Abstract
Soil organic carbon (SOC) is critical for carbon cycling and sequestration in forest ecosystems. However, how stand age affects SOC components and stability still remains poorly understood. Here, soil samples (0–20 cm) were collected from Cryptomeria japonica var. sinensis (L. f.) D. Don plantations of seven stand ages (6, 12, 23, 27, 32, 46, 52 a) in the rainy area of western China. SOC fractions, including soil particulate organic carbon (POC), easily oxidizable carbon (EOC), labile organic carbon (LOC), recalcitrant organic carbon (ROC), and light fraction organic carbon (LFOC), were determined to explore the nature of carbon components and stability across a chronosequence of C. japonica plantation. Soil carbon fractions first increased and then trended to be stable with an increase in stand age. SOC concentrations were the largest in mature forests (27 or 32 a), but the concentrations of other carbon components often peaked in early over-mature forests (46 a). The concentrations of all carbon fractions were the lowest in the young forests (6 a). The ratios of ROC/SOC increased and LOC/SOC decreased with increasing stand age. Almost all carbon fractions were positively correlated with soil bulk density and negatively correlated with soil moisture. The allometric exponent of ROC or HFOC and soil physicochemical properties was higher as compared to LOC and LFOC. The results noted in this study indicate that SOC components often accumulate fast over the first 20 years of afforestation and SOC stability increases with increasing stand age for C. japonica plantation in this specific region.
1. Introduction
Forest soil organic carbon (SOC) storage accounts for approximately two-thirds of the global SOC pool [1,2]. Even a minor fluctuation in the SOC of forest ecosystems may affect the carbon balance of the atmosphere and land [1,3]. Previous studies have shown that forest SOC is thought as an important indicator for sustainable forest management [4,5,6,7]. For example, soil-activated carbon fractions are easily decomposed and utilized by soil microorganisms because of their instability [5]. Furthermore, soil-activated carbon fractions include several carbon sources, such as plant and animal residues, microorganism metabolism of root exudates, and rhizosphere microorganism entry into the soil via litter [1,3]. The litter-derived carbon is easily utilized by microbes and forms a stable structure due to biological activity [3,7]. Although labile organic carbon has a minor percentage of SOC, its oxidation process drives carbon exchange between land and atmosphere [6]. However, recalcitrant soil carbon is one of the vital indicators denoting SOC accumulation and soil fertility due to its stability [7].
SOC fractions are dependent on both abiotic and biotic factors. Studies have indicated that environmental changes (e.g., nitrogen deposition), land use cover change (e.g., forest conversion), and management practices (e.g., affectation) on SOC fractions [8,9,10,11,12]. Stand age is one of the main characteristics of forest growth and development and is closely related to SOC fractions. Firstly, forest growth and development can regulate SOC by affecting stand structure, understory vegetation diversity, and soil properties [13,14]. Secondly, stand-age-associated changes in canopy conditions, stand structure, and understory vegetation could affect litter residue and root exudate, which could in turn affect soil microbial biomass, enzyme activity, and soil physicochemical properties, further leading to changes in SOC accumulation and fractions [15,16]. Thus, the balance between carbon input (e.g., above- and belowground litter) and output (e.g., soil respiration) determines SOC fractions [1,8,11,15,16]. Although a number of studies have reported SOC over stand ages, the stand-age-caused patterns in forest SOC are still highly controversial [13,14,15,16,17]. For example, Meta et al. (2015) showed that SOC increased with increasing stand age [17], but other studies found that SOC decreased first and then increased with increasing stand age [14,16]. Meanwhile, how different carbon components (labile carbon vs. recalcitrant carbon) respond to stand age in forest ecosystems remains poorly understood.
Cryptomeria japonica var. sinensis is a fast-growing coniferous tree that is commonly planted in the Sichuan Basin of southwestern China. There is a 2.0 × 105 hm2 C. japonica plantation in Sichuan province. These plantations play important roles in timber production and ecological protection. There are a series of C. japonica plantations of different ages in the local national forestry farm on the western edge of the Sichuan Basin as a result of frequent afforestation and management. In this study, we selected seven stand ages (6, 12, 23, 27, 32, 46, 52 a) of C. japonica plantations in the rainy area of western China. We aimed to address the following questions: (1) How do SOC fractions and stability vary with stand ages? (2) What are the potential drivers of SOC accumulation along a chronosequence?
2. Materials and Methods
2.1. Study Site
This study was performed at the Long-Term Station of Artificial Forest Ecosystems of Sichuan Agricultural University, which is located at the Hongya National Forestry Farm of Hongya on the western edge of the Sichuan Basin, China (29°24′–30°00′ N, 102°49′–103°32′ E). The mean annual temperature is 16.8 °C, with extreme maximum and minimum temperatures being 36.2 °C and –3.3 °C, respectively. The mean annual precipitation is 1400 mm, and the mean annual relative humidity is 83%. The soil is classified as mountain yellow soil according to the FAO, and its pH value range is 4.8–6.5. C. japonica plantation is the dominant forest type, accounting for 49.6% of the total area. In the local forestry farm, the area of young (≤10 years), middle-aged (11–21 years), near-mature (21–25 years), mature (26–35 years), and over-mature forests (≥35 years) are 2134 hm2, 698 hm2, 577 hm2, 866 hm2, and 1790 hm2, respectively. The understory vegetation was commonly dominated by Fargesia spathacea Franch, Rubus swinhoei Hance, Elatostema involucratum Franch. et Sav, and Pteridophyta in C. japonica plantations [18].
2.2. Experimental Design
In August 2020, we selected seven stand ages (6, 12, 23, 27, 32, 46, 52 a) of C. japonica plantations based on the stand age classification standard of China [14,16]. There were three 20 m × 20 m plots chosen in each stand age forest community. All plots faced west–north and had a slope gradient of less than 30°.
The tree height (H), diameter at breast height (DBH, 1.3 m), and canopy density were recorded for all trees within each plot. Briefly, the basic characteristics of the sites are shown in Table 1.

Table 1.
Basic information of C. japonica plantation in the rainy area of western China.
2.3. Soil Sampling
Before soil collection, the litter layer in each plot was removed. Five soil cores (5 cm in diameter, 20 cm in depth) from each plot were randomly collected and mixed to form composite samples. The soil samples were sieved through 2 mm, and roots and other debris were removed. Each sample was air-dried and stored at room temperature for the determination of the soil physical and chemical properties.
2.4. Soil Nutrient Measurement
The SOC was measured by the dichromate oxidation method [14], and soil easily oxidizable carbon (EOC) was measured according to the potassium permanganate (KMnO4) oxidizable method [19,20]. The soil particulate organic carbon (POC) was determined by the hexametaphosphate separation method [14]. The soil labile organic carbon (LOC) and recalcitrant organic carbon (ROC) were assayed with the acid hydrolysis method [21]. The soil light fraction organic carbon (LFOC) and heavy fraction organic carbon (HFOC) were measured with the 1.70 g·cm−3 NaI solution separation method [3]. The soil pH was measured using a glass electrode [19], and the soil total nitrogen (TN) was determined using the Kjeldahl method [10]. The soil bulk density (g cm−3) was measured using a soil bulk sampler (100 cm3 volume) [19].
2.5. Statistical Analysis
The SOC storage was calculated using the following equation [7]:
where SOC storage is the SOC storage (kg·m−2), C is the soil organic carbon (g·kg−1), BD is the soil bulk density (g·cm−3), H is the soil depth (cm), and G is the proportion of the dry weight of gravel that is greater than 2 mm to the total dry weight of soil (%).
SOC storage = C × BD × H × (1 − G)/100
The POC/SOC, EOC/SOC, LOC/SOC, ROC/SOC, LFOC/SOC, and HFOC/SOC were calculated based on the content ratio. Differences in SOC fractions among stand ages were tested by one-way ANOVA analysis of variance. The Duncan test was used for multiple comparisons (p < 0.05). If the data does not meet the homogeneity of variance, it will be transformed by a logarithm or trigonometric function to assume the normal distribution and improve the homogeneity of variance. Regression analysis was used to fit soil organic carbon with stand age and soil physicochemical properties. Standardized major axis estimation (SMA) was performed to estimate allometric relationships between SOC fractions (EOC and POC, LOC and ROC, LFOC and HFOC) concentrations with soil BD, water content, pH, and TN, and thus to assess the difference in soil carbon fractions in response to environmental change based on the allometric exponents [22]. Statistical analyses were performed using IBM SPSS statistics 26.0 and Standardized Major Axis Tests and Routines (SMATR) 2.0 [23], and the figures were drawn with Origin 2019b.
3. Results
SOC Fractions
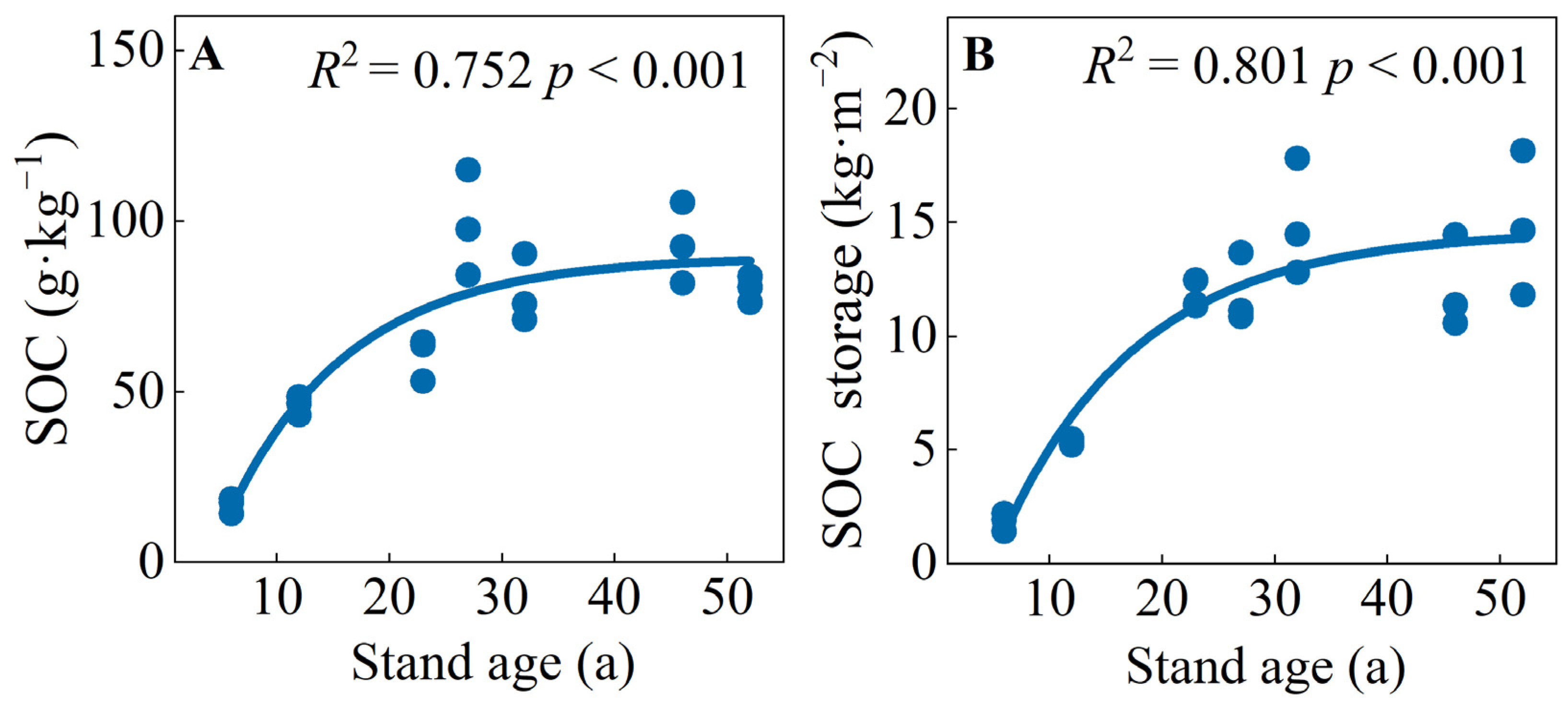
The SOC concentrations and storage of the C. japonica plantation varied from 16.88 to 98.92 g·kg−1 and 1.86 to 15.03 kg·m−2, respectively (Figure S1). Both SOC concentration and storage first increased and then trended to be stable with an increase in stand age, showing a logistic function. Both of them were the largest in mature forests (27 or 32 a) and the lowest in young forests (6 a) (Figure 1 and Figure S1). The SOC concentrations of mature and over-mature stands were significantly higher than those of young, middle-aged, and near-mature forests. The SOC storage of young and middle-aged forests was significantly lower than those of other stand ages (Table 1, Figure S1).
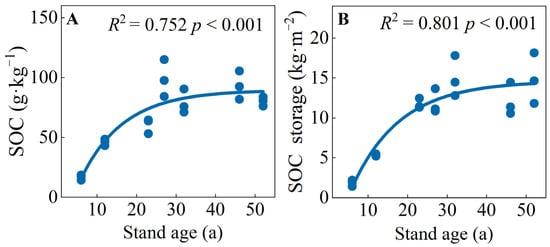
Figure 1.
(A) soil organic carbon concentrations and (B) storage of SOC in C. japonica plantation of different stand ages in the rainy area of western China.
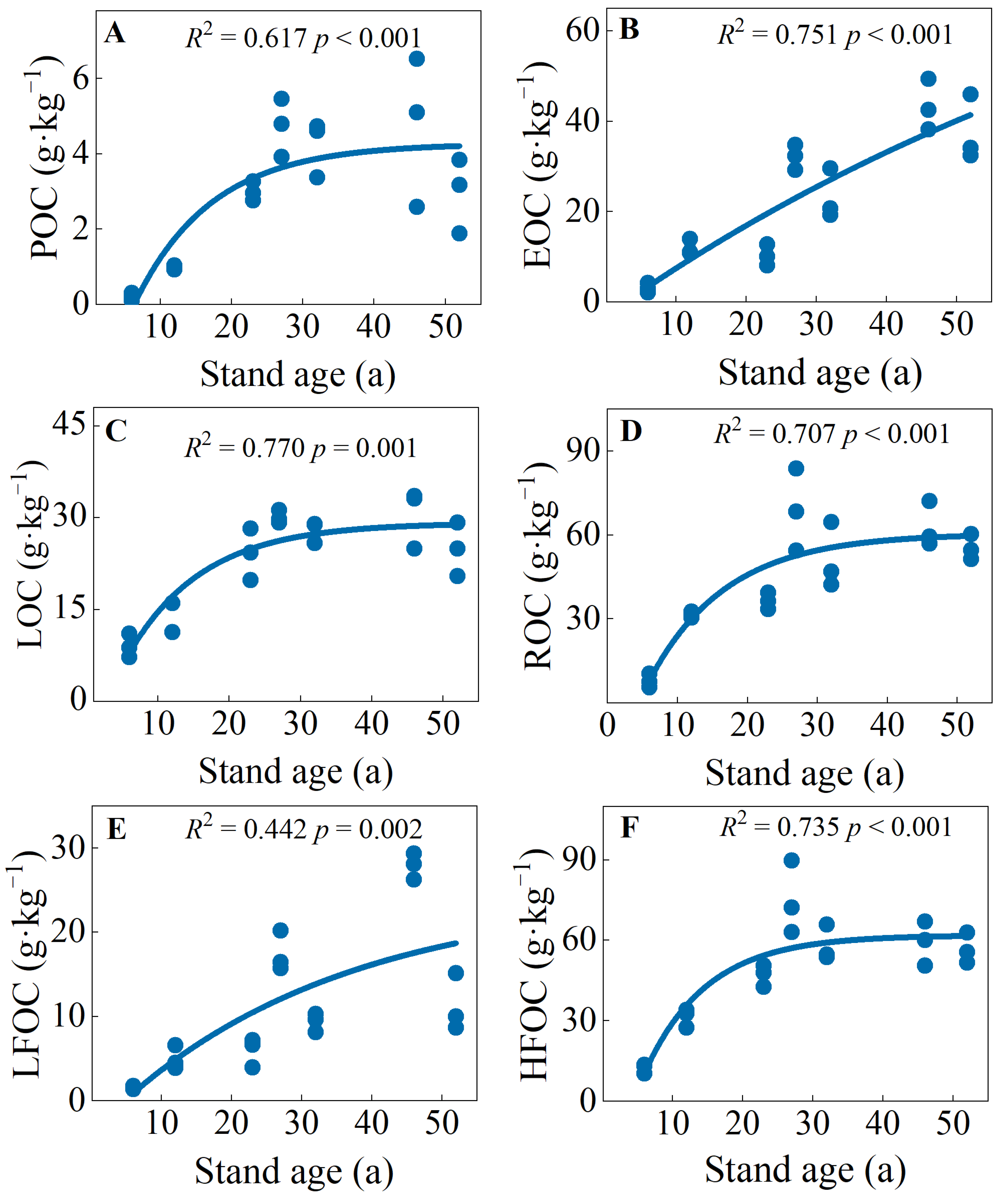
Across the stand ages, the ranges of POC, EOC, LOC, ROC, LFOC, and HFOC concentrations were 0.20–4.74, 1.05–7.38, 9.04–30.55, 7.84–68.83, 1.53–27.90, and 12.38–74.93 g·kg−1, respectively (Figure S2). There were logistic function relationships between soil carbon fractions and stand ages (Figure 2). Specifically, the POC, EOC, LOC, and LFOC were the highest in the early over-mature forests (46 a), and the ROC and HFOC were highest in the early over-mature forest (27 a) (Figure S2). Overall, soil carbon fractions of mature and over-mature forests were significantly higher than those of young, middle-aged, and near-mature forests (Figure 2 and Figure S2).
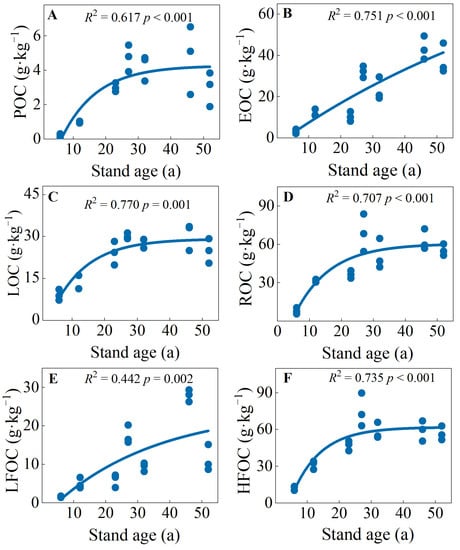
Figure 2.
(A) soil particulate organic carbon (POC), (B) easily oxidizable carbon (EOC), (C) labile organic carbon (LOC), (D) recalcitrant organic carbon (ROC), (E) light fraction organic carbon (LFOC) and (F) heavy fraction organic carbon (HFOC) in C. japonica plantations of different stand ages in the rainy area of western China.
The proportions of the POC, EOC, LOC, ROC, LFOC, and HFOC to SOC were 1.2%–5.4%, 16.8%–46.7%, 30.8%–53.9%, 46.1%–69.1%, 9.1%–30.1%, and 63.3%–77.6%, respectively (Table 2). The proportion of carbon fractions to SOC varied among stand ages (Table 2). Specifically, the POC/SOC of young, middle-aged, and mature forests were significantly lower than those of other stand ages (Table 2). The rank of an average value of the EOC/SOC was over-mature forests > mature forests > near-mature, middle-aged, and young forests. The LOC/SOC of young forests was higher than that of other stand ages, but a contrasting result was observed in ROC/SOC. The highest and lowest proportion of the LFOC/SOC was observed in the early over-mature forests (46 a) and young forests, respectively (Table 2). The HFOC/SOC of young, near-mature, and mature forests were higher than those of middle-aged and over-mature forests (Table 2).

Table 2.
Proportion (%) of carbon fractions to SOC of C. japonica plantation at different stand ages in the rainy area of western China.
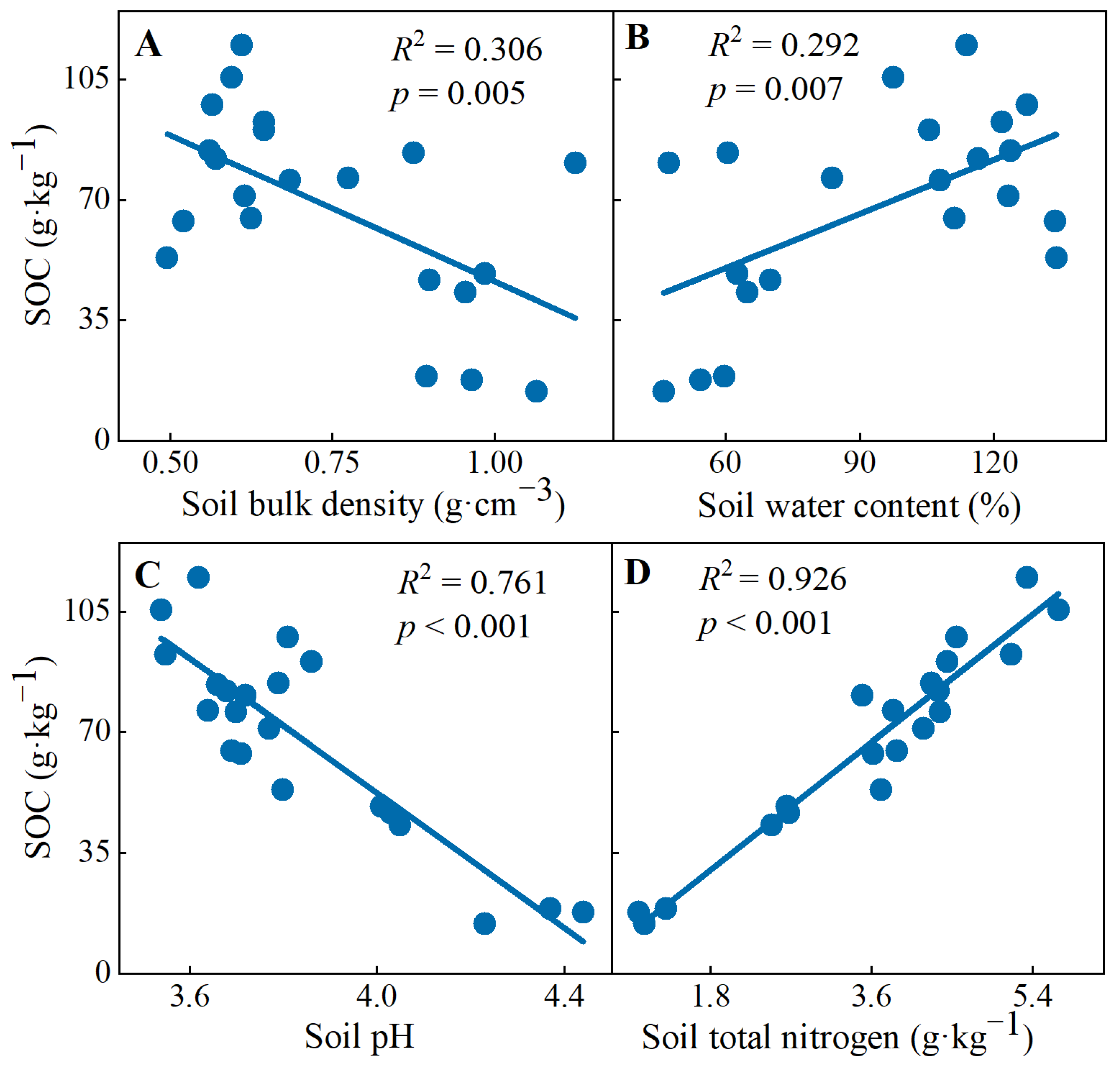
In addition, linear regression analysis showed that the SOC concentrations decreased with increasing soil bulk density (R2 = 0.306, p = 0.005) and soil pH (R2 = 0.761, p < 0.001), but increased with increasing water content (R2 = 0.292, p = 0.007) and nitrogen content (R2 = 0.926, p < 0.001) (Figure 3).
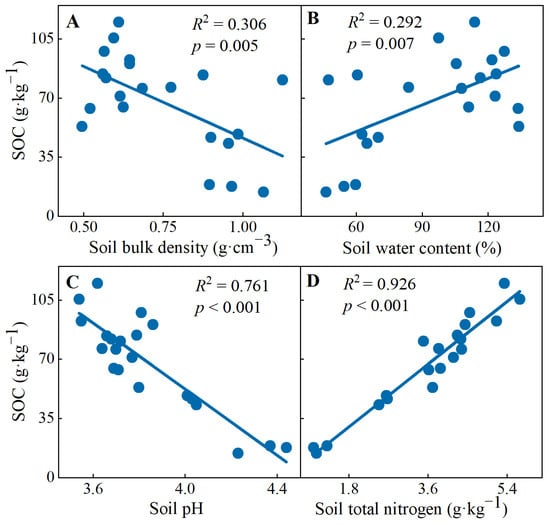
Figure 3.
Linear regression fitting of soil organic carbon concentrations with (A) soil bulk density, (B) soil water content, (C) soil pH and (D) soil total nitrogen.
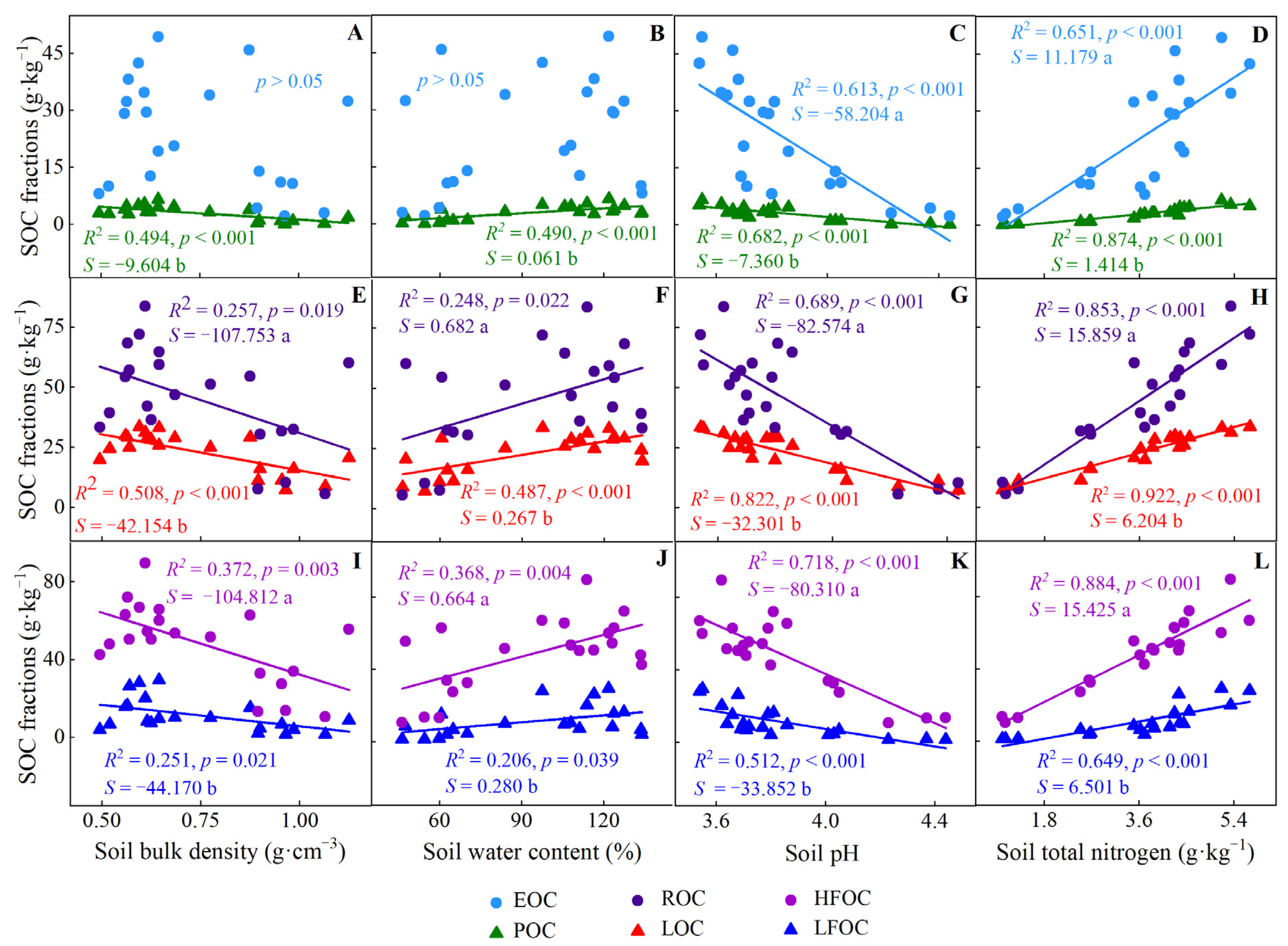
Almost all soil carbon fractions were negatively correlated with soil bulk density and soil pH but positively associated with water content and nitrogen content, respectively (Figure 4). In addition, the allometric exponent between ROC, HFOC, and soil physicochemical properties were higher than those of LOC and LFOC and soil physicochemical properties (Figure 4).
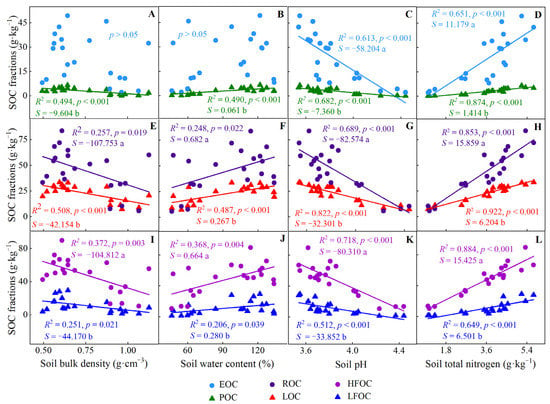
Figure 4.
Estimation and analysis of standardized principal axis between soil physicochemical properties and carbon fractions in C. japonica plantation in the rainy area of western China. (A–D) analysis between EOC and POC with soil bulk density, water content, pH and total nitrogen, respectively; (E–H), analysis between ROC and LOC with soil physicochemical properties, respectively; (I–L), analysis between HFOC and LFOC with soil physicochemical properties, respectively. S: slope. Different lowercase letters denote the correlation between the slope of two straight lines in the same box is significantly different.
4. Discussion
SOC in forest ecosystems is 2–3 times more than that of vegetation and atmospheric carbon, which plays an important role in the global carbon cycle and sequestration [1,24]. Previous studies have found that SOC concentrations and storage are regulated by many abiotic and biotic variables, including litter production, root exudates, understory plant diversity, and microenvironments during forest growth and development [11,25]. For example, Zhu et al. [11] have shown that an increase in litter input stimulates SOC and microbial biomass carbon in Picea schrenkiana forest. Furthermore, fine roots are another important source of SOC fractions. In this study, there were logistic relationships between SOC concentrations and stand ages, where the SOC concentration was the highest in mature forests (98.92 g·kg−1) and the lowest in young forests (16.88 g·kg−1) (Table 1). The results noted in this case were different from the observation of other studies [16,17]. This may be due to the differences in stand age sequence chosen between studies. In general, stand age can promote the accumulation of SOC through the following aspects: Firstly, forest litter is the major source of forest organic carbon. Litter yield often increases with stand age in forest ecosystems [14], which may promote microbial activity and the accumulation of SOC [16]. Secondly, in addition to aboveground litter, belowground root residues and exudates also contribute to SOC [8,15]. For example, Zhang et al. [8] showed that SOC was positively associated with fine root biomass. Thirdly, the better conditions of both underground plant diversity and micro-environments in the old than young forests were beneficial to the accumulated SOC [17,25,26]. In our study, the SOC concentration was lower in over-mature forests compared to mature forests. Similar findings were observed in Chinese fir and Pinus koraiensis forests [14,27]. It may be because of the increased soil bulk density in the over-mature forests, which was not conducive to the extension of plant roots, infiltration of surface water, and microbial activity [28,29]. Additionally, we also found that SOC storage increased by 13.17 kg m−2 from young (6 a) to mature forests (32 a), indicating that soil carbon sinks increased with the development of the C. japonica plantation in this region.
Compared with total SOC, SOC fractions can better indicate the changes in soil quality and stability [19]. In this study, SOC fractions of C. japonica plantations often were the highest in mature (27 a) or early over-mature forests (46 a) and the lowest in young forests (6 a), which also showed logistic functions (Figure 2). The POC consists of plant residues and microbial debris, which is usually at the transition stage of fresh animal and plant residues, transforming into soil humus, and is mainly concentrated in the soil aggregates [14,30]. Previous studies found that forest development could increase POC concentrations by improving the stability of soil aggregates [14]. The EOC has the highest turnover rate, which is directly involved in the nutrient supply for plants and microorganisms [19,24]. The LFOC is a free organic matter with a low relative density in soil, and it is mainly composed of microbial and plant debris [4]. The increase in plant litter soil animals and microorganisms with increasing stand ages can increase the concentrations of EOC and LFOC [4,14,31,32]. A study in Southwest China revealed that soil carbon sequestration depended on stand age [31]. Both EOC and LFOC in topsoil (0–10 cm) increased with stand age [31]. Furthermore, both ROC and HFOC are generally considered to be indicators of soil carbon stability and storage capacity as a result of their high stability, long turnover, and decomposition period [1,21,33]. C. japonica is a coniferous tree species, and its residues contain rich lignin and tannin. Thus, the accumulation of ROC and HFOC may be due to the increasing refractory substances along stand ages. Belay-Tedla et al. [33] demonstrated that there was a positive linear correlation between the accumulation of ROC and litter input.
The SOC stability is mainly dependent on the proportion of SOC fractions [14,24,34,35]. For example, the larger ROC/SOC reflects a more stable carbon pool [34,35]. Our results showed that the proportion of organic carbon fractions to SOC varied with stand ages (Table 2). In this study, the ROC/SOC of young forests was lower than that of other stand ages. The young forests have less carbon input due to the lower vegetation biomass and cover. In addition, the higher solar radiation and precipitation leaching may not be conducive to the accumulation of SOC in the young than in mature forests [30]. However, the relatively high plant and biological biomass in mature forests could increase carbon input, potentially increasing ROC/SOC [16]. Similarly, a high LOC/SOC means low SOC stability [34,35]. In this study, POC/SOC, EOC/SOC, and LFOC/SOC increased with stand age. This might be explained by the fact that soil nitrogen, bulk density, and water content increased with stand age, which could promote microbial activity and the accumulation of LOC, HFOC, and EOC [4,19,24]. However, we found that LOC/SOC decreased with stand age. It could be the variation of LOC/SOC is also affected by other soil LOC fractions, e.g., microbial biomass carbon, dissolved organic carbon water-soluble organic carbon, and mineralized organic carbon [14,24]. These results demonstrate that the SOC stability increased with the development of C. japonica plantations.
In general, there are significant differences in soil physicochemical properties among forest ages [30]. In this case, almost all SOC fractions were negatively correlated with soil bulk density but positively correlated with water content. Similar results were also recorded in other studies [20,36]. As mentioned above, both POC and LFOC mainly include mineral particles, animal, and plant residues, which are mediated by soil bulk density and moisture [4]. However, the conversion and renewal rate of EOC is fast. Consequently, the relationships between EOC and physicochemical properties are not well exhibited as expected [14,19]. Moreover, SOC fractions were negatively correlated with soil pH. This pattern was consistent with the results found in the Chinese Loess Plateau and agricultural soils [20,37]. The increase in soil pH was not beneficial for microorganism growth and enzymatic reactions, which may in turn inhibit the accumulation of SOC [38]. Furthermore, the SOC fractions were positively correlated with nitrogen. It could be explained by the fact that soil carbon is closely coupled with nitrogen in biogeochemical cycles [39]. However, the allometric exponent of ROC and HFOC and physicochemical properties was higher as compared to those of LOC and LFOC, implying that the recalcitrant carbon fractions (ROC and HFOC) may be more responsive to soil physicochemical properties relative to active carbon fractions (LOC and LFOC). Microorganisms can promote the transformation from recalcitrant carbon fractions to active carbon fractions [37]. A previous study has suggested ROC is more sensitive to the priming effect compared to LOC [40]. ROC may trigger great microbial nitrogen demand and ROC decomposition [40].
5. Conclusions
Our results suggest that all carbon components increase with increasing stand age although they peak at different stand ages. The stability of SOC increased with the development of C. japonica plantations. Soil physicochemical properties (e.g., soil bulk density, moisture, pH, and TN) well indicate the changes in carbon fractions. Recalcitrant carbon fractions (ROC and HFOC) are more mediated by soil physicochemical properties relative to active carbon fractions (LOC and LFOC). Taken together, soil carbon pools accumulated fast in the early stage after C. japonica afforestation. Such findings are helpful for the management of C. japonica plantations in this specific region and for modeling soil carbon accumulation.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/f13101663/s1, Figure S1: Soil organic carbon (SOC) concentrations and soil organic carbon storage (SOCS) in C. japonica plantation at different stand ages in the rainy area of western China.; Figure S2: Soil organic carbon fractions of soil particulate organic carbon (POC), easily oxidizable carbon (EOC), labile organic carbon (LOC), recalcitrant organic carbon (ROC), light fraction organic carbon (LFOC) and heavy fraction organic carbon (HFOC) in C. japonica plantation at different stand ages in the rainy area of western China.
Author Contributions
Conceptualization, Z.X., B.T. and C.Y.; methodology, X.L. and X.H.; formal analysis, X.L., X.H. and C.Y.; investigation, X.L., Z.L. and C.Y.; resources, X.L., X.H., Z.L., J.L., Y.Y., H.L., L.Z., S.L., L.W., C.Y., B.T. and Z.X.; data curation, X.L. and X.H.; writing—original draft preparation, X.L., X.H. and Z.L.; writing—review and editing, X.L., X.H., Z.L., C.Y. and Z.X.; visualization, X.L. and X.H.; supervision, Z.X.; project administration, Z.X.; funding acquisition, Z.X. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (32071745, 31870602 and 31901295), the Program of Sichuan Excellent Youth Sci-Tech Foundation (2020JDJQ0052), and the Program of Sichuan Applied Basic Research Foundation (2021YJ0340).
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
We are grateful to the Ecological Security and Protection Key Laboratory of Sichuan Province.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Chen, Z.; Shen, Y.; Tan, B.; Li, H.; You, C.; Xu, Z.; Wei, X.; Ni, X.; Yang, Y.; Zhang, L. Decreased Soil Organic Carbon under Litter Input in Three Subalpine Forests. Forests 2021, 12, 1479. [Google Scholar] [CrossRef]
- Deng, L.; Wang, K.-B.; Chen, M.-L.; Shangguan, Z.-P.; Sweeney, S. Soil organic carbon storage capacity positively related to forest succession on the Loess Plateau, China. CATENA 2013, 110, 1–7. [Google Scholar] [CrossRef]
- Yang, X.; Wang, D.; Lan, Y.; Meng, J.; Jiang, L.; Sun, Q.; Cao, D.; Sun, Y.; Chen, W. Labile organic carbon fractions and carbon pool management index in a 3-year field study with biochar amendment. J. Soils Sediments 2018, 18, 1569–1578. [Google Scholar] [CrossRef]
- Liu, T.; Peng, D.; Tan, Z.; Guo, J.; Zhang, Y. Effects of stand density on soil respiration and labile organic carbon in different aged Larix principis-rupprechtii plantations. Ecol. Process. 2021, 10, 44. [Google Scholar] [CrossRef]
- Benbi, D.K.; Brar, K.; Toor, A.S.; Singh, P.; Singh, H. Soil carbon pools under poplar-based agroforestry, rice-wheat, and maize-wheat cropping systems in semi-arid India. Nutr. Cycl. Agroecosyst. 2012, 92, 107–118. [Google Scholar] [CrossRef]
- Luo, Z.; Feng, W.; Luo, Y.; Baldock, J.; Wang, E. Soil organic carbon dynamics jointly controlled by climate, carbon inputs, soil properties and soil carbon fractions. Glob. Chang. Biol. 2017, 23, 4430–4439. [Google Scholar] [CrossRef]
- Pang, D.; Cui, M.; Liu, Y.; Wang, G.; Cao, J.; Wang, X.; Dan, X.; Zhou, J. Responses of soil labile organic carbon fractions and stocks to different vegetation restoration strategies in degraded karst ecosystems of southwest China. Ecol. Eng. 2019, 138, 391–402. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, Y.; Zhou, Z.; Zhang, Y. Inorganic Nitrogen Addition Affects Soil Respiration and Belowground Organic Carbon Fraction for a Pinus tabuliformis Forest. Forests 2019, 10, 369. [Google Scholar] [CrossRef]
- Su, F.; Xu, S.; Sayer, E.J.; Chen, W.; Du, Y.; Lu, X. Distinct storage mechanisms of soil organic carbon in coniferous forest and evergreen broadleaf forest in tropical China. J. Environ. Manag. 2021, 295, 113142. [Google Scholar] [CrossRef]
- Liu, X.; Zeng, X.; Zou, X.; Lodge, D.J.; Stankavich, S.; González, G.; Cantrell, S.A. Responses of Soil Labile Organic Carbon to a Simulated Hurricane Disturbance in a Tropical Wet Forest. Forests 2018, 9, 420. [Google Scholar] [CrossRef]
- Zhu, H.; Gong, L.; Ding, Z.; Li, Y. Effects of litter and root manipulations on soil carbon and nitrogen in a Schrenk’s spruce (Picea schrenkiana) forest. PLoS ONE 2021, 16, e0247725. [Google Scholar] [CrossRef]
- Monroe, P.H.M.M.; Barreto-Garcia, P.A.B.; Barros, W.T.; de Oliveira, F.G.R.B.; Pereirab, M.G. Physical protection of soil organic carbon through aggregates in different land use systems in the semi-arid region of Brazil. J. Arid. Environ. 2021, 186, 114427. [Google Scholar]
- Sun, Z.; Liu, L.; Peng, S.; Peñuelas, J.; Zeng, H.; Piao, S. Age-Related Modulation of the Nitrogen Resorption Efficiency Response to Growth Requirements and Soil Nitrogen Availability in a Temperate Pine Plantation. Ecosystems 2016, 19, 698–709. [Google Scholar] [CrossRef]
- He, X.X.; Huang, Y.Z.; Zhang, Q.C.; Ye, S.M.; Wang, S.Q. Distribution of organic carbon fractions in soil aggregates in Chinese fir plantations with different stand ages. Ecol. Process. 2021, 10, 49–61. [Google Scholar] [CrossRef]
- Zhang, H.; Deng, Q.; Hui, D.; Wu, J.; Xiong, X.; Zhao, J.; Zhao, M.; Chu, G.; Zhou, G.; Zhang, D. Recovery in soil carbon stock but reduction in carbon stabilization after 56-year forest restoration in degraded tropical lands. For. Ecol. Manag. 2019, 441, 1–8. [Google Scholar] [CrossRef]
- Wang, C.; Xue, L.; Jiao, R. Soil organic carbon fractions, C-cycling associated hydrolytic enzymes, and microbial carbon metabolism vary with stand age in Cunninghamia lanceolate (Lamb.) Hook plantations. For. Ecol. Manag. 2021, 482, 118887. [Google Scholar] [CrossRef]
- Justine, M.F.; Yang, W.; Wu, F.; Tan, B.; Khan, M.N.; Zhao, Y. Biomass Stock and Carbon Sequestration in a Chronosequence of Pinus massoniana Plantations in the Upper Reaches of the Yangtze River. Forests 2015, 6, 3665–3682. [Google Scholar] [CrossRef]
- Liu, X.; Cui, N.; Tan, F.; Hong, Z.; Xiong, S.; Meng, Y.; Tan, B.; Li, H.; Xu, Z.; Zhang, J.; et al. The soil water holding capacity and its indicative effect on soil organic carbon of Cryptomeria japonica plantations in the rainy area of western China. Chin. J. Apple. Environ. Biol. 2023, 29, 1–10. [Google Scholar] [CrossRef]
- Bongiorno, G.; Bünemann, E.K.; Oguejiofor, C.U.; Meier, J.; Gort, G.; Comans, R.; Mäder, P.; Brussaard, L.; de Goede, R. Sensitivity of labile carbon fractions to tillage and organic matter management and their potential as comprehensive soil quality indicators across pedoclimatic conditions in Europe. Ecol. Indic. 2019, 99, 38–50. [Google Scholar] [CrossRef]
- Wang, B.; Liu, D.; Yang, J.; Zhu, Z.; Darboux, F.; Jiao, J.; An, S. Effects of forest floor characteristics on soil labile carbon as varied by topography and vegetation type in the Chinese Loess Plateau. CATENA 2021, 196, 104825. [Google Scholar] [CrossRef]
- Belay-Tedla, A.; Zhou, X.; Bo, S.; Wan, S.; Luo, Y. Labile, recalcitrant, and microbial carbon and nitrogen pools of a tallgrass prairie soil in the US Great Plains subjected to experimental warming and clipping. Soil Biol. Biochem. 2009, 41, 110–116. [Google Scholar] [CrossRef]
- Warton, D.I.; Weber, N.C. Common Slope Tests for Bivariate Errors-in-Variables Models. Biom. J. 2002, 44, 161–174. [Google Scholar] [CrossRef]
- Falster, D.S.; Warton, D.I.; Wright, I.J. User’s Guide to SMATR: Standardized Major Axis Tests and Routines, Copyright 2006. Available online: http://www.bio.mq.edu.au/ecology/SMATR/ (accessed on 28 April 2022).
- Wang, M.; Wang, S.; Cao, Y.; Jiang, M.; Wang, G.; Dong, Y. The effects of hummock-hollow microtopography on soil organic carbon stocks and soil labile organic carbon fractions in a sedge peatland in Changbai Mountain, China. CATENA 2021, 201, 105204. [Google Scholar] [CrossRef]
- Wang, D.; Olatunji, O.A.; Xiao, J. Thinning increased fine root production, biomass, turnover rate and understory vegetation yield in a Chinese fir plantation. For. Ecol. Manag. 2019, 440, 92–100. [Google Scholar] [CrossRef]
- D’Orazio, V.; Traversa, A.; Senesi, N. Forest soil organic carbon dynamics as affected by plant species and their corresponding litters: A fluorescence spectroscopy approach. Plant Soil 2013, 374, 473–484. [Google Scholar] [CrossRef]
- Li, X.; Yi, M.J.; Son, Y.; Park, P.S.; Lee, K.H.; Son, Y.M.; Kim, R.H.; Jeong, M.J. Biomass and Carbon Storage in an Age-Sequence of Korean Pine (Pinus koraiensis) Plantation Forests in Central Korea. J. Plant Biol. 2011, 54, 33–42. [Google Scholar] [CrossRef]
- Meyer, C.; Luscher, P.; Schulin, R. Enhancing the regeneration of compacted forest soils by planting black alder in skid lane tracks. Eur. J. Forest Res. 2014, 133, 453–465. [Google Scholar] [CrossRef]
- Mujuru, L.; Gotora, T.; Velthorst, E.; Nyamangara, J.; Hoosbeek, M.R. Soil carbon and nitrogen sequestration over an age sequence of Pinus patula plantations in Zimbabwean Eastern Highlands. For. Ecol. Manag. 2014, 313, 254–265. [Google Scholar] [CrossRef]
- Johannes, L.; Markus, K. The contentious nature of soil organic matter. Nature 2015, 528, 60–68. [Google Scholar]
- Jia, G.M.; Xi, Y.; Zhang, B.; Chen, F. Soil labile organic carbon and microbial activity changes with age in citrus (Citrus sinensis Osb.) plantations in China. Aust. For. 2014, 77, 153–158. [Google Scholar] [CrossRef]
- Chen, A.; Wang, Z.; Lin, Y.; Wang, X.; Li, Y.; Zhang, Y.; Tao, Z.; Gao, Q.; Tang, G. Temporal variation of soil organic carbon pools along a chronosequence of reforested land in Southwest China. CATENA 2020, 194, 104650. [Google Scholar] [CrossRef]
- Huang, Z.; Clinton, P.; Davis, M.R. Post-harvest residue management effects on recalcitrant carbon pools and plant biomarkers within the soil heavy fraction in Pinus radiata plantations. Soil Biol. Biochem. 2011, 43, 404–412. [Google Scholar] [CrossRef]
- Shen, Y.; Cheng, R.; Xiao, W.; Yang, S.; Guo, Y.; Wang, N.; Zeng, L.; Lei, L.; Wang, X. Labile organic carbon pools and enzyme activities of Pinus massoniana plantation soil as affected by understory vegetation removal and thinning. Sci. Rep. 2018, 8, 1981–1987. [Google Scholar] [CrossRef] [PubMed]
- Gu, C.; Liu, Y.; Mohamed, I.; Zhang, R.; Wang, X.; Nie, X.; Jiang, M.; Brooks, M.; Chen, F.; Li, Z. Dynamic Changes of Soil Surface Organic Carbon under Different Mulching Practices in Citrus Orchards on Sloping Land. PLoS ONE 2016, 11, e0168384. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.H.; Wu, Q.G.; Hu, J.Y.; Yu, L.F.; Bie, P.F.; Wang, H.; Deng, D.Z. Changes in Soil Physical and Chemical Properties During the Process of Alpine Meadow Degradation along the Eastern Qinghai-Tibet Plateau. Eurasian Soil Sci. 2018, 51, 1440–1446. [Google Scholar] [CrossRef]
- Luan, H.; Gao, W.; Huang, S.; Tang, J.; Li, M.; Zhang, H.; Chen, X.; Masiliūnas, D. Organic amendment increases soil respiration in a greenhouse vegetable production system through decreasing soil organic carbon recalcitrance and increasing carbon-degrading microbial activity. J. Soils Sediments 2020, 20, 2877–2892. [Google Scholar] [CrossRef]
- Fujii, K.; Uemura, M.; Hayakawa, C.; Funakawa, S.; Kosaki, T. Environmental control of lignin peroxidase, manganese peroxidase, and laccase activities in forest floor layers in humid Asia. Soil Biol. Biochem. 2013, 57, 109–115. [Google Scholar] [CrossRef]
- Cheng, L.; Leavitt, S.; Kimball, B.; Pinter, P.; Ottman, M.; Matthias, A.; Wall, G.; Brooks, T.; Williams, D.; Thompson, T. Dynamics of labile and recalcitrant soil carbon pools in a sorghum free-air CO2 enrichment (FACE) agroecosystem. Soil Biol. Biochem. 2007, 39, 2250–2263. [Google Scholar] [CrossRef]
- Chen, X.; Lin, J.; Wang, P.; Zhang, S.; Liu, D.; Zhu, B. Resistant soil carbon is more vulnerable to priming effect than active soil carbon. Soil Biol. Biochem. 2022, 168, 108619. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).