Abstract
Biscogniauxia nummularia (Bull.) Kuntze is a fungus which induces strip-cankers on beech, commonly referred to as charcoal canker. The symptoms of infection are visible on the host tree’s bark as elongated, blackish bark lesions on the trunk and branches. Recent years have shown that, due to climate change causing local epidemics, the species is increasing its economic impact in Mediterranean regions. Until recently, B. nummularia was considered rare and uncommon in central Europe. However, in the last few years it has been noticed more often, mostly in coniferous trees, which are out of B. nummularia’s host range. A similar situation has been observed with the closely related species Biscogniauxia mediterranea (De Not.) Kuntze, which prior to 2017 had not been observed in central Europe at all. This study shows the genetic diversity of mid-European strains of Biscogniauxia spp. (based on the ITS, TEF1, TUB2 and ACT regions) and, as the first in Europe, presents a molecular investigation of this species isolated from coniferous trees. It is also the first attempt at estimating the potential impact of this pathogenic fungus on European forestry management in the close future.
1. Introduction
Biscogniauxia nummularia (Bull.) Kuntze (synonym: Hypoxylon nummularium Bull.) is an ascomycetous fungus belonging to the family Xylariaceaes, that induces strip-cankers on beech, commonly called charcoal canker or beech tarcrust (BTC) disease [1,2,3,4,5,6]. The symptoms of infection are visible on the host tree’s bark as elongated, blackish bark lesions on the trunk and branches, and as wood decay in mature trees.
The nature of B. nummularia is complicated and still being discussed. Many authors suggest the fungus generally behaves as a saprotroph in the northern-Mediterranean basin, where the climate is more temperate, but shows the potential for higher impact in Europe as a dangerous pathogen of Fagus sylvatica L. [4,7,8,9]. Despite this, Biscogniauxia spp. were not previously considered as a primary cause of beech decline in Mediterranean regions [10,11]. Observations made in the declining Sicilian and Calabrian beech forests (southern Italy) showed a significant presence of B. nummularia, but did not name it as a direct cause of tree decline [12]. Only pathogenicity tests allowed for the confirmation of this species, whose aggressive strains are able to kill the attacked tree in a short time, as a primary pathogen of beech [2]. Studies have proven its endophytic nature, which allows the fungus to quickly switch strategies from harmless endophyte to primary pathogen [13], which is a well-known phenomenon in the Xylariales group of fungi [13,14,15]. Since the first confirmation of the pathogenicity of B. nummularia, subsequent reports indicate that the problem of beech dieback caused by the mass outbreak of charcoal canker is moving to northern Europe: the problem was observed in 2003 in Hungary [16], in 2014 in northern Spain [9] and in 2017 in the Czech Republic [17]. Disturbing reports have also come from Montenegro [18], where a recent investigation discovered a very aggressive Biscogniauxia population inducing destructive tarcrust formations on F. sylvatica. Molecular studies show that the beech dieback is caused by a new species of Biscogniauxia, which appears to be the hybridization and introgression of B. nummularia and B. anceps (Sacc. J.D. Rogers, Y.M. Ju and Cand). This fact is concerning, and highlights the importance of genetic analysis of these species on future research related to BTC.
Even though B. nummularia seems like a growing threat for European beech forests, the topic still leaves much to be discovered. B. nummularia is considered to be a species closely associated with Fagaceae [13], but it is also known to occur on other, unrelated taxa. Until recently, the fungus was regarded as an endophyte on dicotyledonous angiosperms [19] and some grasses [13]. However, new studies indicate the host range of B. nummularia is still unclear; in 2014, the endophytic stadium of B. nummularia was recorded on Carex brevicollis DC. [9]. In 2019, it was recorded for the first time as an endophyte on representatives of gymnosperms—Abies alba Mill. and Pinus × rhaetica Brūgger in southern Poland [20,21]—although symptoms of massive beech dieback due to charcoal canker have not yet been seen. It is worth considering that the species has recently started to be detected more often on coniferous trees in this area, which raises concerns about the potential impact on native populations of F. sylvatica in central-European beech forests.
An interesting fact can also be seen in the shift in range of related species—such as B. mediterranea (De Not.) Kuntze (synonym: Sphaeria mediterranea De Not.), which normally occurs in the temperate climate of the Mediterranean basin as an endophyte and pathogen of Quercus suber L. [5,6]. For the first time, this species has been found in central Europe: in the Czech Republic in 2017 [17] and three years later on shoots of A. alba in Poland [22], where it constituted 2.8% of all colonies isolated in the study. According to La Porta et al. [8], B. mediterranea is also considered a taxa with increasing economic impact due to climate change. The danger from the range of these species extending can be significant if we consider the importance of the host species in the newly affected areas; B. mediterranea mostly develops in the tissues of genus Quercus, which is one of the most important forest-forming species in this part of central Europe [23,24]. It is also worth noting the fact that B. mediterranea is already known as an important contributing factor in the decline of cork oak (Q. suber) in southern Europe and northern Africa [3]. Furthermore, literature shows that the pathogen can occur in different species of hardwood, e.g., Acer spp., Castanea sativa Miller, F. sylvatica, Platanus acerifolia Willd. and Faxinus excelsior L. [25], which are also important constituents of central European forests and the gardening industry. Similarly to B. nummularia, B. mediterranea has not yet been detected on coniferous trees.
The increasing economic significance of the pathogen causing BTC has already been predicted by some scientists [4,9]. Some authors point to climate change as the most important contributing factor [8]. A decisive factor can be precipitation disturbances, which is one of the elements of ongoing climate change [26,27]. The growth of both Biscogniauxia species is favored by warm temperatures [28] and prolonged summer droughts [4]. According to the literature [2], trees subjected to water stress are particularly susceptible to Biscogniauxia infection. Furthermore, European beech trees are more drought sensitive than other central-European broad-leaved tree species [29,30,31], making them even more vulnerable to BTC. All these factors can affect the pace of extension of Biscogniauxia’s range, which could be much faster than previously suspected. To the best of our knowledge, this is the first report on the genetic diversity of B. nummularia and B. mediterranea populations in central Europe, and one of the first molecular investigations of these species as isolated from coniferous trees.
2. Materials and Methods
2.1. Fieldwork
The study was conducted between June and October of 2019 and 2020 in three spots in Poland (central Europe): Stołowe Mountains National Park (SW Poland), Karkonosze National Park (SW Poland) and Tatra National Park (S Poland) (Table 1). In Stołowe Mountain National Park, the study was carried out on the Great Peat Bog of Batorów, located on a degraded peatbog, surrounded by spruce and pine, under passive protection without any human interference and at an altitude of 750 m a.s.l. The Tatra National Park’s 32 study plots were located at altitudes ranging between 916 and 1249 (mean 1005) meters a.s.l., in multi-age forest stands dominated by Norway spruce (Picea abies (L.) H.Karst) of various protective regimes. Karkonosze National Park’s 32 study plots were at altitudes between 500–982 m a.s.l., on three fir stands and 29 nurseries, established in the years 1995–2015 and located in actively protected areas with low levels of interference.

Table 1.
Characteristics of isolates of Biscogniauxia nummularia and Biscogniauxia mediterranea, isolated from Poland, included in the analysis.
Mycological analysis found individual isolates of Biscogniauxia spp on fresh coniferous tree needles (600 needles collected from 27 individuals of P. × rhaetica from the Great Peat Bog of Batorów in Stołowe Mountains National Park and 450 needles collected from 30 individuals of A. alba on 32 study plots in Tatra and Karkonosze National Parks). In each location from which samples were obtained, symptoms of branch dieback and needle discoloration of coniferous trees were observed. In Karkonosze and Tatra National Parks only the needles and branches in which discoloration covered 50–60% of the surface area were collected for mycological analysis. In Stołowe Mountain National Park, in addition to symptomatic needles, healthy needles were also collected for study. The trees sampled were not in the vicinity of potential hosts of Biscogniauxia spp. (e.g., Fagus sp. or Quercus sp.), which prevents the random appearance of a strain on coniferous trees. During the field work, fresh needles were collected in sterile envelopes and transported to the laboratory, where the isolates underwent mycological analysis in accordance with the method provided by Patejuk et al. [20]. First, needles were disinfected (A. alba for one minute in 0.5% sodium hypochlorite; P × rhaetica for 5 min in 1% sodium hypochlorite) and rinsed in sterile water. They were then cut into 2–4mm fragments and placed on a standard PDA medium (Potato Dextrose Agar, Biocorp, Poland). The growing fungal colonies were subsequently transferred to slants with PDA medium and cultured in order to enable their taxonomic identification. The isolates were identified under a light microscope based on their morphological traits, such as the dimension of spores, color, shape, etc. [32,33]. Isolates initially identified as Biscogniauxia spp. [5,9,34] were sent for molecular analysis.
2.2. Molecular Analysis
The species identification of isolates preliminarily classified based on morphology [5,9,34] as Biscogniauxia spp. was first carried out by analysis of the ITS region of ribosomal DNA (rDNA). Due to the rarity of species of this genus in Poland, to confirm identification, fragments of the TEF1 (translation elongation factor 1-α), TUB2 (β-tubulin) and ACT (actin) genes were also analyzed. In addition to identification, the purpose of the molecular analyses was to compare our isolates with the sequences of other European isolates available from GenBank NCBI.
2.2.1. Preparation of Isolates for Molecular Analysis and DNA Extraction
Prior to molecular analysis, six representative isolates of Biscogniauxia spp. were grown on PDB medium (Potato Dextrose Broth, A&A Biotechnology, Poland) over 10 days at 23 °C in the dark in Petri dishes protected with Parafilm M.
DNA was extracted according to the Doyle and Doyle [35] method, with modifications described by Baturo-Cieśniewska et al. [36]. For extraction of DNA, we used freeze dried mycelium homogenized with quartz beads in a Magna Lyser homogenizer (Roche, Basel, Switzerland) and applied buffer containing CTAB 5.0%, EDTA 0.5 M, NaCl 5.0 M, Tris -HCl (pH 8.0) 1.0 M, 2-mercaptoethanol and PVP 2.0%. After incubation at 65 °C, phenol, chloroform and isoamyl alcohol were used. In the following stages, 95% and 70% ethyl alcohol were used. The obtained DNA was cleaned with an anti-inhibitor kit (A&A Biotechnology, Poland), quantified fluorometrically on a Quantus device (Promega, WI, USA) and diluted in ddH2O to a concentration of 10 ng·L−1 for further analysis.
2.2.2. PCR Assays
Amplification was carried out in 37.5 μL of PCR Mix Plus (A&A Biotechnology, Poland). The final concentration of reagents was as follows: 1× PCR Mix Plus, 0.6 pM of each primer (Table 2) and 2 ng DNA template.

Table 2.
Annealing conditions of PCR assays, primers and their references.
Thermal cycling was performed on an Eppendorf EP Mastercycler using the following parameters: initial denaturation at 94 °C for 5 min, followed by 35 cycles of 94 °C for 1 min, an annealing temperature typical for a specific analysis (Table 2) and 72 °C for 2 min, followed by 10 min at 72 °C for the final extension. The presence of PCR products was verified by electrophoretic separation of 2 μL of the post-reaction mixture in tris-borate-EDTA (TBE) buffered by a 1.2% agarose gel (Pronadisa, Spain) stained with SimplySafe (EURx, Gdańsk, Poland).
2.2.3. Sequencing and Data Analysis
The amplification products were purified and sequenced by Genomed (Poland).
Chromatograms were edited and consensus sequences generated with FinchTV 1.4 (Geospiza Inc., Seattle, WA, USA). The Basic Local Alignment Search Tool (BLAST) [40] of the GenBank NCBI database (The National Center for Biotechnology Information) was used to identify species based on the ITS, TEF1, TUB2 and ACT sequences. Alignment using Clustal W and evolutionary analyses was conducted in MEGAX [41]. The evolutionary history was inferred by using the maximum likelihood method based on the Kimura 2-parameter model [42]. Node support was calculated using 1000 bootstrap replicants.
The sequences of ITS regions of B. nummularia and B. mediterranea were compared to the European sequences of these species available on GenBank (25 randomly taken from each species). For the same isolates, sequences of TEF1, TUB2 and ACT gene fragments were also compared where available. In the case of ACT, all sequences available from GenBank were used for comparison, including those isolates for which ITS was not analyzed.
Additionally, one sequence of the analyzed genes of other Biscogniauxia spp. available in GenBank was included for comparison: ITS, TUB2 and ACT of Austrian B. granmoi Lar.N. Vassiljeva and TEF1 of Chinese B. petrensis Z.F. Zhang, F. Liu and L. Cai, due to the lack of a European sequence. All analyzed sequences of our 6 isolates were deposited in the NCBI GenBank (http://www.ncbi.nlm.nih.gov/genbank/ accessed on 29 November 2021). Their accession numbers and data on isolates used in molecular studies are listed in Table 3.

Table 3.
Details of Biscogniauxia spp. isolates used in the molecular studies.
2.3. Meteorological and Hydrological Data Analysis
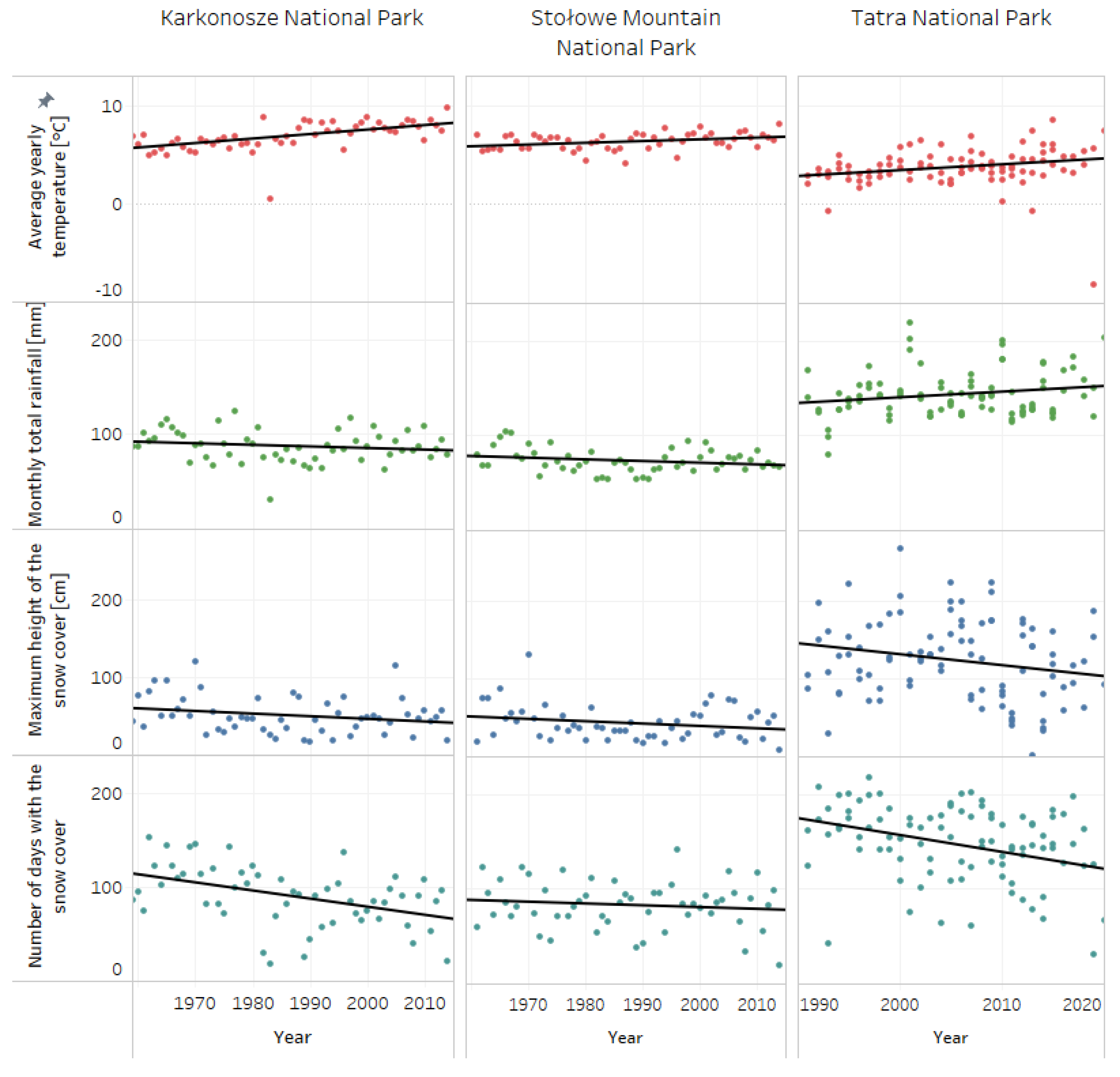
The meteorological and hydrological raw data were extracted from the database of the Institute of Meteorology and Water Management—National Research Institute [43]. Data were processed using Python. The closest measurement stations to the study spots were chosen. For Stołowe Mountain National Park, the meteorological data were extracted from the stations Duszniki-Zdrój in between years 1961–1974 (station code: 250169994) and Słuszów in between 1975–2014 (station code: 250160480)—in 1975 there was a change of the station’s name, for Karkonosze National Park from the station Karpacz (station code: 250150220) and for Tatra National Park from the stations Hala Gąsienicowa (station code: 249200540), Hala Ornak (station code: 249190680), Morskie Oko (station code: 249200910) and Polana Chochołowska (station code: 249190670). Parameters such as: average yearly temperature (°C), monthly total rainfall (mm), maximum height of the snow cover (cm) and number of days with snow cover were analyzed and presented on Figure 1. The changes in meteorological conditions were presented across 55 years, between 1960–2015, for Stołowe Mountain and Karkonosze National Park, and for a 30-year period, between 1990–2020, for Tatra National Park; different periods of time were used for the visualizations due to the availability of data on the IMWM-NRI database. Visualizations of meteorological data and linear fit were made using Tableau software (4 February 2020 Professional Edition).
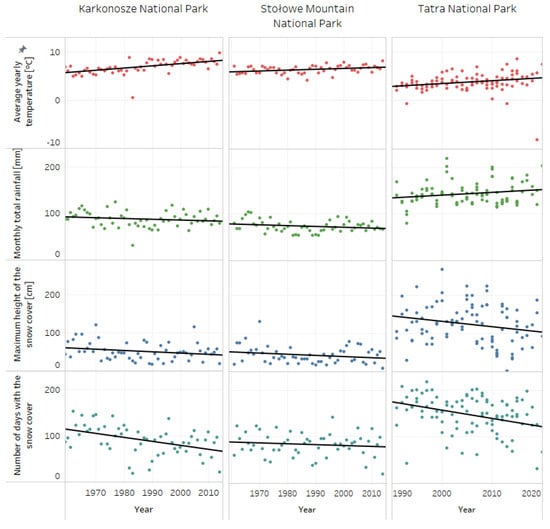
Figure 1.
Annual dynamics of climatic conditions in the studied areas.
3. Results
3.1. Meteorological Data
All of the studied spots were located in mountainous areas, characterized by long periods of snow cover, low annual temperatures and heavy rain. The biggest changes across the last 30–50 years were observed in Karkonosze and Tatra National Parks. In Stołowe Mountain National Park, statistically important differences were detected only for the average annual temperature (Table 4). The parameter with the smallest change was monthly total rainfall, which did not change significantly across the years. On the other hand, as presented in Figure 1, in recent years there has been a visible trend of rising annual temperature and a drop in snow cover. A statistically important decrease in the number of days with snow cover was observed for Karkonosze and Tatra National Parks (Table 4). The most drastic changes in snow cover were observed in Tatra National Park. It is worth highlighting that snow is one of the main reservoirs of water in mountainous areas, and continued decrease can affect local ecosystems in the long term.

Table 4.
Lineal regression parameters.
3.2. Molecular Analysis
The comparative analysis of ITS sequences of our isolates alongside the GenBank database clearly identified our Bm8L-19Aa as B. mediterranea. Its sequence was identical to many European and non-European sequences of this species, e.g., MW132049 and KR909208. In many cases, e.g., KM216761 or KM216784, our isolate differed by only one nucleotide (percent identity >99%), but there were also differences at the level of 30 nucleotides (percent identity ~94%).
The other five of our isolates were classified as B. nummularia. Bn3W-19Pu and Bn6L-19Pu showed 100% identity with e.g., MH860015, MT561409, Bn5L-19Pu, Bn31M-20Aa, Bn56C-20Aa, LN714525 and EF155488. Our first two isolates differed in two nucleotides from the other three. They also differed in one or more nucleotides from numerous sequences, e.g., MT153623 and AJ246231, in GenBank (percent identity >99% and >98%).
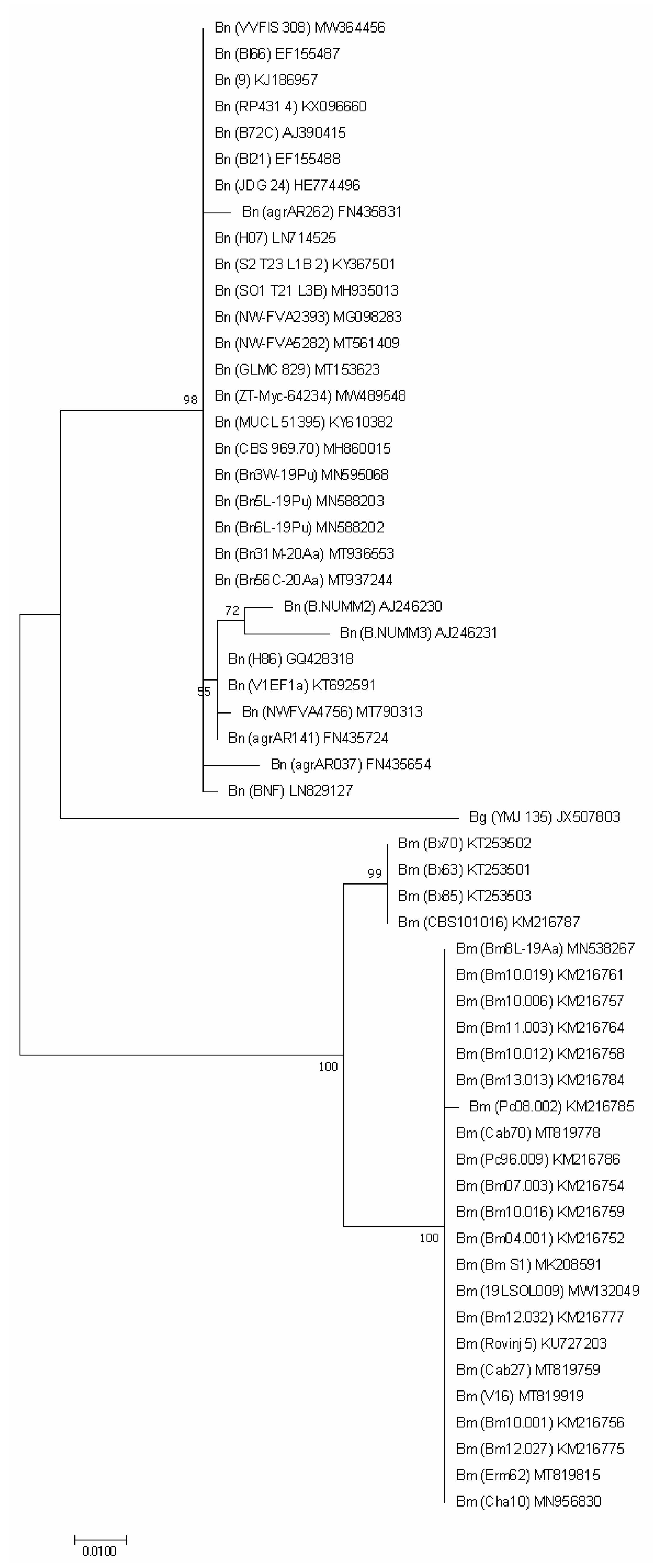
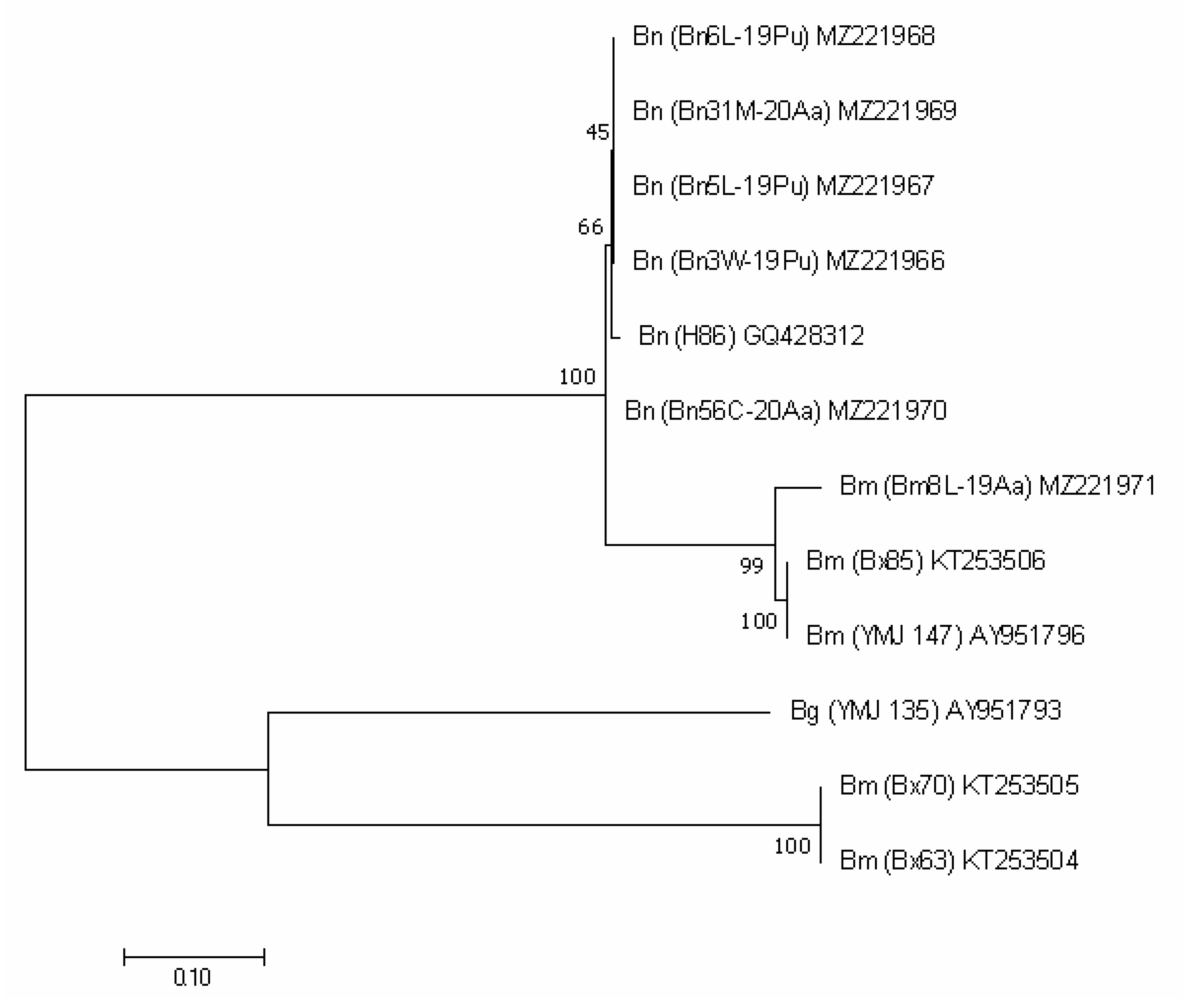
The genetic diversity between our sequences, as well as their comparison with other European sequences is presented in the phylogenetic tree (Figure 2) where the ITS sequences of B. mediterranea and B. nummularia form 2 separate clusters.
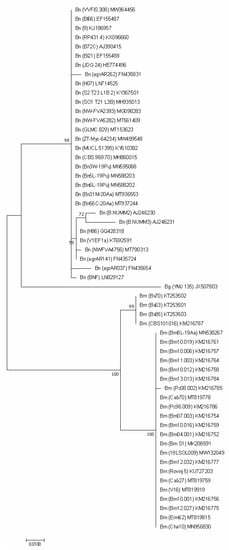
Figure 2.
ITS-based phylogenetic tree of B. mediterranea (Bm) and B. nummularia (Bn) isolates produced using MEGAX. Additional sequence from B. granmoi (Bg) were included for additional comparison. Details on isolates are described in Table 3.
Analysis of the tree shows two main groups of B. mediterranea with a bootstrap value of 99%: first with three samples derived from Q. pubescens in Italy and one from Q. robur in the Netherlands; and the second, with our isolate along with 21 additional including isolates originated from various Quercus species and other plants. This suggests that grouping was not affected by host species.
Our five B. nummularia isolates were grouped together with 17 other isolates of this species. The ITS sequences of our isolates obtained from gymnosperms were genetically identical to four (out of five analyzed) isolates from F. sylvatica. Two isolates from Italy, similar to the Italian B. mediterranea, formed a clearly distinct group. Three isolates from Germany were also grouped separately, while the remaining seven were included in the previously mentioned group of 17 isolates. Host species, like for B. mediterranea, had no effect on grouping.
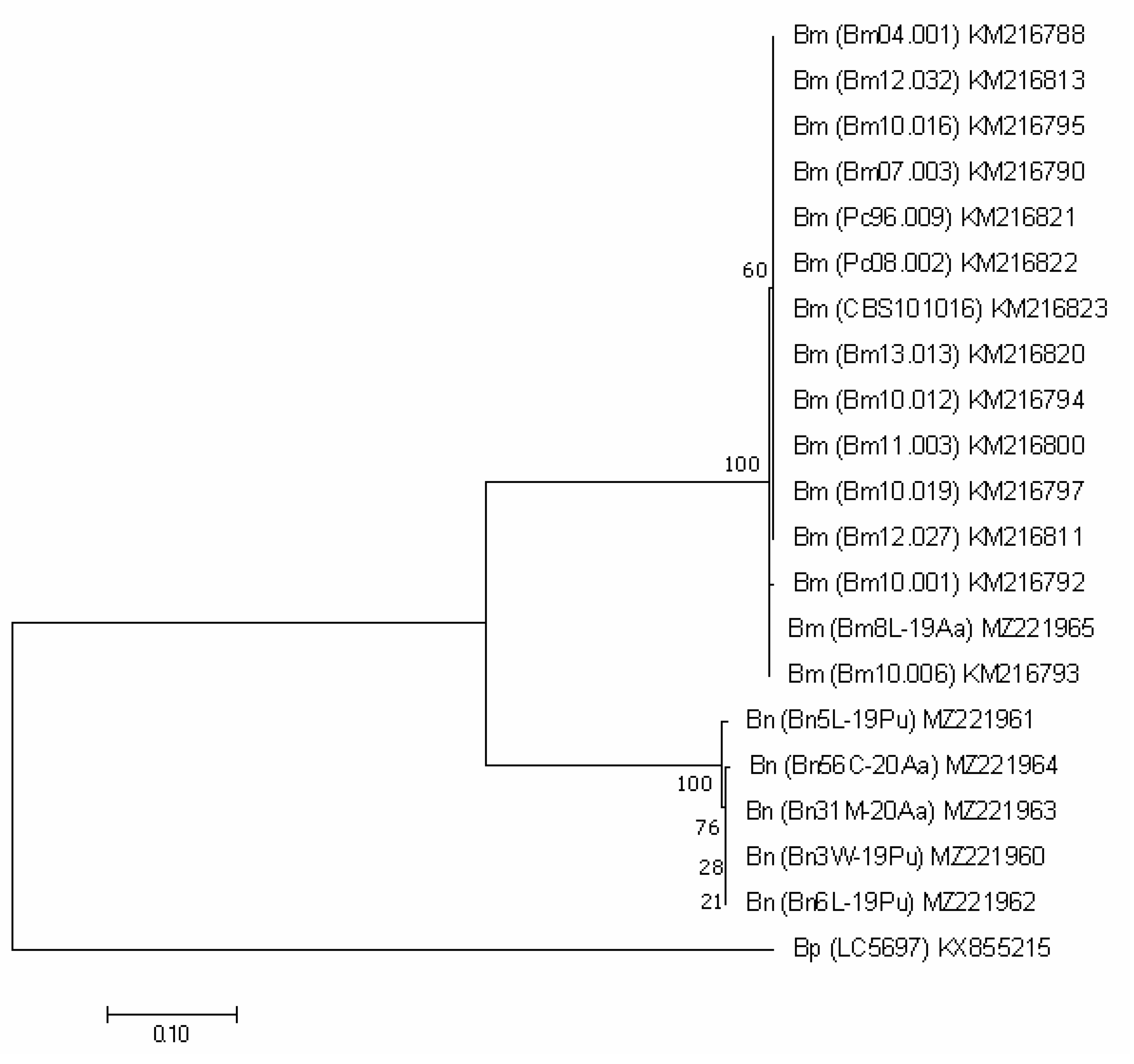
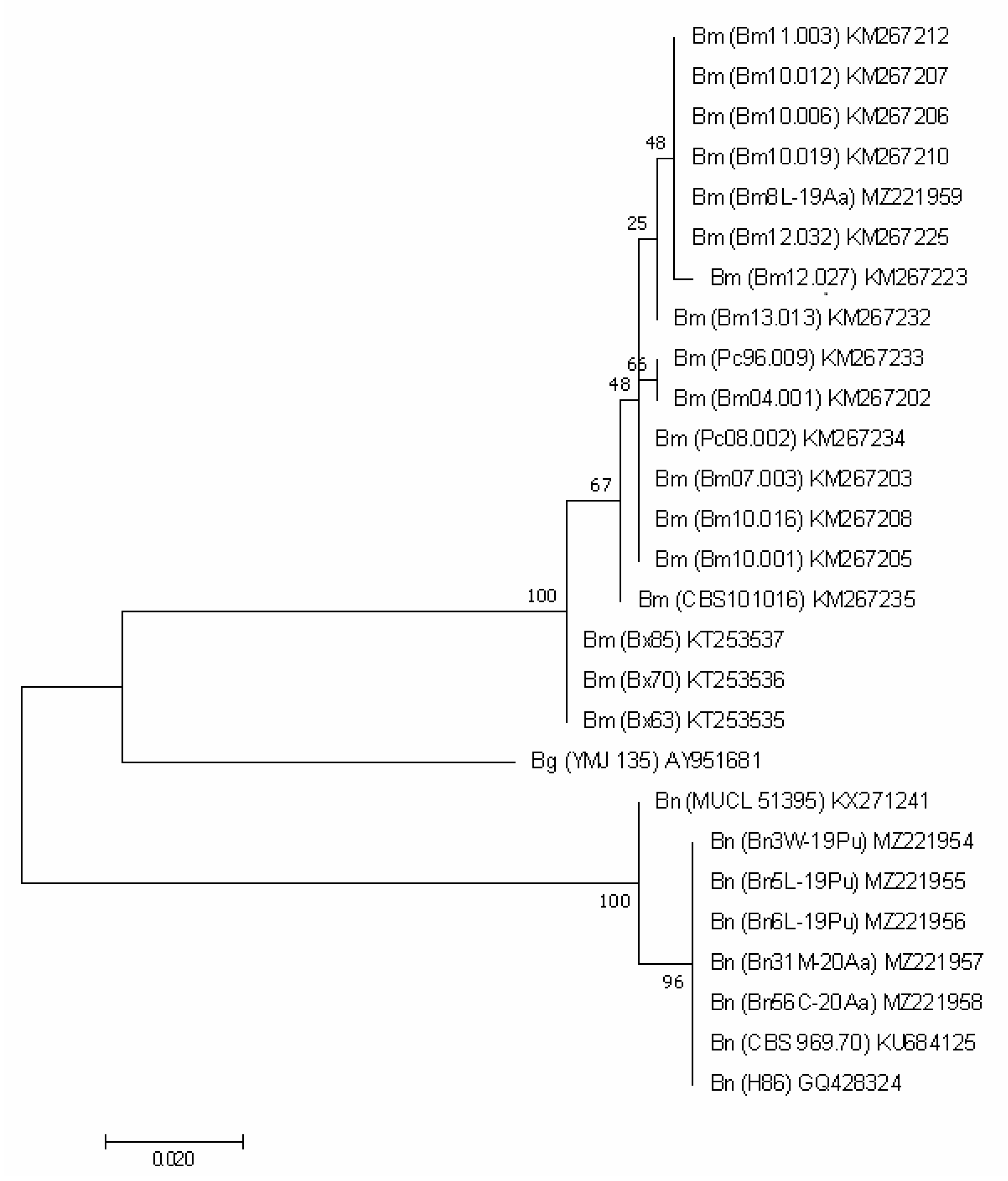
For the protein encoding genes (TEF1, TUB2 and ACT), the analysis imaged of individual phylogenetic trees (Figure 3, Figure 4 and Figure 5) were also effective in the distinction of B. mediterranea isolates from B. nummularia.
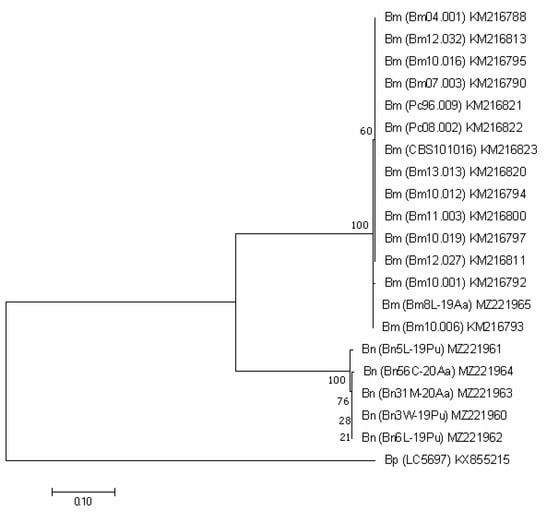
Figure 3.
TEF1-based phylogenetic tree of B. mediterranea (Bm) and B. nummularia (Bn) isolates produced using MEGAX. Additional sequence from B. petrensis (Bp) was included for additional comparison. Details on isolates are described in Table 3.
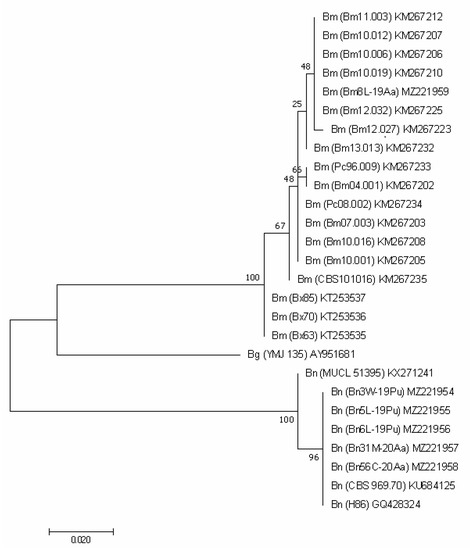
Figure 4.
TUB2-based phylogenetic tree of B. mediterranea (Bm) and B. nummularia (Bn) isolates produced using MEGAX. Additional sequence from B. granmoi (Bg) was included for additional comparison. Details on isolates are described in Table 3.
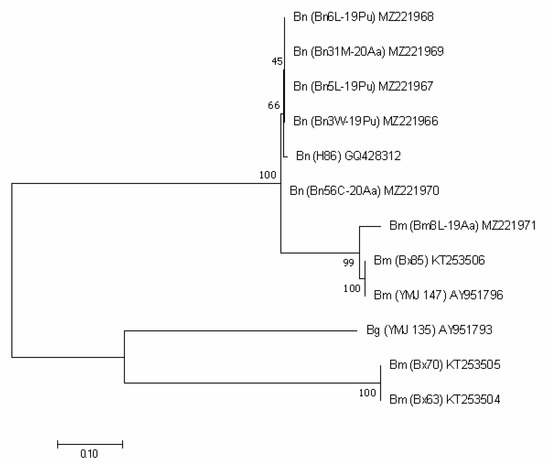
Figure 5.
ACT-based phylogenetic tree of B. mediterranea (Bm) and B. nummularia (Bn) isolates produced using MEGAX. Additional sequence from B. granmoi (Bg) was included for additional comparison. Details on isolates are described in Table 3.
There was much less reference material in GenBank for these sequences, especially for B. nummularia. As a result, comparative analysis for these sequences was sparse or impossible.
As for ITS regions, the comparative analysis of the TEF1 gene fragment with the GenBank database clearly indicated that Bm8L-19Aa is B. mediterranea. Our isolate had a sequence identical (percent identity = 100%) to non-European sequences, e.g., MG456736 or MT361330, and European ones (e.g., KM216793 or KM216820) differed by a maximum of three nucleotides, mostly showing a percent identity close to 99%.
Our B. nummularia TEF1 sequences are the only ones available on GenBank, so it has not been possible to confirm identification and compare them with other isolates. They differed slightly from each other (1 or 2 nucleotides), and Bn5W-19Pu had an additional insertion of 7 nucleotides. Only Bn6L-19Pu and Bn31M-20Aa were identical. This differentiation between our B. nummularia isolates is visible in the dendrogram, where sequences of two analyzed species were grouped into separate clusters (Figure 3).
Also, the analysis of the TUB2 sequence unambiguously qualified our Bm8L-19Aa as B. mediterranea, indicating its 100% identity with the Portuguese KM267210 and KM267225. It differed from other sequences deposited in GenBank in one, a few or a dozen nucleotides, which is reflected in the grouping shown in Figure 4. The sequences of the three Italian isolates (Bx85, Bx70 and Bx63), as well as their ITS regions, turned out to be clearly different (percent identity = 96.08%).
Our five B. nummularia isolates did not differ from each other. They were grouped together with the two isolates available in GenBank. Their sequences were identical with British KU684125 obtained from F. sylvatica and with GQ428324 (Figure 4).
In the sequence analysis of the ACT gene, the two species were also grouped into separate clusters. Bm8L-19Aa fell into one group with two B. mediterranea, however its variation from these sequences was significant, as was that from the remaining three sequences available from GenBank, being a separate subgroup (Figure 5).
Our four B. nummularia isolates differed by one nucleotide from Bn56C-20Aa. They were grouped together with the only available GenBank sequence GQ428312 and differed from it, respectively, by one and two nucleotides (percent identity > 99%).
4. Discussion
The common appearance of Biscogniauxia spp. in Poland is a topic worthy of attention and further observations. Following Ellis and Ellis [34], B. nummularia is considered rare in all of Europe, although current investigations show that this seems to be an outdated view [2,9,16,18,20,21]. Even so, in previous years this information seemed to be accurate in central European countries, e.g., Poland, where prior to 2020 [20,21] B. nummularia was recorded only three times [44,45]. We observe a very similar situation with B. mediterranea [17,22], which in the last five years was reported in central Europe for the first time in history and has now been observed twice. It seems that we are currently witnessing an increasing trend of Biscogniauxia spp. expansion into central Europe, which gives us a good chance to prepare an appropriate BTC monitoring system and to take quick action in case of local outbreaks of charcoal canker. The isolates of both species obtained in the research did not show significant genetic differences in relation to most other European isolates, which suggests the natural drift of the fungus into central Europe, instead of an isolated population. In addition, B. nummularia isolates obtained from gymnosperms have ITS and TUB2 sequences identical to those obtained from F. sylvatica in Germany, Switzerland and the UK (Table 3), which may suggest the separation of the northern populations of this fungus, which better tolerate colder, mid-European climates. It also seems that the northern isolates (from Poland and Germany) may occur on coniferous trees such as pines and firs, on which fungi can find a refugium in areas without potential hosts. It also suggests that gymnosperm plants may be a natural reservoir of the pathogen in the places where coniferous trees coexist with beeches. It should be expected that with progressive climate change, B. nummularia will become a serious pathogen of Fagus sp. and may lead to the emergence of local epidemics in central Europe. It will surely bring long-term changes in forest management, especially, as the research of Dulamsuren et al. [29] shows, as climate change significantly reduces the range of optimal conditions for beech growth in central Europe. Currently, beech is present throughout almost all of central Europe, with high frequency in the Polish part of the Carpathians, the Polish seashore of the Baltic Sea, in Slovakia, in the Alpine belt (Austria and Switzerland) and in southwest Germany [46]. Further warming and regular droughts may contribute to the population decline of beech in lowlands and lower mountain regions of central Europe, leaving mountain to upper mountain elevations as the last places suitable for beech woods [29]. Trees affected by drought could be an easy target for BTC [4,28], encouraging the pathogen’s growth. The potential decline of Fagus and slow disappearance of this species in central Europe would not only be a challenge for the forestry industry, but would also cause the massive extinction of local, treasured species which are tightly coupled to these ecosystems. Due to their uniqueness, plant communities belonging to beech forests are determinants of protected natural habitats requiring designation as Natura 2000 areas [47,48]. However, as some authors suggest [29], climate-change-related growth decline of Fagus is more widespread in the center of the species’ distribution range than previously thought, which is highly relevant for forestry planning. It seems that the expansion of Biscogniauxia may have an important role in these changes and should be regularly monitored as a potentially important threat to the central-European forestry industry and diversity. Research monitoring ongoing changes in forestry ecosystems should be implemented during the planning of long-term forestry industry projects in order to forecast future changes, possible economic consequences and as an integral part of sustainable forest management.
5. Conclusions
This article is the first notification of an increasing number of Biscogniauxia nummularia and Biscogniauxia mediterranea observations in central Europe and their consequences. Genetic analysis of the obtained strains did not show significant genetic differences in relation to most other European isolates, which suggests natural drift of the fungus into central Europe. Climate change, especially rising annual temperatures and regular droughts, are recognized as a direct cause of the increasing distribution range of both types of fungus. Furthermore, research indicates that coniferous trees, such as pine and fir, also act as a natural reservoir of the pathogens. It should be expected that with progressive climate change, B. nummularia will become a serious pathogen of Fagus sp. and may lead to the emergence of local epidemics in central Europe. This type of research should be used during the planning of long-term forestry industry projects and as an integral part of sustainable forest management.
Author Contributions
Conceptualization, K.P. and W.P.; methodology, K.P. and A.B.-C.; investigation, K.P., A.B.-C., W.P. and A.K.-P.; molecular analysis, A.B.-C.; writing—original draft preparation, K.P. and A.B.-C.; writing—review and editing, K.P., A.B.-C. and A.K.-P.; supervision, K.P. All authors have read and agreed to the published version of the manuscript.
Funding
The research was funded by the Forest Fund under the agreement between the State Forests National Forest Holding and the Stołowe Mountains National Park (agreement no. EZ.0290.1.13.2019, entitled “Assessment of Pinus × rhaetica health status on the Great Peat Bog of Batorów in the Stołowe Mountains National Park”), Tatra National Park (agreement no. EZ.0290.1.18.2019, entitled “Assessment of the Herpotrichia needle browning of silver fir occurrence in the Tatra National Park”) and Karkonosze National Park (agreement no. EZ.0290.1.8.2020, entitled “Assessment of silver fir health status in the Karkonosze National Park”).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data sharing not applicable.
Acknowledgments
The authors would like to thank the Forest Fund for funding and financial support. The authors would like to express their gratitude to S. Cano Navarro for assistance with statistical analysis.
Conflicts of Interest
The authors declare that they have no conflict of interest.
Ethics
The samples in the study were collected on the area that belonged to the Stołowe Mountains National Park, Tatra National Park and Karkonosze National Park, and the authors received full permission to conduct the study. The experimental materials did not involve any humans or animals.
References
- Ragazzi, A.; Dellavalle Fedi, I.; Mesturino, L. The oak decline: A new problem in Italy. Eur. J. For. Pathol. 1989, 19, 105–110. [Google Scholar] [CrossRef]
- Granata, G.; Sidoti, A. Biscogniauxia nummularia: Pathogenic agent of a beech decline. For. Pathol. 2004, 34, 363–367. [Google Scholar] [CrossRef]
- Nugent, L.K.; Sihanonth, P.; Thienhirun, S.; Whalley, A.J.S. Biscogniauxia: A genus of latent in-vaders. Mycologist 2005, 19, 40–43. [Google Scholar] [CrossRef]
- Luchi, N.; Capretti, P.; Feducci, M.; Vannini, A.; Ceccarelli, B.; Vettraino, A.M. Latent infection of Biscogniauxia nummularia in Fagus sylvatica: A possible bioindicator of beech health conditions. iForest 2015, 9, 49–54. [Google Scholar] [CrossRef] [Green Version]
- Henriques, J.; Nóbrega, F.; Sousa, E.; Lima, A. Morphological and genetic diversity of Biscogniauxia mediterranea associated to Quercus suber in the Mediterranean Basin. Rev. Ciênc. Agrár. 2015, 38, 166–175. Available online: http://hdl.handle.net/10400.5/10652 (accessed on 29 November 2021).
- Yangui, I.; Zouaoui Boutiti, M.; Vettraino, A.M.; Bruni, N.; Vannini, A.; Ben Jamaâ, M.L.; Boussaid, M.; Messaoud, C. Biscogniauxia mediterranea associated with cork oak (Quercus suber) in Tunisia: Relationships between phenotypic variation, genetic diversity and ecological factors. Fungal Ecol. 2019, 41, 224–233. [Google Scholar] [CrossRef]
- Luchi, N.; Capretti, P.; Vettraino, A.M.; Vannini, A.; Pinzani, P.; Pazzagli, M. Early detection of Biscogniauxia nummularia in symptomless European beech (Fagus sylvatica L.) by TaqMan™real-time PCR. Lett. Appl. Microbiol. 2006, 43, 33–38. [Google Scholar] [CrossRef] [PubMed]
- La Porta, N.; Capretti, P.; Thomsen, I.M.; Kasanen, R.; Hietala, A.M.; Von Weissenberg, K. Forest pathogens with higher damage potential due to climate change in Europe. Can. J. Plant Pathol. 2008, 30, 177–195. [Google Scholar] [CrossRef]
- Zabalgogeazcoa, I.; Pedro, J.; Canals, R.M. Biscogniauxia nummularia infecting beech (Fagus sylvatica) trees and sympatric plants of the sedge Carex brevicollis. For. Pathol. 2015, 45, 346–348. [Google Scholar] [CrossRef]
- Paoletti, E.; Goggioli, V.; Maresi, G. Reperti diB. nummularia su faggio in Italia. [Biscogniauxia nummularia on beech in Italy]. Micol. Ital. 1996, 1, 27–35. (In Italian) [Google Scholar]
- Moriondo, F.; Mugnai, L. A case of group dying of beech in Italy. In Proceedings of the 8th Congress of the Mediterranean Phytopathological Union, Agadir, Morocco, 28 October–3 November 1990; pp. 121–122. [Google Scholar]
- Granata, G.; Whalley, A.J.S. Decline of beech associated to Biscogniauxia nummularia in Italy. Petria 1994, 4, 111–116. [Google Scholar]
- Petrini, L.; Petrini, O. Xylariaceous fungi as endophytes. Sydowia 1985, 38, 216–234. [Google Scholar]
- Sieber, T.N. Endophytic fungi in forest trees: Are they mutualists? Fungal Biol. Rev. 2007, 21, 75–89. [Google Scholar] [CrossRef]
- Osono, T.; Tateno, O.; Masuya, H. Diversity and ubiquity of xylariaceous endophytes in live and dead leaves of temperate forest trees. Mycoscience 2013, 54, 54–61. [Google Scholar] [CrossRef] [Green Version]
- Lakatos, F.; Molnár, M. Mass Mortality of Beech (Fagus sylvatica L.) in South-West Hungary. Acta Silv. Lign. Hung. 2009, 5, 75–82. [Google Scholar]
- Zíbarová, L.; Kout, J. Xylariaceous pyrenomycetes from Bohemia: Species of Biscogniauxia and Hypoxylon new to the Czech Republic, and notes on other rare species. Czech. Mycol. 2017, 69, 77–108. [Google Scholar] [CrossRef]
- Vujanovic, V.; Kim, S.H.; Latinovic, J.; Latinovic, N. Natural Fungicolous Regulators of Biscogniauxia destructiva sp. nov. That Causes Beech Bark Tarcrust in Southern European (Fagus sylvatica) Forests. Microorganisms 2020, 8, 1999. [Google Scholar] [CrossRef]
- Ju, Y.M.; Rogers, J.D.; San Martín, F.; Granmo, A. The genus Biscogniauxia. Mycotaxon 1998, 66, 1–98. [Google Scholar]
- Patejuk, K.; Baturo-Cieśniewska, A.; Kaczmarek-Pieńczewska, A.; Pusz, W. Mycobiota of peat-bog pine (Pinus × rhaetica) needles in the Stołowe Mountains National Park, Poland. Nova Hedwigia 2021, 112, 253–265. [Google Scholar] [CrossRef]
- Pusz, W.; Kaczmarek, A.; Baturo-Cieśniewska, A. Ocena zdrowotności jodły w Karkonoskim Parku Narodowym. Annual Report of Project Funded by Forest Fund; Karkonoski National Park: Jelenia Góra, Poland, 2020. [Google Scholar]
- Pusz, W.; Baturo-Cieśniewska, A.; Kaczmarek-Pieńczewska, A.; Zwijacz-Kozica, T.; Patejuk, K. The mycobiota of needles and shoots of silver fir (Abies alba Mill.) with symptoms of Herpotrichia needle browning in the Tatra Mts. (Poland). Ann. For. Res. 2020, 63, 45–56. [Google Scholar] [CrossRef]
- Vannini, A.; Mazzaglia, A.; Anselmi, N. Use of random amplified polymorphic DNA (RAPD) for detection of genetic variation and proof of the heterothallic mating system in Hypoxylon mediterraneum. Eur. J. Plant Pathol. 1999, 29, 209–218. [Google Scholar] [CrossRef]
- Eaton, E.; Caudullo, G.; Oliveira, S.; de Rigo, D. Quercus robur and Quercus petraea in Europe: Distribution, habitat, usage and threats. In European Atlas of Forest Tree Species; San-Miguel-Ayanz, J., de Rigo, D., Caudullo, G., Houston Durrant, T., Mauri, A., Eds.; Publications Office of the European Union: Luxembourg, 2016; p. e01c6df+. [Google Scholar]
- Ragazzi, A.; Ginetti, B.; Moricca, S. First report of Biscogniauxia mediterranea on English ash in Italy. Plant Dis. 2012, 96, 1694. [Google Scholar] [CrossRef]
- IPCC. Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK, 2013. [Google Scholar]
- Lindner, M.; Fitzgerald, J.B.; Zimmermann, N.E.; Reyer, C.; Delzon, S.; van der Maaten, E.; Schelhaas, M.-J.; Lasch, P.; Eggers, J.; van der Maaten-Theunissen, M.; et al. Climate change and European forests: What do we know, what are the uncertainties, and what are theimplications for forest management? J. Environ. Manag. 2014, 146, 69–83. [Google Scholar] [CrossRef] [Green Version]
- Hendry, S.J.; Boddy, L.; Lonsdale, D. Abiotic variables effect differentialexpression of latent infections in beech (Fagus sylvatica). New Phytol. 2002, 155, 449–460. [Google Scholar] [CrossRef] [PubMed]
- Dulamsuren, C.; Hauck, M.; Kopp, G.; Ruff, M.; Leuschner, C. European beech responds to climate change with growth decline at lower, and growth increase at higher elevations in the center of its distribution range (SW Germany). Trees 2016, 31, 673–686. [Google Scholar] [CrossRef]
- Zang, C.; Hartl-Meier, C.; Dittmar, C.; Rothe, A.; Menzel, A. Patterns of drought tolerance in major European temperate foresttrees: Climatic drivers and levels of variability. Glob. Chang. Biol. 2014, 20, 3767–3779. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, J.; Hauck, M.; Dulamsuren, C.; Leuschner, C. Climate warming-related growth decline affectsFagus sylvatica, but not other broad-leaved tree species in Central Europeanmixed forests. Ecosystems 2015, 18, 560–572. [Google Scholar] [CrossRef]
- Pitt, J.I.; Hocking, A.D. Fungi and Food Spoilage; Springer: New York, NY, USA, 2009; 520p. [Google Scholar] [CrossRef]
- Watanabe, T. Pictorial Atlas of Soil and Seed Fungi: Morphologies of Cultured Fungi and Key to Species, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2010; 426p. [Google Scholar] [CrossRef]
- Ellis, M.B.; Ellis, J.P. Microfungi on Land Plants an Identification Handbook; Richmond Publishing: Slough, UK, 1997. [Google Scholar]
- Doyle, J.J.; Doyle, J.L. Isolation of plant DNA from fresh tissue. Focus 1990, 12, 13–15. [Google Scholar]
- Baturo-Cieśniewska, A.; Pusz, W.; Patejuk, K. Problems, limitations, and challenges in species identification of Ascomycota members on the basis of ITS regions. Acta Mycol. 2020, 55, 5512. [Google Scholar] [CrossRef]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols: A Guide to Methods and Applications; Innis, M.A., Gelfand, D.H., Sninsky, J.J., White, T.J., Eds.; Academic Press: San Diego, CA, USA, 1990; pp. 315–322. [Google Scholar] [CrossRef]
- Carbone, I.; Kohn, L.M. A method for designing sets for speciation studies in filamentous ascomycetes. Mycologia 1999, 91, 553–556. [Google Scholar] [CrossRef]
- Glass, N.L.; Donaldson, G.C. Development of primer sets designed for use with the PCR to amplify conserved genes from filamentous ascomycetes. Appl. Environ. Microbiol. 1995, 61, 1323–1330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- BLAST Database. Available online: https://blast.ncbi.nlm.nih.gov/Blast.cgi?PROGRAM=blastn&PAGE_TYPE=BlastSearch&LINK_LOC=blasthome (accessed on 11 August 2021).
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef] [PubMed]
- Kimura, M. A simple method for estimating evolutionary rate of base substitutions through comparative studies of nucleotide sequences. J. Mol. Evol. 1980, 16, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Institute of Meteorology and Water Management–National Research Institute. Available online: https://danepubliczne.imgw.pl/ (accessed on 22 December 2021).
- Chlebicki, A. Some overlooked and rare Xylariaceous fungi from Poland. Polish Bot. J. 2008, 53, 71–80. [Google Scholar]
- Mułenko, W.; Majewski, T.; Ruszkiewicz-Michalska, M. A preliminary checklist of micromycetes in Poland. In Szafer Institute of Botany; Polish Academy of Sciences: Kraków, Poland, 2008. [Google Scholar]
- Houston Durrant, T.; de Rigo, D.; Caudullo, G. Fagus sylvatica and other beeches in Europe: Distribution, habitat, usage and threats. In European Atlas of Forest Tree Species; San-Miguel-Ayanz, J., de Rigo, D., Caudullo, G., Houston Durrant, T., Mauri, A., Eds.; Publications Office of the European Union: Luxembourg, 2016; p. e012b90+. [Google Scholar]
- Herbich, J. (Ed.) Lasy i Bory. Poradniki Ochrony Siedlisk i Gatunków Natura 2000—Podręcznik Metodyczny; Ministerstwo Środowiska: Warszawa, Poland, 2004; Volume 5, p. 344. [Google Scholar]
- European Commission DG Environment. Interpretation manual of European Union habitats—EUR 28. Nature ENV B.3. 2013. Available online: https://ec.europa.eu/environment/nature/legislation/habitatsdirective/docs/Int_Manual_EU28.pdf (accessed on 5 October 2021).
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).