1. Introduction
Champion Trees are the largest living individuals of their species. In the United States, a Nation Registry of Champion Trees is maintained by American Forests (
https://www.americanforests.org/, accessed on 16 September 2022), a nonprofit conservation organization. Trees are compared using a point calculation based on the tree’s trunk circumference (inches), height (feet) and average crown spread (feet). Champion trees must be remeasured and recertified every 10 years in order to maintain their status. Many states also maintain champion tree registries, typically by state departments of natural resources.
Aesculus glabra Willd., commonly known as
Ohio buckeye, is a member of Hippocastenaceae, the Horse Chestnut family.
Aesculus is a small genus of woody trees and shrubs containing about 15 species with a disjunct distribution in the Northern Hemisphere. Species occur in eastern Asia, eastern and western North America, and Europe, but species delineations and relationships are not well resolved [
1].
Aesculus glabra is a small to medium-sized deciduous tree that grows in the Midwestern and lower Great Plains regions of the United States from western Pennsylvania to Nebraska and south to Oklahoma and Texas. It produces iconic fruits, called buckeyes, that are shiny dark brown with a light tan eye when dried. The seeds contain tannic acid and are poisonous to livestock and humans. The state of Ohio is known as the buckeye state and has adopted the buckeye as its state tree and as the mascot of the state’s largest university, The Ohio State University. Given the popularity of buckeyes in Ohio, one of the authors (JL) began collecting seeds from champion buckeyes to grow and market seedlings. We originally aimed to genetically verify and certify that the seedlings were progeny of champion trees using DNA microsatellite markers. Once evidence of self-pollination emerged, we expanded our objectives to include assessment of genetic variability, population structure and growth rate comparisons.
The registered National Champion
A. glabra is a tree growing in a commercial development in DuPage County, Illinois (
Figure 1) near the village of Oak Brook. Signage on the tree indicates that it is 150 years old and is thought to have been planted by Ben Fuller, an early settler of the region. The last measurements made indicated that the tree has a height of approximately 22.9 m and a circumference of approximately 4.6 m. The Ohio Department of Natural Resources, Division of Forestry, lists the Ohio State Champion tree as one growing in Huron County (
https://Ohiodnr.gov, accessed on 1 July 2022) in a rural area near the town of Greenwich. It is reported to have a height of approximately 23.8 m but is smaller in circumference than the National Champion (~3.8 m). There is no record of this tree being planted, so we assume it grew naturally at this site, but it is possible that it was originally planted by the homeowners. The tree grows approximately 15 m from a house that was built in 1854 (J. Grosswiler, pers. comm.).
3. Results
Overall, the six microsatellite loci originally developed for the congeneric
A. turbinata [
2] were highly variable in
A. glabra, with an overall mean of 7.4 alleles per locus and an overall expected heterozygosity of 0.641. However, as shown in
Table 1, the National Champion tree and its progeny had much lower genetic variability in comparison with the other samples, with an average of only 2.83 alleles per locus and an expected heterozygosity of 0.430. Close inspection of the progeny genotypes of the National Champion revealed that only two of the 44 progeny (4.5%) had non-maternal alleles (
Table S1). This strongly suggests that the other 42 seedlings of the National Champion tree had been produced by self-fertilization. In contrast, the Ohio Champion Tree and its seedlings had an average of 9.83 alleles per locus and an expected heterozygosity of 0.688. Inspection of the genotypes of the progeny of the Ohio Champion tree revealed that all 48 seedlings had a non-maternal allele at one or more loci (
Table S1), indicating that they had all been produced by outcross pollen.
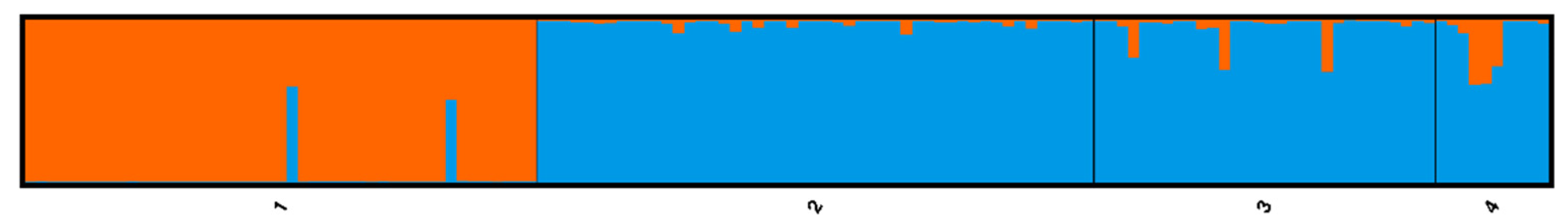
The STRUCTURE analyses found evidence of genetic clusters within the sampled trees. The Evanno Δ
K [
7] approach indicated best
K = 2 whereas MedMedK, MedMeanK, MaxMedK and MaxMeanK [
8] found best
K = 3. For
K = 2 (
Figure 2), the National Champion and its seedlings form one genetic cluster, and the other genetic cluster was comprised of the Ohio State Champion tree and all the naturally growing A. glarus trees from both Ohio and Illinois.
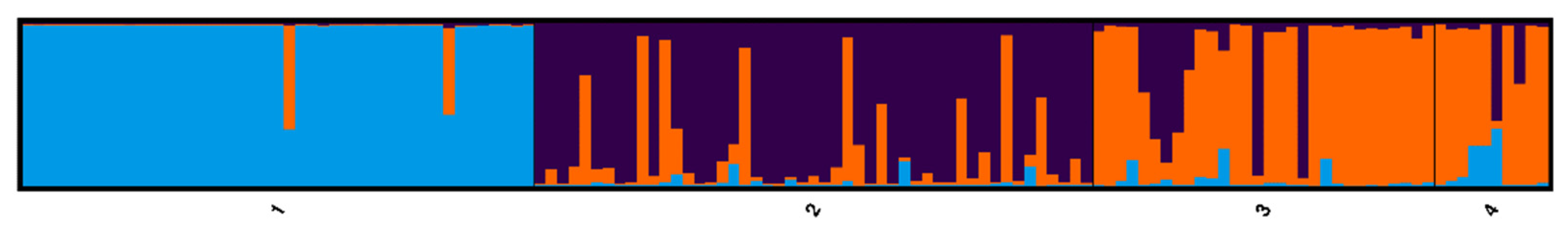
For
K = 3 (
Figure 3), the National Champion Tree and its seedlings remain as a distinct genetic cluster, but the Ohio State Champion and its seedlings comprise an additional cluster distinct from the naturally growing trees in Ohio and Illinois. There are more trees showing mixed ancestry among the Ohio Champion progeny than among the National Champion progeny.
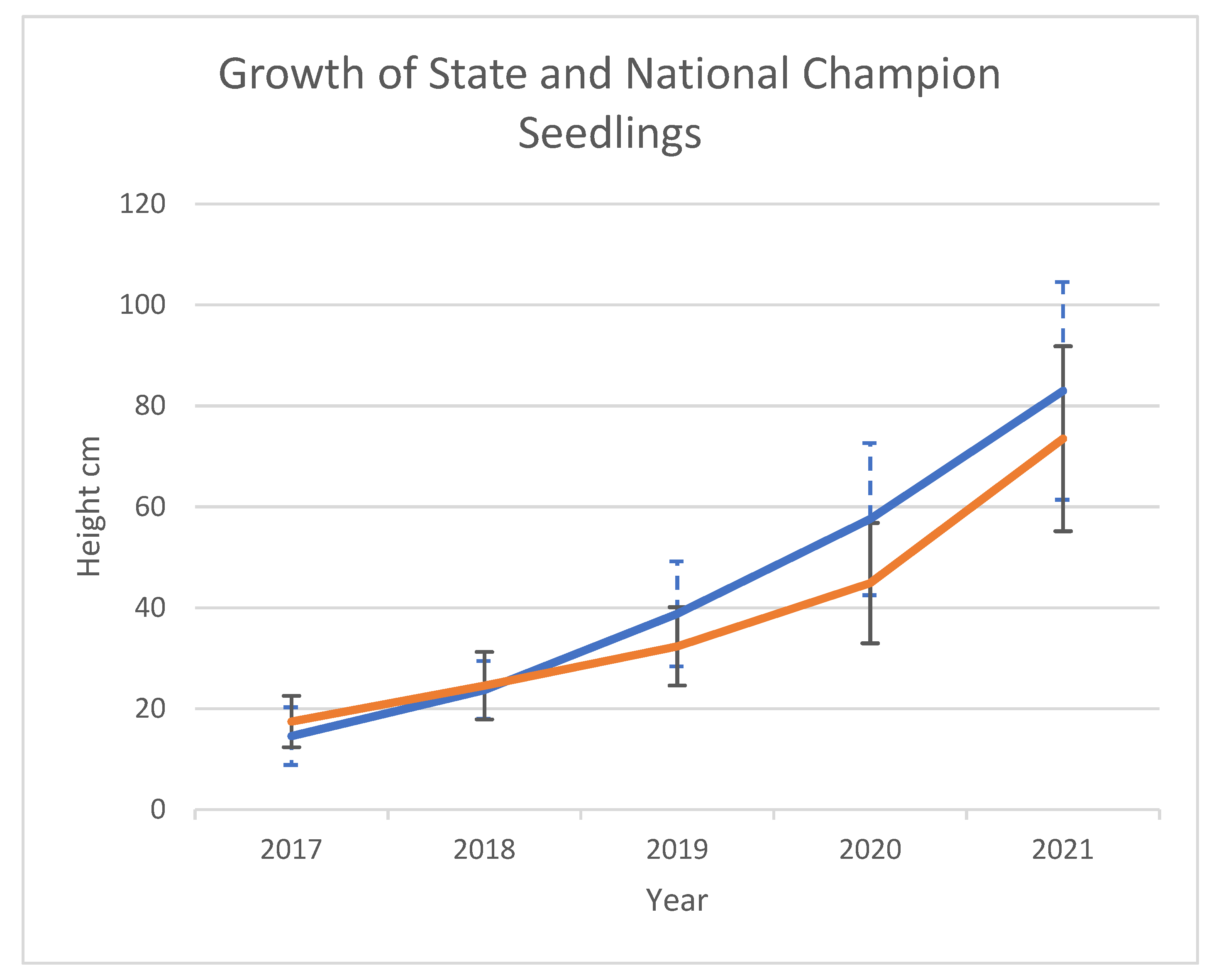
The seeds (buckeyes) collected in 2016 and 2018 from the National and Ohio State Champion trees had similarly high rates (>90%) of germination and early survival (J.L. pers. obs.). However, an early spring frost and high winds in 2022 resulted in the death of 95 of 727 (13.1%) seedlings from the National Champion compared to 23 of 692 seedlings (3.3%) from the Ohio State Champion. Growth measurements (height) taken annually over six years for a random sample of 2016 seedlings from the National Champion and the Ohio State champion show that the Ohio State Champion seedlings began showing evidence for faster growth starting in 2019, after three years of growth (
Figure 4). By 2021, the 15 Ohio Champion seedlings averaged 83.0 cm in height, while the 15 National Champion seedlings averaged 73.5 cm. For seeds collected in 2018, after three years of growth, the average height of the Ohio Champion seedlings (N = 80) was 47.2 cm and the average height of the National Champion seedlings (N = 57) was 41.1 cm, a significant difference (P = 0.00631). While the seedlings used for the growth measurements were not the same seedlings used for genotyping, given the high rate of self-pollination found in both the 2016 and 2018 National Champion seedling cohorts, it is likely that most of these seedlings resulted from self-pollination. The lower growth rate may be indicative of reduced fitness due to inbreeding depression, although more measurements are needed to confirm this possibility.
4. Discussion and Conclusions
Champion trees, the largest living individuals of their species, might generally be expected to be found toward the middle of a species’ range, where environmental conditions are optimal. Conditions on the periphery are more likely to be near the limits of the species’ ecological niche. The range of
Aesculus glabra includes Ohio, Indiana, and central Illinois and Iowa. DuPage County, Illinois, where the National Champion is growing, is just beyond the northern limits of the native range of the species, and is thought to have been planted in the 1800s (
Figure 1). Although the tree obviously survived many years and shows remarkable growth, it may be isolated from other
A. glabra trees. This species is occasionally planted as a street or ornamental tree, although the city of Oak Brook, Illinois (where the National Champion grows) does not list
A. glabra on their tree inventory (M.V.A. pers. comm.). The Morton Arboretum, also in DuPage County, has several
A. glabra, which were sampled for this study. These trees are approximately 11 km from the Champion tree. There is certainly a chance that more
A. glabra are growing closer to the Champion tree, but they are likely scattered and few in number.
Our genotyping results for seedlings of the National Champion tree convincingly demonstrate that nearly all (95%) of the seedlings we genotyped were derived from self-pollination events. Given the variability found at the six microsatellite loci we used for genotyping, the likelihood of outcross pollen contributing only maternal alleles is exceedingly low. The high level of self-pollination is also evidenced by the low observed (0.449) and expected (0.430) heterozygosities, as well as low numbers of alleles among the seedlings (
Table 1). This is in stark contrast to the progeny of the Ohio Champion tree, where we found that all genotyped seedlings had non-maternal alleles and therefore had been produced by outcross pollinations (
Table S1). The reproductive isolation experienced by the National Champion tree could be due to distance from the nearest flowering conspecifics. The seed source for the National Champion is not known, but it might have come from a warmer region where flowering begins earlier in the season. Phenological differences in flowering time could also account for reproductive isolation if the Champion Tree flowered earlier than neighboring conspecifics, or if it flowered before pollinators were active in the spring. Our data do not allow us to distinguish among these possible explanations, and also does not allow us to assess whether self-pollination was autogamous (from the same flower) or geitonogamous (from different flowers on the same plant).
Little has been reported regarding the pollination biology of
A. glabra, but its production of large, showy, nectar-bearing flowers suggests zoophily. Flowers are produced on elongated panicles and have a long yellow to greenish-yellow calyx tube with a reddish base. Flowers can be either staminate (male) or (more commonly) perfect. Flowers are produced quite early in the season and are visited by many types of bees as well as hummingbirds. Self-incompatibility has, to our knowledge, not been assessed for
A. glabra, but our results indicate that it is at least partially self-fertile. For the National Champion Tree, it appears that, at least for the two years that we sampled (2016 and 2018), self-pollination provided reproductive assurance, likely because the delivery of outcross pollen was rare. An important aspect of reproductive assurance is the ability to reproduce when potential mates or pollinators are limited [
10,
11].
Mixed mating systems that produce both selfed and outcrossed seeds are relatively common among flowering plants [
12,
13], and a recent review of plant mating system literature found substantial and prevalent among-population variation in mating systems [
14]. Many factors, both genetic (e.g., floral morphology and display, phenology) and ecological (e.g., pollinator communities, plant population density, habitat fragmentation) influence variation in mating systems in self-compatible species [
14]. We found one tree that was predominantly selfing and one that was predominantly outcrossing, representing an extreme example of mating system variation for
A. glabra. While it is tempting to attribute the high level of selfing in the National Champion to isolation from conspecific potential mates, we cannot eliminate other potential causative factors. Estimates of outcrossing and selfing rates for more individuals and populations are needed to better characterize the mating system of this species.
Cases of pollen limitation leading to selfing for isolated trees have previously been reported. For example, isolated pasture trees of
Pachira quinata, a bat-pollinated species in Costa Rica, showed levels of selfing ranging from 0 to 80%, and self-pollination resulted in reduced fitness [
15]. Investigation of the dipterocarp tree
Shorea acuminata found selfing rates that ranged between 7.6 and 88.4% and was inversely correlated with the number of nearby conspecifics [
16]. In other tree species, frequent long distance pollen dispersal, sometimes at impressive distances, prevents inbreeding in isolated individuals. In the tropical timber tree
Dysoxylum malabaricum, single isolated trees frequently received diverse pollen from over five kilometers away [
17]. Small, relict stands of
Quercus robur in Russia were pollinated from trees at least 80 km away [
18], and substantial levels of long-distance pollination have been reported for other isolated stands of oaks (reviewed in [
19]).
Although not precisely quantified, inbreeding depression, likely to result from self-fertilization in an outcrossing species, was not noted in traits such as seed mass or germination rates for the progeny of the National Champion tree. The buckeyes collected from both the National Champion and the Ohio State Champion showed very high germination rates and seedling survival (>90%) under normal conditions. However, an unseasonal weather event in the spring of 2022 (late frost and high winds) resulted in higher mortality for seedlings from the National Champion. Seed crops for the National Champion also appear to be lower and more variable than for the Ohio State Champion, which consistently produces a large crop each year (J. L., pers. observation). Growth rates after five years were lower for a sample of seedlings of the National Champion compared to the Ohio State Champion (
Figure 4), with the inbred 2016 cohort ~13% shorter in height than the outcrossed 2016 cohort. A larger sample of seedlings from the 2018 cohort showed significant differences in height after three years, with the National Champion seedlings being over 6 cm shorter on average. Inbreeding depression for seedling growth traits has been reported to be greater than those for seed traits for other tree species [
20]. Furthermore, a long-term study of
Eucalyptus regans found that stem diameter at age 4 years was positively associated with seed production at age 29 years, indicating even a small competitive edge in early growth may persist through the entire life cycle [
21]. Indeed, the
E. regans study shows that small but persistent consequences of inbreeding depression may eliminate nearly all inbred progeny over the life cycle of long-lived woody perennials [
21]. The female fitness of trees that predominantly self, such as the National Champion, will likely be extremely low.
STRUCTURE analyses indicated that the National Champion Tree and its progeny formed a genetic cluster. Under the
K = 2 scenario, supported by the Evanno Δ
K [
7] approach, all the other samples, including the Ohio State Champion, its progeny, and the population samples from Ohio and Illinois formed a second cluster (
Figure 2). This indicates that there is no strong evidence for genetic structure or differentiation between populations across this part of the
A. glabra range. The pollen pool sampled by the Ohio State Champion was diverse and not genetically differentiated from the broader population sample. Under the
K = 3 scenario, supported by MedMedK, MedMeanK, MaxMedK and MaxMeanK [
8], the Ohio Champion tree and its progeny formed its own genetic cluster (
Figure 3), with some individuals showing high levels of admixture. These methods of evaluating
K are reported to find more subtle clustering patterns, and it is therefore not surprising that a sample of half-sibs, all having one of two maternal alleles at each locus, would comprise a genetic cluster. Assessing population structure across the range of
A. glabra was not the focus of this study, and both our sampling of natural populations and the number of loci scored were not adequate to achieve this goal. We can only report that our preliminary data does not provide evidence that
A. glabra populations in Ohio and Illinois are genetically differentiated.
In conclusion, we found that the largest recorded individual of Ohio buckeye, A. glabra, has an extremely high level of self-fertilization. Although overall levels of self-fertilization in this species have not been studied, another extremely large tree, a state champion, produced nuts that were exclusively outcrossed. Self-fertilization may provide reproductive assurance for the National Champion tree growing at the periphery of the species’ range, but female fitness may be compromised due to inbreeding depression, as evidenced by slower growth rates of its seedlings, lower seedling survival during extreme events, and smaller seed crops.