Land-Use Change Depletes Quantity and Quality of Soil Organic Matter Fractions in Ethiopian Highlands
Abstract
:1. Introduction
2. Materials and Methods
2.1. Location/Experiment Sites
2.2. Soil Sampling and Analysis
2.2.1. Soil pH and Organic Matter
2.2.2. Bulk Density
The Particle Size
Acid Hydrolysis
Organic Carbon and Total Nitrogen
Stock Calculation
Recalcitrance Index
- RIC (%) = unhydrolyzed C/total organic C × 100
- RIN (%) = unhydrolyzed N/total N × 100
2.3. Statistical Analysis
3. Results
3.1. Variations in C and N Stocks along Soil Depth
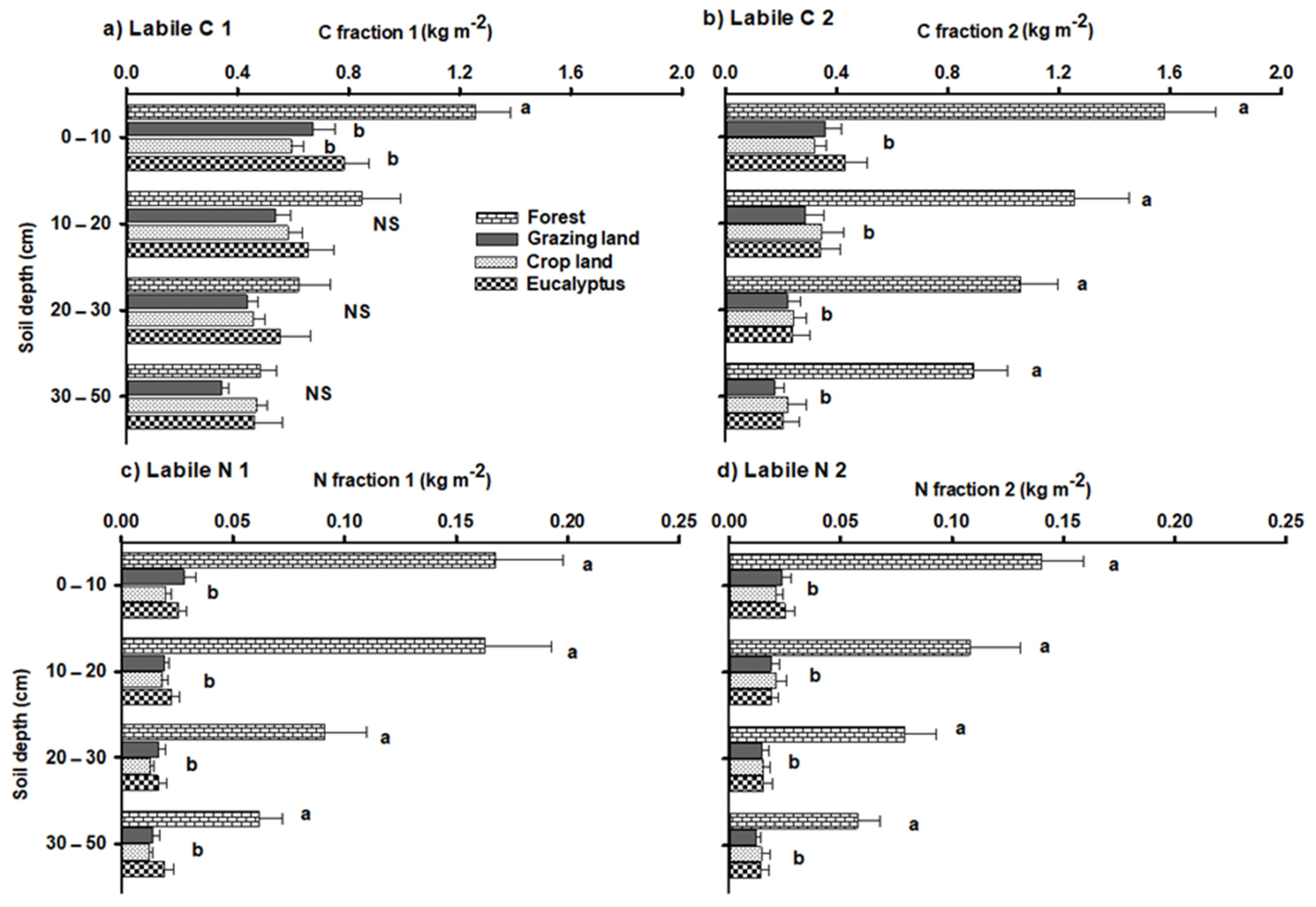
3.2. Labile C and N Stocks in Different Land-Use Types
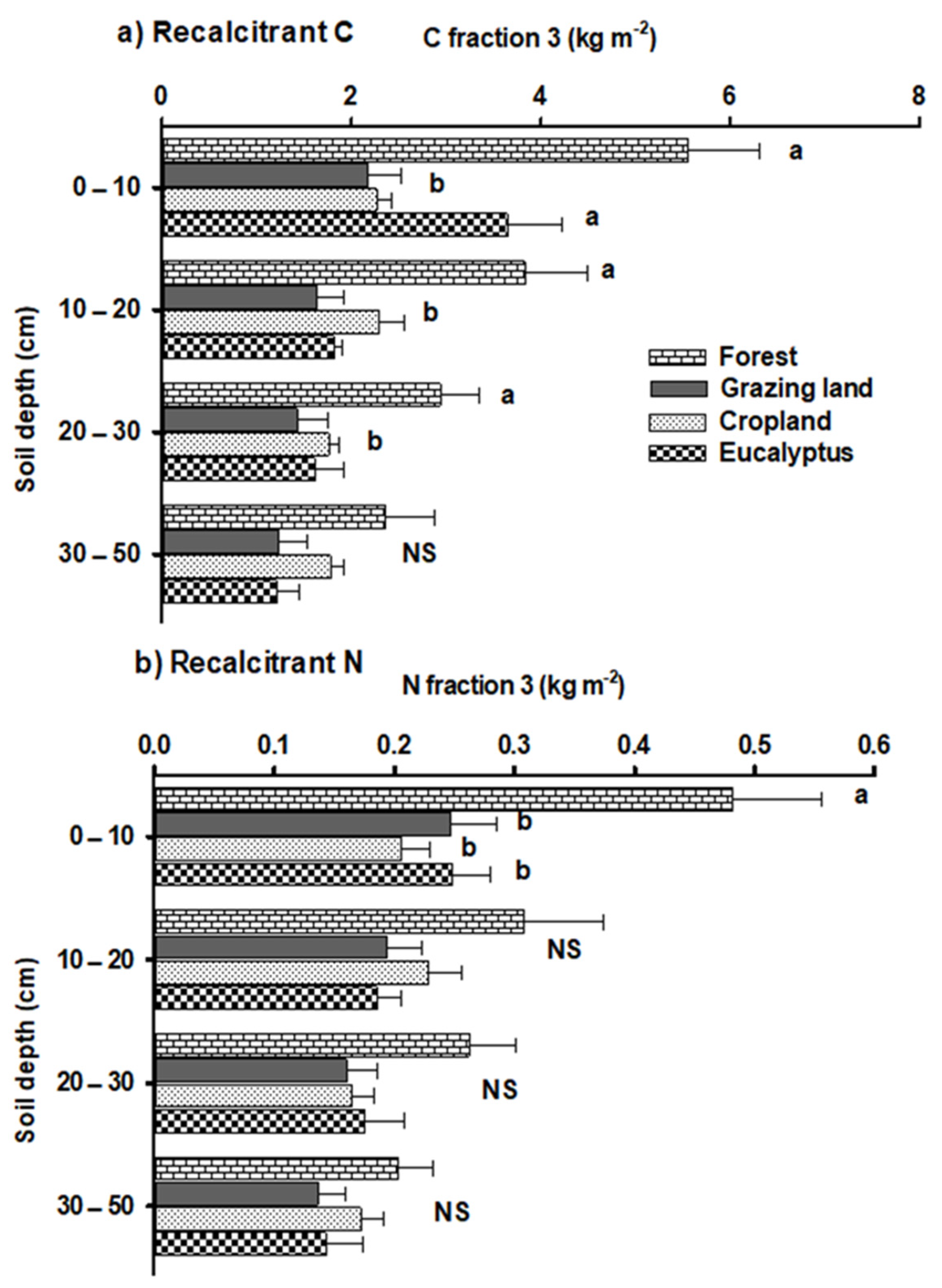
3.3. Recalcitrant C and N Stocks after Land-Use Change
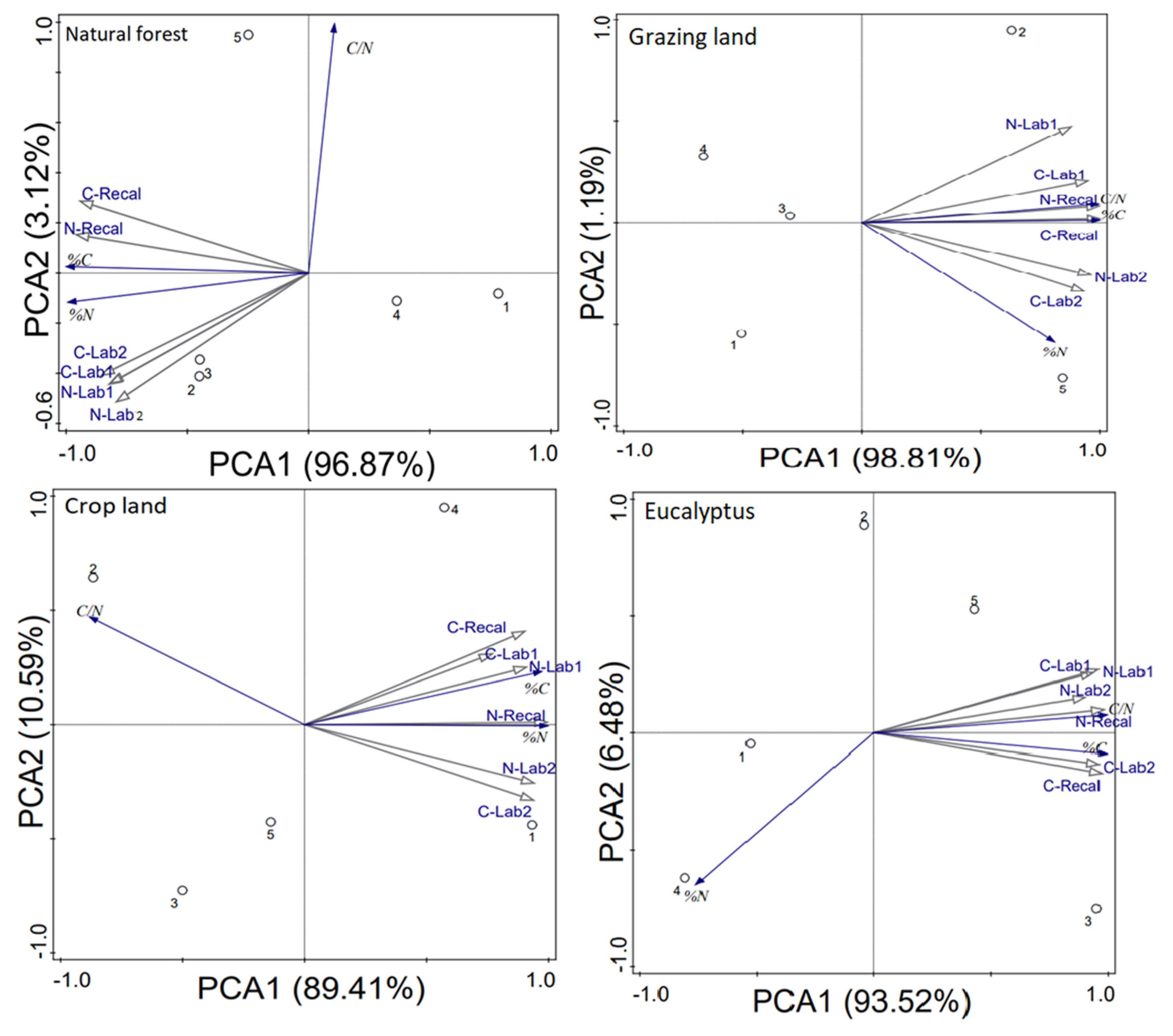
3.4. Effects of Land-Use on SOM Quality
3.4.1. Recalcitrant C and N Index
3.4.2. C:N Ratio of Soil Organic Matter Fractions
4. Discussions
4.1. Depletion of SOC and N Pools
4.2. Labile C and N under Various Land Uses
4.3. Impacts on Recalcitrant C and N
4.4. Quality of SOC and N Stocks
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Smith, P.; House, J.I.; Bustamante, M.; Sobocká, J.; Harper, R.; Pan, G.; West, P.C.; Clark, J.M.; Adhya, T.; Rumpel, C.; et al. Global change pressures on soils from land use and management. Glob. Chang. Biol. 2016, 22, 1008–1028. [Google Scholar] [CrossRef]
- Ito, A.; Hajima, T. Biogeophysical and biogeochemical impacts of land-use change simulated by MIROC-ES2L. Prog. Earth Planet Sci. 2020, 7, 1–15. [Google Scholar] [CrossRef]
- Don, A.; Schumacher, J.; Freibauer, A. Impact of tropical land-use change on soil organic carbon stocks-a meta-analysis. Glob. Chang. Biol. 2011, 17, 1658–1670. [Google Scholar] [CrossRef] [Green Version]
- Guo, L.B.; Gifford, R.M. Soil carbon stocks and land use change: A meta-analysis. Glob. Chang. Biol. 2002, 8, 345–360. [Google Scholar] [CrossRef]
- Sierra, C.A.; Hoyt, A.M.; He, Y.; Trumbore, S.E. Soil organic matter persistence as a stochastic process: Age and transit time distributions of carbon in soils. Glob. Biogeochem. Cycles 2018, 32, 1574–1588. [Google Scholar] [CrossRef]
- Beyene, B.; Pötzelsberger, E.; Sisay, K.; Assefa, D.; Hasenauer, H. The Carbon Dynamics of Dry Tropical Afromontane Forest Ecosystems in the Amhara Region of Ethiopia. Forests 2018, 9, 18. [Google Scholar] [CrossRef] [Green Version]
- Regasa, M.S.; Nones, M.; Adeba, D.A. Review on Land Use and Land Cover Change in Ethiopian Basins. Land 2021, 10, 585. [Google Scholar] [CrossRef]
- Assefa, D.; Rewald, B.; Sandén, H.; Rosinger, C.; Abiyu, A.; Yitaferu, B.; Godbold, D.L. Deforestation and land use strongly effect soil organic carbon and nitrogen stock in Northwest Ethiopia. Catena 2017, 153, 89–99. [Google Scholar] [CrossRef]
- Kassa, H.; Dondeyne, S.; Poesen, J.; Frankl, A.; Nyssen, J. Impact of deforestation on soil fertility, soil carbon and nitrogen stocks: The case of the Gacheb catchment in the White Nile Basin, Ethiopia. Agric. Ecosyst. Environ. 2017, 247, 273–282. [Google Scholar] [CrossRef] [Green Version]
- Amanuel, W.; Yimer, F.; Karltun, E. Soil organic carbon variation in relation to land use changes: The case of Birr watershed, upper Blue Nile River Basin, Ethiopia. J. Ecol. Environ. 2018, 42, 16. [Google Scholar] [CrossRef]
- Villarino, S.H.; Studdert, G.A.; Baldassini, P.; Cendoya, M.G.; Ciuffoli, L.; Mastrángelo, M.; Piñeiro, G. Deforestation impacts on soil organic carbon stocks in the Semiarid Chaco Region, Argentina. Sci. Total Environ. 2017, 575, 1056–1065. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Chen, D.; Yang, T.; Huang, F.; Fu, S.; Li, L. Changes in soil labile and recalcitrant carbon pools after land-use change in a semi-arid agro-pastoral ecotone in Central Asia. Ecol. Ind. 2019, 110, 105925. [Google Scholar] [CrossRef]
- Menichetti, L.; Ågren, G.I.; Barré, P.; Moyano, F.; Kätterer, T. Generic parameters of first-order kinetics accurately describe soil organic matter decay in bare fallow soils over a wide edaphic and climatic range. Sci. Rep. 2019, 9, 20319. [Google Scholar] [CrossRef] [Green Version]
- Rovira, P.; Romanyà, J.; Duguy, B. Long-term effects of wildfires on the biochemical quality of soil organic matter: A study on Mediterranean shrublands. Geoderma 2012, 179–180, 9–19. [Google Scholar] [CrossRef]
- Schmidt, M.W.; Torn, M.S.; Abiven, S.; Dittmar, T.; Guggenberger, G.; Janssens, I.A.; Kleber, M.; Kogel-Knabner, I.; Lehmann, J.; Manning, D.A.C.; et al. Persistence of soil organic matter as an ecosystem property. Nature 2011, 478, 49–56. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Cao, J.; Zhao, J.; Wu, J.; Zou, X.; Fu, S.; Zhang, W. Labile C dynamics reflect soil organic carbon sequestration capacity: Understory plants drive topsoil C process in subtropical forests. Ecosphere 2019, 10, e02784. [Google Scholar] [CrossRef]
- Lehmann, J.; Hansel, C.M.; Kaiser, C.; Kleber, M.; Maher, K.; Manzoni, S.; Nunan, N.; Reichstein, M.; Schimel, J.P.; Torn, M.S.; et al. Persistence of soil organic carbon caused by functional complexity. Nat. Geosci. 2020, 13, 529–534. [Google Scholar] [CrossRef]
- Ukalska-Jaruga, A.; Smreczak, B.; Klimkowicz-Pawlas, A. Soil organic matter composition as a factor affecting the accumulation of polycyclic aromatic hydrocarbons. J. Soils Sediments 2019, 19, 1890–1900. [Google Scholar] [CrossRef] [Green Version]
- Gregorich, E.G.; Gillespie, A.W.; Beare, M.H.; Curtin, D.; Sanei, H.; Yanni, S.F. Evaluating biodegradability of soil organic matter by its thermal stability and chemical composition. Soil Biol. Biochem. 2015, 91, 182–191. [Google Scholar] [CrossRef]
- von Lützow, M.; Kögel-Knabner, I.; Ekschmitt, K.; Flessa, H.; Guggenberger, G.; Matzner, E.; Marschner, B. SOM fractionation methods: Relevance to functional pools and to stabilization mechanisms. Soil Biol. Biochem. 2007, 39, 2183–2207. [Google Scholar] [CrossRef]
- Zhang, D.; Wu, J.; Yang, F.; Chen, Q.; Feng, J.; Li, Q.; Zhang, Q.; Wang, W.; Cheng, X. Linkages between soil organic carbon fractions and carbon-hydrolyzing enzyme activities across riparian zones in the Three Gorges of China. Sci. Rep. 2020, 10, 8433. [Google Scholar] [CrossRef]
- Rovira, P.; Vallejo, V.R. Labile and recalcitrant pools of carbon and nitrogen in organic matter decomposing at different depths in soils an acid hydrolysis approach. Geoderma 2002, 107, 109–141. [Google Scholar] [CrossRef]
- Ferraz de Almeida, R.; Rodrigues Mikhael, J.E.; Oliveira Franco, F.; Fonseca Santana, L.M.; Wendling, B. Measuring the Labile and Recalcitrant Pools of Carbon and Nitrogen in Forested and Agricultural Soils: A Study under Tropical Conditions. Forests 2019, 10, 544. [Google Scholar] [CrossRef] [Green Version]
- Xu, G.; Jiang, H.; Zhang, Y.; Korpelainen, H.; Li, C. Effect of warming on extracted soil carbon pools of Abies faxoniana forest at two elevations. For. Ecol. Manag. 2013, 310, 357–365. [Google Scholar] [CrossRef]
- Dou, C.; Ewanick, S.; Bura, R.; Gustafson, R. Post-treatment mechanical refining as a method to improve overall sugar recovery of steam pretreated hybrid poplar. Bioresour. Technol. 2016, 207, 157–165. [Google Scholar] [CrossRef] [Green Version]
- Feyissa, A.; Yang, F.; Feng, J.; Wu, J.; Chen, Q.; Cheng, X. Soil labile and recalcitrant carbon and nitrogen dynamics in relation to functional vegetation groups along precipitation gradients in secondary grasslands of South China. Environ. Sci. Pollut. Res. Int. 2020, 27, 10528–10540. [Google Scholar] [CrossRef]
- Guo, X.; Luo, Z.; Sun, O.J. Long-term litter type treatments alter soil carbon composition but not microbial carbon utilization in a mixed pine-oak forest. Biogeochemistry 2021, 152, 327–343. [Google Scholar] [CrossRef]
- Krull, E.S.; Swanston, C.W.; Skjemstad, J.O.; McGowan, J.A. Importance of charcoal in determining the age and chemistry of organic carbon in surface soils. J. Geophys. Res. 2006, 111, G04001–G04009. [Google Scholar] [CrossRef]
- Greenfield, L.G.; Gregorich, E.G.; van Kessel, C.; Baldock, J.A.; Beare, M.H.; Billings, S.A.; Clinton, P.W.; Condron, L.M.; Hill, S.; Hopkins, D.W.; et al. Acid hydrolysis to define a biologically-resistant pool is compromised by carbon loss and transformation. Soil Biol. Biochem. 2013, 64, 122–126. [Google Scholar] [CrossRef]
- Taye, M.; Zewdu, F.; Ayalew, D. Characterizing the climate system of Western Amhara, Ethiopia: A GIS approach. Am. J. Res. Commun. 2013, 1, 319–355. [Google Scholar]
- Cardelús, C.L.; Scull, P.; Hair, J.; Baimas-George, M.; Lowman, M.D.; Eshet, A.W. A preliminary assessment of Ethiopian sacred grove status at the landscape and ecosystem scales. Diversity 2013, 5, 320–334. [Google Scholar] [CrossRef]
- Wassie, A.; Sterck, F.J.; Teketay, D.; Bongers, F. Effects of livestock exclusion on tree regeneration in Church forests of Ethiopia. For. Ecol. Manag. 2009, 257, 765–772. [Google Scholar] [CrossRef]
- Meseret, D. Land Degradation in Amhara Region of Ethiopia: Review on Extent, Impacts and Rehabilitation Practices. J. Environ. Earth Sci. 2016, 6, 120–130. [Google Scholar]
- Kettler, T.A.; Doran, J.W.; Gilbert, T.L. Simplified method for soil particle-size determination to accompany soil-quality analyses. Soil Sci. Soc. Am. J. 2001, 65, 849–852. [Google Scholar] [CrossRef] [Green Version]
- Belay-Tedla, A.; Zhou, X.H.; Su, B.; Wan, S.Q.; Luo, Y.Q. Labile, recalcitrant, and microbial carbon and nitrogen pools of a tallgrass prairie soil in the US Great Plains subjected to experimental warming and clipping. Soil Biol. Biochem. 2009, 41, 110–111. [Google Scholar] [CrossRef]
- Schumacher, B.A. Methods for the Determination of Total Organic Carbon (TOC) in Soils and Sediments; U.S. Environmental Protection Agency: Las Vegas, NV, USA, 2002.
- Vesterdal, L.; Leifeld, J. Land-use change and management effects on soil carbon sequestration: Forestry and agriculture. COST 639 project: Greenhouse-gas budget of soils under changing climate and land use (BurnOut). COST Action 2007, 639, 25–32. [Google Scholar]
- Wei, X.; Shao, M.; Gale, W.; Li, L. Global pattern of soil carbon losses due to the conversion of forests to agricultural land. Sci. Rep. 2014, 4, 4062. [Google Scholar] [CrossRef]
- Abegaz, A.; Tamene, L.; Abera, W.; Yaeko, T.; Hailu, H.; Nyawira, S.S.; Da Silva, M.; Sommer, R. Soil organic carbon dynamics along chrono-sequence land-use systems in the highlands of Ethiopia. Agric. Ecosyst. Environ. 2020, 300, 106997. [Google Scholar] [CrossRef]
- Berihu, T.; Girmay, G.; Sebhatleab, M.; Berhane, E.; Zenebe, A.; Sigua, G.C. Soil carbon and nitrogen losses following deforestation in Ethiopia. Agron. Sustain. Dev. 2017, 37, 1. [Google Scholar] [CrossRef] [Green Version]
- Shapiro, B.I.; Gebru, G.; Desta, S.; Negassa, A.; Nigussie, K.; Aboset, G.; Mechale, H. Ethiopia livestock sector analysis. In Proceedings of the ILRI Project Report 2017; International Livestock Research Institute (ILRI): Nairobi, Kenya, 2017. [Google Scholar]
- Adimassu, Z.; Tamene, L.; Degefie, D.T. The influence of grazing and cultivation on runoff, soil erosion, and soil nutrient export in the central highlands of Ethiopia. Ecol. Process 2020, 9, 23. [Google Scholar] [CrossRef]
- Zhang, H.; Xiong, X.; Wu, J.; Zhao, J.; Zhao, M.; Chu, G.; Hui, D.; Zhou, G.; Deng, Q.; Zhang, D. Changes in Soil Microbial Biomass, Community Composition, and Enzyme Activities After Half-Century Forest Restoration in Degraded Tropical Lands. Forests 2019, 10, 1124. [Google Scholar] [CrossRef] [Green Version]
- Bossio, D.A.; Cook-Patton, S.C.; Ellis, P.W.; Fargione, J.; Sanderman, J.; Smith, P.; Wood, S.; Zomer, R.J.; von Unger, M.; Emmer, I.M.; et al. The role of soil carbon in natural climate solutions. Nat. Sustain. 2020, 3, 391–398. [Google Scholar] [CrossRef]
- Dynarski Katherine, A.; Bossio Deborah, A.; Scow Kate, M. Dynamic Stability of Soil Carbon: Reassessing the “Permanence” of Soil Carbon Sequestration. Front. Environ. Sci. 2020, 8, 218. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, X.; Xu, Y.; Jin, M.; Ye, X.; Gao, H.; Chu, W.; Mao, J.; Thompson, M.L. Soil labile organic carbon fractions and soil enzyme activities after 10 years of continuous fertilization and wheat residue incorporation. Sci. Rep. 2020, 10, 11318. [Google Scholar] [CrossRef]
- De Graaff, M.; Classen, A.; Castro, H.; Schadt, C. Labile soil carbon inputs mediate the soil microbial community composition and plant residue decomposition rates. N. Phytol. 2010, 188, 1055–1064. [Google Scholar] [CrossRef] [Green Version]
- Lombardo, L.; Trujillo, C.; Vanwalleghem, T.; Gómez, J.A. Organic carbon fluxes by precipitation, throughfall and stemflow in an olive orchard in Southern Spain. Plant Biosyst. 2018, 152, 1039–1047. [Google Scholar] [CrossRef]
- Leavitt, S.W.; Follett, R.F.; Paul, E.A. Estimation of slow and fast cycling soil organic carbon pools from 6N HCl hydrolysis. Radiocarbon 1997, 38, 231–239. [Google Scholar] [CrossRef] [Green Version]
- Delelegn, Y.T.; Purahong, W.; Blazevic, A. Yitaferu, B.; Wubet, T.; Göransson, H.; Godbold, D.L. Changes in land use alter soil quality and aggregate stability in the highlands of northern Ethiopia. Sci. Rep. 2017, 7, 13602. [Google Scholar] [CrossRef] [Green Version]
- Benjamin, P.; Sandra, M.-G.; Daniel, L.; Jarone, P. Labile Dissolved Organic Matter Compound Characteristics Select for Divergence in Marine Bacterial Activity and Transcription. Front. Microbiol. 2020, 11, 2365. [Google Scholar] [CrossRef]
- Baldrian, P.; Valaskováa, V. Degradation of cellulose by basidiomycetous fungi. FEMS Microbiol. Rev. 2008, 32, 501–521. [Google Scholar] [CrossRef] [Green Version]
- Pulidindi, I.N.; Kimchi, B.B.; and Gedanken, A. Can cellulose be a sustainable feedstock for bioethanol production? Renew. Energy 2014, 71, 77–80. [Google Scholar] [CrossRef]
- Snajdr, J.; Cajthaml, T.; Valaskova, V.; Merhautová, V.; Petránková, M.; Spetz, P.; Leppänen, K.; Baldrian, P. Transformation of Quercus petraea litter: Successive changes in litter chemistry are reflected in differential enzyme activity and changes in the microbial community composition. FEMS Microbiol. Ecol. 2011, 75, 291–303. [Google Scholar] [CrossRef] [Green Version]
- Chang, J.K.; Xavier, D.; Véronique, B.; Hassan, Z.; Lavoie, J. Two-step thermochemical cellulose hydrolysis with partial neutralization for glucose production. Front. Chem. 2018, 6, 117. [Google Scholar] [CrossRef] [Green Version]
- Chmolowska, D.; Hamda, N.; Laskowski, R. Cellulose decomposed faster in fallow soil than in meadow soil due to a shorter lag time. J. Soils Sediments 2017, 17, 299–305. [Google Scholar] [CrossRef] [Green Version]
- Maia Andrade, E.; Valbrun, W.; de Almeida, A.M.; Rosa, G.; da Silva, A.G.R. Land-Use Effect on Soil Carbon and Nitrogen Stock in a Seasonally Dry Tropical Forest. Agronomy 2020, 10, 158. [Google Scholar] [CrossRef] [Green Version]
- Yang, X.; Wei, X.; Xu, X.; Zhang, Y.; Li, J.; Wan, J. Characteristics of Dissolved Organic Nitrogen in the Sediments of Six Water Sources in Taihu Lake, China. Int. J. Environ. Res. Public Health 2019, 16, 929. [Google Scholar] [CrossRef] [Green Version]
- Nie, X.J.; Zhang, H.B.; Su, Y.Y. Soil carbon and nitrogen fraction dynamics affected by tillage erosion. Sci. Rep. 2019, 9, 16601. [Google Scholar] [CrossRef]
- Stamati, F.; Nikolaidis, N.; Venieri, D.; Psillakis, E.; Kalogerakis, N. Dissolved organic nitrogen as an indicator of livestock impacts on soil biochemical quality. Appl. Geochem. 2011, 26. [Google Scholar] [CrossRef]
- Chanie, T.; Collick, A.S.; Adgo, E.; Lehmann, C.J.; Steenhuis, T.S. Ecohydrological impacts of eucalyptus in the semi humid Ethiopian highlands: The Lake tana plain. J. Hydrol. Hydromech. 2013, 61, 21–29b. [Google Scholar] [CrossRef] [Green Version]
- Nath, A.J.; Brahma, B.; Sileshi, G.W.; Das, A.K. Impact of land use changes on the storage of soil organic carbon in active and recalcitrant pools in a humid tropical region of India. Sci. Total Environ. 2018, 624, 908–917. [Google Scholar] [CrossRef]
- Kleber, M.; Nico, P.S.; Plante, A.; Filley, T.; Kramer, M.; Swanston, C.; Sollins, P. Old and stable soil organic matter is not necessarily chemically recalcitrant: Implications for modeling concepts and temperature sensitivity. Glob. Chang. Biol. 2011, 17, 1097–1107. [Google Scholar] [CrossRef] [Green Version]
- Olk, D.C.; Bloom, P.R.; De Nobili, M.; Chen, Y.; McKnight, D.M.; Wells, M.J.M.; Weber, J. Using humic fractions to understand natural organic matter processes in soil and water: Selected studies and applications. J. Environ. Qual. 2019, 48, 1633–1643. [Google Scholar] [CrossRef] [Green Version]
- Rovira, P.; Vallejo, V.R. Labile and recalcitrant and inert organic matter in Mediterranean forest soils. Soil Biol. Biochem. 2007, 39, 202–215. [Google Scholar] [CrossRef]
- Zhen, Y.; Lu, C.; Hennessy, D.A.; Feng, H.; Tian, H. Impacts of tillage practices on soil carbon stocks in the US corn-soybean cropping system during 1998 to 2016. Environ. Res. Lett. 2020, 15, 014008. [Google Scholar]
- Guillaume, T.; Damris, M.; Kuzyakov, Y. Losses of soil carbon by converting tropical forest to plantations: Erosion and decomposition estimated by δ13C. Glob. Chang. Biol. 2015, 21, 3548–3560. [Google Scholar] [CrossRef]
- Sahoo, U.K.; Singh, S.L.; Gogoi, A.; Kenye, A.; Sahoo, S.S. Active and passive soil organic carbon pools as affected by different land use types in Mizoram, Northeast India. PLoS ONE 2019, 14, e0219969. [Google Scholar] [CrossRef] [Green Version]
- Abril, A.; Casado-Murillo, N.; Vázquez, C.; Olivera, P. Labile and Recalcitrant Carbon in Crop Residue and Soil under No-Till Practices in Central Region of Argentina. Open Agric. J. 2013, 7, 32–39. [Google Scholar] [CrossRef] [Green Version]
- Ghafoor, A.; Poeplau, C.; Kätterer, T. Fate of straw- and root-derived carbon in a Swedish agricultural soil. Biol. Fertil. Soils 2017, 53, 257–267. [Google Scholar] [CrossRef] [Green Version]
- Viera, M.; Rodríguez-Soalleiro, R.A. Complete Assessment of Carbon Stocks in Above and Belowground Biomass Components of a Hybrid Eucalyptus Plantation in Southern Brazil. Forests 2019, 10, 536. [Google Scholar] [CrossRef] [Green Version]
- Kögel-Knabner, I. The macromolecular organic composition of plant and microbial residues as inputs to soil organic matter. Soil Biol. Biochem. 2002, 34, 139–162. [Google Scholar] [CrossRef]
- Frey, S.D. Mycorrhizal Fungi as Mediators of Soil Organic Matter Dynamics. Annu. Rev. Ecol. Evol. Syst. 2019, 50, 237–259. [Google Scholar] [CrossRef]
- Compton, J.E.; Boone, R.D. Long-term impacts of agriculture on soil carbon and nitrogen in New England forests. Ecology 2000, 81, 2314–2330. [Google Scholar] [CrossRef]
- Zheng, H.; Liu, W.; Zheng, J.; Luo, Y.; Li, R.; Wang, H.; Qi, H. Effect of long-term tillage on soil aggregates and aggregate-associated carbon in black soil of Northeast China. PLoS ONE 2018, 13, e0199523. [Google Scholar] [CrossRef]
- Lyu, M.; Xie, J.; Ukonmaanaho, L.; Jiang, M.; Li, Y.; Chen, Y.; Yang, Z.; Zhou, Y.; Lin, W.; Yang, Y. Land use change exerts a strong impact on deep soil C stabilization in subtropical forests. J. Soils Sediments 2017, 17, 2305–2317. [Google Scholar] [CrossRef]
- Hicks, L.C.; Meir, P.; Nottingham, A.T.; Reay, D.S.; Stott, A.W.; Salinas, N.; Whitaker, J. Carbon and nitrogen inputs differentially affect priming of soil organic matter in tropical lowland and montane soils. Soil Biol. Biochem. 2019, 129, 212–222. [Google Scholar] [CrossRef]
- Zhang, H.; Zhou, Z. Recalcitrant carbon controls the magnitude of soil organic matter mineralization in temperate forests of northern China. For. Ecosyst. 2018, 5, 17. [Google Scholar] [CrossRef]
- Batjes, N.H. Total carbon and nitrogen in the soils of the world. Eur. J. Soil Sci. 2014, 65, 10–21. [Google Scholar] [CrossRef]
- Veldkamp, E.; Schmidt, M.; Powers, J.S.; Corre, M.D. Deforestation and reforestation impact on soils in the tropics. Nat. Rev. Earth Environ. 2020, 1, 590–605. [Google Scholar] [CrossRef]
- Tolessa, T.; Senbeta, F. The extent of soil organic carbon and total nitrogen in forest fragments of the central highlands of Ethiopia. J. Ecol. Environ. 2018, 42, 20. [Google Scholar] [CrossRef]
- Fan, J.; Zhang, C.; Jin, H.; Zhang, J.; Han, G. Grazing accelerates labile and recalcitrant soil carbon loss driving by rare microbial taxa in a desert steppe. Land Degrad. Dev. 2021, 1–13. [Google Scholar] [CrossRef]
- Loke, P.F.; Kotzé, E.; Du Preez, C.C.; Twigge, L. Cross-rangeland comparisons on soil carbon dynamics in the Pedoderm of semi-arid and arid South African commercial farms. Geoderma 2021, 381, 114689. [Google Scholar] [CrossRef]
- Pineiro, G.; Oesterheld, M.; Batista, W.B.; Paruelo, J.M. Opposite changes of whole-soil vs. pools C:N ratios: A case of Simpson’s paradox with implications on nitrogen cycling. Glob. Chang. Biol. 2006, 12, 804–809. [Google Scholar] [CrossRef]
- Silveira, M.L.; Comerford, N.B.; Reddy, K.R.; Cooper, W.T.; El-Rifai, H. Characterization of soil organic carbon pools by acid hydrolysis. Geoderma 2008, 144, 405–414. [Google Scholar] [CrossRef]
- Rovira, P.; Jorba, M.; Romanyà, J. Active and passive organic matter fractions in Mediterranean forest soils. Biol. Fertil. Soils 2010, 46, 355–369. [Google Scholar] [CrossRef]
- Olson, B.M.; Lowe, L.E. Effects of intensive vegetable production on the hydrolyzable carbon, nitrogen and sulphur of a British Columbia humisol. Can. J. Soil Sci. 1989, 70, 621–628. [Google Scholar]
- Fang, S.; Xie, B.; Zhang, H. Nitrogen dynamics and mineralization in degraded agricultural soil mulched with fresh grass. Plant Soil 2007, 300, 269–280. [Google Scholar] [CrossRef]
- Mahal, N.K.; Osterholz, W.R.; Miguez, F.E.; Poffenbarger, H.J.; Sawyer, J.E.; Olk, D.C.; Archontoulis, S.V.; Castellano, M.J. Nitrogen Fertilizer Suppresses Mineralization of Soil Organic Matter in Maize Agroecosystems. Front. Ecol. Evol. 2019, 7, 59. [Google Scholar] [CrossRef] [Green Version]
- Paré, T.; Dinel, H.; Schnitzer, M.; Dumontet, S. Transformations of carbon and nitrogen during composting of animal manure and shredded paper. Biol. Fertil. Soils 1998, 26, 173–178. [Google Scholar] [CrossRef]


| Land-Use | ||||
|---|---|---|---|---|
| Natural Forest | Grazing Land | Crop Land | Eucalyptus Planta | |
| Sand-Silt-clay (%) | 9-39-52 | 24-48-28 | 3-50-46 | 10-41-49 |
| Textural class | Clay | Clay loam | Silty clay | Silty clay |
| Bulk density (g cm−3) | 0.74 ± 0.01 | 1.22 ± 0.06 | 1.23 ± 0.03 | 1.2 4 ± 0.03 |
| pH (H2O) | 6.26 ± 0.08 | 5.95 ± 0.12 | 5.99 ± 0.05 | 6.12 ± 0.15 |
| pH (CaCl2) | 5.94 ± 0.11 | 5.37 ± 0.07 | 5.13 ± 0.06 | 5.08 ± 0.13 |
| Organic matter (%)(loss on ignition) | 20.9 ± 3.6 | 10.5 ± 0.6 | 9.3 ± 0.7 | 11.5 ± 0.4 |
| C (%) | 11.3 ± 1.4 | 2.7 ± 0.4 | 2.7 ± 0.2 | 4.0 ± 0.6 |
| N (%) | 1.07 ± 0.13 | 0.24 ± 0.03 | 0.21 ± 0.02 | 0.3 ± 0.04 |
| C:N Ratio | 10.6 ± 0.1 | 16.2 ± 1.1 | 13.3 ± 1.1 | 10.8 ± 0.2 |
| Vegetation (dominant species) | Albizia gummifera, Dombeya torrida, Croton macrostachus | Bare land with sparse grass tufts | Teff (Eragrostis tef) | Eucalyptus globulus (with few understory shrubs) |
| Sources | C Fraction 1 | C Fraction 2 | C Fraction 3 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| df | Mean Square | F Value | Sig. | Mean Square | F Value | Sig. | Mean Square | F Value | Sig. | |
| Corrected Model | 15 | 0.08 | 5.64 | ** | 0.505 | 11.736 | ** | 0.182 | 6.502 | ** |
| Intercept | 1 | 46.54 | 3266.01 | ** | 15.633 | 363.60 | ** | 7.526 | 268.83 | ** |
| Land-use | 3 | 0.12 | 8.50 | ** | 2.172 | 50.511 | ** | 0.456 | 16.273 | ** |
| Depth | 3 | 0.23 | 15.86 | ** | 0.331 | 7.696 | ** | 0.377 | 13.483 | ** |
| Land-use × Depth | 9 | 0.02 | 1.28 | NS | 0.007 | 0.157 | NS | 0.026 | 0.918 | NS |
| Error | 64 | 0.02 | 0.043 | 0.028 | ||||||
| N fraction 1 | N fraction 2 | N fraction 3 | ||||||||
| Corrected Model | 15 | 0.70 | 19.53 | ** | 0.602 | 15.756 | ** | 0.100 | 3.610 | ** |
| Intercept | 1 | 197.52 | 5516.73 | ** | 208.439 | 5456.02 | ** | 39.724 | 1427.06 | ** |
| Land-use | 3 | 3.09 | 86.38 | ** | 2.652 | 69.428 | ** | 0.218 | 7.843 | ** |
| Depth | 3 | 0.33 | 9.14 | ** | 0.335 | 8.768 | ** | 0.238 | 8.550 | ** |
| Land-use × Depth | 9 | 0.03 | 0.71 | NS | 0.007 | 0.195 | NS | 0.015 | 0.552 | NS |
| Error | 64 | 0.04 | 0.038 | 0.028 | ||||||
| C Fraction (mg g−1 C) | N Fraction (mg g−1 N) | |||||||
|---|---|---|---|---|---|---|---|---|
| Soil Depth (cm) | Natural Forest | Grazing Land | Crop Land | Eucalyptus | Natural Forest | Grazing Land | Cropland | Eucalyptus |
| Fraction 1 | ||||||||
| 0–10 | 152 ± 7 a | 213 ± 13 b | 187 ± 10 ab | 164 ± 11 a | 218 ± 34 a | 92 ± 7 b | 81 ± 4 b | 85 ± 5 b |
| 10–20 | 145 ± 13a | 226 ± 17 b | 184 ± 14 ab | 229 ± 18 b | 288 ± 44 a | 85 ± 5 b | 70 ± 6 b | 96 ± 7 b |
| 20–30 | 132 ± 13 a | 228 ± 26 b | 184 ± 8 ab | 227 ± 19 b | 207 ± 20 a | 87 ± 4 b | 67 ± 3 b | 75 ± 9 b |
| 30–50 | 134 ± 15 a | 218 ± 31 b | 190 ± 12 ab | 240 ± 23 b | 192 ± 20 a | 88 ± 8 b | 63 ± 5 b | 126 ± 29 ab |
| Fraction 2 | ||||||||
| 0–10 | 190 ± 9 a | 113 ± 8 b | 99 ± 11 b | 87 ± 5 b | 178 ± 10 a | 79 ± 5 b | 83 ± 5 b | 82 ± 6 b |
| 10–20 | 212 ± 2 a | 112 ± 8 b | 103 ± 15 b | 119 ± 17 b | 182 ± 7 a | 79 ± 4 b | 76 ± 7 b | 83 ± 7 b |
| 20–30 | 236 ± 28 a | 106 ± 6 b | 99 ± 15 b | 104 ± 18 b | 187 ± 27 a | 75 ± 6 b | 78 ± 10 b | 72 ± 8 b |
| 30–50 | 252 ± 35 a | 101 ± 4 b | 85 ± 20 b | 108 ± 14 b | 181 ± 24 a | 74 ± 3 b | 68 ± 9 b | 84 ± 13 b |
| Fraction 3 | ||||||||
| 0–10 | 658 ± 15 a | 674 ± 11 ab | 713 ± 15 b | 748 ± 11 b | 604 ± 41 a | 829 ± 3 b | 836 ± 5 b | 833 ± 10 b |
| 10–20 | 643 ± 14 a | 661 ± 12 a | 713 ± 14 a | 657 ± 32 a | 529 ± 40 a | 837 ± 3 b | 854 ± 8 b | 820 ± 7 b |
| 20–30 | 632 ± 37 a | 666 ± 27 a | 718 ± 20 a | 669 ± 16 a | 608 ± 44 a | 837 ± 4 b | 855 ± 12 b | 854 ± 10 b |
| 30–50 | 614 ± 50 a | 681 ± 30 a | 725 ± 20 a | 652 ± 18 a | 627 ± 44 a | 838 ± 5 b | 868 ± 10 b | 790± 41 b |
| Depth (cm) | Forest | Grazing Land | Crop Land | Eucalyptus |
|---|---|---|---|---|
| Recalcitrant C Index (RIC) | ||||
| 0–10 | 43 ± 2 a | 45 ± 1 ab | 51 ± 2 bc | 56 ± 2 c |
| 10–20 | 41 ± 2 a | 44 ± 2 a | 51 ± 2 a | 43 ± 4 a |
| 20–30 | 40 ± 5 a | 45 ± 4 a | 52 ± 3 a | 45 ± 2 a |
| 30–50 | 39 ± 6 a | 47 ± 4 a | 53 ± 3 a | 43 ± 2 a |
| Recalcitrant N Index (RIN) | ||||
| 0–10 | 32 ± 2 a | 68 ± 1 b | 70 ± 1 b | 69 ± 2 b |
| 10–20 | 32 ± 1 a | 70 ± 1 bc | 73 ± 1 b | 67 ± 1 c |
| 20–30 | 33 ± 4 a | 70 ± 1 b | 73 ± 2 b | 73 ± 2 b |
| 30–50 | 35 ± 4 a | 70 ± 1 b | 75 ± 2 b | 63 ± 6 b |
| Land-Use & Soil Depth (cm) | C:N Ratio | |||
|---|---|---|---|---|
| Whole Soil | Fraction 1 | Fraction 2 | Fraction 3 | |
| Natural Forest | ||||
| 0–10 | 11 ± 0.1 | 6.5± 0.1 | 11.4 ± 0.2 | 11.7 ± 0.5 |
| 10–20 | 10 ± 0.2 | 6.6 ± 0.2 | 12.1 ± 0.6 | 13.1 ± 1.6 |
| 20–30 | 10 ± 0.2 | 6.9 ± 0.2 | 14.0 ± 1.0 | 11.3 ± 0.3 |
| 30–50 | 12 ± 0.6 | 7.9 ± 0.3 | 15.9 ± 1.0 | 11.2 ± 0.8 |
| Mean | 11 ± 0.3 a | 7.0 ± 0.2 a | 13.3 ± 0.5 a | 11.8 ± 0.4 a |
| Grazing Land | ||||
| 0–10 | 11 ± 0.3 | 25.6 ± 2.0 | 15.4 ± 0.5 | 8.8 ± 0.1 |
| 10–20 | 11 ± 0.1 | 28.2 ± 0.9 | 15.0 ± 0.4 | 8.4 ± 0.3 |
| 20–30 | 10 ± 0.2 | 27.6 ± 2.6 | 15.0 ± 0.4 | 8.5 ± 0.7 |
| 30–50 | 11 ± 0.5 | 26.6 ± 3.2 | 14.3 ± 0.5 | 8.6 ± 0.7 |
| Mean | 11 ± 0.3 a | 26.0 ± 1.0 b | 15.0 ± 0.2 ab | 8.6 ± 0.2 b |
| Crop Land | ||||
| 0–10 | 12 ± 1.0 | 30.8 ± 2.3 | 15.4 ± 0.2 | 11.4 ± 1.1 |
| 10–20 | 13 ± 0.8 | 32.3 ± 1.9 | 16.1 ± 1.2 | 10.2 ± 0.6 |
| 20–30 | 13 ± 0.5 | 36.7 ± 2.4 | 16.3 ± 0.7 | 11.1 ± 1.0 |
| 30–50 | 13 ± 0.4 | 38.0 ± 2.0 | 14.8 ± 2.1 | 10.5 ± 0.4 |
| Mean | 13 ± 0.4 b | 34.3 ± 1.1 c | 15.6 ± 0.6 b | 10.8 ± 0.4 a |
| Eucalyptus | ||||
| 0–10 | 14 ± 1.0 | 31.3 ± 1.1 | 17.3 ± 1.1 | 14.6 ± 1.2 |
| 10–20 | 12 ± 1.0 | 30.1 ± 1.8 | 18.0 ± 2.4 | 10.2 ± 1.2 |
| 20–30 | 13 ± 2.0 | 35.6 ± 2.7 | 16.0 ± 1.0 | 9.0 ± 0.8 |
| 30–50 | 12 ± 1.0 | 25.4 ± 5.1 | 14.5 ± 0.6 | 9.5 ± 1.3 |
| Mean | 13 ± 1.0 b | 30.6 ± 1.5 bc | 16.4 ± 0.7 b | 10.8 ± 0.7 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmed, I.U.; Assefa, D.; Godbold, D.L. Land-Use Change Depletes Quantity and Quality of Soil Organic Matter Fractions in Ethiopian Highlands. Forests 2022, 13, 69. https://doi.org/10.3390/f13010069
Ahmed IU, Assefa D, Godbold DL. Land-Use Change Depletes Quantity and Quality of Soil Organic Matter Fractions in Ethiopian Highlands. Forests. 2022; 13(1):69. https://doi.org/10.3390/f13010069
Chicago/Turabian StyleAhmed, Iftekhar U., Dessie Assefa, and Douglas L. Godbold. 2022. "Land-Use Change Depletes Quantity and Quality of Soil Organic Matter Fractions in Ethiopian Highlands" Forests 13, no. 1: 69. https://doi.org/10.3390/f13010069
APA StyleAhmed, I. U., Assefa, D., & Godbold, D. L. (2022). Land-Use Change Depletes Quantity and Quality of Soil Organic Matter Fractions in Ethiopian Highlands. Forests, 13(1), 69. https://doi.org/10.3390/f13010069








