Improvement of Ex Vitro Growing Completion of Highbush Blueberry (Vaccinium corymbosum L.) in Containers
Abstract
:1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Agirbov, Y.I.; Mukhametzyanov, R.R.; Romanyuk, M.A.; Dzhancharova, G.K.; Platonovskiy, N.G. Russia and other countries in the global fruit and berry market. Izv. Timiryazevskoy Sel’skokhozyaystvennoy Akad. 2021, 6, 129–147. [Google Scholar] [CrossRef]
- Bläsing, D. Performance of highbush blueberries on sites previously used for agricultural crops. Acta Hortic. 1989, 241, 213–220. [Google Scholar] [CrossRef]
- Kalinichenko, V.P.; Glinushkin, A.P.; Minkina, T.M.; Mandzhieva, S.S.; Sushkova, S.N.; Sukovatov, V.A.; Iljina, L.P.; Makarenkov, D.A.; Zavalin, A.A.; Dudnikova, T.S.; et al. Intra-soil waste recycling provides safety of environment. Environ. Geochem. Health 2022, 44, 1355–1376. [Google Scholar] [CrossRef] [PubMed]
- Klavins, M.; Klavina, L. Vaccinium Genus Berry Waxes and Oils. In Fruit Oils: Chemistry and Functionality; Ramadan, M.F., Ed.; Springer Nature: Cham, Switzerland, 2019; pp. 419–431. [Google Scholar] [CrossRef]
- Ostroluchká, M.G.; Gajdošová, A.; Libiaková, G.; Hrubíková, K.; Bežo, M. Protocol for micropropagation of selected Vaccinium spp. In Protocols for Micropropagation of Woody Trees and Fruits; Jain, S.M., Häggman, H., Eds.; Springer: Dordrecht, The Netherlands, 2007; pp. 445–455. [Google Scholar] [CrossRef]
- Smolarz, K.; Pula, S. Cultivation of the High-Bush blueberry in Poland. Acta Hortic. 2014, 1017, 199–204. [Google Scholar] [CrossRef]
- Titok, V.V.; Reshetnikov, V.N.; Volodko, I.K.; Pavlonskij, N.B. History and results of the introduction of berry plants of the family Ericaceae Juss. in the Republic of Belarus. In Experience and Prospects of Growing Non-Traditional Berry Crops on the Territory of Belarus and Neighboring Countries: Materials of the International Scientific and Practical Seminar; Titok, V.V., Rupasova, Z.A., Goncharova, L.V., Pavlovskij, N.B., Lenkovets, T.I., Kuzmenkova, S.M., Eds.; Medisont Limited Liability Company: Minsk, Belarus, 2021; pp. 3–14. [Google Scholar]
- Rupasova, Z.A.; Reshetnikov, V.N. Tall Blueberry: Evaluation of Adaptive Potential during Introduction in the Conditions of Belarus: Monograph; LAP LAMBERT Acad. Publ.: Saarbrucken, Germany, 2011; p. 474. [Google Scholar]
- Zaytseva, Y.; Ambros, E.; Novikova, T. Rooting and acclimatization to ex vitro conditions of regenerants of frost-resistant members of Rhododendron. Turczaninowia 2018, 21, 144–152. [Google Scholar] [CrossRef]
- Schuchovski, C.S.; Biasi, L.A. In vitro establishment of ‘Delite’ rabbiteye blueberry microshoots. Horticulturea 2019, 5, 24. [Google Scholar] [CrossRef]
- Brilkina, A.A.; Pavlova, E.E. Peculiarities of microclonal reproduction of representatives of the lingonberry subfamily. In Proceedings of the Biology of Plant Cells In Vitro and Biotechnology: Abstract of the International Conference, Zvenigorod, Russia, 8–12 September 2008; Nosov, A.V., Ed.; FBK-PRESS ID: Moscow, Russia, 2008; pp. 52–53. [Google Scholar]
- Vysotsky, V.A. Clonal micropropagation of fruit plants and ornaments shrubs. In Micropropagation and Improvement of Plants in Industrial Horticulture and Floriculture: Collection of Scientific Papers of VNIIS Named after I.V. Michurin; VNIISSOK: Michurinsk, Russia, 1989; pp. 3–8. [Google Scholar]
- Vysotsky, V.A. Morphogenesis and clonal micropropagation of plants. In Plant Cell Culture and Biotechnology; Nauka: Moscow, Russia, 1986; pp. 91–102. [Google Scholar]
- Vysotsky, V.A. Some results and prospects of using the methods of culture of isolated tissues and organs in horticulture. In History, Modernity and Prospects for the Development of Horticulture in Russia, Proceedings of the Materials of the International Conference, Moscow, Russia, 15–17 November 2000; VSTISP: Moscow, Russia, 2000; pp. 163–191. [Google Scholar]
- Demenko, V.I.; Lebedev, V.G.; Shestibratov, K.A. Adaptation of plants obtained in vitro to non-sterile conditions. Izv. Timiryazev Agric. Acad. (TAA) 2010, 1, 73–85. [Google Scholar]
- Demenko, V.I.; Akimova, S.V.; Kirkach, V.V.; Vikulina, A.N. Biological bases for the development of technologies for vegetative propagation of horticultural crops: A textbook. ANO Editor. Off. J. ‘MESH’ Mosc. 2019, 156. [Google Scholar]
- Kataeva, N.V.; Butenko, R.G. Clonal Micropropagation of Plants; Science: Moscow, Russia, 1983; p. 96. [Google Scholar]
- Cassells, A.C. Contamination and its impact in tissue culture. Acta Hortic. 2001, 560, 353–359. [Google Scholar] [CrossRef]
- Litwińczuk, M. Micropropagation of Vaccinium sp. by in vitro axillary shoot proliferation. In Protocols for Micropropagation of Selected Economically-Important Horticultural Plants; Lambardi, M., Ozudogru, E.A., Jain, S.M., Eds.; Humana Totowa; Springer: New York, NY, USA; Heidelberg, Germany; Dordrecht, The Netherlands; London, UK, 2013; pp. 63–76. [Google Scholar] [CrossRef]
- Pliego-Alfare, F.J. Development of in vitro rooting bioassay using juvenile stem cuttings of Persea americane Mill. Hortic. Sci. 1988, 63, 295–301. [Google Scholar] [CrossRef]
- Akimova, S.V.; Radjabov, A.K.; Bukhtin, D.A.; Kirkach, V.V.; Aladina, O.N.; Demenko, V.I.; Beloshapkina, O.O. Adaptation to non-sterile conditions of grape plants rooted in vitro on a nutrient medium enriched with organosilicon compounds. Izv. Timiryazev Agric. Acad. (TAA) 2019, 5, 34–53. [Google Scholar] [CrossRef]
- Dutta Cupta, S.; Agarwal, A. Influence of LED lighting on in vitro plant regeneration and associated cellular rebox balance. In Light Emitting Diodes for Agriculture: Smart Light; Dutta Gupta, S., Ed.; Springer: Singapore, 2017; pp. 273–303. [Google Scholar] [CrossRef]
- Kozai, T. Acclimatization of micropropagated plants. In High-Tech and Micropropagation I; Bajaj, Y.P.S., Ed.; Spinger: Berlin/Heidelberg, Germany, 1991; Volume 17, pp. 127–140. [Google Scholar] [CrossRef]
- Teixeira da Silva, J.A.; Hossain, M.; Sharma, M.; Dobránszki, J.; Cardoso, J.C.; Zeng, S. Acclimatization of in vitro-derived Dendrobium. Hortic. Plant J. 2017, 3, 110–124. [Google Scholar] [CrossRef]
- Kumar, K.; Rao, I.U. Morphophysiologicals problems in acclimatization of micropropagated plants in—Ex vitro conditions—A reviews. J. Ornam. Hortic. Plants 2012, 2, 271–283. [Google Scholar]
- Hazarika, B.N. Acclimatization of tissue-cultured plants. Curr. Sci. 2003, 85, 1704–1712. [Google Scholar]
- Hazarika, B.N. Morpho-physiological disorders in in vitro culture of plants. Sci. Hortic. 2006, 108, 105–120. [Google Scholar] [CrossRef]
- Wright, R.D.; Niemiera, A.X. Nutrition of container-grown woody nursery crops. In Horticultural Reviews; Janick, J., Ed.; Van Nostrand Reinhold Company: New York, NY, USA, 1987; Volume 9, pp. 75–101. [Google Scholar]
- Skovorodnikov, D.N.; Raikov, I.A.; Chelyaev, D.N. Adaptation of raspberry plants obtained in vitro to non-sterile conditions. Bull. Oryol State Agrar. Univ. 2012, 2, 70–73. [Google Scholar]
- Sutter, E. Use of Humidity tends and antitranspirants in the acclimatization of tissue cultured plants to greenhouse. Sci. Hortic. 1984, 23, 303–312. [Google Scholar] [CrossRef]
- Gresshoff, P. Sindicate Methods employed in planting aut Tissue culture. The horizons of tissue culture propagation. In A Seminar Directed by Dr. R.A. de Fossard for the N.S.W. Association of Nurserymen Ltd. At the University of Sydney, 3–4 Desember; University of Sydney: Sydney, Australia, 1977; pp. 106–108. [Google Scholar]
- Kornatskiy, S.A. Tissue culture as a model for study adaptation processes in ontogeny of fruit and berry plants. Fruit Berry Grow. Russ. 1996, 3, 84–89. [Google Scholar]
- Sutter, E.G.; Shackel, K.; Diaz, J.C. Acclimatization of tissue cultured plants. Acta Hortic. 1992, 314, 115–119. [Google Scholar] [CrossRef]
- Hazarika, B.N.; Teixeira da Silva, J.A.; Talukdar, A. Effective acclimatization of in vitro cultured plants: Methods, physiology and genetics. In Floriculture, Ornamental and Plant Biotechnology: Advances and Topical Issues; Teixeira da Silva, J.A., Ed.; Global Science Books, Ltd.: Middlesex, UK, 2006; Volume II, pp. 427–438. [Google Scholar]
- Shchubakova, N.V.; Khapaeva, O.V. Peculiarities of propagation of blackcurrant with a closed root system. Sci. Tech. Bull. VIR 1991, 207, 34–35. [Google Scholar]
- Demina, T.G. Growing seedlings with an isolated root system. In 75th Anniversary of the Tatar Research Institute of Agriculture; Foliant: Kazan, Russia, 1996; p. 223. [Google Scholar]
- Bezukh, E.P. Growing seedlings of fruit and berry crops with a closed root system as an environmentally friendly technology. In Scientific and Methodological, Organizational and Innovative Aspects of Seed Production of Agricultural Crops in the Northwestern Region of the Russian Federation; IAEP: St. Petersburg, Russia, 1999; pp. 59–60. [Google Scholar]
- Preece, J.E.; Sutter, E.G. Acclimatization of micropropagated plants to the greenhouse and field. In Micropropagation. Technology and Application; Debergh, P.C., Zimmerman, R.H., Eds.; Springer: Dordrecht, The Netherlands, 1991; pp. 71–93. [Google Scholar] [CrossRef]
- Hung, C.D.; Hong, C.H.; Kim, S.K.; Lee, K.H.; Park, J.Y.; Nam, M.W.; Choi, D.H.; Lee, H.I. LED light for in vitro and ex vitro efficient growth of economically important highbush blueberry (Vaccinium corymbosum L.). Acta Physiol. Plant. 2016, 38, 1–9. [Google Scholar] [CrossRef]
- Hung, C.D.; Hong, C.H.; Kim, S.K.; Lee, K.H.; Park, J.Y.; Cao, D.D.; Nam, M.W.; Choi, D.H.; Lee, H.I. In vitro proliferation and ex vitro rooting of microshoots of commercially important rabbiteye blueberry (Vaccinium ashei Reade) using spectral lights. Sci. Hortic. 2016, 211, 248–254. [Google Scholar] [CrossRef]
- Sethi, D.; Subudhi, S.; Rajput, V.D.; Kusumavathi, K.; Sahoo, T.R.; Dash, S.; Mangaraj, S.; Nayak, D.K.; Pattanayak, S.K.; Minkina, T.; et al. Exploring the role of mycorrhizal and rhizobium inoculation with organic and inorganic fertilizers on the nutrient uptake and growth of Acacia mangium saplings in acidic soil. Forests 2021, 12, 1657. [Google Scholar] [CrossRef]
- Chizhik, O.V.; Reshetnikov, V.N.; Filipenya, V.L.; Gorbatzevich, V.I.; Kartyzhova, L.E.; Aleschenkova, Z.M. The microorganisms influence on adaptation of clonal planting stock of hardy-shrub species of Vaccinium. Physiol. Biochem. Cultiv. Plants 2013, 45, 254–259. [Google Scholar]
- Demenko, V.I. Biological and Technological Features of Vegetative Reproduction in the Production of Healthy Planting Material. Ph.D. Thesis, RSAU-MTAA, Moscow, Russia, 2006; p. 329. [Google Scholar]
- Chandra, S.; Bandopadhyay, R.; Kumar, V.; Chandra, R. Acclimatization of tissue cultured plantlets: From laboratory to land. Biotechnol. Lett. 2010, 32, 1199–1205. [Google Scholar] [CrossRef] [PubMed]
- Darnell, R.L.; Stutte, G.W.; Martin, G.C.; Lang, G.A.; Early, D. Development physiology of rabbiteye blueberry. In Horticultural Reviews; Janick, J., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 1992; Volume 13, pp. 339–406. [Google Scholar] [CrossRef]
- Kim, J.K.; Shawon, M.d.R.A.; An, J.H.; Yun, Y.J.; Park, S.J.; Na, J.K.; Choi, K.Y. Influence of substrate composition and container size on the growth of tissue culture propagated apple rootstock plants. Agronomy 2021, 11, 2450. [Google Scholar] [CrossRef]
- Brazaitytė, A.; Ulinskaitė, R.; Duchovskis, P.; Samuolienė, G.; Šikšnianienė, J.B.; Jankauskienė, J.; Šabajevienė, G.; Baranauskis, K.; Stanienė, G.; Tamulaitis, G.; et al. Optimization of lighting spectrum for photosynthetic system and productivity of lettuce by using light-emitting. Acta Hortic. 2006, 711, 183–188. [Google Scholar] [CrossRef]
- Yakovtseva, M.; Govorova, G.; Tarakanov, I. Supplemental lighting for greenhouse-grown strawberries: Effects of different ratios of red to blue radiation. Acta Hortic. 2017, 1170, 1011–1018. [Google Scholar] [CrossRef]
- Jaakola, L.; Tolyanen, A.; Laine, K.; Hohtola, A. Effect of N6-isopentenyladenine concentration on growth initiation in vitro and rooting of bilberry and lingonberry. Plant Cell Tiss. Org. Cult. 2001, 66, 73–77. [Google Scholar] [CrossRef]
- Kirilovich, Y.u.G.; Latkova, E.V.; Latkov, N.Y. Influence of lighting on growing plants in greenhouses with a closed ground system. Innov. Sci. 2021, 2, 37–40. [Google Scholar]
- Xiaoying, L.; Mingjuan, Y.; Xiaodong, X.; Abm, K.; Atak, A.; Caihong, Z.; Dawei, L. Effect of light on growth and chlorophyll development in kiwifruit ex vitro and in vitro. Sci. Hortic. 2022, 291, 110599. [Google Scholar] [CrossRef]
- Singh, S.; Agrawal, S.B.; Agrawal, M. Role of light in plant development. Int. J. Plant Environ. 2015, 1, 43–56. [Google Scholar] [CrossRef]
- Pospóšilová, J.; Tichá, I.; Kadleček, P.; Haisel, D.; Plzáková, Š. Acclimatization of micropropagated plants to ex vitro conditions. Biol. Plant. 1999, 42, 481–497. [Google Scholar] [CrossRef]
- Pacholczak, A.; Nowakowska, K. The ex vitro rooting of blueberry (Vaccinium corymbosum L.) microcuttings. Folia Hortic. 2015, 27, 145–150. [Google Scholar] [CrossRef]
- Lloyd, G. Commercially-feasible micropropagation of mountain laurel, Kalmia latifolia, by use of shoot-tip culture. Proc. Int. Plant Propagator’s Soc. 1980, 30, 421–426. [Google Scholar]
- Akimova, S.V.; Kirkach, V.V.; Demenko, V.I.; Malevannaya, N.N. Recultivation of micro-plants of perpetual raspberry after deposition in the culture room conditions on the nutrient media with the use of modifications of the Superstim product in low and ultra0low doses. IOP Conf. Ser. Earth Environ. Sci. 2021, 663, 1–6. [Google Scholar] [CrossRef]
- Erst, A.; Gorbunov, A.; Karakulov, A. Rooting and acclimatization of in vitro propagated microshoots of the Ericacea. J. Appl. Hortic. 2018, 20, 177–181. [Google Scholar] [CrossRef]
- Bal, J.J.M.; Balkhoven, J.; Peppelman, G. Results of testing highbush blueberry cultivars in the Netherlands. Acta Hortic. 2006, 715, 157–162. [Google Scholar] [CrossRef]
- Kozos, K.; Ochmian, I.; Chelpiński, P. The effect of rapid chilling and storage conditions on the quality of Brigitta Blue cultivar highbush blueberries (Vaccinium corymbosum L.). Folia Hortic. 2014, 26, 147–153. [Google Scholar] [CrossRef]
- Chen, L.; Xue, X.; Yang, Y.; Chen, F.; Zhao, J.; Wang, X.; Khan, A.T.; Hu, Y. Effect of red and blue LEDs on in vitro growth and microtuberization of potato single-node cutting. Front. Agric. Sci. Eng. 2018, 5, 197–205. [Google Scholar] [CrossRef]
- Nhut, D.T.; Takamura, T.; Watanabe, H.; Okamoto, K.; Tanaka, M. Response of strawberry plantlets cultured in vitro under superbright red and blue light-emitting diodes (LEDs). Plant Cell Tissue Organ Cult. 2003, 73, 43–52. [Google Scholar] [CrossRef]
- Isutsa, D.K.; Pritts, M.P.; Mudge, K.W. Rapid propagation of blueberry plants using ex vitro rooting and controlled acclimatization of micropagules. HortScience 1994, 29, 1124–1126. [Google Scholar] [CrossRef]
- Abiyan, M.V.; Gish, R.A.; Podushin, Y.V. Influence of the period of artificial lighting on the formation of lettuce seedlings. Sci. J. KubSAU 2014, 101, 2199–2210. [Google Scholar]
- Isachkin, A.V. Fundamentals of Scientific Research in Horticulture: A Textbook for Universities; Isachkin, A.V., Kryuchkova, V.A., Eds.; Lan: St. Petersburg, Russia, 2020; p. 420. [Google Scholar]
- Kalinitchenko, V.P.; Glinushkin, A.P.; Kudeyarov, V.N.; Minkina, T.M.; Chernenko, V.V.; Sushkova, S.N.; Mandzhieva, S.S.; Makarenkov, D.A.; Ilyina, L.P. Biogeosystem Technique for Sustainable Agriculture, Water Scarcity Overcoming, Healthy Soil and Environment. In Proceedings of the ACS Fall Meeting, San Francisco, CA, USA, 17–20 August 2020. [Google Scholar] [CrossRef]
- Zinchenko, V.V.; Grishina, E.V.; Kalinitchenko, V.P.; Glinushkin, A.P.; Kudeyarov, V.N.; Gudkov, S.V.; Savostyanov, A.P.; Minkina, T.M.; Ilyin, V.B.; Mandzhieva, S.S. Biogeosystem Technique methodology as a new chemical soil-biological engineering foundation for the safe expanded technological development in the noosphere. In Proceedings of the 23rd EGU General Assembly, Online. 19–30 April 2021. [Google Scholar] [CrossRef]
- Severina, V.; Proklin, V.; Rykhlik, A.; Kalinitchenko, V.; Glinushkin, A.; Dubenok, N.; Minkina, T.; Nesvat, A.; Deryabkina, I.; Zamulina, I. Biogeosystem technique water paradigm for prevention of the world water scarcity and cardinal transformation of current irrigation practice. In Proceedings of the EGU General Assembly 2021, Online. 19–30 April 2021. [Google Scholar] [CrossRef]
- Bunkin, N.F.; Glinushkin, A.P.; Shkirin, A.V.; Ignatenko, D.N.; Chirikov, S.N.; Savchenko, I.V.; Meshalkin, V.P.; Samarin, G.N.; Maleki, A.; Kalinitchenko, V.P. Identification of organic matter dispersions based on light scattering matrices focusing on soil organic matter management. ACS Omega 2020, 5, 33214–33224. [Google Scholar] [CrossRef]
- Konchekov, E.M.; Glinushkin, A.P.; Kalinitchenko, V.P.; Artem’ev, K.V.; Burmistrov, D.E.; Kozlov, V.A.; Kolik, L.V. Properties and Use of Water Activated by Plasma of Piezoelectric Direct Discharge. Front. Phys. 2021, 8, 616385. [Google Scholar] [CrossRef]
- Gudkov, S.V.; Shafeev, G.A.; Glinushkin, A.P.; Shkirin, A.V.; Barmina, E.V.; Rakov, I.I.; Simakin, A.V.; Kislov, A.V.; Astashev, M.E.; Vodeneev, V.A.; et al. Production and use of selenium nanoparticles as fertilizers. ACS Omega 2020, 5, 17767–17774. [Google Scholar] [CrossRef]
- Belov, S.V.; Glinushkin, A.P.; Danyleiko, Y.u.K.; Kalinitchenko, V.P.; Egorov, A.V.; Sidorov, V.A.; Gudkov, S.V.; Dorokhov, A.S.; Lobachevsky, Y.P.; Izmailov, A.Y. Activated potassium phosphate fertilizer solution for agricultural plants growth stimulation. Front. Phys. 2021, 8, 618320. [Google Scholar] [CrossRef]
- Wallace, C.; Both, A.J. Evaluating operating characteristics of light sources for horticultural application. Acta Hortic. 2016, 1134, 434–444. [Google Scholar] [CrossRef]
- Tarakanov, I.; Yakovleva, O.; Konovalova, I.; Paliutina, G.; Anisimov, A. Light-emitting diodes: On the way to combinatorial lighting technologies for basic research and crop production. Acta Hortic. 2012, 956, 171–178. [Google Scholar] [CrossRef]
- Mitchell, C.A.; Dzakovich, M.P.; Gomez, C.; Lopez, R.; Burr, J.F.; Hernández, R.; Kubota, C.; Currey, C.J.; Meng, Q.; Runkle, E.S.; et al. Light-Emitting Diodes in Horticulture. In Horticultural Reviews; Janick, J., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2015; Volume 43, pp. 1–88. [Google Scholar] [CrossRef]
- Paradiso, R.; Proietti, S. Light-quality manipulation to control plant growth and photomorphogenesis in greenhouse horticulture: The state of the art and the opportunities of modern LED systems. J. Plant Growth Regul. 2022, 41, 742–780. [Google Scholar] [CrossRef]
- Bickford, E.; Dunn, S. Incandescent lamps: Advantages and disadvantages. In Light Sources, Lighting for Plant Growth; Kent State University Press: Kent, OH, USA, 1972; p. 221. [Google Scholar]
- Nelson, J.A.; Bugbee, B. Economic analysis of greenhouse lighting: Light emitting diodes vs. high intensity discharge fixtures. PLoS ONE 2014, 9, e99010. [Google Scholar] [CrossRef] [PubMed]
- Konstantinova, T.N.; Aksenova, N.P.; Sergeeva, L.I.; Chailakhyan, M.K. Mutual influence of light and hormones on the regulation of morphogenetic processes in in vitro culture. Plant Physiol. 1998, 34, 795–802. [Google Scholar]
- Makarov, S.S.; Kuznetsova, I.B.; Surov, V.V. Organogenesis of half-highbush blueberry during clonal micropropagation depending on lighting conditions. Izv. Orenbg. State Agrar. Univ. 2021, 90, 76–79. [Google Scholar] [CrossRef]
- Tallman, G.; Zhu, J.; Mawson, B.T.; Amodeo, G.; Nouhi, Z.; Levy, K.; Zeiger, E. Induction of CAM in Mesembryanthemum crystallinum abolishes the stomatal response to blue light and light-dependent zeaxanthin formation in guard cell chloroplasts. Plant Cell Physiol. 1997, 38, 236–242. [Google Scholar] [CrossRef]
- Ceusters, J.; Borland, A.M.; Taybi, T.; Frans, M.; Godts, C.; De Proft, M.P. Light quality modulates metabolic synchronization over the diel phases of crassulacean acid metabolism. J. Exp. Bot. 2014, 65, 3705–3714. [Google Scholar] [CrossRef]
- Brix, H.; van den Driessche, R. Mineral nutrition of container-grown tree seedlings. In Great Plains Agricultural Council Publication 68, Proceedings of the North American Containerized Forest Tree Seedling Symposium, Denver, CO, USA, 26–29 August 1974; Tinus, R.W., Stein, W.I., Balmer, W.E., Eds.; Pacific Forestry Centre: Denver, CO, USA, 1974; pp. 77–84. [Google Scholar]
- Kornova, K.M.; Popov, S.K. Effect of the in vitro container type on growth characteristics of the microplants in in vitro propagation of GF 677. Acta Hortic. 2009, 825, 277–282. [Google Scholar] [CrossRef]
- Tasmin, R.; Galderwood, L.; Tooley, B.; Wang, L.; Zhang, Y.J. Are Foliar Fertilizers Beneficial to Growth and Yield of Wild Lowbush Blueberries? Agronomy 2022, 12, 470. [Google Scholar] [CrossRef]
- Grimashevich, V.V. Complexity of measures to increase the productivity of swamp blueberries. In Vaccinioidae in USSR: Resources, Introduction, and Selection; Gorbunov, A.B., Cherkasov, A.F., Eds.; Nauka: Novosibirsk, Russia, 1990; pp. 92–97. [Google Scholar]
- Esposti, M.D.D.; Siqueria, D.L.; Pereira, P.R.G.; Venegas, V.H.A.; Salomão, L.C.C.; Filho, J.A.M. Assessment of nitrogenized nutrition of citrus rootstock using chlorophyll concentration in the leaf. J. Plant Nutr. 2003, 26, 1287–1299. [Google Scholar] [CrossRef]
- Pirata, M.S.; Correia, S.; Canhoto, J. Ex vitro simultaneous acclimatization and rooting of in vitro propagated tamarillo plants (Solanum betaceum Cav.): Effect of the substrate and mineral nutrition. Agronomy 2022, 12, 1082. [Google Scholar] [CrossRef]
- Sidorovich, E.A.; Ruban, N.N.; Sherstenikina, A.V. Introduction and Experience in Growing Large-Fruited Cranberries, High Blueberries and Lingonberries; BelRIISTIFSSEP: Minsk, Belarus, 1991; p. 52. [Google Scholar]
- Szwonek, E.; Laszlovszky-Zmarlicka, A. Reaction of renovated blueberry (Vaccinium corymbosum L.) plants on nitrophoska 12-12-17 and wigor fertilizers. Acta Hortic. 2009, 810, 747–752. [Google Scholar] [CrossRef]

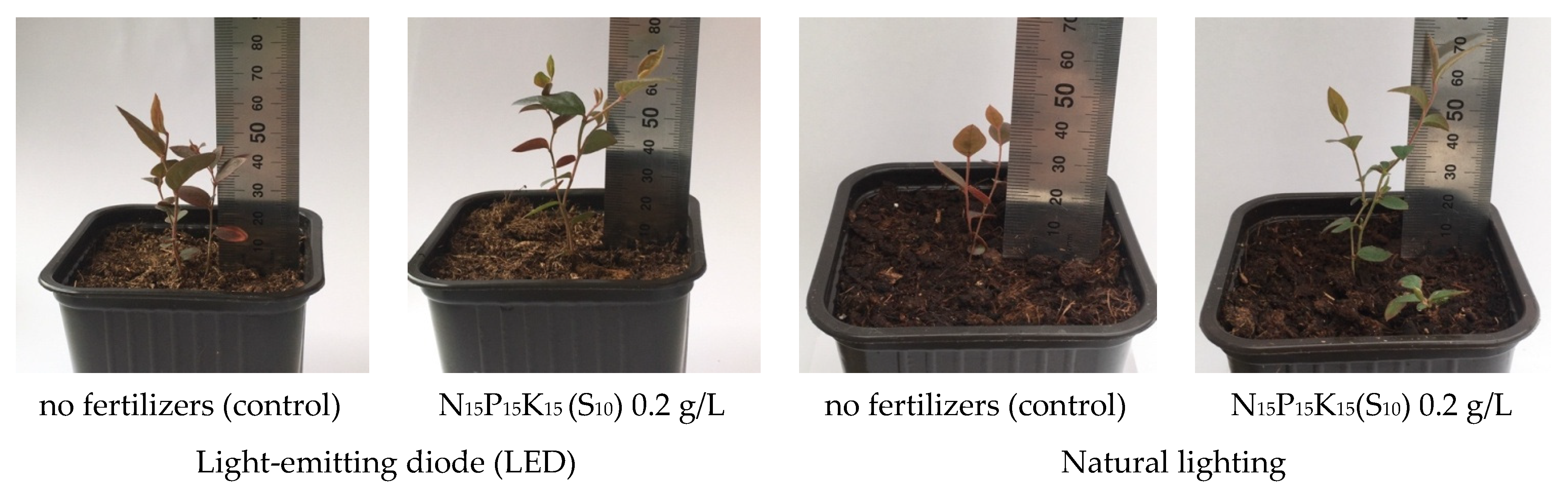

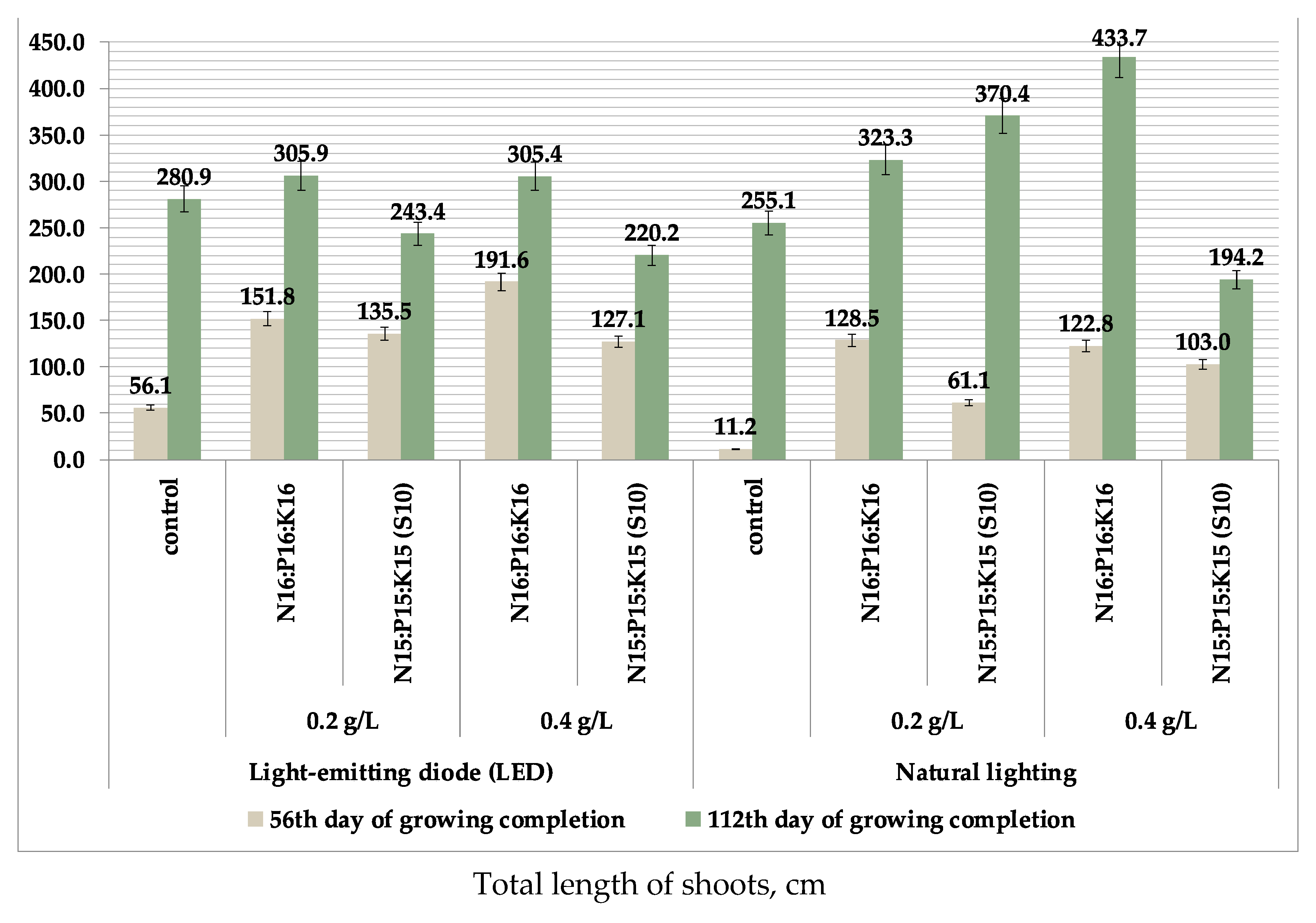
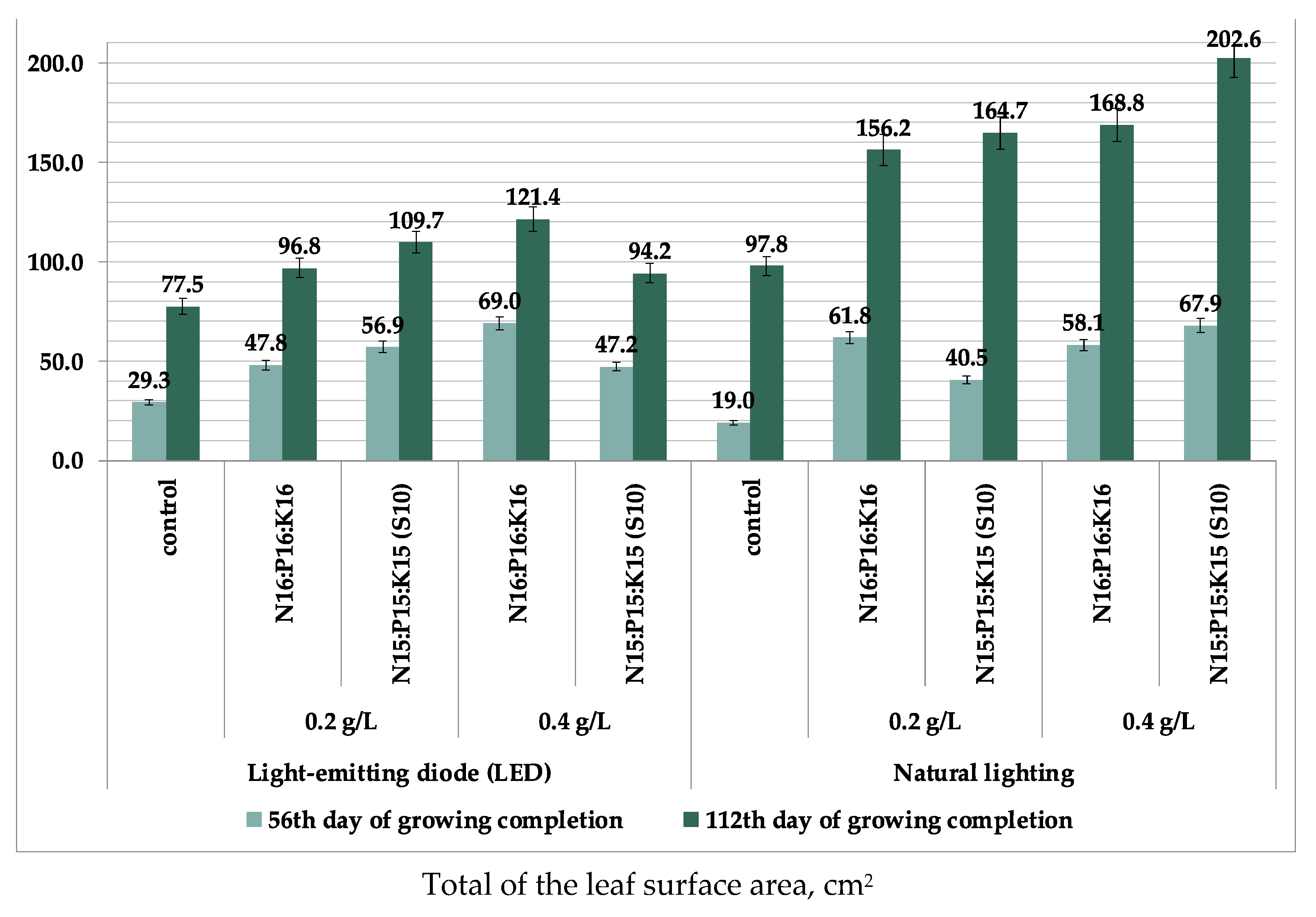
| Type of Fertilizer (Factor B) | Illumination (Factor A) | Factor Average B | |
|---|---|---|---|
| Natural Lighting + SD * | Light-Emitting Diode (LED) + SD | ||
| Average number of the 0th order, pcs. | LSD05 b = Fe < Ft *** | ||
| no fertilizers (control) | 3.0 ± 1.41 | 2.2 ± 0.45 | 2.6 |
| N16P16K16 0.2 g/L | 2.4 ± 0.55 | 2.4 ± 0.55 | 2.4 |
| N16P16K16 0.4 g/L | 2.8 ± 0.84 | 3.2 ± 0.84 | 3.0 |
| N15P15K15(S10) 0.2 g/L | 2.8 ± 0.45 | 3.2 ± 1.10 | 3.0 |
| N15P15K15(S10) 0.4 g/L | 3.0 ± 0.71 | 2.8 ± 0.84 | 2.9 |
| Factor average A LSD05 a = Fe < Ft | 2.8 | 2.8 | |
| LSD05 ab = Fe < Ft | |||
| Average number of the 1st order, pcs. | LSD05 b = Fe < Ft | ||
| no fertilizers (control) | 0 | 0.2 ± 0.00 | 0.1 |
| N16P16K16 0.2 g/L | 0.6 ± 0.71 | 0.8± 0.58 | 0.7 |
| N16P16K16 0.4 g/L | 0.8 ± 0.00 | 1.2 ± 0.58 | 1.0 |
| N15P15K15(S10) 0.2 g/L | 0 | 1.2 ± 2.83 | 0.6 |
| N15P15K15(S10) 0.4 g/L | 3.2 ± 2.16 ab, ** | 0.4 ± 0.00 | 1.8 |
| Factor average A LSD05 a = Fe < Ft | 1.5 | 0.8 | |
| LSD05 ab = 2.8 | |||
| The total length of the shoots, cm | LSD05 b = Fe < Ft | ||
| no fertilizers (control) | 11.8 ± 4.93 | 8.5 ± 1.35 | 10.15 |
| N16P16K16 0.2 g/L | 11.3 ± 1.52 | 8.3 ± 1.00 | 9.8 |
| N16P16K16 0.4 g/L | 11.9 ± 2.01 | 12.7 ± 1.80 b | 12.3 |
| N15P15K15(S10) 0.2 g/L | 11.2 ± 1.71 | 16.4 ± 6.73 b, ab | 13.8 |
| N15P15K15(S10) 0.4 g/L | 13.9 ± 0.63 | 13.0 ± 2.31 b | 13.5 |
| Factor average A LSD05 a = Fe < Ft | 12.0 | 11.8 | |
| LSD05 ab = 6.3 | |||
| Leaf surface area, cm2 | LSD 05 b = 3.7 | ||
| no fertilizers (control) | 9.2 ± 5.53 | 3.6 ± 1.11 | 6.4 |
| N16P16K16 0.2 g/L | 5.6 ± 1.60 | 5.0 ± 1.79 | 5.3 |
| N16P16K16 0.4 g/L | 5.1 ± 1.68 | 7.9 ± 2.15 b | 6.5 |
| N15P15K15(S10) 0.2 g/L | 4.8 ± 0.55 | 13.0 ± 5.80 b, ab | 8.9 |
| N15P15K15(S10) 0.4 g/L | 3.8 ± 2.01 | 5.2 ± 2.02 | 4.5 |
| Factor average A LSD05 a = Fe < Ft | 5.7 | 6.9 | |
| LSD05 ab = 6.2 | |||
| Type of Fertilizer (Factor B) | Illumination (Factor A) | Factor Average B | |
|---|---|---|---|
| Natural Lighting + SD * | Light-Emitting Diode (LED) + SD | ||
| Average number of the 0th order, pcs. | LSD05 b = Fe < Ft *** | ||
| no fertilizers (control) | 3.0 ± 1.41 | 2.4 ± 0.55 | 2.7 |
| N16P16K16 0.2 g/L | 2.6 ± 0.55 | 2.6 ± 0.55 | 2.6 |
| N16P16K16 0.4 g/L | 2.8 ± 0.84 | 3.2 ± 0.84 | 3.0 |
| N15P15K15(S10) 0.2 g/L | 2.8 ± 0.55 | 3.2 ± 1.22 | 3.0 |
| N15P15K15(S10) 0.4 g/L | 3.0 ± 0.84 | 2.8 ± 0.84 | 2.9 |
| Factor average A LSD05 a = Fe < Ft | 2.8 | 2.8 | |
| LSD05 ab = Fe < Ft | |||
| Average number of the 1st order, pcs. | LSD05 b = 1.7 | ||
| no fertilizers (control) | 0.0 ± 0.00 | 1.0 ± 0.00 | 0.5 |
| N16P16K16 0.2 g/L | 3.0 ± 1.58 b ** | 1.7 ± 0.58 | 2.6 |
| N16P16K16 0.4 g/L | 2.0 ± 1.15 b | 2.0 ± 0.71 | 2.0 |
| N15P15K15(S10) 0.2 g/L | 2.8 ± 2.87 b | 2.0 ± 1.22 | 2.4 |
| N15P15K15(S10) 0.4 g/L | 3.2 ± 1.64 b | 1.5 ± 0.58 | 2.6 |
| Factor average A LSD05 a = Fe < Ft | 2.2 | 1.6 | |
| LSD05 ab = 2.8 | |||
| The total length of the shoots, cm | LSD05 b = 6.7 | ||
| no fertilizers (control) | 14.5 ± 7.17 | 10.4 ± 0.93 | 12.5 |
| N16P16K16 0.2 g/L | 20.3 ± 4.24 | 16.1 ± 4.64 | 18.2 |
| N16P16K16 0.4 g/L | 17.9 ± 6.01 | 26.0 ± 4.09 b, ab | 22.3 |
| N15P15K15(S10) 0.2 g/L | 14.7 ± 2.28 | 25.0 ± 9.02 b, ab | 19.9 |
| N15P15K15(S10) 0.4 g/L | 23.4 ± 5.53 b | 18.5 ± 4.48 b | 21.0 |
| Factor average A LSD05 a = Fe < Ft | 18.16 | 19.3 | |
| LSD05 ab = 11.2 | |||
| Leaf surface area, cm2 | LSD05 b = 12.0 | ||
| no fertilizers (control) | 11.3 ± 4.55 | 6.6 ± 2.61 | 8.9 |
| N16P16K16 0.2 g/L | 21.6 ± 6.67 | 18.3 ± 9.80 | 20.0 |
| N16P16K16 0.4 g/L | 16.7 ± 7.22 | 26.0 ± 15.29 b | 21.4 |
| N15P15K15(S10) 0.2 g/L | 10.3 ± 2.71 | 35.5 ± 10.64 b, ab | 23.0 |
| N15P15K15(S10) 0.4 g/L | 26.9 ± 17.40 b | 20.2 ± 4.54 b | 23.6 |
| Factor average A LSD05 a = Fe < Ft | 17.4 | 21.3 | |
| LSD05 ab = 19.9 | |||
| Type of Fertilizer (Factor B) | Illumination (Factor A) | Factor Average B | |
|---|---|---|---|
| Natural Lighting + SD * | Light-Emitting Diode (LED) + SD | ||
| Average number of the 0th order, pcs. | LSD05 b = Fe < Ft *** | ||
| no fertilizers (control) | 3.0 ± 1.41 | 2.6 ± 0.55 | 2.8 |
| N16P16K16 0.2 g/L | 2.6 ± 0.55 | 2.6 ± 0.55 | 2.6 |
| N16P16K16 0.4 g/L | 3.0 ± 0.71 | 3.2 ± 0.84 | 3.1 |
| N15P15K15(S10) 0.2 g/L | 2.8 ± 0.55 | 3.2 ± 1.22 | 3.0 |
| N15P15K15(S10) 0.4 g/L | 3.0 ± 0.71 | 2.8 ± 0.84 | 2.9 |
| Factor average A LSD05 a = Fe < Ft | 2.9 | 2.9 | |
| LSD05 ab = Fe < Ft | |||
| Average number of the 1st order, pcs. | LSD05 b = 2.8 | ||
| no fertilizers (control) | 0.6 ± 0.00 | 1.0 ± 0.50 | 0.8 |
| N16P16K16 0.2 g/L | 4.6 ± 1.52 a, b ** | 2.2 ± 1.30 | 3.4 |
| N16P16K16 0.4 g/L | 3.2 ± 1.30 | 3.4 ± 1.34 a | 3.3 |
| N15P15K15(S10) 0.2 g/L | 3.8 ± 5.56 a, b | 2.6 ± 2.51 a | 3.2 |
| N15P15K15(S10) 0.4 g/L | 7.2 ± 1.79 a, b | 3.0 ± 2.00 a | 5.1 |
| Factor average A LSD05 a = 1.3 | 3.9 | 2.4 | |
| LSD05 ab = Fe < Ft | |||
| The total length of the shoots, cm | LSD05 b = 24.7 | ||
| no fertilizers (control) | 16.1 ± 8.43 | 15.4 ± 2.45 | 15.8 |
| N16P16K16 0.2 g/L | 40.2 ± 10.42 b, ab | 27.0 ± 9.26 | 33.6 |
| N16P16K16 0.4 g/L | 38.1 ± 13.12 b, ab | 45.6 ± 8.49 b, ab | 41.9 |
| N15P15K15(S10) 0.2 g/L | 26.3 ± 10.02 | 37.7 ± 8.73 b, ab | 32.0 |
| N15P15K15(S10) 0.4 g/L | 43.2 ± 13.79 b, ab | 32.7 ± 7.90 b | 38.0 |
| Factor average A LSD05 a = Fe < Ft | 32.8 | 31.7 | |
| LSD05 ab = 41.0 | |||
| Leaf surface area, cm2 | LSD05 b = 24.7 | ||
| no fertilizers (control) | 9.0 ± 5.66 | 12.2 ± 3.50 | 10.5 |
| N16P16K16 0.2 g/L | 60.2 ± 19.30 b, ab | 42.4 ± 19.45 b | 51.3 |
| N16P16K16 0.4 g/L | 58.9 ± 34.89 b, ab | 70.6 ± 16.87 b, ab | 64.8 |
| N15P15K15(S10) 0.2 g/L | 22.2 ± 5.28 | 65.0 ± 20.60 b, ab | 43.6 |
| N15P15K15(S10) 0.4 g/L | 49.7 ± 28.51 b | 48.9 ± 15.48 b | 49.3 |
| Factor average A LSD05 a = Fe < Ft | 40.0 | 47.8 | |
| LSD05 ab = 41.0 | |||
| Type of Fertilizer (Factor B) | Illumination (Factor A) | Factor Average B | |
|---|---|---|---|
| Natural Lighting + SD * | Light-Emitting Diode (LED) + SD | ||
| Average number of the 0th order, pcs. | LSD05 b = Fe < Ft *** | ||
| no fertilizers (control) | 3.0 ± 1.30 | 2.6 ± 0.55 | 2.8 |
| N16P16K16 0.2 g/L | 2.8 ± 0.84 | 2.6 ± 0.55 | 2.7 |
| N16P16K16 0.4 g/L | 3.0 ± 0.89 | 3.2 ± 0.84 | 3.1 |
| N15P15K15(S10) 0.2 g/L | 2.8 ± 0.55 | 3.2 ± 1.22 | 3.0 |
| N15P15K15(S10) 0.4 g/L | 3.0 ± 0.71 | 3.0 ± 1.00 | 3.0 |
| Factor average A LSD05 a = Fe < Ft | 2.9 | 2.9 | |
| LSD05 ab = Fe < Ft | |||
| Average number of the 1st order, pcs. | LSD05 b = 2.9 | ||
| no fertilizers (control) | 1.0 ± 0.00 | 2.0 ± 0.71 | 1.5 |
| N16P16K16 0.2 g/L | 4.6 ± 1.52 | 2.6 ± 1.14 | 3.6 |
| N16P16K16 0.4 g/L | 3.8 ± 1.64 | 4.2 ± 1.48 | 4.0 |
| N15P15K15(S10) 0.2 g/L | 4.8 ± 5.56 | 3.4 ± 2.70 | 4.1 |
| N15P15K15(S10) 0.4 g/L | 7.6 ± 2.19 b ** | 3.0 ± 2.00 | 5.3 |
| Factor average A LSD05 a = Fe < Ft | 4.4 | 3.0 | |
| LSD05 ab = Fe < Ft | |||
| The total length of the shoots, cm | LSD05 b = 16.5 | ||
| no fertilizers (control) | 19.0 ± 7.84 | 29.3 ± 5.28 | 24.2 |
| N16P16K16 0.2 g/L | 61.8 ± 15.73 b, ab | 47.8 ± 18.92 b | 54.8 |
| N16P16K16 0.4 g/L | 58.1 ± 14.91 b, ab | 69.0 ± 11.60 b, ab | 63.6 |
| N15P15K15(S10) 0.2 g/L | 40.5 ± 15.99 b | 56.9 ± 7.09 b, ab | 48.7 |
| N15P15K15(S10) 0.4 g/L | 67.9 ± 16.98 b, ab | 47.2 ± 8.04 b | 57.6 |
| Factor average A LSD05 a = Fe < Ft | 49.5 | 50.0 | |
| LSD05 ab = 27.5 | |||
| Leaf surface area, cm2 | LSD05 b = 55.5 | ||
| no fertilizers (control) | 11.2 ± 7.05 | 56.1 ± 22.50 | 33.7 |
| N16P16K16 0.2 g/L | 128.5 ± 22.55 a, b | 151.8 ± 65.76 a, b | 140.2 |
| N16P16K16 0.4 g/L | 122.8 ± 80.72 a, b | 191.6 ± 50.80 a, b | 157.2 |
| N15P15K15(S10) 0.2 g/L | 61.1 ± 25.53 a | 135.5 ± 6.63 a, b | 98.3 |
| N15P15K15(S10) 0.4 g/L | 103.0 ± 48.39 a, b | 127.1 ± 41.85 a, b | 115.1 |
| Factor average A LSD05 a = 25.5 | 85.3 | 132.4 | |
| LSD05 ab = Fe < Ft | |||
| Type of Fertilizer (Factor B) | Illumination (Factor A) | Factor average BLSD05 b = 59.4 | |
|---|---|---|---|
| Natural Lighting + SD * | Light-Emitting Diode (LED) + SD | ||
| no fertilizers (control) | 273.3 ± 10.14 | 333.7 ± 12.68 | 353.5 |
| N16P16K16 0.2 g/L | 331.0 ± 46.07 a ** | 414.3 ± 38.31 a, b | 372.6 |
| N16P16K16 0.4 g/L | 391.3 ± 30.92 a, b, ab | 343.7 ± 4.71 | 367.5 |
| N15P15K15(S10) 0.2 g/L | 426.0 ± 25.96 a, b, ab | 378.3 ± 35.91 a | 402.1 |
| N15P15K15(S10) 0.4 g/L | 365.3 ± 20.42 a, b | 452.7 ± 27.93 a, b, ab | 409.0 |
| Factor average A LSD05 a = 26.8 | 357.4 | 384.5 | |
| LSD05 ab = 100.0 | |||
| Type of Fertilizer (Factor B) | Illumination (Factor A) | Factor Average B | |
|---|---|---|---|
| Natural Lighting + SD * | Light-Emitting Diode (LED) + SD | ||
| Average number of the 0th order, pcs. | LSD05 b = 2.4 *** | ||
| no fertilizers (control) | 5.0 ± 2.35 | 3.6 ± 1.52 | 4.3 |
| N16P16K16 0.2 g/L | 5.8 ± 0.84 | 5.0 ± 1.22 a | 5.4 |
| N16P16K16 0.4 g/L | 8.4 ± 2.30 a, b ** | 4.2 ± 1.30 | 6.3 |
| N15P15K15(S10) 0.2 g/L | 7.2 ± 2.77 a | 5.0 ± 2.55 | 6.1 |
| N15P15K15(S10) 0.4 g/L | 10.2 ± 1.64 a, b | 6.4 ± 1.14 a, b | 8.3 |
| Factor average A LSD05 a = 1.1 | 7.3 | 4.8 | |
| LSD05 ab = Fe < Ft | |||
| Average number of the 1st order, pcs. | LSD05 b = 2.9 | ||
| no fertilizers (control) | 3.2 ± 1.92 | 3.6 ± 0.55 | 3.4 |
| N16P16K16 0.2 g/L | 8.6 ± 4.16 a, b | 4.8 ± 1.30 | 6.7 |
| N16P16K16 0.4 g/L | 8.2 ± 2.17 a, b | 5.8 ± 2.05 a | 7.0 |
| N15P15K15(S10) 0.2 g/L | 7.4 ± 1.82 a, b | 4.4 ± 1.52 | 5.9 |
| N15P15K15(S10) 0.4 g/L | 10.6 ± 3.58 b | 4.6 ± 1.34 | 7.6 |
| Factor average A LSD05 a = 1.3 | 7.6 | 4.6 | |
| LSD05 ab = Fe < Ft | |||
| The total length of the shoots, cm | LSD05 b = 37.2 | ||
| no fertilizers (control) | 97.8 ± 35.66 | 77.5 ± 23.90 | 87.7 |
| N16P16K16 0.2 g/L | 156.2 ± 32.70 a, b | 96.8 ± 28.72 a | 126.5 |
| N16P16K16 0.4 g/L | 168.8 ± 29.36 a, b, ab | 121.4 ± 14.31 a, b | 145.1 |
| N15P15K15(S10) 0.2 g/L | 164.7 ± 51.37 a, b, ab | 109.7 ± 24.82 a | 137.2 |
| N15P15K15(S10) 0.4 g/L | 202.6 ± 22.94 a, b, ab | 94.2 ± 8.94 | 148.4 |
| Factor average A LSD05 a = 17.1 | 158.0 | 99.9 | |
| LSD05 ab = 61.9 | |||
| Leaf surface area, cm2 | LSD05 b = 116.2 | ||
| No fertilizers (control) | 255.1 ± 93.93 | 280.9 ± 61.63 | 268.0 |
| N16P16K16 0.2 g/L | 323.3 ± 37.13 | 305.9 ± 120.04 | 314.6 |
| N16P16K16 0.4 g/L | 433.7 ± 131.71 b | 305.4 ± 138.92 | 369.6 |
| N15P15K15(S10) 0.2 g/L | 370.4 ± 97.23 | 243.4 ± 59.52 | 306.9 |
| N15P15K15(S10) 0.4 g/L | 194.2 ± 55.28 | 220.2 ± 57.28 | 207.2 |
| Factor average A LSD05 a = Fe < Ft | 315.3 | 271.2 | |
| LSD05 ab = Fe < Ft | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akimova, S.; Radzhabov, A.; Esaulko, A.; Samoshenkov, E.; Nechiporenko, I.; Kazakov, P.; Voskoboinikov, Y.; Matsneva, A.; Zubkov, A.; Aisanov, T. Improvement of Ex Vitro Growing Completion of Highbush Blueberry (Vaccinium corymbosum L.) in Containers. Forests 2022, 13, 1550. https://doi.org/10.3390/f13101550
Akimova S, Radzhabov A, Esaulko A, Samoshenkov E, Nechiporenko I, Kazakov P, Voskoboinikov Y, Matsneva A, Zubkov A, Aisanov T. Improvement of Ex Vitro Growing Completion of Highbush Blueberry (Vaccinium corymbosum L.) in Containers. Forests. 2022; 13(10):1550. https://doi.org/10.3390/f13101550
Chicago/Turabian StyleAkimova, Svetlana, Agamagomed Radzhabov, Aleksandr Esaulko, Egor Samoshenkov, Ivan Nechiporenko, Pavel Kazakov, Yurii Voskoboinikov, Anna Matsneva, Aleksandr Zubkov, and Timur Aisanov. 2022. "Improvement of Ex Vitro Growing Completion of Highbush Blueberry (Vaccinium corymbosum L.) in Containers" Forests 13, no. 10: 1550. https://doi.org/10.3390/f13101550
APA StyleAkimova, S., Radzhabov, A., Esaulko, A., Samoshenkov, E., Nechiporenko, I., Kazakov, P., Voskoboinikov, Y., Matsneva, A., Zubkov, A., & Aisanov, T. (2022). Improvement of Ex Vitro Growing Completion of Highbush Blueberry (Vaccinium corymbosum L.) in Containers. Forests, 13(10), 1550. https://doi.org/10.3390/f13101550








