Effects of Buried Wood on the Development of Populus tremuloides on Various Oil Sands Reclamation Soils
Abstract
:1. Introduction
2. Materials and Methods
2.1. Soil Characteristics
2.2. Experimental Design
2.3. Statistical Analysis
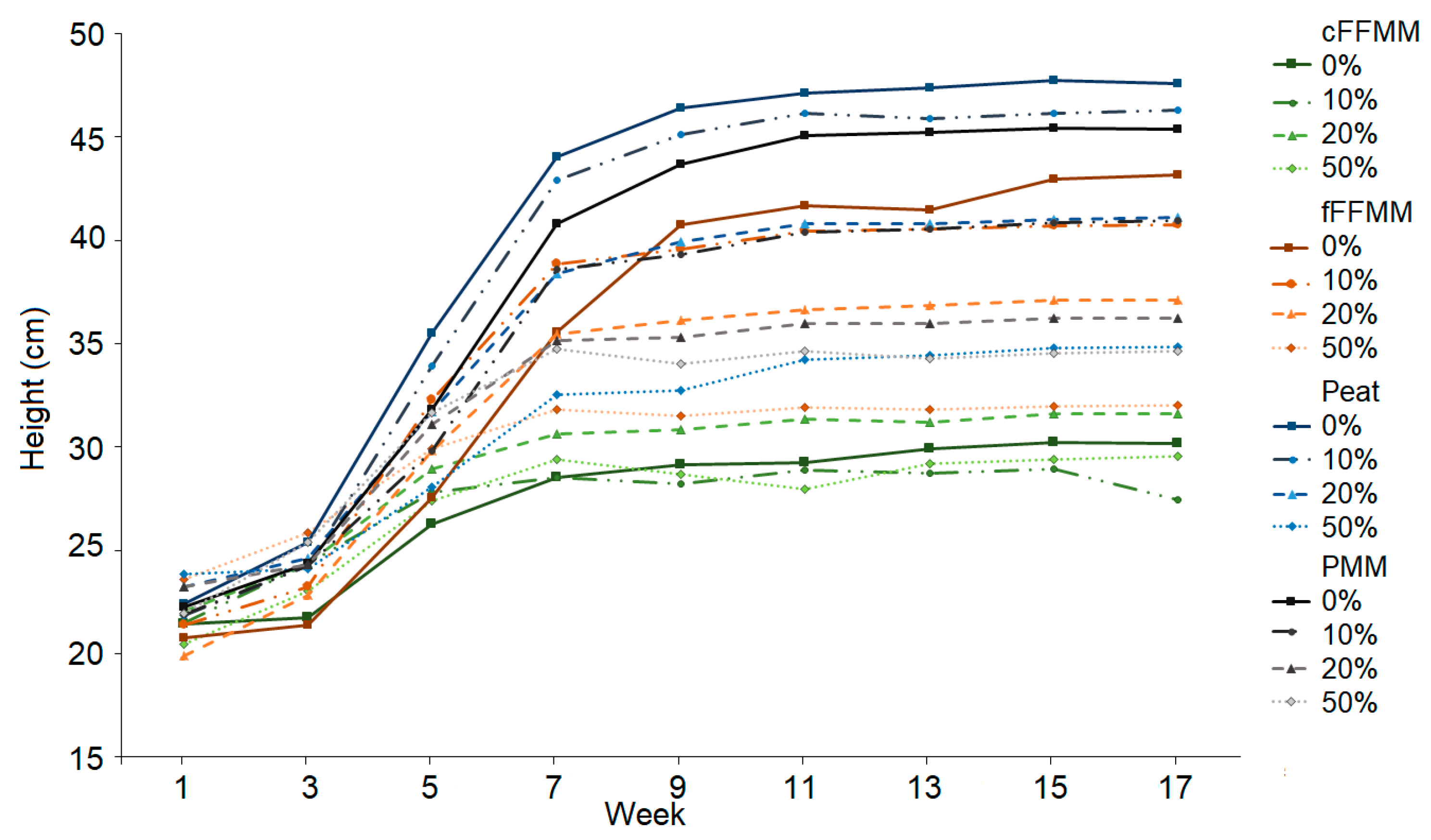
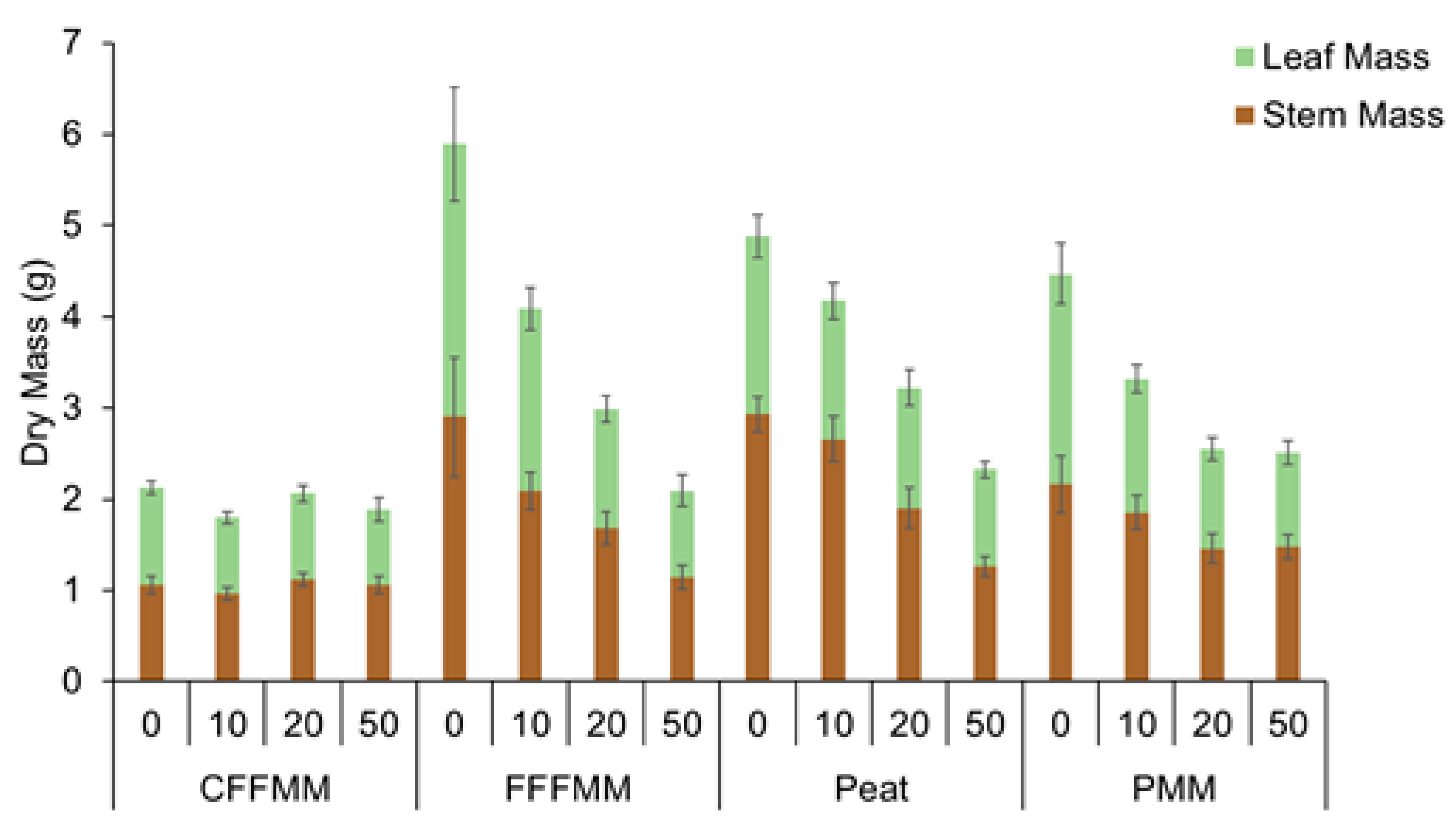
3. Results
4. Discussion
5. Management Implications
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Government of Alberta. Environmental Protection and Enhancement Act; Alberta Queen’s Printer: Edmonton, AB, Canada, 2018.
- Government of Alberta. Oil Sands Facts and Statistics. Available online: https://www.alberta.ca/oil-sands-facts-and-statistics.aspx (accessed on 7 September 2021).
- Government of Alberta. Oil Sands Mine Reclamation and Disturbance Tracking by Year. Available online: https://osip.alberta.ca/library/Dataset/Details/27 (accessed on 7 September 2021).
- Alberta Environment. Guidelines for Reclamation to Forest Vegetation in the Athabasca Oil Sands Region; Prepared by the Terrestrial Subgroup of the Reclamation Working Group of the Cumulative Environmental Management Association; Alberta Environment: Edmonton, AB, Canada, 2009. [Google Scholar]
- Dhar, A.; Comeau, P.G.; Karst, J.; Pinno, B.D.; Chang, S.X.; Naeth, A.M.; Vassov, R.; Bampfylde, C. Plant Community Development Following Reclamation of Oil Sands Mine Sites in the Boreal Forest: A Review. Environ. Rev. 2018, 298, 1–13. [Google Scholar] [CrossRef]
- Macdonald, S.E.; Quideau, S.; Landhäusser, S.M. Rebuilding Boreal Forest Ecosystems after Industrial Disturbance. Restor. Reclam. Boreal Ecosyst. 2012, 123–160. [Google Scholar] [CrossRef]
- Macdonald, S.E.; Fenniak, T.E. Understory Plant Communities of Boreal Mixedwood Forests in Western Canada: Natural Patterns and Response to Variable-Retention Harvesting. For. Ecol. Manag. 2007, 242, 34–48. [Google Scholar] [CrossRef]
- Macdonald, S.E.; Snively, A.E.K.; Fair, J.M.; Landhäusser, S.M. Early Trajectories of Forest Understory Development on Reclamation Sites: Influence of Forest Floor Placement and a Cover Crop. Restor. Ecol. 2015, 23, 698–706. [Google Scholar] [CrossRef]
- Kwak, J.H.; Chang, S.X.; Naeth, M.A.; Schaaf, W. Coarse Woody Debris Increases Microbial Community Functional Diversity but Not Enzyme Activities in Reclaimed Oil Sands Soils. PLoS ONE 2015, 10, e0143857. [Google Scholar] [CrossRef] [PubMed]
- Kwak, J.H.; Chang, S.X.; Naeth, M.A.; Schaaf, W. Coarse Woody Debris Extract Decreases Nitrogen Availability in Two Reclaimed Oil Sands Soils in Canada. Ecol. Eng. 2015, 84, 13–21. [Google Scholar] [CrossRef]
- Stokland, J.N. Wood Decomposition. In Biodiversity in Deadwood; Cambridge University Press: Cambridge, UK, 2012; pp. 10–28. [Google Scholar]
- Moroni, M.T.; Morris, D.M.; Shaw, C.; Stokland, J.N.; Harmon, M.E.; Fenton, N.J.; Merganičová, K.; Merganič, J.; Okabe, K.; Hagemann, U. Buried Wood: A Common Yet Poorly Documented Form of Deadwood. Ecosystems 2015, 18, 605–628. [Google Scholar] [CrossRef]
- Zeng, N.; King, A.W.; Zaitchik, B.; Wullschleger, S.D.; Gregg, J.; Wang, S.; Kirk-Davidoff, D. Carbon Sequestration via Wood Harvest and Storage: An Assessment of Its Harvest Potential. Clim. Chang. 2013, 118, 245–257. [Google Scholar] [CrossRef]
- Eden, W.J. Buried Soil Profile under Apron of an Earth Flow. Bull. Geol. Soc. Am. 1967, 78, 1183–1184. [Google Scholar] [CrossRef]
- Hagemann, U.; Moroni, M.T.; Shaw, C.H.; Kurz, W.A.; Makeschin, F. Comparing Measured and Modelled Forest Carbon Stocks in High-Boreal Forests of Harvest and Natural-Disturbance Origin in Labrador, Canada. Ecol. Model. 2010, 221, 825–839. [Google Scholar] [CrossRef]
- Alberta Environment and Water. Best Management Practices for Conservation of Reclamation Materials in the Mineable Oil Sands Region of Alberta; Prepared by MacKenzie, D. for the Terrestrial Subgroup, Best Management Practices Task Group of the Reclamation Working Group of the Cumulative Environmental Management Association; Alberta Environment and Water: Edmonton, AB, Canada, 2012; ISBN 9781460100486. [Google Scholar]
- Alberta Sustainable Resource Development. Management of Wood Chips on Public Land; Alberta Sustainable Resource Development: Edmonton, AB, Canada, 2009. [Google Scholar]
- McMillan, R.; Quideau, S.A.; MacKenzie, M.D.; Biryukova, O. Nitrogen Mineralization and Microbial Activity in Oil Sands Reclaimed Boreal Forest Soils. J. Environ. Qual. 2007, 36, 1470–1478. [Google Scholar] [CrossRef]
- MacKenzie, M.D.; Quideau, S.A. Laboratory-Based Nitrogen Mineralization and Biogeochemistry of Two Soils Used in Oil Sands Reclamation. Can. J. Soil Sci. 2012, 92, 131–142. [Google Scholar] [CrossRef]
- Quideau, S.A.; Norris, C.E.; Rees, F.; Dyck, M.; Samadi, N.; Oh, S.W. Carbon, Nitrogen, and Phosphorus Release from Peat and Forest Floor-Based Cover Soils Used during Oil Sands Reclamation. Can. J. Soil Sci. 2017, 97, 757–768. [Google Scholar] [CrossRef] [Green Version]
- Mukhortova, L.V. Carbon and Nutrient Release during Decomposition of Coarse Woody Debris in Forest Ecosystems of Central Siberia. Folia For. Pol. Ser. A 2012, 54, 71–83. [Google Scholar] [CrossRef]
- Smyth, C.E.; Titus, B.; Trofymow, J.A.; Moore, T.R.; Preston, C.M.; Prescott, C.E.; the CIDET Working Group Patterns of Carbon. Nitrogen and Phosphorus Dynamics in Decomposing Wood Blocks in Canadian Forests. Plant Soil 2016, 409, 459–477. [Google Scholar] [CrossRef]
- Ulyshen, M.D. Saproxylic Insects Diversity, Ecology and Conservation; Springer: Berlin, Germany, 2018; Volume 1, ISBN 978-3-319-75936-4. [Google Scholar]
- Howell, D.M.; MacKenzie, M.D. Using Bioavailable Nutrients and Microbial Dynamics to Assess Soil Type and Placement Depth in Reclamation. Appl. Soil Ecol. 2017, 116, 87–95. [Google Scholar] [CrossRef]
- Burns, R.M.; Honkala, B.H. Silvics of North America: Volume 2. Hardwoods; United States Department of Agriculture (USDA), Forest Service: Washington, DC, USA, 1990; Volume 2, p. 877.
- Pinno, B.D.; Errington, R.C. Maximizing Natural Trembling Aspen Seedling Establishment on a Reclaimed Boreal Oil Sands Site. Ecol. Restor. 2015, 33, 43–50. [Google Scholar] [CrossRef] [Green Version]
- Jean, S.A.; Pinno, B.D.; Nielsen, S.E. Trembling Aspen Root Suckering and Stump Sprouting Response to above Ground Disturbance on a Reclaimed Boreal Oil Sands Site in Alberta, Canada. New For. 2019, 50, 771–784. [Google Scholar] [CrossRef]
- Pinno, B.D.; Landhäusser, S.M.; Derek MacKenzie, M.; Quideau, S.A.; Chow, P.S. Trembling Aspen Seedling Establishment, Growth and Response to Fertilization on Contrasting Soils Used in Oil Sands Reclamation. Can. J. Soil Sci. 2012, 92, 143–151. [Google Scholar] [CrossRef] [Green Version]
- Ste-Marie, C.; Paré, D.; Gagnon, D. The Contrasting Effects of Aspen and Jack Pine on Soil Nutritional Properties Depend on Parent Material. Ecosystems 2007, 10, 1299–1310. [Google Scholar] [CrossRef]
- Chen, H.Y.H.; Klinka, K.; Kabzems, R.D. Site Index, Site Quality, and Foliar Nutrients of Trembling Aspen: Relationships and Predictions. Can. J. For. Res. 1998, 28, 1743–1755. [Google Scholar] [CrossRef]
- Hogberg, J.I.; Pinno, B.D.; MacKenzie, M.D. Evaluating Foliar Nutrient Concentration as an Indicator of Soil Nutrients in Reclaimed and Natural Forests in Alberta, Canada. Int. J. Min. Reclam. Environ. 2020, 34, 75–87. [Google Scholar] [CrossRef]
- das Gupta, S.; Pinno, B.D. Drivers of Understory Species Richness in Reconstructed Boreal Ecosystems: A Structural Equation Modeling Analysis. Sci. Rep. 2020, 10, 11555. [Google Scholar] [CrossRef]
- Silva, J.A.; Uchida, R.S.; Silva, J.A. Plant Nutrient Management in Hawaii’s Soils Approaches for Tropical and Subtropical Agriculture From: Plant Nutrient Management in Hawaii’s Soils, Approaches for Tropical and Subtropical Agriculture; College of Tropical Agriculture and Human Resources: Honolulu, HI, USA, 2000. [Google Scholar]
- Evans, J.R.; Clarke, V.C. The Nitrogen Cost of Photosynthesis. J. Exp. Bot. 2019, 70, 7–15. [Google Scholar] [CrossRef]
- Kalra, Y.P.; Maynard, D.G. Methods Manual for Forest Soil and Plant Analysis; Northern Forestry Centre: Edmonton, AB, Canada, 1991. [Google Scholar]
- Janssen, B.H. Nitrogen Mineralization in Relation to C:N Ratio and Decomposability of. Plant Soil 1996, 39–45. [Google Scholar] [CrossRef]
- Swift, M.J. The Ecology of Wood Decomposition. Sci. Prog. 1977, 64, 175–199. [Google Scholar]
- Omari, K.; Kranabetter, J.M.; de Montigny, L. Productivity of Coastal Douglas-Fir and Western Redcedar in Response to Species Mixture, Planting Density, and Soil Carbon: Nitrogen Ratio. Can. J. For. Res. 2021, 51, 668–674. [Google Scholar] [CrossRef]
- Lotfiomran, N.; Köhl, M.; Fromm, J. Interaction Effect between Elevated CO2 and Fertilization on Biomass, Gas Exchange and C/N Ratio of European Beech (Fagus sylvatica L.). Plants 2016, 5, 38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wan, X.; Huang, Z.; He, Z.; Yu, Z.; Wang, M.; Davis, M.R.; Yang, Y. Soil C:N Ratio Is the Major Determinant of Soil Microbial Community Structure in Subtropical Coniferous and Broadleaf Forest Plantations. Plant Soil 2015, 387, 103–116. [Google Scholar] [CrossRef]
- Kirk, T.K.; Cowling, E.B. Biological Decomposition of Solid Wood; American Chemical Society: Washington, DC, USA, 1984. [Google Scholar]
- Zabel, R.; Morrell, J. Wood Microbiology, 2nd ed.; Elsecier: London, UK, 2020. [Google Scholar]
- Yan, H.; Hu, X.; Li, F. Leaf Photosynthesis, Chlorophyll Fluorescence, Ion Content and Free Amino Acids in Caragana Korshinskii Kom Exposed to NaCl Stress. Acta Physiol. Plant. 2012, 34, 2285–2295. [Google Scholar] [CrossRef]
- Jamro, G.M.; Chang, S.X.; Naeth, M.A. Organic Capping Type Affected Nitrogen Availability and Associated Enzyme Activities in Reconstructed Oil Sands Soils in Alberta, Canada. Ecol. Eng. 2014, 73, 92–101. [Google Scholar] [CrossRef]
- Hemstock, S.S.; Quideau, S.A.; Chanasyk, D.S. Nitrogen Availability from Peat Amendments Used in Boreal Oil Sands Reclamation. Can. J. Soil Sci. 2010, 90, 165–175. [Google Scholar] [CrossRef]
- Jalali, M.; Merrikhpour, H. Effects of Poor Quality Irrigation Waters on the Nutrient Leaching and Groundwater Quality from Sandy Soil. Environ. Geol. 2008, 53, 1289–1298. [Google Scholar] [CrossRef]
- Erickson, J.E.; Cisar, J.L.; Snyder, G.H.; Volin, J.C.; Park, D.M. Erratum: Phosphorus and Potassium Leaching under Contrasting Residential Landscape Models Established on a Sandy Soil. Crop Sci. 2005, 45, 1179. [Google Scholar] [CrossRef]
- Carlyle, J.C.; Bligh, M.W.; Sadanandan Nambiar, E.K. Woody Residue Management to Reduce Nitrogen and Phosphorus Leaching from Sandy Soil after Clear-Felling Pinus Radiata Plantations. Can. J. Forest Res. 1998, 28, 1222–1232. [Google Scholar] [CrossRef]
- Rees, F.; Quideau, S.; Dyck, M.; Hernandez, G.; Yarmuch, M. Water and Nutrient Retention in Coarse-Textured Soil Profiles from the Athabasca Oil Sand Region. Appl. Geochem. 2020, 114, 104526. [Google Scholar] [CrossRef]
- Shepherd, M.A.; Bennett, G. Nutrient Leaching Losses from a Sandy Soil in Lysimeters. Commun. Soil Sci. Plant Anal. 1998, 29, 931–946. [Google Scholar] [CrossRef]
- Tahir, S.; Marschner, P. Clay Addition to Sandy Soil Reduces Nutrient Leaching—Effect of Clay Concentration and Ped Size. Commun. Soil Sci. Plant Anal. 2017, 48, 1813–1821. [Google Scholar] [CrossRef]
- Vassov, R.; (Canadian Natural Resources Limited, Fort McMurray, AB, Canada). Personal Communication, 2021.


| Soil Type | TIN Supply Rate (µg/10 cm2/7 Days) | Phosphates Supply Rate (µg/10 cm2/7 Days) | K Supply Rate (µg/10 cm2/7 Days) | pH | Electrical Conductivity (µS/cm) | FC (%) | TN (w/w%) | TOC (w/w%) | C:N Ratio |
|---|---|---|---|---|---|---|---|---|---|
| cFFMM | d 9.39 (1.21) | a 29.92 (3.54) | a 253.31 (61.52) | b 6.32 (0.11) | d 161.60 (0.43) | c 15.11 (0.88) | b 0.23 (0.14) | c 4.27 (0.40) | 18.57 |
| fFFMM | a 718.23 (39.40) | b 0.72 (0.07) | b 14.18 (7.08) | ab 6.54 (0.49) | c 641.0 (0.0) | b 26.91 (0.32) | b 0.18 (0.14) | c 3.55 (0.40) | 19.72 |
| Peat | c 27.25 (1.10) | c 0.38 (0.14) | c 2.62 (0.15) | c 4.42 (0.014) | a 1221.0 (2.16) | a 194.38 (1.64) | a 2.31 (0.14) | a 47.25 (0.40) | 20.45 |
| PMM | b 232.82 (66.91) | bc 0.51 (0.32) | b 7.47 (2.93) | a 6.84 (0.024) | b 764.67 (1.25) | b 30.47 (8.89) | b 0.22 (0.14) | b 8.92 (0.40) | 40.54 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trepanier, K.E.; Manchola-Rojas, L.; Pinno, B.D. Effects of Buried Wood on the Development of Populus tremuloides on Various Oil Sands Reclamation Soils. Forests 2022, 13, 42. https://doi.org/10.3390/f13010042
Trepanier KE, Manchola-Rojas L, Pinno BD. Effects of Buried Wood on the Development of Populus tremuloides on Various Oil Sands Reclamation Soils. Forests. 2022; 13(1):42. https://doi.org/10.3390/f13010042
Chicago/Turabian StyleTrepanier, Kaitlyn E., Laura Manchola-Rojas, and Bradley D. Pinno. 2022. "Effects of Buried Wood on the Development of Populus tremuloides on Various Oil Sands Reclamation Soils" Forests 13, no. 1: 42. https://doi.org/10.3390/f13010042
APA StyleTrepanier, K. E., Manchola-Rojas, L., & Pinno, B. D. (2022). Effects of Buried Wood on the Development of Populus tremuloides on Various Oil Sands Reclamation Soils. Forests, 13(1), 42. https://doi.org/10.3390/f13010042






