Anatomical and Morphological Features of Scots Pine Heartwood Formation in Two Forest Types in the Middle Taiga Subzone
Abstract
1. Introduction
2. Materials and Methods
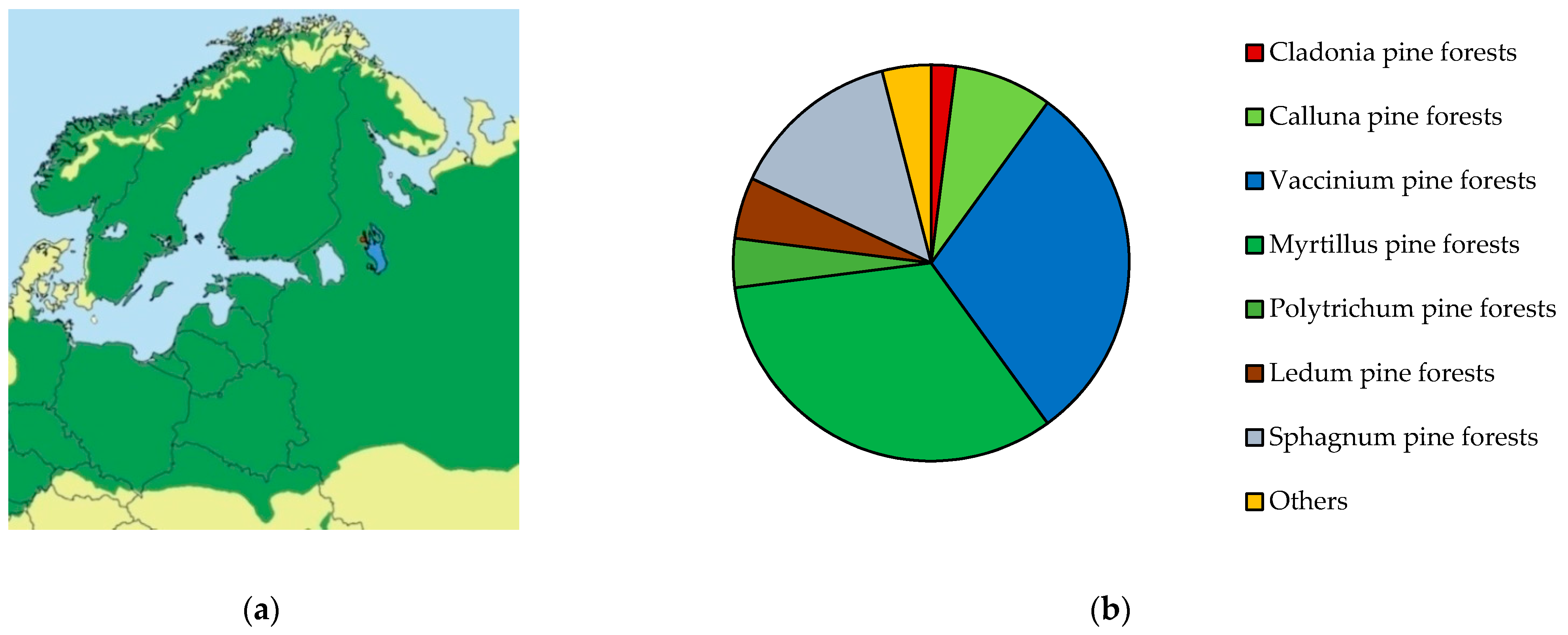
2.1. Sample Plot Characteristics
2.1.1. Selection and Description
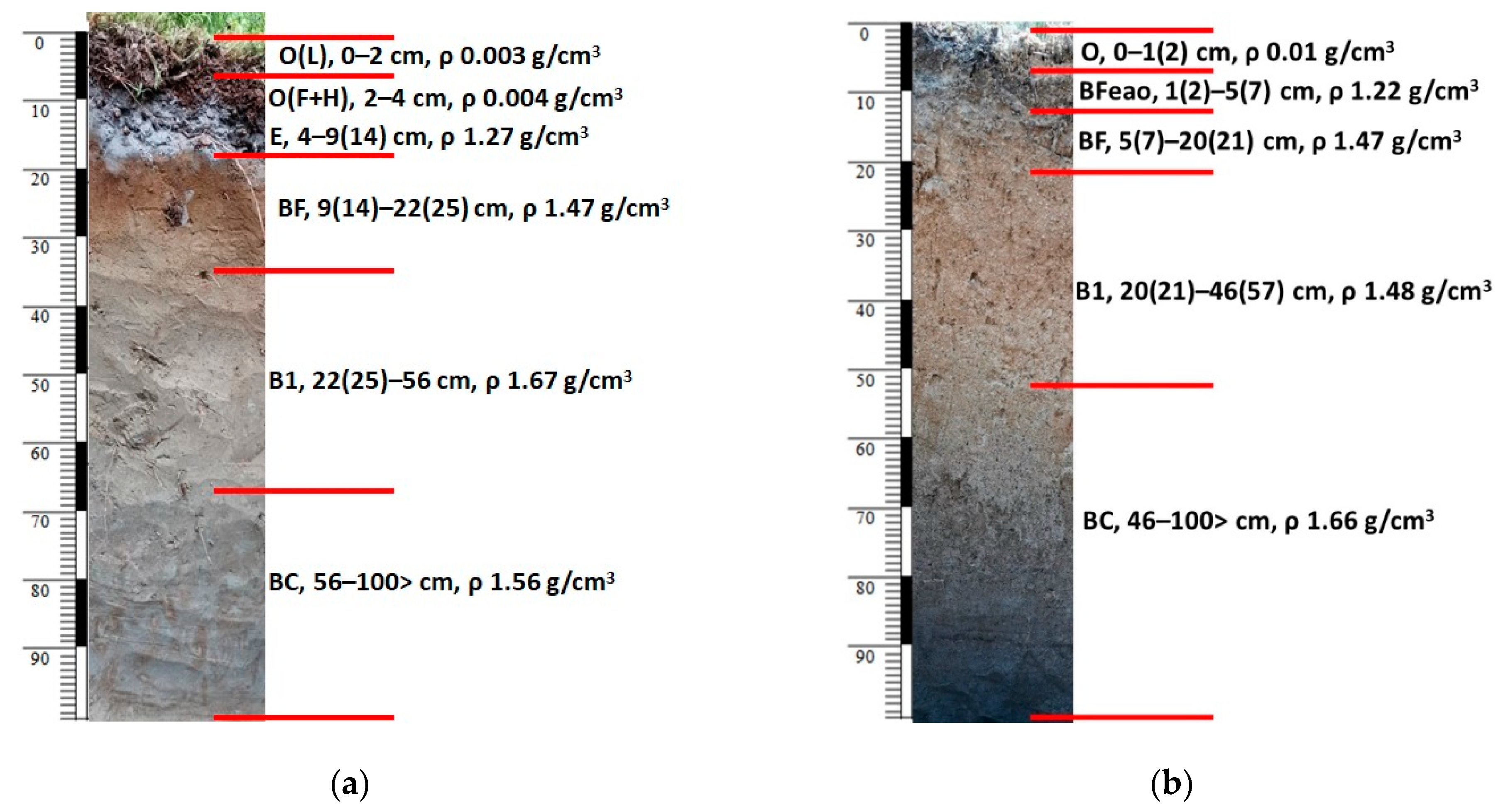
2.1.2. Soil Sampling and Chemical Soil Analysis
2.2. Model Trees Characteristics
2.3. Cores Sampling and Measurement
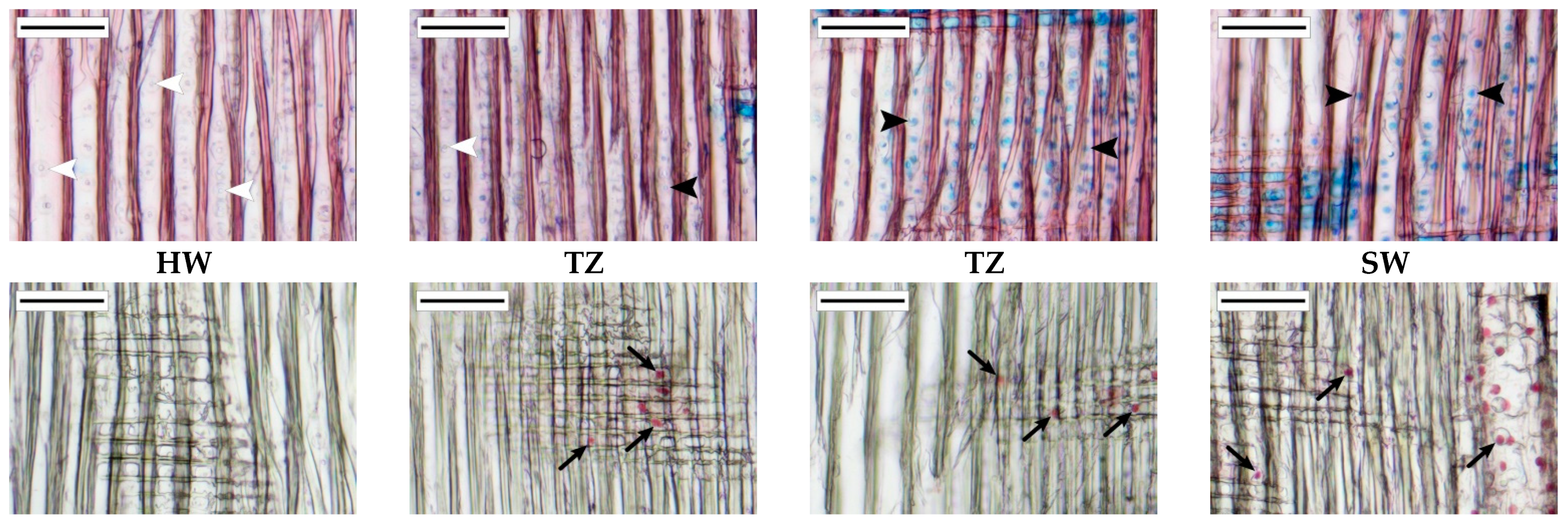
2.4. Sampling, Preparation and Microscopy Processing of the Transition Zone
2.5. Statistical Analyses
3. Results
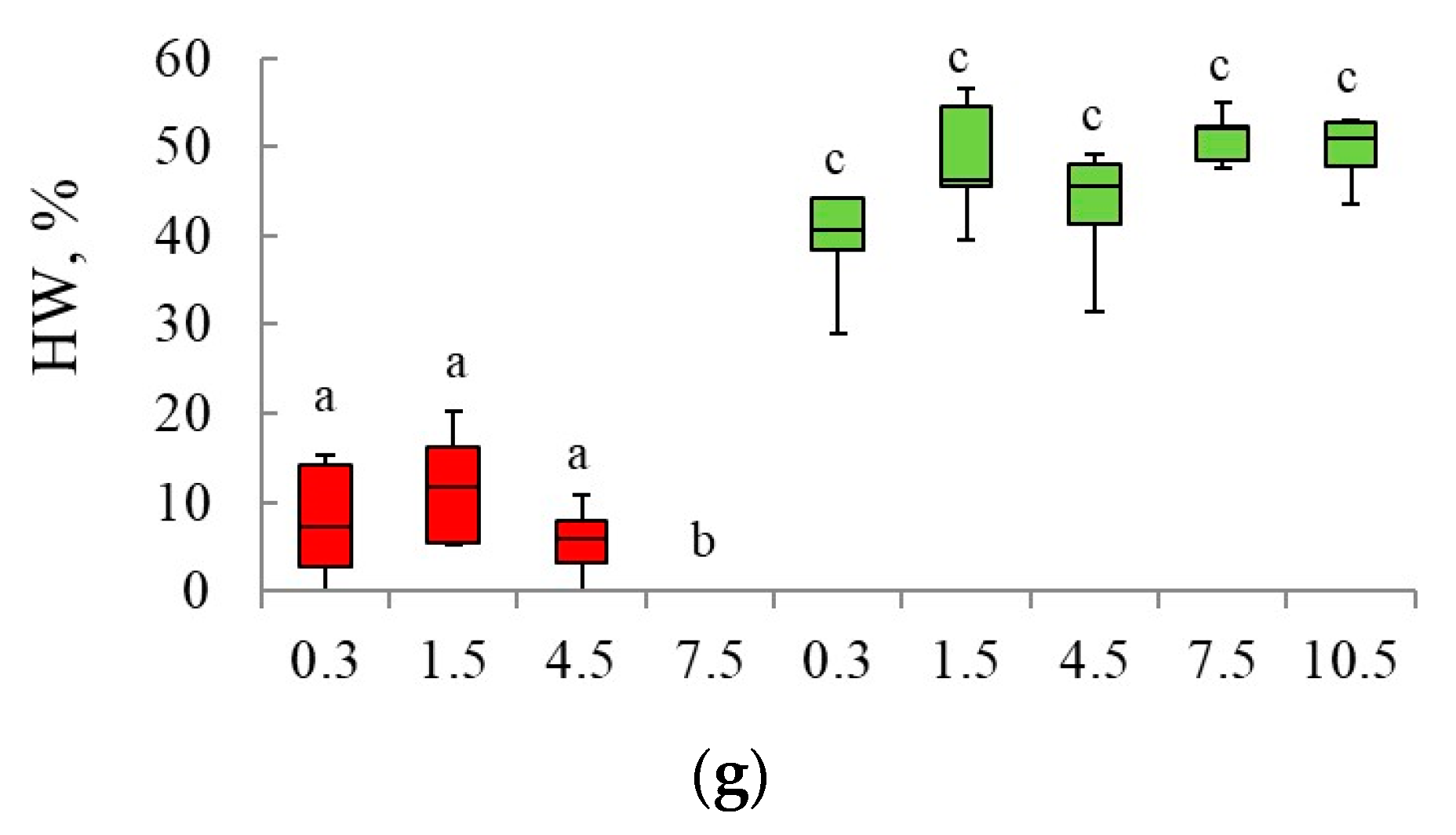
3.1. Spatial Distribution of Heartwood in Scots Pine Trees Trunks Growing in Blueberry and Lichen Pine Stands
3.2. Validation of the Models Developed for the Investigated Forest Types
3.3. Anatomical Characteristics of the Transition Zone
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Praciak, A.; Pasiecznik, N.; Sheil, D.; van Heist, M.; Sassen, M.; Correia, C.S.; Dixon, C.; Fyson, C.; Rushford, K.; Teeling, C. The CABI Encyclopedia of Forest Trees; CABI: Oxfordshire, UK, 2013. [Google Scholar]
- Climent, J.; Gil, L.; Pardos, J. Heartwood and Sapwood Development and Its Relationship to Growth and Environment in Pinus Canariensis Chr.Sm Ex DC. For. Ecol. Manag. 1993, 59, 165–174. [Google Scholar] [CrossRef]
- Climent, J.; Chambel, M.R.; Gil, L.; Pardos, J.A. Vertical Heartwood Variation Patterns and Prediction of Heartwood Volume in Pinus Canariensis Sm. For. Ecol. Manag. 2003, 174, 203–211. [Google Scholar] [CrossRef]
- Björklund, L. Identifying Heartwood-Rich Stands or Stems of Pinus sylvestris by Using Inventory Data. Silva Fenn. 1999, 33. [Google Scholar] [CrossRef]
- Pinto, I.; Pereira, H.; Usenius, A. Heartwood and Sapwood Development within Maritime Pine (Pinus pinaster Ait.) Stems. Trees-Struct. Funct. 2004, 18, 284–294. [Google Scholar] [CrossRef]
- Metsä-Kortelainen, S. Differences between Sapwood and Heartwood of Thermally Modified Norway Spruce (Picea abies) and Scots Pine (Pinus sylvestris) under Water and Decay Exposure; VTT: Espoo, Finland, 2011; ISBN 978-951-38-7752-1. [Google Scholar]
- Esteves, B.; Nunes, L.; Domingos, I.; Pereira, H. Comparison between Heat Treated Sapwood and Heartwood from Pinus Pinaster. Eur. J. Wood Prod. 2014, 72, 53–60. [Google Scholar] [CrossRef]
- Bamber, R.; Fukazawa, K. Sapwood and Heartwood: A Review. For. Prod. Abstr. 1985, 46, 567–580. [Google Scholar]
- Hillis, W.E. Heartwood and Tree Exudates; Springer Series in Wood Science; Springer: Berlin/Heidelberg, Germany, 1987; Volume 4, ISBN 978-3-642-72536-4. [Google Scholar]
- Smith, A.L.; Campbell, C.L.; Walker, D.B.; Hanover, J.W. Extracts from Black Locust as Wood Preservatives: Extraction of Decay Resistance from Black Locust Heartwood. Holzforschung 1989, 43, 293–296. [Google Scholar] [CrossRef]
- Onuorah, E.O. The Effects of Some Manufacturing Variables on the Properties of Particleboard. Niger. J. Technol. 2011, 20, 19–40. [Google Scholar]
- Panshin, A.J.; de Zeeuw, C. Textbook of Wood Technology; McGraw-Hill: Toronto, ON, Canada, 1980. [Google Scholar]
- Yamamoto, K. Yearly and Seasonal Process of Maturation of Ray Parenchyma Cells in Pinus Species. Researh Bull. Coll. Exp. For.-Hokkaido Univ. 1982, 39, 245–296. [Google Scholar]
- Sperry, J.S.; Perry, A.H.; Sullivan, J.E.M. Pit Membrane Degradation and Air-Embolism Formation in Ageing Xylem Vessels of Populus Tremuloides Michx. J. Exp. Bot. 1991, 42, 1399–1406. [Google Scholar] [CrossRef]
- Fujii, T.; Suzuki, Y.; Kuroda, N. Bordered Pit Aspiration in the Wood of Cryptomeria Japonica in Relation to Air Permeability. IAWA J. 1997, 18, 69–76. [Google Scholar] [CrossRef]
- Magel, E.A.; Höll, W. Storage Carbohydrates and Adenine Nucleotides in Trunks of Fagus sylvatica L. in Relation to Discolored Wood. Holzforschung 1993, 47, 19–24. [Google Scholar] [CrossRef]
- Magel, E.; Einig, W.; Hampp, R. Carbohydrates in trees. In Developments in Crop Science; Elsevier: Amsterdam, The Netherlands, 2000; Volume 26, pp. 317–336. ISBN 978-0-444-50269-8. [Google Scholar]
- Meerts, P. Mineral Nutrient Concentrations in Sapwood and Heartwood: A Literature Review. Ann. For. Sci. 2002, 59, 713–722. [Google Scholar] [CrossRef]
- Saito, K.; Mitsutani, T.; Imai, T.; Matsushita, Y.; Fukushima, K. Discriminating the Indistinguishable Sapwood from Heartwood in Discolored Ancient Wood by Direct Molecular Mapping of Specific Extractives Using Time-of-Flight Secondary Ion Mass Spectrometry. Anal. Chem. 2008, 80, 1552–1557. [Google Scholar] [CrossRef] [PubMed]
- Houle, D.; LaFlèche, M.R.; Duchesne, L. Sequential Extractions of Elements in Tree Rings of Balsam Fir and White Spruce. Commun. Soil Sci. Plant Anal. 2008, 39, 1138–1146. [Google Scholar] [CrossRef]
- Andrews, J.A.; Siccama, T.G.; Vogt, K.A. The Effect of Soil Nutrient Availability on Retranslocation of Ca, Mg and K from Senescing Sapwood in Atlantic White Cedar. Plant Soil 1999, 208, 117–123. [Google Scholar] [CrossRef]
- Taylor, A.M.; Gartner, B.L.; Morre, J.I. Heartwood Formation and Natural Durability- a Review. Wood Fib. Sci. 2002, 34, 587–611. [Google Scholar]
- Boratyński, A. Range of Natural Distribution. In Genetics of Scots Pine; Developments in Plant Genetics and Breeding; Elsevier: Amsterdam, The Netherlands, 1991; Volume 3, pp. 19–30. ISBN 978-0-444-98724-2. [Google Scholar]
- Krakau, U.-K.; Liesebach, M.; Aronen, T.; Lelu-Walter, M.-A.; Schneck, V. Scots Pine (Pinus sylvestris L.). In Forest Tree Breeding in Europe; Pâques, L.E., Ed.; Managing Forest Ecosystems; Springer: Dordrecht, The Netherlands, 2013; Volume 25, pp. 267–323. ISBN 978-94-007-6145-2. [Google Scholar]
- Kazimirov, N.I.; Volkov, A.D.; Zjabchenko, S.S.; Ivanchikov, A.A.; Morozova, R.M. Metabolism and Energy Exchange in Pine Forests of the European North; Nauka: Leningrad, Russia, 1977. [Google Scholar]
- Gromtsev, A.N.; Kitaev, S.P.; Krutov, V.I.; Kuznetsov, O.L.; Lindholm, T.; Yakovlev, E.B. Biotic Diversity of Karelia: Conditions of Formation, Communities and Species; Karelian Research Centre of RAS: Petrozavodsk, Russia, 2003. [Google Scholar]
- Cajander, A.K. Forest Types and Their Significance. Acta For. Fenn. 1949, 56, 1–71. [Google Scholar] [CrossRef]
- Tkachenko, M.E. General Forestry; Goslesbumizdat: Moscow, Russia, 1952. [Google Scholar]
- Koperin, F.I. Dependence of the Structure and Physical and Mechanical Properties of Wood of Coniferous Species on Forest Growing Conditions. Proc. Arkhangelsk For. Inst. 1955, 16, 156–168. [Google Scholar]
- Rigling, A.; Waldner, P.O.; Forster, T.; Bräker, O.U.; Pouttu, A. Ecological Interpretation of Tree-Ring Width and Intraannual Density Fluctuations in Pinus sylvestris on Dry Sites in the Central Alps and Siberia. Can. J. For. Res. 2001, 31, 18–31. [Google Scholar] [CrossRef]
- Kishchenko, I.T. Anatomical Structure of the Annual Ring of Pinus sylvestris L. in Different Forest Types of the Taiga Zone. For. J. 2016, 4, 61–70. [Google Scholar] [CrossRef]
- Wieruszewski, M.; Mydlarz, K. The Influence of Habitat Conditions on the Properties of Pinewood. Forests 2021, 12, 1311. [Google Scholar] [CrossRef]
- Kampe, A.; Magel, E. New Insights into Heartwood and Heartwood Formation. In Cellular Aspects of Wood Formation; Fromm, J., Ed.; Plant Cell Monographs; Springer: Berlin/Heidelberg, Germany, 2013; Volume 20, pp. 71–95. ISBN 978-3-642-36490-7. [Google Scholar]
- Moya, R.; Bond, B.; Quesada, H. A Review of Heartwood Properties of Tectona Grandis Trees from Fast-Growth Plantations. Wood Sci. Technol. 2014, 48, 411–433. [Google Scholar] [CrossRef]
- Moya, R.; Berrocal, A. Wood Colour Variation in Sapwood and Heartwood of Young Trees of Tectona Grandis and Its Relationship with Plantation Characteristics, Site, and Decay Resistance. Ann. For. Sci. 2010, 67, 109. [Google Scholar] [CrossRef]
- Moya, R.; Calvo-Alvarado, J. Variation of Wood Color Parameters of Tectona Grandis and Its Relationship with Physical Environmental Factors. Ann. For. Sci. 2012, 69, 947–959. [Google Scholar] [CrossRef]
- Miranda, I.; Gominho, J.; Lourenço, A.; Pereira, H. The Influence of Irrigation and Fertilization on Heartwood and Sapwood Contents in 18-Year-Old Eucalyptus Globulus Trees. Can. J. For. Res. 2006, 36, 2675–2683. [Google Scholar] [CrossRef]
- Gjerdrum, P. Heartwood in Relation to Age and Growth Rate in Pinus sylvestris L. in Scandinavia. Forestry 2003, 76, 413–424. [Google Scholar] [CrossRef]
- Uusitalo, J. Heartwood and Extractive Content of Scots Pine in Southern Finland: Models to Apply at Harvest. Wood Fiber Sci. 2004, 36, 3–8. [Google Scholar]
- Mörling, T.; Valinger, E. Effects of Fertilization and Thinning on Heartwood Area, Sapwood Area and Growth in Scots Pine. Scand. J. For. Res. 1999, 14, 462–469. [Google Scholar] [CrossRef]
- Pikk, J.; Kask, R.; Peterson, P. The Wood Quality of Fertilized Scots Pine (Pinus sylvestris L.) Stands on Vaccinium Vitis-Idaea and Cladonia Site Type. Metsanduslikud Uurim. (For. Stud.) 2006, 44, 9–19. [Google Scholar]
- Splawa-Neyman, S.; Pazdrowskì, W. Effect of Site Fertility upon Formation of Heartwood in Timber of Scots Pine [Pinus sylvestris L.]. Folia For. Polonica. Ser. B-Drzew. 2001, 32, 83–87. [Google Scholar]
- Jakubowski, M.; Kaluzinski, D.; Tomczak, A.; Jelonek, T. Proportion of Heartwood and Sapwood in Scots Pine (Pinus sylvestris L.) Stems Grown in Different Site Conditions. Ann. Wars. Univ. Life Sci.-SGGW. For. Wood Technol. 2015, 92, 122–126. [Google Scholar]
- Anan’ev, V.A.; Moshnikov, S.A. Structure and Dynamics of the Forest Reserves of the Republic of Karelia. Lesn. Zhurnal 2016, 19–29. [Google Scholar] [CrossRef]
- Galibina, N.A.; Moshnikov, S.A.; Nikerova, K.M.; Afoshin, N.V.; Ershova, M.A.; Ivanova, D.S.; Kharitonov, V.A.; Romashkin, I.V.; Semenova, L.I.; Serkova, A.A.; et al. Changes in the Intensity of Heartwood Formation in Scots Pine (Pinus sylvestris L.) Ontogenesis. IAWA J. 2022, accepted.
- Krylov, G.V. Forests of Western Siberia; Publishing House of the Academy of Sciences of the USSR: Moscow, Russia, 1961. [Google Scholar]
- Rysin, L.P.; Savel’eva, L.I. Pine Forests of Russia; Scientific Publications Partnership KMK: Moscow, Russia, 2008. [Google Scholar]
- Spicer, R. Senescence in Secondary Xylem: Heartwood Formation as an Active Developmental Program. In Vascular Transport in Plants; Holbrook, N.M., Zwieniecki, M.A., Eds.; Elsevier: Amsterdam, The Netherlands, 2005; pp. 457–475. ISBN 978-0-12-088457-5. [Google Scholar]
- Fengel, D. Ultrastructural Changes during Aging of Wood Cells. Wood Sci. Technol. 1970, 4, 176–188. [Google Scholar] [CrossRef]
- Bauch, J.; Berndt, H. Variability of the Chemical Composition of Pit Membranes in Bordered Pits of Gymnosperms. Wood Sci. Technol. 1973, 7, 6–19. [Google Scholar] [CrossRef]
- Bauch, J.; Schweers, W.; Berndt, H. Lignification during heartwood formation: Comparative study of rays and bordered pit membranes in coniferous woods. Holzforschung 1974, 28, 86–91. [Google Scholar] [CrossRef]
- Felhofer, M.; Prats-Mateu, B.; Bock, P.; Gierlinger, N. Antifungal Stilbene Impregnation: Transport and Distribution on the Micron-Level. Tree Physiol. 2018, 38, 1526–1537. [Google Scholar] [CrossRef]
- Rust, S. Comparison of Three Methods for Determining the Conductive Xylem Area of Scots Pine (Pinus sylvestris). Forestry 1999, 72, 103–108. [Google Scholar] [CrossRef][Green Version]
- Bieker, D.; Rust, S. Non-Destructive Estimation of Sapwood and Heartwood Width in Scots Pine (Pinus sylvestris L.). Silva Fenn. 2010, 44, 267–273. [Google Scholar] [CrossRef]
- Millers, M. The Proportion of Heartwood in Conifer (Pinus sylvestris L., Picea Abies [L.] H. Karst.) Trunks and Its Influence on Trunk Wood Moisture. J. For. Sci. 2013, 59, 295–300. [Google Scholar] [CrossRef]
- Frey-Wyssling, A.; Bosshard, H.H. Cytology of the Ray Cells in Sapwood and Heartwood. Holzforschung 1959, 13, 129–137. [Google Scholar] [CrossRef]
- Saranpaa, P. Plastids and Glycolipids in the Stemwood of Pinus sylvestris L. Trees 1988, 2, 180–187. [Google Scholar] [CrossRef]
- Bergstrom, B. Chemical and Structural Changes during Heartwood Formation in Pinus Sylvestris. Forestry 2003, 76, 45–53. [Google Scholar] [CrossRef]
- Esteban, L.G.; Gasson, P.; Climent, J.M.; de Palacios, P.; Guindeo, A. The Wood of Pinus Canariensis and Its Resinous Heartwood. IAWA J. 2005, 26, 69–78. [Google Scholar] [CrossRef]
- Saranpaa, P.; Holl, W. Soluble Carbohydrates of Pinus sylvestris L. Sapwood and Heartwood. Trees 1989, 3, 138–143. [Google Scholar] [CrossRef]
- Fischer, C.; Höll, W. Food Reserves of Scots Pine (Pinus sylvestris L.). Trees 1991, 5, 187–195. [Google Scholar] [CrossRef]
- Gustafsson, G.; Bergström, B.; Gref, R.; Ericsson, A. Changes in Chemical Constituents in the Sapwood of Pinus sylvestris Due to Debarking. Scand. J. For. Res. 2003, 18, 90–96. [Google Scholar] [CrossRef]
- Bert, D.; Danjon, F. Carbon Concentration Variations in the Roots, Stem and Crown of Mature Pinus Pinaster (Ait.). For. Ecol. Manag. 2006, 222, 279–295. [Google Scholar] [CrossRef]
- Tulik, M.; Jura-Morawiec, J.; Bieniasz, A.; Marciszewska, K. How Long Do Wood Parenchyma Cells Live in the Stem of a Scots Pine (Pinus sylvestris L.)? Studies on Cell Nuclei Status along the Radial and Longitudinal Stem Axes. Forests 2019, 10, 977. [Google Scholar] [CrossRef]
- Industrial Standard 56-69-83. Forest Surveying Sample Plots. Coupe Demarcation Method; TsBPTI Gosleskhoza USSR: Moscow, Russia, 1984.
- Fadeeva, M.A.; Golubkova, N.S.; Vitikajnen, O.; Ahti, T. Abstract of Lichens and Lichenophilic Fungi of the Republic of Karelia; Forest Research Institute of the Karelian Research Centre of the Russian Academy of Sciences: Petrozavodsk, Russia, 2007. [Google Scholar]
- IUSS Working Group WRB 2015. World Reference Base for Soil Resources 2014, Update 2015. International Soil Classification System for Naming Soils and Creating Legends for Soil Maps; World Soil Resources Reports; FAO: Rome, Italy.
- Soil Diversity and Biodiversity in Forest Ecosystems of the Middle Taiga; Fedorec, N.G., Ed.; Nauka: Moscow, Russia, 2006. [Google Scholar]
- Galibina, N.A.; Novitskaya, L.L.; Nikerova, K.M.; Moshkina, E.V.; Moshchenskaya, Y.L.; Borodina, M.N.; Sofronova, I.N.; Nikolaeva, N.N. Labile nitrogen availability in soil influences the expression of wood pattern in Karelian birch. Bot. Zhurnal 2019, 104, 1598–1609. [Google Scholar] [CrossRef]
- Oliva, J.; Camarero, J.J.; Stenlid, J. Understanding the Role of Sapwood Loss and Reaction Zone Formation on Radial Growth of Norway Spruce (Picea Abies) Trees Decayed by Heterobasidion Annosum s.l. For. Ecol. Manag. 2012, 274, 201–209. [Google Scholar] [CrossRef]
- Plant Microtechniques and Protocols; Yeung, E.C.T., Stasolla, C., Sumner, M.J., Huang, B.Q., Eds.; Springer International Publishing: Cham, Switerzerland, 2015; ISBN 978-3-319-19943-6. [Google Scholar]
- Shinozaki, K.; Yoda, K.; Hozumi, K.; Kira, T. A Quantitative Analysis of Plant Form—The Pipe Model Theory: I. Basic Analyses. Jpn. J. Ecol. 1964, 14, 97–105. [Google Scholar] [CrossRef]
- Bamber, R.K. Heartwood, Its Function and Formation. Wood Sci. Technol. 1976, 10, 1–8. [Google Scholar] [CrossRef]
- Celedon, J.M.; Bohlmann, J. An Extended Model of Heartwood Secondary Metabolism Informed by Functional Genomics. Tree Physiol. 2018, 38, 311–319. [Google Scholar] [CrossRef]
- Rigling, A.; Bräker, O.; Schneiter, G.; Schweingruber, F. Intra-Annual Tree-Ring Parameters Indicating Differences in Drought Stress of Pinus sylvestris Forests within the Erico-Pinion in the Valais (Switzerland). Plant Ecol. 2002, 163, 105–121. [Google Scholar] [CrossRef]
- Sterck, F.J.; Zweifel, R.; Sass-Klaassen, U.; Chowdhury, Q. Persisting Soil Drought Reduces Leaf Specific Conductivity in Scots Pine (Pinus Sylvestris) and Pubescent Oak (Quercus pubescens). Tree Physiol. 2008, 28, 529–536. [Google Scholar] [CrossRef]
- Plavcová, L.; Jansen, S. The Role of Xylem Parenchyma in the Storage and Utilization of Nonstructural Carbohydrates. In Functional and Ecological Xylem Anatomy; Hacke, U., Ed.; Springer International Publishing: Berlin/Heidelberg, Germany, 2015; pp. 209–234. ISBN 978-3-319-15782-5. [Google Scholar]
- Belt, T.; Venäläinen, M.; Altgen, M.; Harju, A.; Rautkari, L. Extractive Concentrations and Cellular-Level Distributions Change Radially from Outer to Inner Heartwood in Scots Pine. Tree Physiol. 2021, 41, 1034–1045. [Google Scholar] [CrossRef]
- Ojansuu, R.; Maltamo, M. Sapwood and Heartwood Taper in Scots Pine Stems. Can. J. For. Res. 1995, 25, 1928–1943. [Google Scholar] [CrossRef]
- Vanninen, P.; Ylitalo, H.; Sievänen, R.; Mäkelä, A. Effects of Age and Site Quality on the Distribution of Biomass in Scots Pine (Pinus sylvestris L.). Trees 1996, 10, 231–238. [Google Scholar] [CrossRef]
- Mäkelä, A.; Vanninen, P. Impacts of Size and Competition on Tree Form and Distribution of Aboveground Biomass in Scots Pine. Can. J. For. Res. 1998, 28, 216–227. [Google Scholar] [CrossRef]
- Krause, C.; Gagnon, R. The Relationship between Site and Tree Characteristics and the Presence of Wet Heartwood in Black Spruce in the Boreal Forest of Quebec, Canada. Can. J. For. Res. 2006, 36, 1519–1526. [Google Scholar] [CrossRef]
- Thulasidas, P.K.; Bhat, K.M. Log Characteristics and Sawn Timber Recovery of Home-Garden Teak from Wet and Dry Localities of Kerala, India. Small-Scale For. 2009, 8, 15–24. [Google Scholar] [CrossRef]
- Pérez Cordero, L.; Kanninen, M. Heartwood, Sapwood and Bark Content, and Wood Dry Density of Young and Mature Teak (Tectona grandis) Trees Grown in Costa Rica. Silva Fenn. 2003, 37, 45–54. [Google Scholar] [CrossRef]
- Crespo, R.; Jiménez, E.; Suatuance, P.; Law, G.; Sánchez, C. Comparative Analysis of Physical-Mechanical Properties of Teak (Tectona grandis LF) from Quevedo and Baltazar. Cienc. Y Tecnol. 2008, 1, 55–63. [Google Scholar]
- Climent, J.; Chambel, M.; Pérez, E.; Gil, L.; Pardos, J. Relationship between Heartwood Radius and Early Radial Growth, Tree Age, and Climate in Pinus Canariensis. Can. J. For. Res. 2002, 32, 103–111. [Google Scholar] [CrossRef]
- Bektas, I.; Alma, M.H.; Goker, Y.; Yuksel, A.; Gundogan, R. Influence of Site on Sapwood and Heartwood Ratios of Turkish Calabrian Pine. For. Prod. J. 2003, 53, 48–50. [Google Scholar]
- Moreno Chan, J.; Raymond, C.A.; Walker, J.C.F. Development of Heartwood in Response to Water Stress for Radiata Pine in Southern New South Wales, Australia. Trees 2013, 27, 607–617. [Google Scholar] [CrossRef]
- Anda, R.R.; Koch, G.; Richter, H.-G.; Talavera, F.J.F.; Guzmán, J.A.S.; Satyanarayana, K.G. Formation of Heartwood, Chemical Composition of Extractives and Natural Durability of Plantation-Grown Teak Wood from Mexico. Holzforschung 2019, 73, 547–557. [Google Scholar] [CrossRef]
- de Almeida, M.N.F.; Vidaurre, G.B.; Pezzopane, J.E.M.; Lousada, J.L.P.C.; Silva, M.E.C.M.; Câmara, A.P.; Rocha, S.M.G.; de Oliveira, J.C.L.; Campoe, O.C.; Carneiro, R.L.; et al. Heartwood Variation of Eucalyptus Urophylla Is Influenced by Climatic Conditions. For. Ecol. Manag. 2020, 458, 117743. [Google Scholar] [CrossRef]



| SP | Age (Years) | FSC 1 | DBH 2/H 3 (cm)/(m) | D 4 (Trees × 1000/ha) | V 5 (m3/ha) | Basal Area per Hectare (m2) | UTSC 6 (Trees × 1000/ha) | NE 7 |
|---|---|---|---|---|---|---|---|---|
| 2 | 80 | 95% Pinus sylvestris 1% Picea 4% Betula | 22.9/23.3 | 0.96 | 377/25 | 35.6 | 70% Spruce 30% Birch/0.33 | 62°17′48.3″ N 34°00′31.7″ E |
| 8 | 70–80 | 100% Pinus sylvestris | 15.0/11.6 | 1.80 | 109/0 | 18.4 | 100% Pine/43 | 62°28′41.6″ N 33°48′21.6″ E |
| SP Geobotanical Characteristics | SP Photo |
|---|---|
| SP 2, Blueberry pine forest, 80 years General projective coverage—80%, moss-lichen layer—70%, herb-dwarf shrub layer—60%. In the living ground cover, 11 species were recorded, including Vaccinium myrtillus, V. vitis-idaea, Calluna vulgaris, Lycopodium annotinum L., Melampyrum pretense L., Deschampsia flexuosa (L.) Trin. and others. In the moss-lichen layer there are Pleurozium schreberi, Hylocomium splendens, Dicranum polysetum Sw. |  |
| SP 8, Lichen pine forest, 70–80 years General projective coverage—90%, moss-lichen layer—90%, herb-dwarf shrub layer—20%. In the living ground cover, there are Calluna vulgaris, Vaccinium vitis-idaea, Arctostaphylos uva-ursi, Diphasiastrum complanatum, Empetrum nigrum and Vaccinium myrtillus. In the moss-lichen layer there are Pleurozium schreberi and Cladonia rangiferina, Cl. arbuscula. |  |
| Soil Horizon | Horizon Thickness, cm | C, tons/ha | N, kg/ha | Mineral N, kg/ha | Hydrolysable N, kg/ha | Humus, tons/ha | P2O5, kg/ha | K2O, kg/ha | Water Supply (m3/ha) |
|---|---|---|---|---|---|---|---|---|---|
| SP 2, blueberry pine forest | |||||||||
| Forest litter | 4 | 11.7 | 344 | 1.09 | 0.03 | 20.1 | 8.5 | 28 | 27 |
| Mineral layer | 50 | 11.4 | 945 | 3.94 | 0.69 | 19.7 | 2357 | 115 | 359 |
| In total | 23.1 | 1289 | 5.03 | 0.73 | 39.8 | 2366 | 144 | 386 | |
| SP 8, lichen pine forest | |||||||||
| Forest litter | 2 | 3.5 | 83 | 0.19 | 0.01 | 6.0 | 1.8 | 5 | 9 |
| Mineral layer | 50 | 15.6 | 1700 | 3.18 | 0.62 | 27.0 | 2400 | 201 | 166 |
| In total | 19.1 | 1783 | 3.37 | 0.62 | 32.9 | 2402 | 206 | 174 | |
| Site | № | Age | DBH 1 | H 2 | LCr 3 | LCr |
|---|---|---|---|---|---|---|
| (Years) | (cm) | (m) | (m) | (%) | ||
| Blueberry pine forest | 1 | 78 | 31.1 | 28.0 | 10.0 | 36 |
| 2 | 80 | 32.7 | 27.0 | 9.5 | 35 | |
| 3 | 77 | 34.8 | 29.0 | 6.0 | 21 | |
| 4 | 77 | 31.6 | 30.0 | 10.5 | 35 | |
| 5 | 78 | 31.7 | 29.2 | 6.7 | 23 | |
| Lichen pine forest | 1 | 74 | 22.6 | 11.6 | 7.9 | 68 |
| 2 | 82 | 25.4 | 13.4 | 10.2 | 76 | |
| 3 | 79 | 19.6 | 11.8 | 9.2 | 78 | |
| 4 | 66 | 22.6 | 14.6 | 10.3 | 71 | |
| 5 | 71 | 24.3 | 14.1 | 11.0 | 78 |
| Studied Stand | Model | Slop | SE | t | p | R2 | SEE |
|---|---|---|---|---|---|---|---|
| Lichen pine forest | SW = 0.36 × C.A. + 11.88 | 0.91 | 0.02 | 49.8 | <0.001 | 0.90 | 2.5 |
| HW = 0.64 × C.A. − 11.74 | 1.12 | 0.04 | 29.6 | <0.001 | 0.95 | 3.1 | |
| √(HW) = 0.95 × √(C.A.) − 2.45 | 1.01 | 0.04 | 25.7 | <0.001 | 0.90 | 3.3 | |
| Lg(HW) = 1.61 × Lg(C.A.) − 1.55 | 0.88 | 0.04 | 23.6 | <0.001 | 0.94 | 3.1 | |
| Blueberry pine forest | SW = 0.36 × C.A. + 11.88 | 0.96 | 0.02 | 61.8 | <0.001 | 0.54 | 2.8 |
| HW = 0.64 × C.A. − 11.74 | 1.03 | 0.02 | 50.4 | <0.001 | 0.65 | 2.9 | |
| √(HW) = 0.95 × √(C.A.) − 2.45 | 0.94 | 0.02 | 49.7 | <0.001 | 0.65 | 2.7 | |
| Lg(HW) = 1.61 × Lg(C.A.) − 1.55 | 0.81 | 0.02 | 47.6 | <0.001 | 0.65 | 2.5 |
| Studied Stand | Model | Slop | SE | t | p | R2 | SEE |
|---|---|---|---|---|---|---|---|
| Lichen pine forest | √(HW) = √(C.A.) − 3 [38] | 0.95 | 0.03 | 28 | <0.001 | 0.95 | 2.8 |
| Blueberry pine forest | 0.89 | 0.02 | 48 | <0.001 | 0.65 | 2.7 | |
| Lichen pine forest | HW = 0.0021 × C.A.^2. + 0.3232 × C.A. − 4.8297 [4] | 0.94 | 0.04 | 26.71 | <0.001 | 0.95 | 2.9 |
| Blueberry pine forest | 0.87 | 0.02 | 48.6 | <0.001 | 0.65 | 2.6 | |
| Lichen pine forest | HW = 0.669 × C.A. − 13.650 [39] | 1.11 | 0.04 | 30.3 | <0.001 | 0.95 | 3.0 |
| Blueberry pine forest | 1.03 | 0.02 | 49.8 | <0.001 | 0.65 | 3.0 |
| Studied Stand | Slop | SE | t | p | R2 | SEE |
|---|---|---|---|---|---|---|
| R(HW) = −37.0691 + 0.3746 × C.A. + 4.8351 × Lring + 0.5593 × R(W) | ||||||
| Lichen pine forest | 0.96 | 0.18 | 5.3 | <0.001 | 0.53 | 34.3 |
| Blueberry pine forest | 0.79 | 0.02 | 40.3 | <0.001 | 0.34 | 8.7 |
| Lichen and blueberry pine forests | 0.84 | 0.04 | 23.1 | <0.001 | 0.79 | 17.25 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tarelkina, T.V.; Galibina, N.A.; Moshnikov, S.A.; Nikerova, K.M.; Moshkina, E.V.; Genikova, N.V. Anatomical and Morphological Features of Scots Pine Heartwood Formation in Two Forest Types in the Middle Taiga Subzone. Forests 2022, 13, 91. https://doi.org/10.3390/f13010091
Tarelkina TV, Galibina NA, Moshnikov SA, Nikerova KM, Moshkina EV, Genikova NV. Anatomical and Morphological Features of Scots Pine Heartwood Formation in Two Forest Types in the Middle Taiga Subzone. Forests. 2022; 13(1):91. https://doi.org/10.3390/f13010091
Chicago/Turabian StyleTarelkina, Tatiana V., Natalia A. Galibina, Sergei A. Moshnikov, Kseniya M. Nikerova, Elena V. Moshkina, and Nadezhda V. Genikova. 2022. "Anatomical and Morphological Features of Scots Pine Heartwood Formation in Two Forest Types in the Middle Taiga Subzone" Forests 13, no. 1: 91. https://doi.org/10.3390/f13010091
APA StyleTarelkina, T. V., Galibina, N. A., Moshnikov, S. A., Nikerova, K. M., Moshkina, E. V., & Genikova, N. V. (2022). Anatomical and Morphological Features of Scots Pine Heartwood Formation in Two Forest Types in the Middle Taiga Subzone. Forests, 13(1), 91. https://doi.org/10.3390/f13010091







