The Properties of Black Locust Robinia pseudoacacia L. to Selectively Accumulate Chemical Elements from Soils of Ecologically Transformed Areas
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sampling Sites
2.2. Soil and Plant Sampling
2.3. Estimating Pollutant Impact
2.3.1. Index of Geoaccumulation
- If Igeo < 0, unpolluted;
- If 0 ≤ Igeo < 1, unpolluted to moderately polluted;
- If 1 ≤ Igeo < 2, moderately polluted;
- If 2 ≤ Igeo < 3, moderately to strongly polluted;
- If 3 ≤ Igeo < 4, strongly polluted;
- If 4 ≤ Igeo < 5, strongly to very strongly polluted;
- If 5 ≤ Igeo, very strongly polluted.
2.3.2. Enrichment Factor
- If EF < 2, deficiency to minimal enrichment;
- If 2 ≤ EF < 5, moderate enrichment;
- If 5 ≤ EF < 20, significant enrichment;
- If 20 ≤ EF < 40, very high enrichment;
- If 40 ≤ EF, extremely high enrichment.
2.3.3. Contamination Factor
- If CF < 1, low contamination factor;
- If 1 ≤ CF < 3, moderate contamination factor;
- If 3 ≤ CF < 6, considerable contamination factor;
- If 6 ≤ CF, very high contamination factor.
2.3.4. Pollution Load Index
2.3.5. Potential Ecological Risk Index
- If < 40, low;
- If 40 ≤ < 80, moderate;
- If 80 ≤ < 160, considerable;
- If 160 ≤ < 320, high;
- If 320 ≤ , very high.
- If RI <150, low risk;
- If 150 ≤ RI < 300, moderate risk;
- If 300 ≤ RI < 600, considerable risk;
- If 600 ≤ RI, very high risk.
2.3.6. Bioaccumulation Factor
3. Results
3.1. Soil Morphology
3.2. Chemical Properties of Soils and Level of Disturbance
3.3. Element Concentration in R. pseuodacacia Leaves, Seeds, and Branches
3.3.1. Major Element Concentration
3.3.2. Heavy Metal Concentration
3.3.3. Concentration of Minor Elements
3.3.4. Trace Elements Content
3.4. The Level of Heavy Metal Contamination in Soil and Plant (Robinia pseudacacia)
3.5. Comparison of Heavy Metal Content in the Soil–Plant–Soil System
- -
- For leaves: Cu > Zn > Hg > Cd > Mn > Ni > Cr > Pb > Fe,Co,As;
- -
- For branches: Cu > Zn > Cd > Ni > Cr > Hg > Pb > Mn > Co > Fe,As;
- -
- For seeds: Cu > Zn > Ni > Cd > Cr > Mn,Hg > Pb > Co,Fe.
4. Discussion
5. Conclusions
- -
- By colonizing the transformed areas, R. pseudoacacia initiates soil formation processes on anthropogenic substrates characterized by different granulometric compositions, which significantly impacts the soil’s physicochemical properties;
- -
- Clear differentiation in terms of the main elements (Fe, Ca, P, Mg, Al, K, S) content was found in the chemical composition of black locust leaves, branches, seeds, and soil materials. These elements in the initial stages of ecosystem development are important nutrients for invading organisms. The results reveal a significant influence of R. pseudoacacia on the restoration of disturbed habitats;
- -
- The content of heavy metals concentration was higher in soil than in the R. pseudoacacia analyzed tissues. In the case of plant tissues, the highest concentrations were in the leaves and branches, whereas seeds contained reduced zinc content;
- -
- Based on the index of geoaccumulation (Igeo), it was determined that the content of Cd had the most significant impact on the level of contamination of the studied soils. This statement also confirmed the results of the enrichment factor (EF) and contamination factor (CF);
- -
- Despite the anthropogenic character of soil material and location within the investigated urban region, the potential of ecological risk for most studied elements was low—higher values were only found for Hg and Cd;
- -
- BAF analysis indicates that R. pseudoacacia is a weak accumulator of heavy metals. Within the analyzed elements, Cu and Zn accumulated the most.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pełka-Gościniak, J. Restoring Nature in Mining Areas of the Silesian Upland (Poland). Earth Surf. Proc. Land. 2006, 31, 1685–1691. [Google Scholar] [CrossRef]
- Dulias, R. The Impact of Mining on the Landscape: A Study of the Upper Silesian Coal Basin in Poland; Environmental Science and Engineering; Springer International Publishing: Cham, Switzerland, 2016; ISBN 978-3-319-29539-8. [Google Scholar]
- Ciesielczuk, J. Coal Mining and Combustion in the Coal Waste Dumps of Poland. In Coal and Peat Fires: A Global Perspective; Elsevier: Amsterdam, The Netherlands, 2015; pp. 463–473. [Google Scholar]
- Abramowicz, A.; Rahmonov, O.; Chybiorz, R. Environmental Management and Landscape Transformation on Self-Heating Coal-Waste Dumps in the Upper Silesian Coal Basin. Land 2021, 10, 23. [Google Scholar] [CrossRef]
- Krzysztofik, R.; Dulias, R.; Kantor-Pietraga, I.; Spórna, T.; Dragan, W. Paths of Urban Planning in a Post-Mining Area. A Case Study of a Former Sandpit in Southern Poland. Land Use Policy 2020, 99, 104801. [Google Scholar] [CrossRef]
- Różkowski, J.; Rahmonov, O.; Zarychta, R.; Zarychta, A. Environmental Transformation and the Current State of Hydrogeological Condition in the Wojkowice Area—Southern Poland. Resources 2021, 10, 54. [Google Scholar] [CrossRef]
- Rahmonov, O.; Krzysztofik, R.; Środek, D.; Smolarek-Lach, J. Vegetation- and Environmental Changes on Non-Reclaimed Spoil Heaps in Southern Poland. Biology 2020, 9, 164. [Google Scholar] [CrossRef] [PubMed]
- Worsztynowicz, A.; Mill, W. Potential Ecological Risk Due to Acidification of Heavy Industrialized Areas—the Upper Silesia Case. In Studies in Environmental Science. Acid Rain Research: Do We Have Enough Answers? Heij, G.J., Erisman, J.W., Eds.; Elsevier: Amsterdam, The Netherlands, 1995; Volume 64, pp. 353–365. [Google Scholar]
- Abramowicz, A.K.; Rahmonov, O.; Fabiańska, M.J.; Nádudvari, Á.; Chybiorz, R.; Michalak, M. Changes in Soil Chemical Composition Caused by Self-Heating of a Coal-Waste Dump. Land Degradn. Dev. 2021, 32, 4340–4349. [Google Scholar] [CrossRef]
- Dzwonko, Z.; Loster, S. Effects of Dominant Trees and Anthropogenic Disturbances on Species Richness and Floristic Composition of Secondary Communities in Southern Poland. J. App. Ecol. 1997, 34, 861–870. [Google Scholar] [CrossRef]
- Tokarska-Guzik, B. The Establishment and Spread of Alien Plant Species (Kenophytes) in the Flora of Poland; University of Silesia: Katowice, Poland, 2005. [Google Scholar]
- Rahmonov, O. The Chemical Composition of Plant Litter of Black Locust (Robinia pseudoacacia L.) and Its Ecological Role in Sandy Ecosystems. Acta Ecol. Sinica 2009, 29, 237–243. [Google Scholar] [CrossRef]
- Rahmonov, O.; Cabała, J.; Krzysztofik, R. Vegetation and Environmental Changes on Contaminated Soil Formed on Waste from an Historic Zn-Pb Ore-Washing Plant. Biology 2021, 10, 1242. [Google Scholar] [CrossRef]
- Huntley, J. Robinia Pseudoacacia L. Black Locust. Silvics of North America. In Hardwoods. Agriculture Handbook; Department of Agriculture: Washington, DC, USA, 1990; Volume 2, pp. 755–761. [Google Scholar]
- Palowski, B.; Małkowska, E.; Kurtyka, R.; Szymanowska-Pulka, J.; Gucwa-Przepióra, E.; Małkowski, Ł.; Woźnica, A.; Małkowski, E. Bioaccumulation of Heavy Metals in Selected Organs of Black Locust (Robinia pseudoacacia) and Their Potential Use as Air Contamination Bioindicators. Pol. J. Environ. Stud. 2016, 25, 1–12. [Google Scholar] [CrossRef]
- Lambdon, P.W.; Pyšek, P.; Basnou, C.; Hejda, M.; Arianoutsou, M.; Essl, F.; Jarosik, V.; Pergl, J.; Winter, M.; Anastasiu, P.; et al. Alien Flora of Europe: Species Diversity, Temporal Trends, Geographical Patterns and Research Needs. Preslia 2008, 80, 101–149. [Google Scholar]
- Vítková, M.; Müllerová, J.; Sádlo, J.; Pergl, J.; Pyšek, P. Black Locust (Robinia pseudoacacia) Beloved and Despised: A Story of an Invasive Tree in Central Europe. For. Ecol. Manag. 2017, 384, 287–302. [Google Scholar] [CrossRef] [PubMed]
- Kidawa, J.; Chmura, D.; Molenda, T. The Hydrological-Hydrochemical Factors That Control the Invasion of the Black Locust (Robinia pseudoacacia L.) in Succession in Areas with Opencast Mines. Plants 2021, 10, 40. [Google Scholar] [CrossRef]
- Cierjacks, A.; Kowarik, I.; Joshi, J.; Hempel, S.; Ristow, M.; von der Lippe, M.; Weber, E. Biological Flora of the British Isles: Robinia pseudoacacia. J. Ecol. 2013, 101, 1623–1640. [Google Scholar] [CrossRef]
- Grünewald, H.; Böhm, C.; Quinkenstein, A.; Grundmann, P.; Eberts, J.; von Wühlisch, G. Robinia pseudoacacia L.: A Lesser Known Tree Species for Biomass Production. Bioenerg. Res. 2009, 2, 123–133. [Google Scholar] [CrossRef]
- Mantovani, D.; Veste, M.; Freese, D. Black Locust (Robinia pseudoacacia L.) Ecophysiological and Morphological Adaptations to Drought and Their Consequence on Biomass Production and Water-Use Efficiency. N. Z. J. For. Sci. 2014, 44, 29. [Google Scholar] [CrossRef] [Green Version]
- Kraszkiewicz, A. Productivity of Black Locust (Robinia pseudoacacia L.) Grown on a Varying Habitats in Southeastern Poland. Forests 2021, 12, 470. [Google Scholar] [CrossRef]
- Boring, L.R.; Swank, W.T. The Role of Black Locust (Robinia pseudoacacia) in Forest Succession. J. Ecol. 1984, 72, 749–766. [Google Scholar] [CrossRef] [Green Version]
- Tilman, D.; Lehman, C. Human-Caused Environmental Change: Impacts on Plant Diversity and Evolution. Proc. Natl. Acad. Sci. USA 2001, 98, 5433–5440. [Google Scholar] [CrossRef] [Green Version]
- Ussiri, D.A.N.; Lal, R.; Jacinthe, P.A. Soil Properties and Carbon Sequestration of Afforested Pastures in Reclaimed Minesoils of Ohio. Soil Sci. Soc. Am. J. 2006, 70, 1797–1806. [Google Scholar] [CrossRef]
- Yüksek, T.; Yüksek, F. The Effects of Restoration on Soil Properties in Degraded Land in the Semi-Arid Region of Turkey. Catena 2011, 84, 47–53. [Google Scholar] [CrossRef]
- Shi, Z.; Bai, Z.; Guo, D.; Chen, M. Develop a Soil Quality Index to Study the Results of Black Locust on Soil Quality below Different Allocation Patterns. Land 2021, 10, 785. [Google Scholar] [CrossRef]
- Kraszkiewicz, A. Chemical Composition and Selected Energy Properties of Black Locust Bark (Robinia pseudoacacia L.). Agr. Eng. 2016, 20, 117–124. [Google Scholar] [CrossRef]
- Rahmonov, O.; Pukowiec-Kurda, K.; Banaszek, J.; Brom, K. Floristic Diversity in Selected City Parks in Southern Poland. Environ. Prot. Nat. Res. 2019, 30, 8–17. [Google Scholar] [CrossRef]
- Heymann, Y.; Steenmans, C.; Croisille, G.; Bissard, M. CORINE Land Cover Technical Guide, Part I.; Official Publications of the European Communities: Luxembourg, 1994. [Google Scholar]
- Rahmonov, O.; Czylok, A.; Orczewska, A.; Majgier, L.; Parusel, T. Chemical Composition of the Leaves of Reynoutria Japonica Houtt. and Soil Features in Polluted Areas. Open Life Sci. 2014, 9, 320–330. [Google Scholar] [CrossRef] [Green Version]
- Banaszek, J.; Gajos, M.; Karkosz, D.; Rahmonov, O.; Parusel, T. Using GIS Methods to Investigate Urban Parks within Industrial Regions. Pol. J. Environ. Stud. 2014, 23, 609–617. [Google Scholar]
- Banaszek, J.; Leksy, M.; Rahmonov, O. The Ecological Diversity of Vegetation within Urban Parks in the Dabrowski Basin (Southern Poland). In Proceedings of the 10th International Conference Environmental Engineering, Vilnius, Lithuania, 27–28 April 2017; Vilnuis Gediminas Technical University (VGTU) Press: Vilnius, Lithuania, 2017. [Google Scholar] [CrossRef]
- Szymczyk, A.; Rahmonov, O.; Parusel, T. The ecological differentiations of flora and vegetation in the area of Siemonia sandpit. Acta Geogr. Sile 2011, 9, 63–74. [Google Scholar]
- Bednarek, R.; Dziadowiec, H.; Pokojowska, U. Pedological Aspect of Variability. Ecol. Quest. 2002, 1, 35–41. [Google Scholar]
- MacNaeidhe, F. Procedures and Precautions Used in Sampling Techniques and Analysis of Trace Elements in Plant Matrices. Sci. Total Environ. 1995, 176, 25–31. [Google Scholar] [CrossRef]
- Markert, B. Sample Preparation (Cleaning, Drying, Homogenization) for Trace Element Analysis in Plant Matrices. Sci. Total Environ. 1995, 176, 45–61. [Google Scholar] [CrossRef]
- Solgi, E.; Esmaili-Sari, A.; Riyahi-Bakhtiari, A.; Hadipour, M. Soil Contamination of Metals in the Three Industrial Estates, Arak, Iran. Bull. Environ. Contam. Toxicol. 2012, 88, 634–638. [Google Scholar] [CrossRef]
- Okedeyi, O.O.; Dube, S.; Awofolu, O.R.; Nindi, M.M. Assessing the Enrichment of Heavy Metals in Surface Soil and Plant (Digitaria Eriantha) around Coal-Fired Power Plants in South Africa. Environ. Sci. Pollut. Res. 2014, 21, 4686–4696. [Google Scholar] [CrossRef]
- Napoletano, P.; Colombo, C.; Di Iorio, E.; Memoli, V.; Panico, S.C.; Ruggiero, A.G.; Santorufo, L.; Maisto, G.; De Marco, A. Integrated Approach for Quality Assessment of Technosols in Experimental Mesocosms. Sustainability 2021, 13, 9101. [Google Scholar] [CrossRef]
- Wedepohl, K. The Composition of the Continental Crust. Geochim. Cosmochim. Acta 1995, 59, 1217–1232. [Google Scholar] [CrossRef]
- Schwikowski, M.; Barbante, C.; Doering, T.; Gaeggeler, H.W.; Boutron, C.; Schotterer, U.; Tobler, L.; Van de Velde, K.; Ferrari, C.; Cozzi, G.; et al. Post-17th-Century Changes of European Lead Emissions Recorded in High-Altitude Alpine Snow and Ice. Environ. Sci. Technol. 2004, 38, 957–964. [Google Scholar] [CrossRef]
- Boës, X.; Rydberg, J.; Martinez-Cortizas, A.; Bindler, R.; Renberg, I. Evaluation of Conservative Lithogenic Elements (Ti, Zr, Al, and Rb) to Study Anthropogenic Element Enrichments in Lake Sediments. J. Paleolimnol. 2011, 46, 75–87. [Google Scholar] [CrossRef]
- Samhan, S.; Friese, K.; von Tümpling, W.; Pöllmann, H.; Hoetzl, H.; Ghanem, M. Anthropogenic Influence of Trace Metals in Sediments of the Al-Qilt Catchment, West Bank, Palestine: 1. Contamination Factor and Bonding Forms. Environ. Earth Sci. 2014, 71, 1533–1539. [Google Scholar] [CrossRef] [Green Version]
- Chien, C.-T.; Allen, B.; Dimova, N.T.; Yang, J.; Reuter, J.; Schladow, G.; Paytan, A. Evaluation of Atmospheric Dry Deposition as a Source of Nutrients and Trace Metals to Lake Tahoe. Chem. Geol. 2019, 511, 178–189. [Google Scholar] [CrossRef]
- Gruszecka-Kosowska, A.; Baran, A.; Wdowin, M.; Mazur-Kajta, K.; Czech, T. The Contents of the Potentially Harmful Elements in the Arable Soils of Southern Poland, with the Assessment of Ecological and Health Risks: A Case Study. Environ. Geochem. Health 2020, 42, 419–442. [Google Scholar] [CrossRef] [Green Version]
- Lizárraga-Mendiola, L.; Durán-Domínguez-de-Bazúa, M.D.C.; González-Sandoval, M.-R. Environmental Assessment of an Active Tailings Pile in the State of Mexico (Central Mexico). Res. J. Environ. Sci. 2008, 2, 197–208. [Google Scholar] [CrossRef] [Green Version]
- Ho, H.H.; Rudy, S.; Damme, A. Distribution and Contamination Status of Heavy Metals in Estuarine Sediments near CauOng Harbor, Ha Long Bay, Vietnam. Geol. Belg. 2010, 13, 37–47. [Google Scholar]
- Ghrefat, H.A.; Abu-Rukah, Y.; Rosen, M.A. Application of Geoaccumulation Index and Enrichment Factor for Assessing Metal Contamination in the Sediments of Kafrain Dam, Jordan. Environ. Monit. Assess. 2011, 178, 95–109. [Google Scholar] [CrossRef]
- Schiff, K.; Weisberg, S. Iron as a Reference Element for Determining Trace Metal Enrichment in Southern California Coastal Shelf Sediments. Mar. Environ. Res. 1999, 48, 161–176. [Google Scholar] [CrossRef]
- Meza-Figueroa, D.; Maier, R.M.; de la O-Villanueva, M.; Gómez-Alvarez, A.; Moreno-Zazueta, A.; Rivera, J.; Campillo, A.; Grandlic, C.J.; Anaya, R.; Palafox-Reyes, J. The Impact of Unconfined Mine Tailings in Residential Areas from a Mining Town in a Semi-Arid Environment: Nacozari, Sonora, Mexico. Chemosphere 2009, 77, 140–147. [Google Scholar] [CrossRef] [Green Version]
- Antić-Mladenović, S.; Kresović, M.; Čakmak, D.; Perović, V.; Saljnikov, E.; Ličina, V.; Rinklebe, J. Impact of a Severe Flood on Large-Scale Contamination of Arable Soils by Potentially Toxic Elements (Serbia). Environ. Geochem. Health 2019, 41, 249–266. [Google Scholar] [CrossRef] [Green Version]
- Sutherland, R.A. Bed Sediment-Associated Trace Metals in an Urban Stream, Oahu, Hawaii. Environ. Geol. 2000, 39, 611–627. [Google Scholar] [CrossRef]
- Uriah, L.A.; Shehu, U. Environmental Risk Assessment of Heavy Metals Content of Municipal Solid Waste Used as Organic Fertilizer in Vegetable Gardens on the Jos Plateau, Nigeria. Am. J. Environ. Prot. 2014, 3, 1. [Google Scholar] [CrossRef]
- Tomlinson, D.L.; Wilson, J.G.; Harris, C.R.; Jeffrey, D.W. Problems in the Assessment of Heavy-Metal Levels in Estuaries and the Formation of a Pollution Index. Helgolander. Meeresun. 1980, 33, 566–575. [Google Scholar] [CrossRef] [Green Version]
- Zhu, H.; Yuan, X.; Zeng, G.; Jiang, M.; Liang, J.; Zhang, C.; Yin, J.; Huang, H.; Liu, Z.; Jiang, H. Ecological Risk Assessment of Heavy Metals in Sediments of Xiawan Port Based on Modified Potential Ecological Risk Index. Trans. Nonferrous Met. Soc. 2012, 22, 1470–1477. [Google Scholar] [CrossRef]
- Ahamad, M.I.; Song, J.; Sun, H.; Wang, X.; Mehmood, M.S.; Sajid, M.; Su, P.; Khan, A.J. Contamination Level, Ecological Risk, and Source Identification of Heavy Metals in the Hyporheic Zone of the Weihe River, China. Int. J. Environ. Res. Public Health 2020, 17, 1070. [Google Scholar] [CrossRef] [Green Version]
- Sekabira, K.; Oryem-Origa, H.; Mutumba, G.M.; Kakudidi, E.; Basamba, T.A. Heavy Metal Phytoremediation by Commelina benghalensis (L) and Cynodon dactylon (L) Growing in Urban Stream Sediments. Int. J. Plant Physiol. Biochem. 2011, 3, 133–142. [Google Scholar]
- Xu, M.; Zhang, J.; Liu, G.B.; Yamanaka, N. Soil Properties in Natural Grassland, Caragana Korshinskii Planted Shrubland, and Robinia Pseudoacacia Planted Forest in Gullies on the Hilly Loess Plateau, China. Catena 2014, 119, 116–124. [Google Scholar] [CrossRef]
- Yuan, Y.; Zhao, Z.; Niu, S.; Li, X.; Wang, Y.; Bai, Z. Reclamation Promotes the Succession of the Soil and Vegetation in Opencast Coal Mine: A Case Study from Robinia Pseudoacacia Reclaimed Forests, Pingshuo Mine, China. Catena 2018, 165, 72–79. [Google Scholar] [CrossRef]
- Samecka-Cymerman, A.; Stankiewicz, A.; Kolon, K.; Kempers, A.J. Self-Organizing Feature Map (Neural Networks) as a Tool to Select the Best Indicator of Road Traffic Pollution (Soil, Leaves or Bark of Robinia pseudoacacia L.). Environ. Pollut. 2009, 157, 2061–2065. [Google Scholar] [CrossRef]
- Lis, J.; Pasieczna, A. Geochemical Atlas of Upper Silesia 1:200000, 48th ed.; Polish Geological Institute: Warsaw, Poland, 1995. [Google Scholar]
- Cadmium. Environmental Health Criteria; WHO: Geneva, Switzerland, 1992; Volume 134.
- Kabata-Pendias, A. Trace Elements in Soil and Plants, 3rd ed.; CRC Press LLC: Washington, DC, USA, 2001. [Google Scholar]
- Spychalski, W. Trace elements in soils developed from post-mining land. Protect Environ. Nat. Res. 2007, 33. (In Polish) [Google Scholar]
- Klojzy-Karczmarczyk, B.; Mazurek, J.; Staszczak, J. Analysis of the quality of waste from a closed hard coal mining heap in relation to the requirements for inert mining waste. Sci. J. Inst. Min. Energy Eco. Pol. Aca Sci. 2016, 95, 227–242. (In Polish) [Google Scholar]
- Klatka, S.; Malec, M.; Kruk, E.; Ryczek, M. Evaluation of possibility of natural utilisation of coal mine waste used for surface levelling. Acta Agroph. 2017, 24, 253–262. [Google Scholar]
- Li, X.; Yang, H.; Zhang, C.; Zeng, G.; Liu, Y.; Xu, W.; Wu, Y.; Lan, S. Spatial Distribution and Transport Characteristics of Heavy Metals around an Antimony Mine Area in Central China. Chemosphere 2017, 170, 17–24. [Google Scholar] [CrossRef]
- Kabata-Pendias, A.; Mukherjee, A. Trace Elements from Soil to Human; Springer: Berlin, Germany, 2007. [Google Scholar]
- Nieminen, T.; Derome, J.; Saarsalmi, A. The Applicability of Needle Chemistry for Diagnosing Heavy Metal Toxicity to Trees. Water Air Soil Poll. 2004, 157, 269–279. [Google Scholar] [CrossRef]
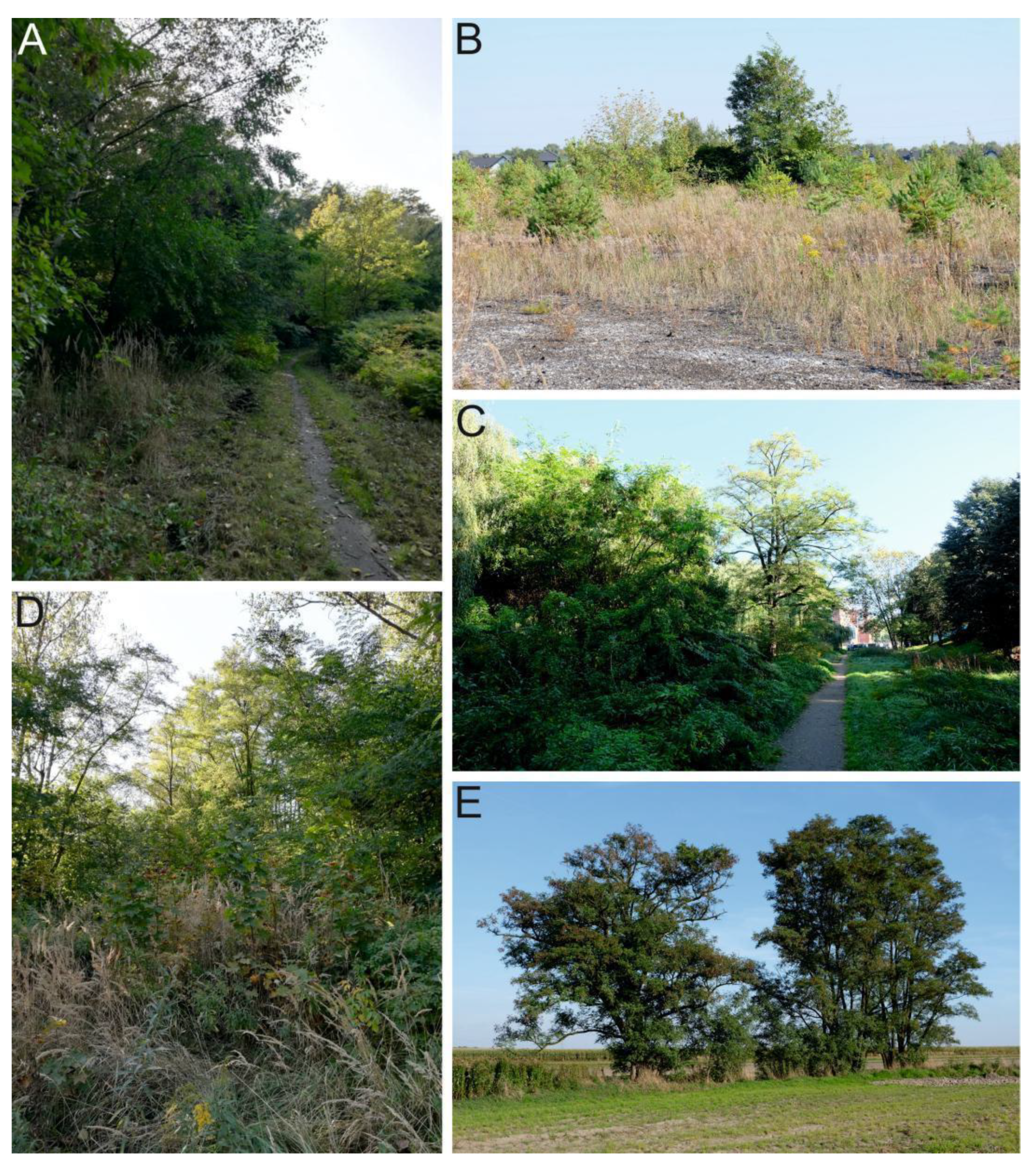

| Site No. | Site Name | Geographical Coordination | Site Attributes | ||||
|---|---|---|---|---|---|---|---|
| Habitat | Level of Disturbance | Land Use Classification | Method of Restoration | Soil Type | |||
| 1 | Sosnowiec–Dębowa Góra | 50°15’29.71” N 19°08’56.37” E | Dumping site with building materials and used electronic equipment | Extreme | Urban and industrial site | Spontaneous succession | Technic regosol, technic anthrosol |
| 2 | Sosnowiec–Kazimierz Górniczy | 50°17’02.40” N 19°14’24.11” E | Spoil heap of coal mining excavation material, with a high share of fine-grained material | Very high | Mine, dump, and construction site | Spontaneous succession | Technic regosol, technic anthrosol |
| 3 | Sosnowiec–Sielec | 50°17’10.43” N 19°08’34.59” E | Artificial surfaces, urban parks with decorative plant species | High | Green urban areas | Planting | Urbic technosol, mollic technosol |
| 4 | Rogoźnik | 50°24’01.35” N 19°02’02.82” E | Reclaimed area with artificial plantings | Medium | Former mineral extraction sites, green urban areas | Spontaneous succession | Mollic antroposol, arenosole |
| 5 | Zawada | 50°23’10.64” N 18°40’02.71” E | Edge of an arable field | Low | Non-irrigated arable land | Spontaneous succession | Haplic luvisols, albic luvisols |
| Percentage Share (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| >10.00 | 10.00–5.00 | 5.00–2.00 | 2.00–1.00 | 1.00–0.50 | 0.50–0.25 | 0.25–0.10 | 0.10–0.05 | <0.05 | |
| Wild landfill | 2.8 | 1.4 | 2.9 | 2.5 | 25.0 | 33.8 | 18.8 | 6.5 | 6.3 |
| Spoil heap | 5.2 | 8.7 | 17.7 | 17.4 | 10.3 | 16.5 | 10.8 | 5.1 | 8.3 |
| City park | 0.0 | 0.0 | 0.0 | 1.2 | 11.0 | 56.9 | 24.5 | 3.8 | 2.6 |
| Old sandpit | 0.0 | 0.0 | 0.7 | 1.3 | 5.4 | 46.1 | 33.9 | 8.7 | 3.9 |
| Fields edge | 0.0 | 0.0 | 1.0 | 1.2 | 15.0 | 41.9 | 22.5 | 6.1 | 12.3 |
| Wild Landfill | Spoil Heap | City Park | Old Sandpit | Fields Edge | |
|---|---|---|---|---|---|
| pH (H2O) | 5.94 | 6.81 | 6.29 | 5.70 | 5.48 |
| pH (KCl) | 5.16 | 6.37 | 5.37 | 5.02 | 4.70 |
| Loss ignition | 5.72 | 29.67 | 9.53 | 5.01 | 2.83 |
| Corg(%) | 3.52 | 19.61 | 5.28 | 2.04 | 1.42 |
| Ntot(%) | 0.109 | 0.528 | 0.273 | 0.304 | 0.098 |
| Mgavail (mg/kg) | 108.50 | 401.00 | 143.50 | 99.00 | 54.20 |
| Pavail. (mg P2O5)/kg) | 26.77 | 14.30 | 40.81 | 17.27 | 36.10 |
| PT (mg/kg) | 375.00 | 362.00 | 379.00 | 202.40 | 276.40 |
| Kavail. (mg/kg) | 86.00 | 212.50 | 205.00 | 153.00 | 166.50 |
| Kavail. (mg K2O/kg) | 103.63 | 256.06 | 247.03 | 184.37 | 200.63 |
| Hh (cmol(+)·kg−1) | 11.80 | 7.72 | 8.52 | 11.88 | 7.48 |
| Al3+ (cmol(+)·kg−1) | 0.36 | 0.60 | 1.28 | 0.56 | 1.52 |
| H+ (cmol(+)·kg−1) | 1.04 | 0.72 | 1.52 | 0.36 | 1.36 |
| Fe | Pb | Cd | Zn | Cu | Ni | Cr | As | Hg | ||
|---|---|---|---|---|---|---|---|---|---|---|
| % | ppm | ppm | ppm | ppm | ppm | ppm | ppm | ppb | ||
| Soils | Wild landfill | 2.24 | 89.71 | 2.50 | 305.37 | 50.29 | 24.97 | 56.60 | 7.07 | 144.00 |
| Spoil heap | 2.07 | 78.17 | 1.30 | 188.03 | 37.11 | 28.27 | 18.73 | 4.23 | 262.33 | |
| City park | 1.07 | 157.99 | 3.91 | 543.97 | 32.69 | 9.80 | 13.03 | 11.77 | 155.33 | |
| Sandpit area | 0.70 | 152.86 | 3.24 | 200.03 | 9.33 | 7.60 | 8.70 | 8.87 | 47.67 | |
| Field edge | 0.79 | 103.06 | 1.41 | 129.77 | 11.24 | 7.27 | 12.80 | 8.97 | 66.67 | |
| Leaves | Wild landfill | 0.03 | 2.70 | 0.58 | 103.37 | 8.47 | 3.17 | 1.40 | 0.23 | 33.67 |
| Spoil heap | 0.02 | 3.28 | 0.26 | 30.33 | 6.10 | 1.03 | 1.47 | 0.10 | 24.33 | |
| City park | 0.02 | 2.93 | 0.20 | 61.67 | 3.93 | 0.30 | 1.53 | 0.27 | 29.67 | |
| Sandpit area | 0.01 | 3.46 | 0.37 | 44.40 | 6.39 | 0.40 | 1.33 | <0.01 | 15.00 | |
| Field edge | 0.02 | 4.12 | 0.77 | 94.53 | 9.04 | 2.57 | 1.30 | 0.03 | 29.67 | |
| Branches | Wild landfill | 0.01 | 3.17 | 0.63 | 94.93 | 9.48 | 4.63 | 1.60 | <0.01 | 3.00 |
| Spoil heap | 0.01 | 3.51 | 0.15 | 27.60 | 7.07 | 1.53 | 1.23 | 0.03 | 3.00 | |
| City park | 0.01 | 1.74 | 0.19 | 80.63 | 7.21 | 0.90 | 1.77 | 0.03 | 2.33 | |
| Sandpit area | 0.01 | 8.02 | 0.89 | 61.93 | 6.47 | 0.67 | 1.73 | <0.01 | 4.33 | |
| Field edge | 0.03 | 16.35 | 1.18 | 103.80 | 8.69 | 4.93 | 1.60 | 0.13 | 14.67 | |
| Seeds | Wild landfill | 0.01 | 0.75 | 0.18 | 60.90 | 10.60 | 6.20 | 1.17 | 0.03 | 3.33 |
| Spoil heap | 0.01 | 0.52 | 0.06 | 23.77 | 6.83 | 3.17 | 1.63 | <0.01 | 1.67 | |
| City park | 0.01 | 1.21 | 0.09 | 49.67 | 4.98 | 0.57 | 1.30 | <0.01 | 4.67 | |
| Sandpit area | 0.01 | 2.53 | 0.37 | 46.37 | 6.96 | 0.47 | 1.17 | <0.01 | 3.67 | |
| Field edge | 0.01 | 4.91 | 0.51 | 54.27 | 6.52 | 1.93 | 1.67 | 0.03 | 9.33 | |
| Pb | Cd | Zn | Fe | Cu | Ni | Cr | Hg | As | |
|---|---|---|---|---|---|---|---|---|---|
| Wild landfill | 1.81 | 4.03 | 1.97 | −1.05 | 1.23 | −0.16 | 0.11 | 0.78 | 1.24 |
| Spoil heap | 1.61 | 3.09 | 1.27 | −1.16 | 0.79 | 0.02 | −1.49 | 1.64 | 0.50 |
| City park | 2.63 | 4.68 | 2.80 | −2.11 | 0.61 | −1.51 | −2.01 | 0.89 | 1.97 |
| Sandpit area | 2.58 | 4.40 | 1.36 | −2.73 | −1.20 | −1.88 | −2.59 | −0.82 | 1.56 |
| Field edge | 2.01 | 3.20 | 0.73 | −2.55 | −0.93 | −1.94 | −2.04 | −0.34 | 1.58 |
| Pb | Cd | Zn | Fe | Cu | Ni | Cr | Hg | As | |
|---|---|---|---|---|---|---|---|---|---|
| Wild landfill | 7.28 | 33.80 | 8.10 | 1.00 | 4.5 | 1.85 | 2.23 | 3.55 | 4.87 |
| Spoil heap | 6.86 | 19.02 | 5.40 | 1.00 | 3.87 | 2.27 | 0.80 | 6.99 | 3.16 |
| City park | 26.83 | 110.67 | 30.20 | 1.00 | 6.60 | 1.52 | 1.07 | 8.01 | 16.99 |
| Sandpit area | 39.68 | 140.17 | 16.98 | 1.00 | 2.88 | 1.80 | 1.10 | 3.76 | 19.57 |
| Field edge | 23.70 | 54.05 | 9.76 | 1.00 | 3.07 | 1.53 | 1.43 | 4.61 | 17.54 |
| Pb | Cd | Zn | Fe | Cu | Ni | Cr | Hg | As | PLI | |
|---|---|---|---|---|---|---|---|---|---|---|
| Wild landfill | 5.28 | 24.51 | 5.87 | 0.73 | 3.52 | 1.34 | 1.62 | 2.57 | 3.54 | 7.57 |
| Spoil heap | 4.60 | 12.75 | 3.62 | 0.67 | 2.60 | 1.52 | 0.54 | 4.68 | 2.12 | 7.80 |
| City park | 9.29 | 38.33 | 10.46 | 0.35 | 2.29 | 0.53 | 0.37 | 2.77 | 5.89 | 9.29 |
| Sandpit area | 8.99 | 31.76 | 3.85 | 0.23 | 0.65 | 0.41 | 0.25 | 0.85 | 4.44 | 7.07 |
| Field edge | 6.06 | 13.82 | 2.50 | 0.26 | 0.79 | 0.39 | 0.37 | 1.18 | 4.49 | 5.56 |
| Pb | Cd | Zn | Fe | Cu | Ni | Cr | Hg | As | RI | |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 5 | 30 | 1 | 1 | 5 | 5 | 2 | 40 | 10 | |
| Wild landfill | 26.39 | 735.29 | 5.87 | 0.73 | 17.58 | 6.71 | 3.23 | 102.86 | 35.35 | 934.01 |
| Spoil heap | 22.99 | 382.35 | 3.62 | 0.67 | 12.96 | 7.60 | 1.07 | 187.38 | 21.15 | 639.80 |
| City park | 46.47 | 1150.00 | 10.46 | 0.35 | 11.43 | 2.63 | 0.74 | 110.95 | 58.85 | 1391.88 |
| Sandpit area | 44.96 | 952.94 | 3.85 | 0.23 | 3.26 | 2.04 | 0.50 | 34.05 | 44.35 | 1086.18 |
| Field edge | 30.31 | 414.71 | 2.50 | 0.26 | 3.93 | 1.95 | 0.73 | 47.14 | 44.85 | 546.38 |
| Cu | Pb | Ni | Co | As | Cd | Cr | Hg | Zn | Mn | Fe | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Leaves | 0.39 | 0.03 | 0.12 | 0.02 | 0.02 | 0.23 | 0.10 | 0.26 | 0.31 | 0.19 | 0.02 |
| Branches | 0.41 | 0.06 | 0.22 | 0.02 | 0.01 | 0.31 | 0.11 | 0.07 | 0.34 | 0.05 | 0.01 |
| Seeds | 0.37 | 0.02 | 0.15 | 0.01 | 0.00 | 0.12 | 0.09 | 0.06 | 0.21 | 0.06 | 0.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Środek, D.; Rahmonov, O. The Properties of Black Locust Robinia pseudoacacia L. to Selectively Accumulate Chemical Elements from Soils of Ecologically Transformed Areas. Forests 2022, 13, 7. https://doi.org/10.3390/f13010007
Środek D, Rahmonov O. The Properties of Black Locust Robinia pseudoacacia L. to Selectively Accumulate Chemical Elements from Soils of Ecologically Transformed Areas. Forests. 2022; 13(1):7. https://doi.org/10.3390/f13010007
Chicago/Turabian StyleŚrodek, Dorota, and Oimahmad Rahmonov. 2022. "The Properties of Black Locust Robinia pseudoacacia L. to Selectively Accumulate Chemical Elements from Soils of Ecologically Transformed Areas" Forests 13, no. 1: 7. https://doi.org/10.3390/f13010007
APA StyleŚrodek, D., & Rahmonov, O. (2022). The Properties of Black Locust Robinia pseudoacacia L. to Selectively Accumulate Chemical Elements from Soils of Ecologically Transformed Areas. Forests, 13(1), 7. https://doi.org/10.3390/f13010007






