S.; Chapman, J.; Lugo, A. Wood Densities of Tropical Tree Species; US Department of Agriculture: New Orleans, LA, USA, 1992.
- Muller-Landau, H.C. Interspecific and Inter-Site Variation in Wood Specific Gravity of Tropical Trees. Biotropica 2004, 36, 20–32. [Google Scholar] [CrossRef]
- Sotelo Montes, C.; Beaulieu, J.; Hernández, R.E. Genetic Variation in Wood Shrinkage and Its Correlations with Tree Growth and Wood Density of Calycophyllum spruceanum at an Early Age in the Peruvian Amazon. Can. J. For. Res. 2007, 37, 966–976. [Google Scholar] [CrossRef]
- Bossu, J.; Beauchêne, J.; Estevez, Y.; Duplais, C.; Clair, B. New Insights on Wood Dimensional Stability Influenced by Secondary Metabolites: The Case of a Fast-Growing Tropical Species Bagassa Guianensis Aubl. PLoS ONE 2016, 11, e0150777. [Google Scholar] [CrossRef]
- Lima, I.; Longui, E.; Freitas, M.L.; Zanatto, A.; Zanata, M.; Florsheim, S.; Jr, G. Physical-Mechanical and Anatomical Characterization in 26-Year-Old Eucalyptus resinifera Wood. Floresta E Ambiente 2013, 21, 91–98. [Google Scholar] [CrossRef][Green Version]
- Sotelo Montes, C.; Hernández, R.E.; Beaulieu, J.; Weber, J.C. Genetic Variation in Wood Color and Its Correlations with Tree Growth and Wood Density of Calycophyllum spruceanum at an Early Age in the Peruvian Amazon. New For. 2008, 35, 57–73. [Google Scholar] [CrossRef]
- Sotelo Montes, C.; Weber, J.C.; Garcia, R.A.; Silva, D.A.; Muñiz, G.I.B. Variation in Wood Color among Natural Populations of Five Tree and Shrub Species in the Sahelian and Sudanian Ecozones of Mali. Can. J. For. Res. 2013, 43, 552–562. [Google Scholar] [CrossRef]
- Moya, R.; Calvo-Alvarado, J. Variation of Wood Color Parameters of Tectona Grandis and Its Relationship with Physical Environmental Factors. Ann. For. Sci. 2012, 69, 947–959. [Google Scholar] [CrossRef]
- Bakali, I.; Yagi, S.; Merlin, A.; Deglise, X. A Screening Study of Natural Colour of Wood from Different Geographical Regions. Res. J. For. 2011, 5, 162–168. [Google Scholar] [CrossRef]
- Rungwattana, K.; Hietz, P. Radial Variation of Wood Functional Traits Reflect Size-Related Adaptations of Tree Mechanics and Hydraulics. Funct. Ecol. 2018, 32, 260–272. [Google Scholar] [CrossRef]
- Bhat, K.M.; Priya, P.B. Influence of provenance variation on wood properties of teak from the Western Ghat region in India. IAWA J. 2004, 25, 273–282. [Google Scholar] [CrossRef]
- Sousa, V.B.; Cardoso, S.; Quilhó, T.; Pereira, H. Growth Rate and Ring Width Variability of Teak, Tectona grandis (Verbenaceae) in an Unmanaged Forest in East Timor. Rev. Biol. Trop. 2012, 60, 483–494. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, L.; Dayal, R. Wood Anatomy of Indian Albizias. IAWA J. 1985, 6, 213–218. [Google Scholar] [CrossRef]
- Odiye, M.; Owolabi, S.; Akinloye, A.; Folorunso, A.; Ayodele, A. Comparative Wood Anatomical Studies in the Genus albizia Durazz in Nigeria and Their Potential for Papermaking. Plants Environ. 2019, 1, 70–82. [Google Scholar]
- Nithaniyal, S.; Parani, M. Evaluation of Chloroplast and Nuclear DNA Barcodes for Species Identification in Terminalia L. Biochem. Syst. Ecol. 2016, 68, 223–229. [Google Scholar] [CrossRef]
- InsideWood. Published on the Internet 2004. Available online: http://insidewood.lib.ncsu.edu/search (accessed on 1 May 2020).
- Wheeler, E.A. Inside Wood—A Web Resource for Hardwood Anatomy. IAWA J. 2011, 32, 199–211. [Google Scholar] [CrossRef]
- Bolza, E.; Keating, W.G. African Timbers—The Properties, Uses and Characteristics of 700 Species; Division of Building Research CSIRO: Melbourne, Australia, 1972. [Google Scholar]
- Keating, W.G.; Bolza, E. Characteristics, Properties and Uses of Timber Southeast Asia, Northern Australia and the Pacific; Inkata Press: Melbourne, Australia, 1982; Volume 1. [Google Scholar]
- Affre, A.; Kathe, W.; Raymakers, C. Looking Under the Veneer. Implementation Manual on EU Timber Trade Control: Focus on CITES-Listed Trees; Report to the European Commission; TRAFFIC Europe: Brussels, Belgium, 2004. [Google Scholar]
| Code | Species | Code | Species |
|---|---|---|---|
| I1 | Acacia catechu Willd. | T1 | Albizialebbeckoides (DC) Benth. |
| I2 | Aegle marmelos Corrêa | T2 | Aleurites moluccana Willd. |
| I3 | Albizialebbeck Benth. | T3 | Alstonia scholaris (L.) R. Br. |
| I4 | Artocarpus integrifoliaL. | T4 | Artocarpus integrifolia L. |
| I5 | Bombax malabaricum DC. | T5 | Bischofia javanica Blume |
| I6 | Careya arborea Roxb. | T6 | Calophyllum inophyllum L. |
| I7 * | Dalbergiasissoo Roxb. | T7 | Canarium commune L. |
| I8 | Eugenia jambolana Lam. | T8 | Cassia fistula L. |
| I9 | Ficus indica Roxb. | T9 | Casuarina junghuhniana Miq. |
| I10 | Lagerstroemia parviflora Roxb. | T10 * | Cedrela toona var australis Roxb. C. DC. |
| I11 | Mangifera indica L. | T11 | Decaspermum paniculatum Kurz |
| I12 | Polyalthia fragans Benth. and Hook | T12 | Elaeocarpus sphaericus K. Schum. |
| I13 | Tectona grandisL. | T13 | Ficus macrophylla Roxb. |
| I14 | Terminalia bellirica Roxb. | T14 | Ganophyllum falcatum Blume |
| I15 | Terminalia paniculata Roth | T15 | Hibiscus tiliaceus L. |
| I16 | Terminalia tomentosa W. et Arn. | T16 | Homalium tomentosum Benth. |
| I17 | Xylia dolabriformis Benth. | T17 | Intsia bijuga O. K. |
| M1 | Adina microcephala (del.) Hiern | T18 | Macaranga tanarius Muell. |
| M2 | Afrormosia angolensis (Bak.) Harms | T19 | Melaleuca leucadendron L. |
| M3 | Afzelia quanzensis Welw. | T20 | Pometia pinnata Forst. |
| M4 | Albizia adianthifolia W. F. Wight | T21 | Pterocarpusindicus Willd. |
| M5 | Albizia versicolor Welw. ex Oliv.als | T22 | Pterospermum acerifolium Will. |
| M6 | Amblygonocarpus obtusangulus Harms | T23 | Pygeum sp. |
| M7 | Androstachys johnsonii Prain | T24 | Santalum album L. |
| M8 | Bombax rhodognaphalon K. Schum. ex. Engl. | T25 | Sarcocephalus cordatus Miq. |
| M9 | Burkea africana Hook. | T26 | Schleichera oleosa Merr. |
| M10 | Celtis durandii Engl. | T27 | Sterculiafoetida L. |
| M11 | Celtis kraussiana Bernh. | T28 | Tamarindus indica L. |
| M12 | Chlorophora excelsa (Milicia excelsa) (Welw.) Benth. Hook | T29 | Tectona grandis L. |
| M13 | Colophospermum mopane Kirk. | T30 | Terminalia catappa L. |
| M14 | Combretum imberbe Wawra | T31 | Thespesia populnea Soland, ex Corrêa |
| M15 | Cordyla africana Lour. | T32 | Timonius rumphii DC. |
| M16 * | Dalbergia melanoxylon Guill. and Perr | T33 | Vitex pubescens Vahl |
| M17 | Dialium schlechteri Harms | N1 | Acacia robusta Burch |
| M18 | Diospyros mespiliformis Hochst. ex A. DC. | N2 | Amblygonocarpusandongensis (Welw. ex Oliv.) Excell and Torre |
| M19 | Erythrophleum africanum (Benth.) Harms | N3 | Berchemia discolor (Klotzsch) Hemsl. |
| M20 | Erythrophleum guineense Don | N4 * | Cedrelaodorata L. |
| M21 | Khaya sp. | N5 | Cleistanthus schlechteri (Pax) Hutch. |
| M22 | Khaya sp. | N6 | Combretumzeyheri Sond. |
| M23 | Millettia stuhlmannii Taub. | N7 | Diplorhynchus condylocarpon (Mull. Arg.) Pichon |
| M24 | Morus lactea Mildbr. (Celtis lactea Sim.) | N8 | Melaleuca leucadendron(L.) L. |
| M25 | Ostryoderris stuhlmannii Dunn ex Baker f. | N9 | Morusmesozygia Stapf |
| M26 | Piliostigma thonningii (Schumach.) Milne-Redhead | N10 | Pterocarpusantunesii (Tab.) Harms |
| M27 | Piptadenia buchananii Bak. (Newtonia buchananii) | N11 | Rhodognaphalon schumannianum A. Robyns |
| M28 | Pteleopsis myrtifolia (Lawson) Engl. and Diels | N12 | Schrebera trichoclada Welw. |
| M29 | Pterocarpus angolensis DC. | N13 | Syncarpia glomulifera (Sm.) Wield. |
| M30 | Ricinodendron rautanenii (Schinz) Radcl-Sm | N14 | Syringa vulgaris L. |
| M31 | Spirostachys africana Sond. | N15 | Xyliatorreana Brenan |
| M32 | Sterculia quinqueloba (Garcke) K. Schum. | ||
| M33 | Swartzia madagascariensis Desv. |
| Code | Character Description (Units) | Code | Character Description (Units) |
|---|---|---|---|
| V1 | Mean number (Nr) of vessels/mm2 | FIS1 | Wood density, 12% MC (g/cm3) |
| V4 | Mean vessel pit diameter (µm) | FIS2 | Oven-dry wood density (g/cm3) |
| V5 | Mean vessel wall thickness (µm) | FIS3 | MC, dry basis (%) |
| V6 | Mean vessel element length (µm) | FIS4 | MC, wet basis (%) |
| V7 | Mean vessel diameter (µm) | FIS5 | Volumetric shrinkage (%) |
| R1 | Mean Nr of rays/mm | FIS6 | Tangential shrinkage (%) |
| R3 | Ray height in cell numbers (#) | FIS7 | Radial shrinkage (%) |
| R5 | Mean ray height (µm) | FIS8 | Axial shrinkage (%) |
| R7 | Ray width in cell numbers (#) | FIS9 | Coefficient of volumetric shrinkage (%) |
| R8 | Mean ray width (µm) | FIS10 | Coefficient of tangential shrinkage (%) |
| F1 | Mean fiber wall thickness (µm) | FIS11 | Coefficient of radial shrinkage (%) |
| F2 | Mean fiber length (µm) | FIS12 | Coefficient of axial shrinkage (%) |
| F4 | Mean fiber width (µm) | C4 | L* polished sample/natural wood |
| C5 | a* polished sample/natural wood | ||
| C6 | b* polished sample/natural wood | ||
| C7 | Color intensity |
| Species (#) | Tropical Species Code | Variable Code |
|---|---|---|
| 81 | I1 to I17, M1 to M33, T1 to T17, T20 to T33 | V1, V4, V5, V6, V7, R1, R3, R5, R7, R8, F1, F2, F4, C4, C5, C6, C7, FIS1, FIS3, FIS5 |
| 87 | I1 to I17, M1 to M6, M8 to M21, M23 to M29, M31, M33, T2 to T23, T25, T26, T28 to T33, N2 to N9, N11 to N13 | V1, V4, V5, V6, V7, R1, R3, R5, R7, R8, F1, F2, F4 |
| 54 | I1 to I17, M1 to M6, M8 to M30, M33, T9, T10, T17, T20, T21, T25, T28 | FIS1, FIS4 to FIS12, C4, C5, C6, C7 |
| Character (Units) | Min–Max Values/Species Name/Species Code |
|---|---|
| V1 (Nr vessels/mm2) | 1–193/Bombax malabaricum–Androstachys jobnsonii/I5–M7 |
| V4 (µm) | 1.16–15.85/Cleistanthus schlechteri–Ricinodendron rautanenii/N5–M30 |
| V5 (µm) | 3.1–15.6/Khaya–Xylia torreana/M21–N15 |
| V6 (µm) | 150–850/Dalbergia sissoo–Aleurites moluccana/I7–T2 |
| V7 (µm) | 45–285/A. jobnsonii–R. rautanenii/M7–M30 |
| V8 (µm) | 5–85/Schrebera trichoclata–Sterculia quinqueloba/N12–M32 |
| R1 (Nr of rays/mm) | 2–23/Acacia robusta–Pterocarpus antunesii/N1–N10 |
| R3 (#) | 5–67/Calophyllum inophyllum–A. robusta/T6–N1 |
| R5 (µm) | 101–1500/Dalbergia sissoo–S. quinqueloba/I7–M32 |
| R7 (#) | 1–11/Lagerstromia parviflora–S. quinqueloba/I10–M32 |
| R8 (µm) | 13–215/Ganophyllum falcatum–Albizia lebbeckoides/T14–T1 |
| F1 (µm) | 2.4–7.2/Elaeocarpus sphaericus–O. stuhlmannii/T12–M25 |
| F2 (µm) | 700–3780/Dalbergia melanoxylon–Syringa vulgaris/M16–N14 |
| F4 (µm) | 12–46/Colophospermum mopane–R. rautanenii/M13–M30 |
| C4 | 25.91–85.11/D. melanoxylon–A. moluccana/M16 –T2 |
| C5 | 1.86–20.29/D. melanoxylon–P. indicus/M16–T21 |
| C6 | 0.97–34.89/D. melanoxylon–Morus lactea/M16–M24 |
| C7 | 1–27/Diospyros mespiliformis–D. melanoxylon/M18–T2 |
| FIS1 (g/cm3) | 0.23–1.37/R. rautanenii–Tamarindus indica/M30–T28 |
| FIS2 (g/cm3) | 0.21–1.31/R. rautanenii–T. indica/M30–T28 |
| FIS3 (%) | 10.0–16.9/P. indicus–Bischofia javanica/T21–T5 |
| FIS4 (%) | 9.1–31.0/P. indicus–Ficus indica/T21–I9 |
| FIS5 (%) | 3.50–14.33/Cordyla africana–Terminalia tomentosa/M15–I16 |
| FIS6 (%) | 1.6–9.17/Cordyla africana–Aegle marmelos/M15–I2 |
| FIS7 (%) | 1.20–5.17/Albizia adianthifolia–Terminalia tomentosa/M4–I16 |
| FIS8 (%) | 0.01–0.65/Aegle marmelos–Terminalia bellirica/I2–I14 |
| FIS9 (%) | 0.26–0.77/R. rautanenii–Casuarina junghuniana/M30–T9 |
| FIS10 (%) | 0.14–0.50/R. rautanenii–C. junghuniana/M30–T9 |
| FIS11(%) | 0.09–0.30/R. rautanenii–T. indica/M30–T28 |
| FIS12 (%) | 0.00–0.04/Eugenia jambolana–P. indicus/I8–T21 |
| 81 Species | 87 Species | 54 Species | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Variable | PC1 | PC2 | PC3 | PC1 | PC2 | PC3 | PC1 | PC2 | PC3 |
| E.V. | 4.606 | 3.079 | 2.240 | 3.433 | 2.217 | 2.065 | 4.213 | 3.925 | 1.992 |
| P.E. (%) | 23.0 | 15.4 | 11.2 | 26.7 | 17.1 | 15.9 | 30.1 | 28.0 | 14.2 |
| C.P. (%) | 23.0 | 38.4 | 49.6 | 26.7 | 43.8 | 59.7 | 30.1 | 58.1 | 72.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bessa, F.; Sousa, V.; Quilhó, T.; Pereira, H. An Integrated Similarity Analysis of Anatomical and Physical Wood Properties of Tropical Species from India, Mozambique, and East Timor. Forests 2022, 13, 1675. https://doi.org/10.3390/f13101675
Bessa F, Sousa V, Quilhó T, Pereira H. An Integrated Similarity Analysis of Anatomical and Physical Wood Properties of Tropical Species from India, Mozambique, and East Timor. Forests. 2022; 13(10):1675. https://doi.org/10.3390/f13101675
Chicago/Turabian StyleBessa, Fernanda, Vicelina Sousa, Teresa Quilhó, and Helena Pereira. 2022. "An Integrated Similarity Analysis of Anatomical and Physical Wood Properties of Tropical Species from India, Mozambique, and East Timor" Forests 13, no. 10: 1675. https://doi.org/10.3390/f13101675
APA StyleBessa, F., Sousa, V., Quilhó, T., & Pereira, H. (2022). An Integrated Similarity Analysis of Anatomical and Physical Wood Properties of Tropical Species from India, Mozambique, and East Timor. Forests, 13(10), 1675. https://doi.org/10.3390/f13101675








