Arthrinium arundinis, a Novel Causal Agent of Moso Bamboo (Phyllostachys edulis) Culm Rhomboid Rot and Its Sensitivity to Fungicides
Abstract
1. Introduction
2. Materials and Methods
2.1. Field survey and Fungal Isolation
2.2. Pathogenicity Assay
2.2.1. Inoculation under Laboratory Conditions
2.2.2. Inoculation under Field Conditions
2.3. Morphological Characterization
2.4. Molecular and Phylogenetic Analysis
2.5. Sensitivity of Isolate BBB1 to Fungicides
3. Results
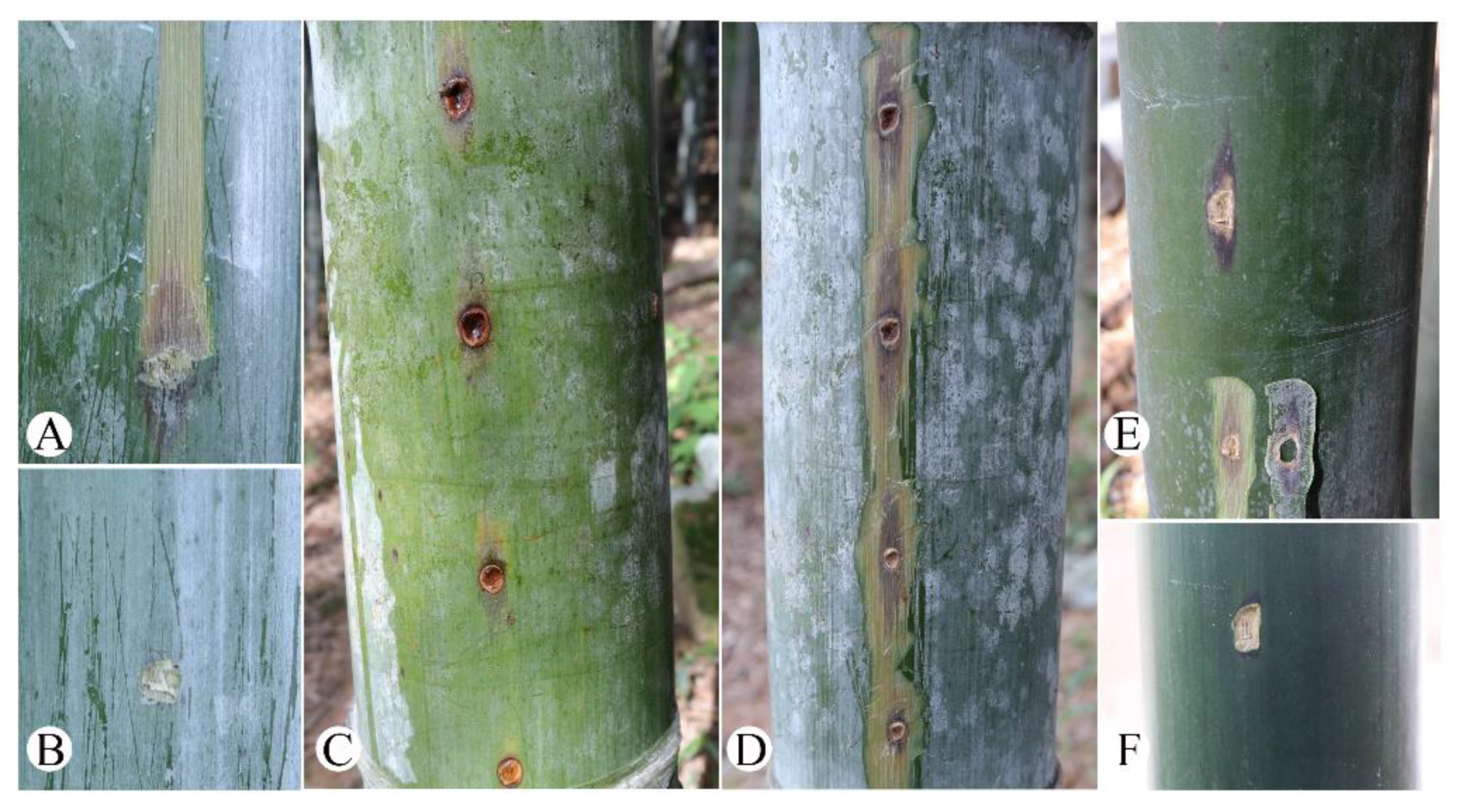
3.1. Symptoms Description, Fungal Isolation, and Pathogenicity
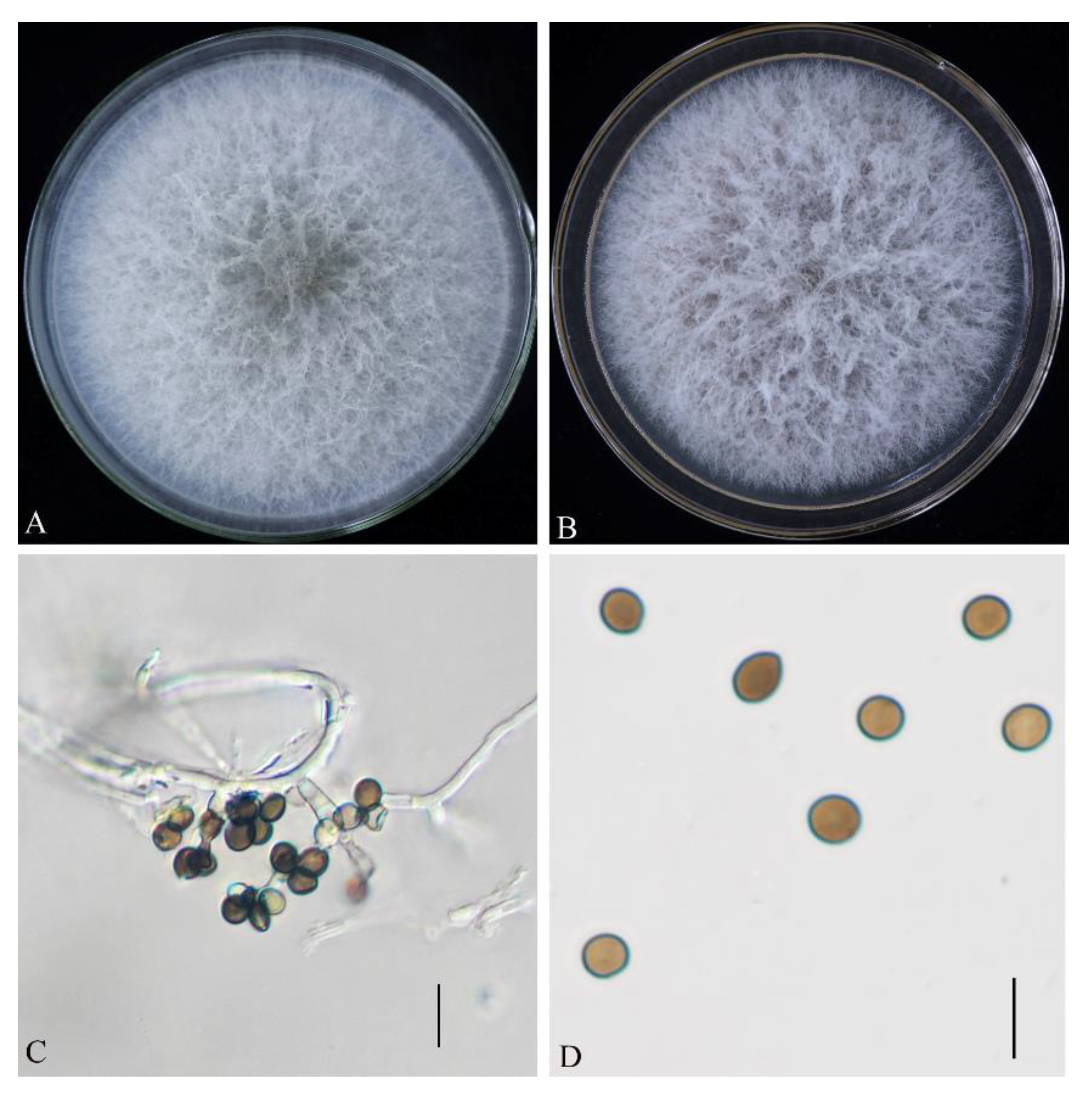
3.2. Morphological Characteristics of Pathogenic Fungus
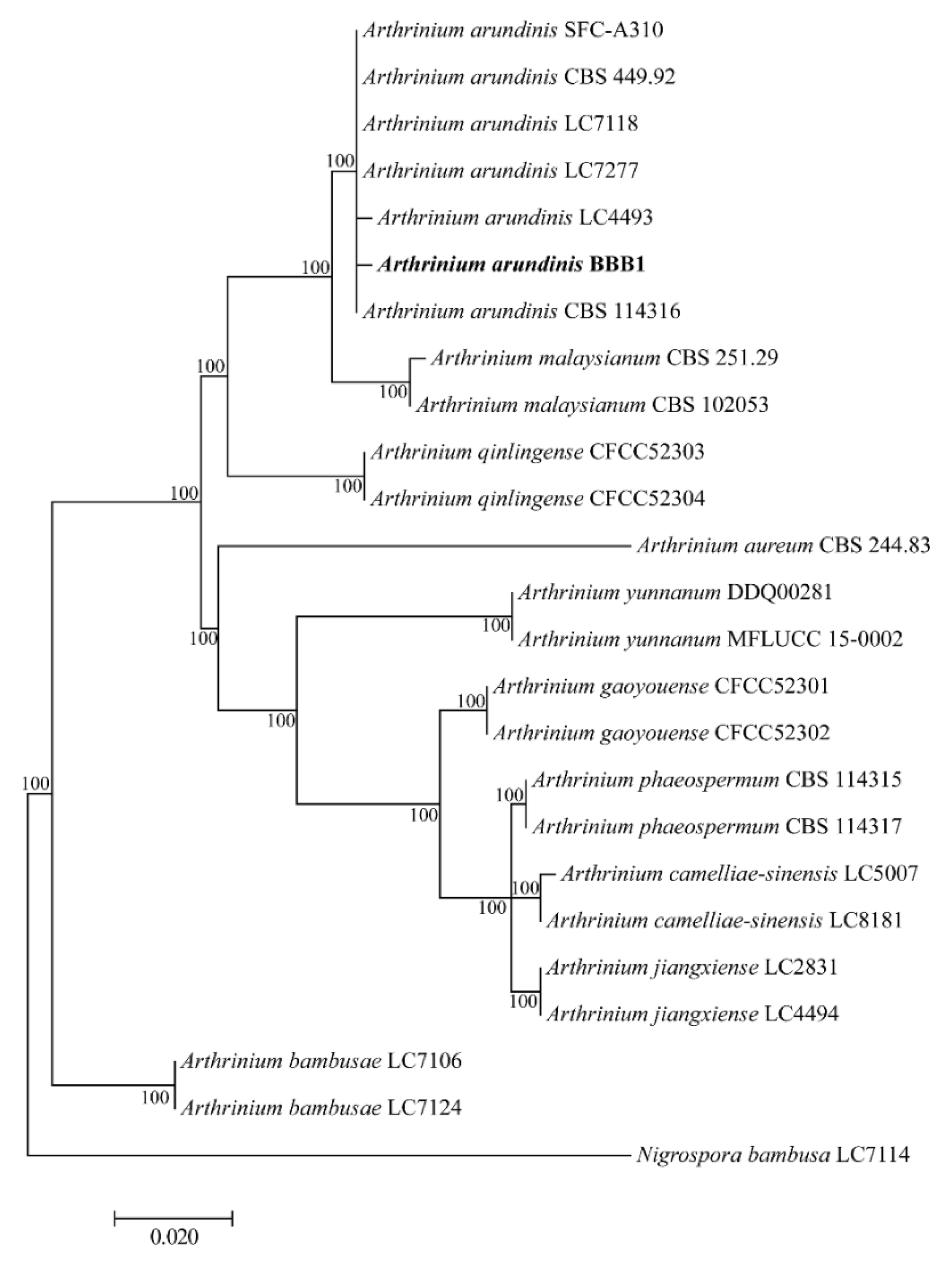
3.3. Phylogenetic Analysis of the Pathogenic Fungus
3.4. Sensitivity of A. arundinis BBB1 to Fungicides
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kellogg, E.A. Flowering Plants. Monocots; Kubitzki, K., Ed.; Springer: Hamburg, Germany, 2015; pp. 87–91. [Google Scholar] [CrossRef]
- Wang, S.; Chen, T.H.; Liu, E.U.; Liu, C.P. Accessing the nursing behaviour of moso bamboo (Phyllostachys edulis) on carbohydrates dynamics and photosystems. Sci. Rep. 2020, 10, 1015. [Google Scholar] [CrossRef]
- Ramakrishnan, M.; Yrjl, K.; Vinod, K.K.; Sharma, A.; Zhou, M. Genetics and genomics of moso bamboo (Phyllostachys edulis): Current status, future challenges, and biotechnological opportunities toward a sustainable bamboo industry. Food Energy Secur. 2020, 9, e229. [Google Scholar] [CrossRef]
- Song, X.; Chen, X.; Zhou, G.; Jiang, H.; Peng, C. Observed high and persistent carbon uptake by moso bamboo forests and its response to environmental drivers. Agr. Forest Meteorol. 2017, 247, 467–475. [Google Scholar] [CrossRef]
- Xu, M.Q.; Dai, Y.C.; Fan, S.H.; Jin, L.X.; Lu, Q.; Tian, G.Z.; Wang, L.F. Records of bamboo diseases and the taxonomy of their pathogens in China (I). For. Res. 2006, 19, 692–699. (In Chinese) [Google Scholar]
- Yang, C.L.; Xu, X.; Liu, Y. Podonectria Sichuanensis, a potentially mycopathogenic fungus from Sichuan Province in China. Phytotaxa 2019, 402, 219–231. [Google Scholar] [CrossRef]
- Yang, C.L.; Xu, X.L.; Wanasinghe, D.N.; Jeewon, R.; Phookamsak, R.; Liu, Y.G.; Liu, L.J.; Hyde, K.D. Neostagonosporella sichuanensis gen. et sp. nov. (Phaeosphaeriaceae, Pleosporales) on Phyllostachysheteroclada (Poaceae) from Sichuan Province, China. MycoKeys 2019, 46, 119–150. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.L.; Baral, H.O.; Xu, X.; Liu, Y. Parakarstenia phyllostachydis, a new genus and species of non-lichenized Odontotremataceae (Ostropales, Ascomycota). Mycol. Prog. 2019, 18, 833–845. [Google Scholar] [CrossRef]
- Wang, H.X.; Xu, X.J.; Peng, J.S.; Liu, Y.X.; Zhang, Z.B. Isolation and identification of pathogen infecting moso bamboo in Jinggangshan. J. Bamboo Res. 2012, 31, 42–46. (In Chinese) [Google Scholar]
- Ramos, H.P.; Braun, G.H.; Pupo, M.T.; Said, S. Antimicrobial activity from endophytic fungi Arthrinium state of Apiospora montagnei Sacc. and Papulaspora immersa. Braz. Arch. Biol. Technol. 2010, 53, 629–632. [Google Scholar] [CrossRef]
- Crous, P.W.; Groenewald, J.Z. A phylogenetic re-evaluation of Arthrinium. IMA Fungus 2013, 4, 133–154. [Google Scholar] [CrossRef]
- Wang, M.; Tan, X.M.; Liu, F.; Cai, L. Eight new Arthrinium species from China. Mycokeys 2018, 34, 1–24. [Google Scholar] [CrossRef]
- Pintos, A.; Alvarado, P.; Planas, J.; Jarling, R. Six new species of Arthrinium from Europe and notes about A. caricicola and other species found in Carex spp. hosts. Mycokeys 2019, 49, 15–48. [Google Scholar] [CrossRef] [PubMed]
- Kallol, D.; Lee, S.Y.; Choi, H.W.; Eom, A.H.; Cho, Y.J.; Jung, H.Y. Taxonomy of Arthrinium minutisporum sp. nov., Pezicula neosporulosa, and Acrocalymma pterocarpi: New Records from Soil in Korea. Mycobiology 2020, 6, 450–463. [Google Scholar] [CrossRef]
- Senanayake, I.C.; Bhat, J.D.; Cheewangkoon, R.; Xie, N. Bambusicolous Arthrinium Species in Guangdong Province, China. Front. Microbiol. 2020, 11, 602773. [Google Scholar] [CrossRef]
- Kwon, S.L.; Park, M.S.; Jang, S.; Lee, Y.M.; Kim, J.J. The genus Arthrinium (Ascomycota, Sordariomycetes, Apiosporaceae) from marine habitats from Korea, with eight new species. IMA Fungus 2021, 12, 1–26. [Google Scholar] [CrossRef]
- Chen, K.; Wu, X.Q.; Huang, M.X.; Han, Y.Y. First report of brown culm streak of Phyllostachys praecox caused by Arthrinium arundinis in Nanjing, China. Plant Dis. 2014, 98, 1274. [Google Scholar] [CrossRef]
- Li, B.J.; Liu, P.Q.; Jiang, Y.; Weng, Q.Y.; Chen, Q.H. First report of culm rot caused by Arthrinium phaeospermum on Phyllostachys viridis in China. Plant Dis. 2016, 100, 1013. [Google Scholar] [CrossRef]
- Dai, D.Q.; Phookamsak, R.; Wijayawardene, N.N.; Li, W.J.; Bhat, D.J.; Xu, J.C.; Taylor, J.E.; Hyde, K.D.; Chukeatirote, E. Bambusicolous fungi. Fungal Divers. 2017, 82, 1–105. [Google Scholar] [CrossRef]
- Yang, C.L.; Xu, X.; Liu, Y. First report of bamboo blight disease caused by Arthrinium yunnanum on Phyllostachys heteroclada in Sichuan, China. Plant Dis. 2018, 103, 1026. [Google Scholar] [CrossRef]
- Yang, C.L.; Xu, X.; Liu, Y. First report of bamboo blight disease caused by Arthrinium pseudoparenchymaticum on Dendrocalamus latiflorus in Sichuan, China. Plant Dis. 2019, 103, 1411. [Google Scholar] [CrossRef]
- Feng, Y.; Liu, J.K.J.; Lin, C.G.; Chen, Y.Y.; Liu, Z.Y. Article additions to the genus Arthrinium (Apiosporaceae) from bamboos in China. Front. Microbiol. 2021, 12, 661281. [Google Scholar] [CrossRef] [PubMed]
- Ellis, M.B. Dematiaceous Hyphomycetes. Commonwealth Mycological Institute. Kew. 1971, pp. 608. Available online: https://www.scirp.org/(S(lz5mqp453edsnp55rrgjct55))/reference/ReferencesPapers.aspx?ReferenceID=2024742 (accessed on 28 September 2022).
- Hyde, K.D.; Fröhlich, J.; Taylor, J.E. Fungi from palms. XXXVI. Reflections on unitunicate Ascomycetes with Apiospores. Sydowia 1998, 50, 21–80. [Google Scholar]
- Ji, Z.L.; Zhang, S.W.; Zhu, F.; Wan, B.X.; Liang, R.Z. First report of Arthrinium arundinis causing leaf edge spot of peach in China. Plant Dis. 2020, 104, 11. [Google Scholar] [CrossRef]
- Kuberan, T.; Cheng, L.L.; Deng, C.; Deng, W.W.; Zhang, Z. First report of leaf blight caused by Arthrinium arundinis on tea plants in China. Plant Dis. 2019, 103, 12. [Google Scholar] [CrossRef]
- Cano, C.M. First report of Arthrinium arundinis causing kernel blight on barley. Plant Dis. 1992, 76, 1077B. [Google Scholar] [CrossRef]
- Bagherabadi, S.; Zafari, D.; Anvar, F.G. First report of leaf spot caused by Arthrinium arundinis on rosemary in Iran. J. Plant Pathol. 2014, 96, 126. [Google Scholar] [CrossRef]
- Koch, R. Ueber den augenblicklichen Stand der bakteriologischen Choleradiagnose. Z. Für Hyg. Und Infekt. 1893, 14, 319–338. [Google Scholar] [CrossRef][Green Version]
- White, T.J.; Bruns, T.; Lee, S.J.W.T.; Taylor, J.L. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols: A Guide to Methods and Applications; Innis, M.A., Gelfand, D.H., Sninsky, J.J., White, T.J., Eds.; Academic Press: San Diego, CA, USA, 1990; pp. 315–322. [Google Scholar] [CrossRef]
- Carbone, I.; Kohn, L.M. A method for designing primer sets for speciation studies in filamentous Ascomycetes. Mycologia 1999, 91, 553–556. [Google Scholar] [CrossRef]
- Glass, N.L.; Donaldson, G.C. Development of primer sets designed for use with the PCR to amplify conserved genes from Filamentous Ascomycetes. Appl. Environ. Microb. 1995, 61, 1323–1330. [Google Scholar] [CrossRef]
- Huang, L.; Yang, J.Y.; Zhu, Y.N.; Zhu, L.H.; Zhang, Y.F.; Ye, J.R.; Li, D.W. Canker on culm of Bambusa multiplex (Lour.) Raeusch. ex Schult. caused by Fusarium incarnatum (Roberge) Sacc. J. Phytopathol. 2019, 167, 91–97. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Tamura, K. Mega7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2015, 33, 1870–1874. [Google Scholar] [CrossRef] [PubMed]
- Gerin, D.; Nigro, F.; Faretra, F.; Pollastro, S. Identification of Arthrinium marii as causal agent of Olive tree dieback in Apulia (Southern Italy). Plant Dis. 2020, 104, 694–701. [Google Scholar] [CrossRef] [PubMed]
- Franco, F.P.; Túler, A.C.; Gallan, D.Z.; Gonçalves, F.G.; Favaris, A.P.; Peñaflor, M.F.G.V.; Leal, W.S.; Moura, D.S.; Bento, J.M.S.; Silva-Filho, M.C. Fungal phytopathogen modulates plant and insect responses to promote its dissemination. ISME J. 2021, 15, 3522–3533. [Google Scholar] [CrossRef] [PubMed]
- Rossman, A.Y. Podonectria, a genus in the Pleosporales on scale insects. Mycotaxon 1978, 7, 163–182. [Google Scholar]
- Dao, H.T.; Beattie, G.; Rossman, A.Y.; Burgess, L.W.; Holford, P. Four putative entomopathogenic fungi of armoured scale insects on Citrus in Australia. Mycol. Prog. 2016, 15, 47. [Google Scholar] [CrossRef]
- Xu, X.L.; Zeng, Q.; Lv, Y.C.; Jeewon, R.; Yang, C.L. Insight into the systematics of novel entomopathogenic fungi associated with armored scale insect, Kuwanaspis howardi (Hemiptera: Diaspididae) in China. J. Fungi. 2021, 7, 628. [Google Scholar] [CrossRef]
- Qi, R.; Yang, C.; Li, L.; Liu, C.; Xu, X.; Liu, Y.; Zeng, Q.; Liu, L.; Liu, Y. Pathogenic characteristics, biological characteristics and drug control test of pathogen causing rhombic-spot of Phyllostachys heteroclada. J. Northeast. For. Univ. 2021, 49, 131–135, 141. (In Chinese) [Google Scholar]
- Ma, Z.; Michailides, T.J. Advances in understanding molecular mechanisms of fungicide resistance and molecular detection of resistant genotypes in phytopathogenic fungi. Crop Prot. 2005, 24, 853–863. [Google Scholar] [CrossRef]
- Mora-Aguilera, J.A.; Ríos-López, E.G.; Yáez-Zúiga, M.; Rebollar-Alviter, A.; Tovar-Pedraza, J.M. Sensitivity to MBC fungicides and prochloraz of Colletotrichum gloeosporioides species complex isolates from mango orchards in Mexico. J. Plant Dis. Protect. 2021, 128, 481–491. [Google Scholar] [CrossRef]

| Species | Strain * | Substrates | Origin | GeneBank Accession Number | ||
|---|---|---|---|---|---|---|
| ITS | TEF | TUB | ||||
| Arthirinium arundinis | BBB1 | Bamboo | China | OK639113 | OL944302 | OL944301 |
| A. arundinis | LC4493 | Phyllostachys sp. | China | KY494689 | KY705088 | KY806202 |
| A. arundinis | LC7277 | Bamboo | China | KY494750 | KY705146 | KY705218 |
| A. arundinis | LC7118 | Bamboo | China | KY494723 | KY705120 | KY705191 |
| A. arundinis | SFC-A310 | Beach Sand | Korea | MH498551 | MH544682 | MH498509 |
| A. arundinis | CBS 449.92 | Bamboo | Canada | KF144887 | KF145019 | KF144977 |
| A. arundinis | CBS 114316 | Hordeum vulgare | Iran | KF144884 | KF145016 | KF144974 |
| A. aureum | CBS 244.83 | Air | Spain | AB220251 | KF145023 | KF144981 |
| A. bambusae | LC7106 | Bamboo | China | KY494718 | KY806204 | KY705186 |
| A. bambusae | LC7124 | Bamboo | China | KY494727 | KY806206 | KY705195 |
| A. camelliaesinensis | LC5007 | Camellia sinensis | China | KY494704 | KY705103 | KY705173 |
| A. camelliaesinensis | LC8181 | Brassica rapa | China | KY494761 | KY705157 | KY705229 |
| A. gaoyouense | CFCC 52301 | Phragmites australis | China | MH197124 | MH236793 | MH236789 |
| A. gaoyouense | CFCC 52302 | Phragmites australis | China | MH197125 | MH236794 | MH236790 |
| A. jiangxiense | LC2831 | Bamboo | China | KY494686 | KY705085 | KY806201 |
| A. jiangxiense | LC4494 | Phyllostachys sp. | China | KY494690 | KY705089 | KY705160 |
| A. malaysianum | CBS 251.29 | Cinnamomum camphora | Malaysia | KF144897 | KF145031 | KF144989 |
| A. malaysianum | CBS 102053 | Macaranga hullettii | Malaysia | KF144896 | KF145030 | KF144988 |
| A. phaeospermum | CBS 114315 | Hordeum vulgare | Iran | KF144905 | KF145039 | KF144997 |
| A. phaeospermum | CBS 114317 | Hordeum vulgare | Iran | KF144906 | KF145040 | KF144998 |
| A. qinlingense | CFCC 52303 | Fargesia qinlingensis | China | MH197120 | MH236795 | MH236791 |
| A. qinlingense | CFCC 52304 | Fargesia qinlingensis | China | MH197121 | MH236796 | MH236792 |
| A. yunnanum | DDQ00281 | Phyllostachys nigra | China | KU940148 | N/A | N/A |
| A. yunnanum | MFLUCC15-1002 | Phyllostachys nigra | China | KU940147 | N/A | N/A |
| Nigrospora Bambusa | LC7114 | Bamboo | China | KY385307 | KY385313 | KY385319 |
| Fungicide | Regression Equation | EC50 * (95% CL)/mg/L | Correlation Coefficient (r) |
|---|---|---|---|
| Difenoconazole | y = 1.05 + 1.48 x | 0.195 (0.130–0.263) | 0.991 |
| Hymexazol | y = −9.09 + 4.07 x | 170.520 (152.558–193.500) | 0.988 |
| Iprodione | y = −8.3 + 11.06 x | 5.627 (5.377–5.873) | 0.992 |
| Mancozeb | y = −10.87 + 4.48 x | 267.514 (240.548–296.180) | 0.971 |
| Prochloraz | y = 2.69 + 1.56 x | 0.019 (0.014–0.025) | 0.989 |
| Propiconazole | y = −1.27 + 2 x | 4.324 (3.462–5.659) | 0.977 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, S.; Zhang, Q.; Song, Z.; Zhou, H.; Liao, Y.; Zhang, F. Arthrinium arundinis, a Novel Causal Agent of Moso Bamboo (Phyllostachys edulis) Culm Rhomboid Rot and Its Sensitivity to Fungicides. Forests 2022, 13, 1616. https://doi.org/10.3390/f13101616
Zheng S, Zhang Q, Song Z, Zhou H, Liao Y, Zhang F. Arthrinium arundinis, a Novel Causal Agent of Moso Bamboo (Phyllostachys edulis) Culm Rhomboid Rot and Its Sensitivity to Fungicides. Forests. 2022; 13(10):1616. https://doi.org/10.3390/f13101616
Chicago/Turabian StyleZheng, Shuzhao, Qinghua Zhang, Zhang Song, Huixia Zhou, Yiran Liao, and Feiping Zhang. 2022. "Arthrinium arundinis, a Novel Causal Agent of Moso Bamboo (Phyllostachys edulis) Culm Rhomboid Rot and Its Sensitivity to Fungicides" Forests 13, no. 10: 1616. https://doi.org/10.3390/f13101616
APA StyleZheng, S., Zhang, Q., Song, Z., Zhou, H., Liao, Y., & Zhang, F. (2022). Arthrinium arundinis, a Novel Causal Agent of Moso Bamboo (Phyllostachys edulis) Culm Rhomboid Rot and Its Sensitivity to Fungicides. Forests, 13(10), 1616. https://doi.org/10.3390/f13101616





