Fungal Perspective of Pine and Oak Colonization in Mediterranean Degraded Ecosystems
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Sites and Design
2.2. Soil Sampling
2.3. Fungal Community Analyses
2.4. Bioinformatic Analysis
2.5. Taxonomic and Functional Identification
2.6. Statistical Analyses
3. Results
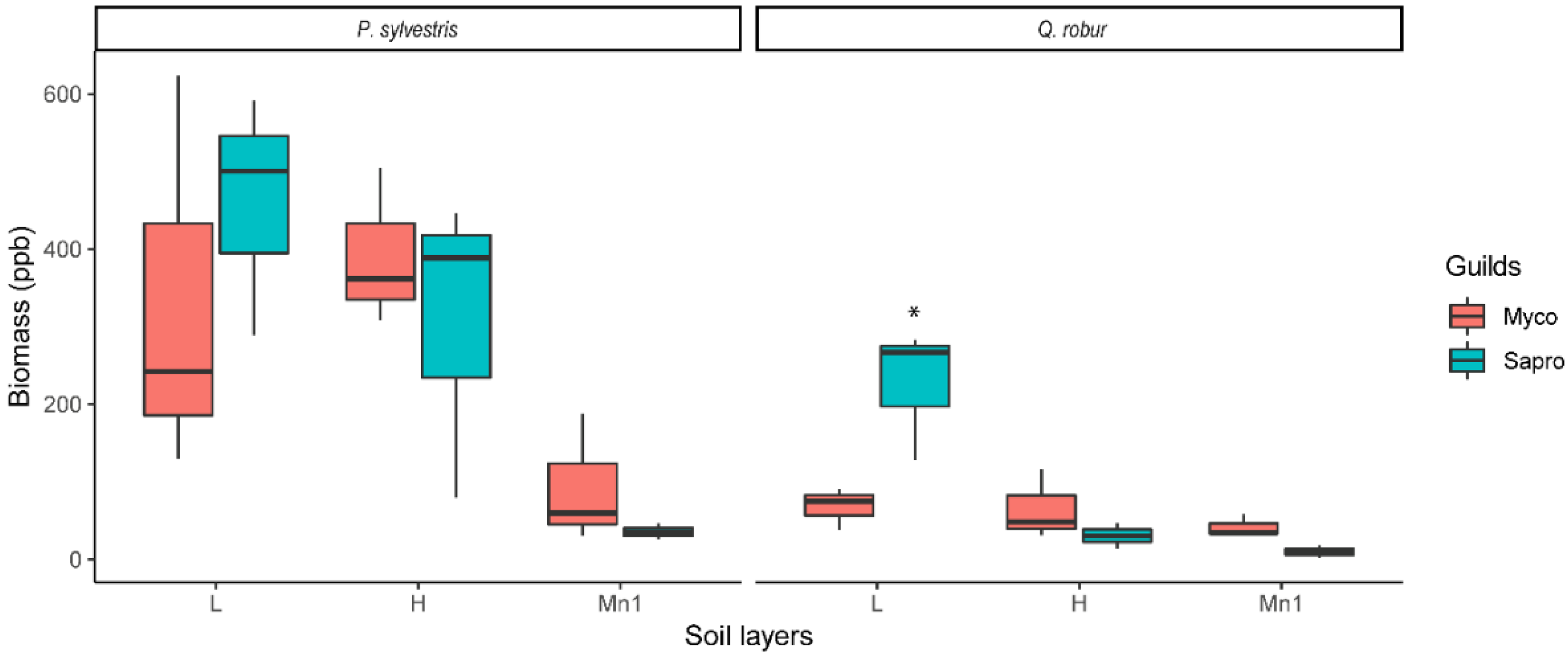
3.1. Soil Fungi Differences between Pine and Oak Stands (Forest Experiment)
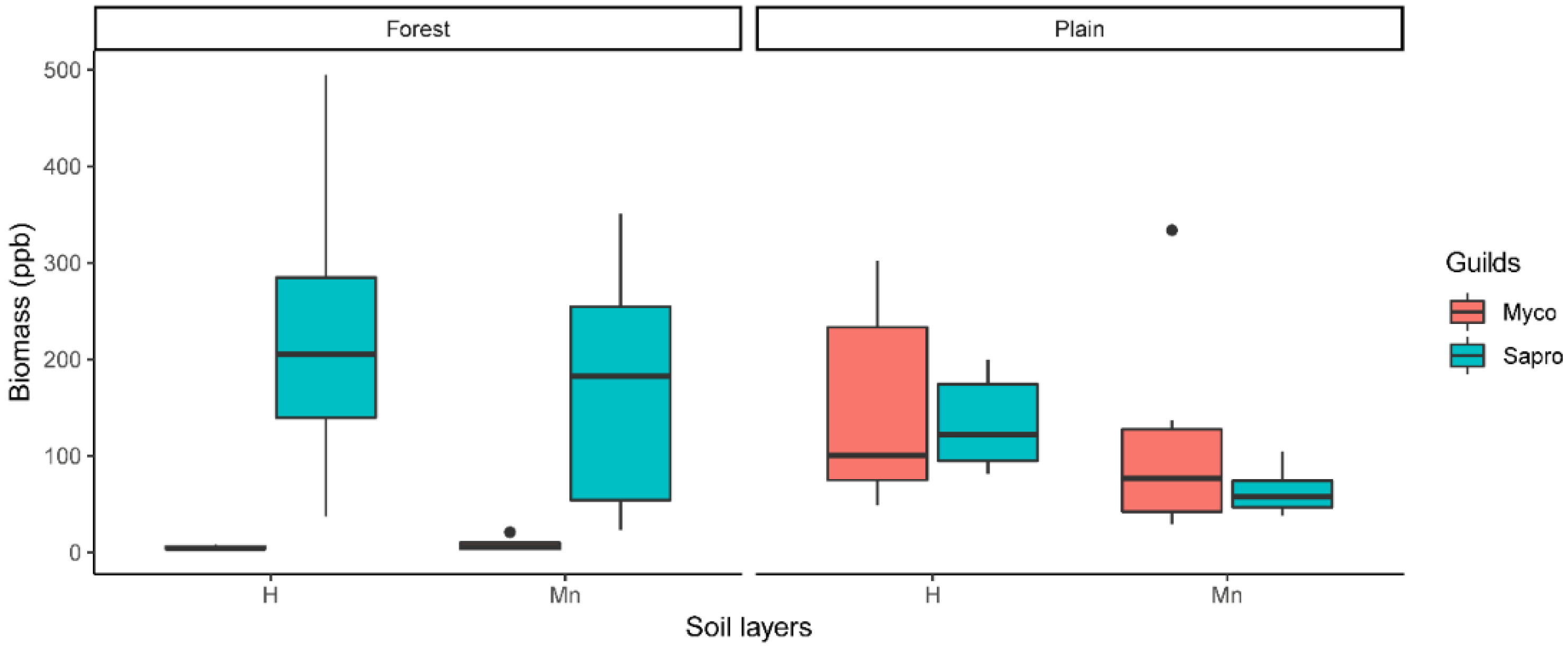
3.2. Soil Fungi Differences between Q. ilex and Revegetated Quarry (Mine Experiment)
4. Discussion
4.1. Soil Fungi Differences between Pine and Oak Stands (Forest Study)
4.2. Soil Fungi Differences between Q. ilex and Revegetated Quarry (Mine Study)
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Watson, J.E.M.; Evans, T.; Venter, O.; Williams, B.; Tulloch, A.; Stewart, C.; Thompson, I.; Ray, J.C.; Murray, K.; Salazar, A.; et al. The exceptional value of intact forest ecosystems. Nat. Ecol. Evol. 2018, 2, 599–610. [Google Scholar] [CrossRef] [PubMed]
- Paustian, K.; Lehmann, J.; Ogle, S.; Reay, D.; Robertson, G.P.; Smith, P. Climate-smart soils. Nat. Cell Biol. 2016, 532, 49–57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, S.; MacKenzie, R.A.; Tieng, T.; Soben, K.; Tulyasuwan, N.; Resanond, A.; Blate, G.; Litton, C.M. The impacts of degradation, deforestation and restoration on mangrove ecosystem carbon stocks across Cambodia. Sci. Total. Environ. 2020, 706, 135416. [Google Scholar] [CrossRef] [PubMed]
- Alday, J.G.; Zaldívar, P.; Torroba-Balmori, P.; Fernández-Santos, B.; Martínez-Ruiz, C. Natural forest expansion on re-claimed coal mines in Northern Spain: The role of native shrubs as suitable microsites. Environ. Sci. Pollut. Res. 2016, 23, 13606–13616. [Google Scholar] [CrossRef]
- Brooker, R.W.; Maestre, F.T.; Callaway, R.M.; Lortie, C.L.; Cavieres, L.A.; Kunstler, G.; Liancourt, P.; Tielborger, K.; Travis, J.M.J.; Anthel-me, F.; et al. Facilitation in plant communities: The past, the present, and the future. J. Ecol. 2008, 96, 18–34. [Google Scholar] [CrossRef] [Green Version]
- Onaindia, M.; Ametzaga, I.; San Sebastián, M.; Mitxelena, A.; Rodríguez-Loinaz, G.; Peña, L.; Alday, J.G. Can understorey na-tive woodland plant species regenerate under exotic pine plantations using natural succession? For. Ecol. Man-Agement 2013, 308, 136–144. [Google Scholar] [CrossRef]
- van der Putten, W.H.; Bardgett, R.D.; Bever, J.D.; Martijn Bezemer, T.; Casper, B.B.; Fukami, T.; Kardol, P.; Klironomos, J.N.; Kulmatiski, A.; Schweitzer, J.A.; et al. Plant-soil feed-backs: The past, the present and future challenges. J. Ecol. 2013, 101, 265–276. [Google Scholar] [CrossRef]
- Phillips, R.P.; Brzostek, E.; Midgley, M.G. The mycorrhizal-associated nutrient economy: A new framework for predicting carbon–nutrient couplings in temperate forests. New Phytol. 2013, 199, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Bardgett, R.D.; van der Putten, W. Belowground biodiversity and ecosystem functioning. Nat. Cell Biol. 2014, 515, 505–511. [Google Scholar] [CrossRef]
- Tedersoo, L.; Bahram, M.; Põlme, S.; Kõljalg, U.; Yorou, N.S.; Wijesundera, R.L.C.; Ruiz, L.V.; Vasco-Palacios, A.M.; Thu, P.Q.; Suija, A.; et al. Global diversity and geography of soil fungi. Science 2014, 346, 1256688. [Google Scholar] [CrossRef] [Green Version]
- Adamo, I.; Piñuela, Y.; Bonet, J.A.; Castaño, C.; de Aragón, J.M.; Parladé, J.; Pera, J.; Alday, J.G. Sampling forest soils to describe fungal diversity and composition. Which is the optimal sampling size in mediterranean pure and mixed pine oak forests? Fungal Biol. 2021, 125, 469–476. [Google Scholar] [CrossRef]
- Urbina, I.; Grau, O.; Sardans, J.; Ninot, J.M.; Peñuelas, J. Encroachment of shrubs into subalpine grasslands in the Pyrenees changes the plant-soil stoichiometry spectrum. Plant Soil 2020, 448, 37–53. [Google Scholar] [CrossRef]
- Lourenço, K.S.; Suleiman, A.K.A.; Pijl, A.; Cantarella, H.; Kuramae, E.E. Dynamics and resilience of soil myco-biome under multiple organic and inorganic pulse disturbances. Sci. Total Environ. 2020, 733, 139173. [Google Scholar] [CrossRef]
- Rodriguez-Ramos, J.C.; Cale, J.A.; Cahill Jr, J.F.; Simard, S.W.; Karst, J.; Erbilgin, N. Changes in soil fungal community composition depend on functional group and forest disturbance type. New Phytol. 2021, 229, 1105–1117. [Google Scholar] [CrossRef] [PubMed]
- Barnes, A.D.; Allen, K.; Kreft, H.; Corre, M.D.; Jochum, M.; Veldkamp, E.; Clough, Y.; Daniel, R.; Darras, K.; Denmead, L.H.; et al. Direct and cascading impacts of tropi-cal land-use change on multi-trophic biodiversity. Nat. Ecol. Evol. 2017, 1, 1511–1519. [Google Scholar] [CrossRef]
- Alday, J.G.; Marrs, R.H.; Martínez-Ruiz, C. Vegetation succession on reclaimed coal wastes in Spain: The influence of soil and environmental factors. Appl. Veg. Sci. 2010, 14, 84–94. [Google Scholar] [CrossRef]
- Alday, J.G.; Marrs, R.H.; Martínez-Ruiz, C. Vegetation convergence during early succession on coal wastes: A 6-year permanent plot study. J. Veg. Sci. 2011, 22, 1072–1083. [Google Scholar] [CrossRef]
- Zhou, L.; Li, H.; Shen, H.; Xu, Y.; Wang, Y.; Xing, A.; Fang, J. Shrub-encroachment induced alterations in input chemistry and soil microbial community affect topsoil organic carbon in an Inner Mongolian grassland. Biogeochemistry 2017, 136, 311–324. [Google Scholar] [CrossRef]
- Qiang, W.; He, L.; Zhang, Y.; Liu, B.; Liu, Y.; Liu, Q.; Pang, X. Aboveground vegetation and soil physicochemical properties jointly drive the shift of soil microbial community during subalpine secondary succession in southwest China. Catena 2021, 202, 105251. [Google Scholar] [CrossRef]
- Krüger, C.; Kohout, P.; Janoušková, M.; Püschel, D.; Frouz, J.; Rydlová, J. Plant communities rather than soil prop-erties structure arbuscular mycorrhizal fungal communities along primary succession on a mine spoil. Front. Micro-Biol. 2017, 8, 719. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kolaříková, Z.; Kohout, P.; Krüger, C.; Janoušková, M.; Mrnka, L.; Rydlová, J. Root-associated fungal communities along a primary succession on a mine spoil: Distinct ecological guilds assemble differently. Soil Biol. Biochem. 2017, 113, 143–152. [Google Scholar] [CrossRef]
- Gil-Martínez, M.; López-García, Á.; Dominguez, M.T.; Navarro-Fernández, C.M.; Kjøller, R.; Tibbett, M.; Marañón, T. Ectomycorrhizal Fungal Communities and Their Functional Traits Mediate Plant–Soil Interactions in Trace Element Contaminated Soils. Front. Plant Sci. 2018, 9, 1682. [Google Scholar] [CrossRef]
- Berendse, F.; Jonasson, S. Nutrient Use and Nutrient Cycling in Northern Ecosystems. In Arctic Ecosystems in a Changing Climate; Elsevier: Alpharetta, GA, USA, 1992; pp. 337–356. [Google Scholar]
- Tkacz, A.; Cheema, J.; Chandra, G.; Grant, A.; Poole, P.S. Stability and succession of the rhizosphere microbiota depends upon plant type and soil composition. ISME J. 2015, 9, 2349–2359. [Google Scholar] [CrossRef]
- Bogar, L.; Peay, K.; Kornfeld, A.; Huggins, J.; Hortal, S.; Anderson, I.; Kennedy, P. Plant-mediated partner discrim-ination in ectomycorrhizal mutualisms. Mycorrhiza 2019, 29, 97–111. [Google Scholar] [CrossRef]
- Blažková, A.; Jansa, J.; Püschel, D.; Vosátka, M.; Janoušková, M. Is mycorrhiza functioning influenced by the quan-titative composition of the mycorrhizal fungal community? Soil Biol. Biochem. 2021, 157, 108249. [Google Scholar] [CrossRef]
- Werner, G.D.A.; Zhou, Y.; Pieterse, C.M.J.; Kiers, E.T. Tracking plant preference for higher-quality mycorrhizal sym-bionts under varying CO2 conditions over multiple generations. Ecol. Evol. 2018, 8, 78–87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quinto, L.; Navarro-Cerrillo, R.M.; Palacios-Rodriguez, G.; Ruiz-Gómez, F.; Duque-Lazo, J. The current situation and future perspectives of Quercus ilex and Pinus halepensis afforestation on agricultural land in Spain under climate change scenarios. New For. 2021, 52, 145–166. [Google Scholar] [CrossRef]
- Fernandez, C.W.; See, C.R.; Kennedy, P.G. Decelerated carbon cycling by ectomycorrhizal fungi is controlled by substrate quality and community composition. New Phytol. 2020, 226, 569–582. [Google Scholar] [CrossRef] [PubMed]
- Teste, F.P.; Kardol, P.; Truner, B.L.; Wardle, D.A.; Zemunik, G.; Renton, M.; Laliberté, E. Plant-soil feedback and the maintainence of diversity in Mediterranean-climate shrublands. Science 2017, 355, 173–176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Segnitz, R.M.; Russo, S.E.; Davies, S.J.; Peay, K.G. Ectomycorrhizal fungi drive positive phylogenetic plant–soil feedbacks in a regionally dominant tropical plant family. Ecology 2020, 101, e03083. [Google Scholar] [CrossRef]
- Hagenbo, A.; Piñuela, Y.; Castaño, C.; de Aragón, J.M.; de-Miguel, S.; Alday, J.G.; Bonet, J.A. Production and turnover of mycorrhizal soil mycelium relate to variation in drought conditions in Mediterranean Pinus pinaster, Pinus sylvestris and Quercus ilex forests. New Phytol. 2021, 230, 1609–1622. [Google Scholar] [CrossRef] [PubMed]
- Smith, G.R.; Peay, K.G. Stepping forward from relevance in mycorrhizal ecology. New Phytol. 2020, 226, 292–294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alday, J.G.; De Aragón, J.M.; de-Miguel, S.; Bonet, J.A. Mushroom biomass and diversity are driven by different spatio-temporal scales along Mediterranean elevation gradients. Sci. Rep. 2017, 7, 1–11. [Google Scholar]
- Ihrmark, K.; Bödeker, I.T.; Cruz-Martinez, K.; Friberg, H.; Kubartova, A.; Schenck, J.; Strid, Y.; Stenlid, J.; Brandström-Durling, M.; Clemmensen, K.; et al. New primers to amplify the fungal ITS2 region—Evaluation by 454-sequencing of artificial and natural communities. FEMS Microbiol. Ecol. 2012, 82, 666–677. [Google Scholar] [CrossRef] [PubMed]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. PCR Protoc. Guide Methods Appl. 1990, 18, 315–322. [Google Scholar]
- Edgar, R.C. Search and clustering orders of magnitude faster than BLAST. Bioinformatics 2010, 26, 2460–2461. [Google Scholar] [CrossRef] [Green Version]
- Kõljalg, U.; Nilsson, R.H.; Abarenkov, K.; Tedersoo, L.; Taylor, A.F.; Bahram, M.; Larsson, K.H. Towards a uni-fied paradigm for sequence-based identification of fungi. Wiley Online Libr. 2013, 22, 5271–5277. [Google Scholar]
- Somervuo, P.; Koskela, S.; Pennanen, J.; Nilsson, R.H.; Ovaskainen, O. Unbiased probabilistic taxonomic classification for DNA barcoding. Bioinformatics 2016, 32, 2920–2927. [Google Scholar] [CrossRef] [Green Version]
- Abarenkov, K.; Nilsson, R.H.; Larsson, K.-H.; Alexander, I.J.; Eberhardt, U.; Erland, S.; Høiland, K.; Kjøller, R.; Larsson, E.; Pennanen, T.; et al. The UNITE database for molecular identification of fungi–recent updates and future perspectives. New Phytol. 2010, 186, 281–285. [Google Scholar] [CrossRef]
- Nguyen, N.H.; Song, Z.; Bates, S.T.; Branco, S.; Tedersoo, L.; Menke, J.; Schilling, J.S.; Kennedy, P.G. FUNGuild: An open annotation tool for parsing fungal community datasets by ecological guild. Fungal Ecol. 2016, 20, 241–248. [Google Scholar] [CrossRef]
- Wickham, H. Ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016; ISBN 978-3-319-24277-4. [Google Scholar]
- Pinheiro, J.C.; Bates, D.M.; DebRoy, S.; Sakar, D. The Nlme Package: Linear and nonlinear mixed efects models, R Version 3. 2012. Available online: https://svn.r-project/R-packages/trunk/nlme. (accessed on 7 September 2021).
- Lenth, R. Estimated Marginal Means, Aka Least-Squares Means, 1.4.8; R Package. Available online: https://CRAN.R-project.org/package=emmeans (accessed on 5 June 2021).
- Castaño, C.; Alday, J.G.; Lindahl, B.D.; de Aragón, J.M.; de-Miguel, S.; Colinas, C.; Bonet, J.A. Lack of thinning effects over inter-annual changes in soil fungal community and diversity in a Mediterranean pine forest. For. Ecol. Manag. 2018, 424, 420–427. [Google Scholar] [CrossRef]
- Querejeta, J.I.; Roldán, A.; Albaladejo, J.; Castillo, V. The role of mycorrhizae, site preparation, and organic amendment in the afforestation of a semi-arid Mediterranean site with Pinus halepensis. For. Sci. 1998, 44, 203–211. [Google Scholar]
- Fernandez, C.W.; Kennedy, P.G. Revisiting the ‘Gadgil effect’: Do interguild fungal interactions control carbon cycling in forest soils? New Phytol. 2016, 209, 1382–1394. [Google Scholar] [CrossRef]
- Shah, F.; Nicolás, C.; Bentzer, J.; Ellström, M.; Smits, M.; Rineau, F.; Canbäck, B.; Floudas, D.; Carleer, R.; Lackner, G. Ectomycorrhizal fungi decompose soil organic matter using oxidative mechanisms adapted from saprotrophic ancestors. New Phytol. 2016, 209, 1705–1719. [Google Scholar] [CrossRef]
- Nicolás, C.; Martin-Bertelsen, T.; Floudas, D.; Bentzer, J.; Smits, M.; Johansonn, T.; Troein, C.; Persson, P.; Tulind, A. The soil organic matter decomposition mechanisms in ectomycorrhizal fungi are tuned for liberating soil organic nitrogen. ISME J. 2019, 13, 977–988. [Google Scholar] [CrossRef] [Green Version]
- Berg, B.; Meentemeyer, V. Litter quality in a north European transect versus carbon storage potential. Plant Soil 2002, 242, 83–92. [Google Scholar] [CrossRef]
- Voříšková, J.; Brabcová, V.; Cajthaml, T.; Baldrian, P. Seasonal dynamics of fungal communities in a temperate oak forest soil. New Phytol. 2014, 201, 269–278. [Google Scholar] [CrossRef]
- Guénon, R.; Day, T.A.; Velazco-Ayuso, S.; Gros, R. Mixing of Aleppo pine and Holm oak litter increases biochem-ical diversity and alleviates N limitations of microbial activity. Soil Biol. Biochem. 2017, 105, 216–226. [Google Scholar] [CrossRef]
- Terradas, J. Holm Oak and Holm Oak Forests: An Introduction. En Ecology of Mediterranean Evergreen Oak Forests; Springer: Berlin/Heidelberg, Germany, 1999; pp. 3–14. [Google Scholar]
- Allen, E.B.; Allen, M.F. Competition between plants of different successional stages: Mycorrhizae as regulators. Can. J. Bot. 1984, 62, 2625–2629. [Google Scholar] [CrossRef]
- Liu, L.; Zhu, K.; Krause, S.M.; Li, S.; Wang, X.; Zhang, Z.; Zhang, J. Changes in assembly processes of soil micro-bial communities during secondary succession in two subtropical forests. Soil Biol. Biochem. 2021, 154, 108144. [Google Scholar] [CrossRef]
- Harantová, L.; Mudrák, O.; Kohout, P.; Elhottová, D.; Frouz, J.; Baldrian, P. Development of microbial community during primary succession in areas degraded by mining activities. Land Degrad. Dev. 2017, 28, 2574–2584. [Google Scholar] [CrossRef]
- Urbanová, M.; Šnajdr, J.; Baldrian, P. Composition of fungal and bacterial communities in forest litter and soil is largely determined by dominant trees. Soil Biol. Biochem. 2015, 84, 53–64. [Google Scholar] [CrossRef]
- Wallander, H.; Ekblad, A.; Godbold, D.L.; Johnson, D.; Bahr, A.; Baldrian, P.; Björkf, R.G.; Kieliszewska-Rokickag, B.; Kjøllerh, R.; Kraigher, H.; et al. Evaluation of methods to estimate production, biomass and turnover of ectomycorrhizal mycelium in forests soils–A review. Soil Biol. Biochem. 2013, 57, 1034–1047. [Google Scholar] [CrossRef]



| Forest Type (No. of Plots) | Range | pH | OM % | N % | P mg/kg |
|---|---|---|---|---|---|
| Ps | Min. | 6.65 | 4.56 | 0.19 | 9.80 |
| (n = 3) | Mean | 7.38 | 11.12 | 0.5 | 17.07 |
| Max. | 7.89 | 35.54 | 0.99 | 30.70 | |
| Qr | Min. | 7.56 | 4.24 | 0.20 | 6.70 |
| (n = 3) | Mean | 7.69 | 9.14 | 0.38 | 10.05 |
| Max. | 7.99 | 16.98 | 0.88 | 15.50 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adamo, I.; Dashevskaya, S.; Alday, J.G. Fungal Perspective of Pine and Oak Colonization in Mediterranean Degraded Ecosystems. Forests 2022, 13, 88. https://doi.org/10.3390/f13010088
Adamo I, Dashevskaya S, Alday JG. Fungal Perspective of Pine and Oak Colonization in Mediterranean Degraded Ecosystems. Forests. 2022; 13(1):88. https://doi.org/10.3390/f13010088
Chicago/Turabian StyleAdamo, Irene, Svetlana Dashevskaya, and Josu G. Alday. 2022. "Fungal Perspective of Pine and Oak Colonization in Mediterranean Degraded Ecosystems" Forests 13, no. 1: 88. https://doi.org/10.3390/f13010088
APA StyleAdamo, I., Dashevskaya, S., & Alday, J. G. (2022). Fungal Perspective of Pine and Oak Colonization in Mediterranean Degraded Ecosystems. Forests, 13(1), 88. https://doi.org/10.3390/f13010088







