Carbon, Nutrients and Methylmercury in Water from Small Catchments Affected by Various Forest Management Operations
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sampling Sites
2.2. Water Sampling and Chemical Analyses
2.3. Statistical Analysis
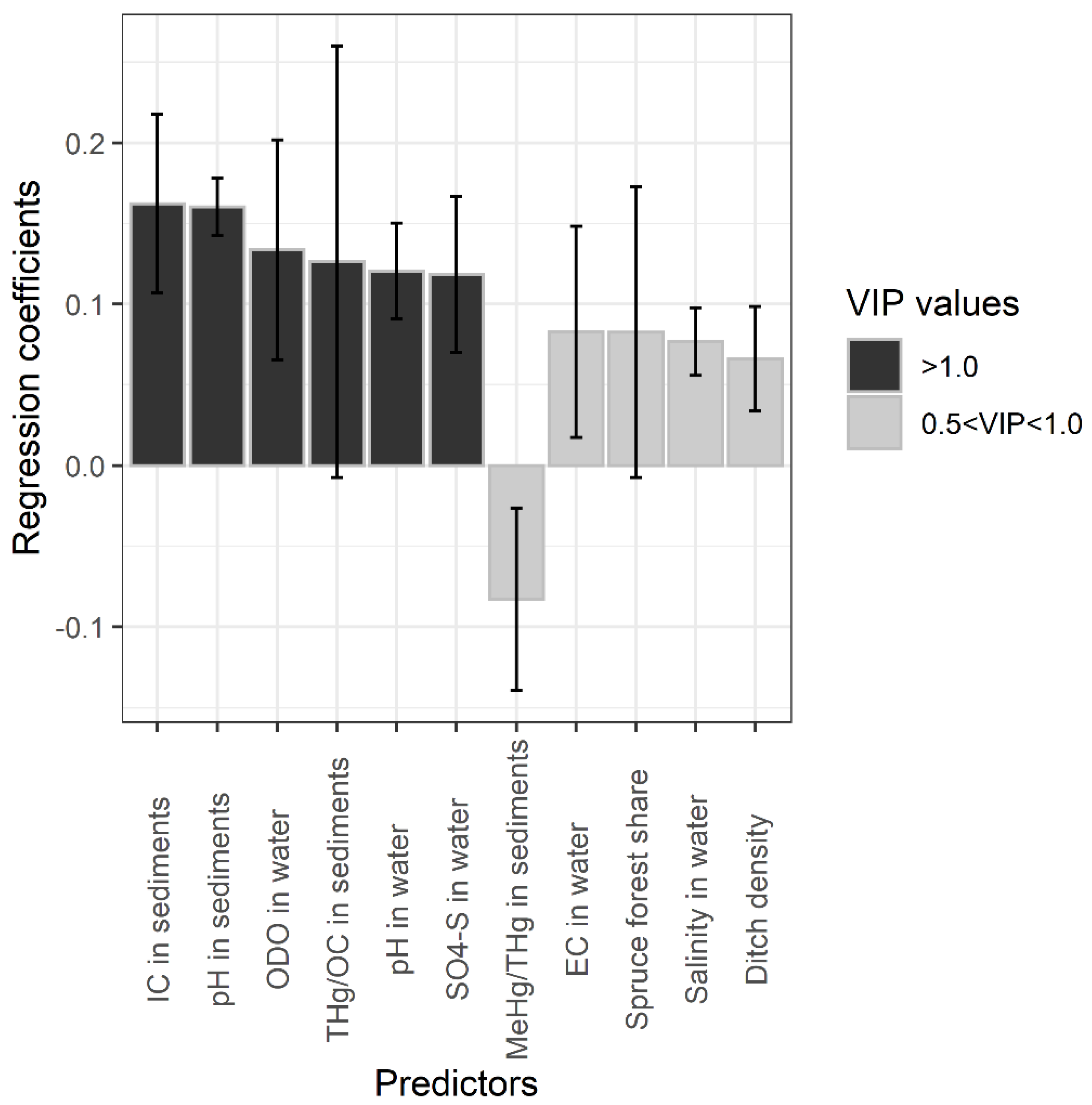
3. Results
3.1. Temporal Variation in Water Chemistry
3.2. Effects of Management-Induced Disturbances on Water Chemistry
3.3. Correlations between Water Physico-Chemical Parameters
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Parameter, Unit | Sampling Site | |||||||
|---|---|---|---|---|---|---|---|---|
| Site 1 | Site 2 | Site 3 | Site 4 | Site 5 | Site 6 | Site 7 | Site 8 | |
| Catchment area, ha | 27.8 | 54.6 | 11.0 | 70.6 | 758.5 | 256.4 | 314.1 | 665.2 |
| Elevation (min), m | 95.8 | 95.8 | 99.1 | 93.1 | 93.4 | 92.9 | 96.4 | 114.3 |
| Elevation (max), m | 98.7 | 101.7 | 99.6 | 106.8 | 132.3 | 99.9 | 132.3 | 169.1 |
| Total ditch length, km | 0.05 | 0.58 | 0.00 | 3.03 | 63.65 | 18.69 | 24.60 | 12.34 |
| Ditch density, m ha−1 | 1.9 | 10.7 | 0.0 | 42.9 | 83.9 | 72.9 | 78.3 | 18.6 |
| Forest area, ha | 25.10 | 51.37 | 9.42 | 65.93 | 717.51 | 242.37 | 304.87 | 452.60 |
| Share of forest area of total catchment area, % | 90.1 | 94.0 | 85.5 | 93.3 | 94.6 | 94.5 | 97.1 | 68.0 |
| Share of spruce-dominated forest from the total forest area, % | 27.7 | 25.8 | 0.0 | 13.1 | 41.2 | 44.6 | 31.8 | 21.0 |
| Share of pine-dominated forest from the total forest area, % | 63.5 | 69.0 | 74.1 | 54.8 | 39.4 | 38.9 | 45.8 | 41.0 |
| Share of broadleaves-dominated forest from the total forest area, % | 8.8 | 5.2 | 25.9 | 32.2 | 16.7 | 16.5 | 16.2 | 30.8 |
| Share of clearfellings from the total forest area, % | 0.0 | 0.0 | 0.0 | 0.0 | 2.6 | 0.0 | 6.2 | 3.2 |
| Share of drained peatlands from the total forest area, % | 12.0 | 9.6 | 0.0 | 19.1 | 35.8 | 21.7 | 39.8 | 2.4 |
| Share of peatland forests from the total forest area, % | 40.2 | 29.6 | 51.8 | 0.0 | 1.1 | 0.0 | 2.5 | 5.0 |
| Share of wetlands from the total forest area, % | 0.0 | 0.0 | 16.0 | 0.0 | 0.5 | 0.0 | 0.7 | 1.0 |
| Parameter, Unit | Value | Sampling Site | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Site 1 | Site 2 | Site 3 | Site 4 | Site 5 | Site 6 | Site 7 | Site 8 | ||
| pH | mean ± S.E. | 7.79 ± 0.07 | 7.84 ± 0.04 | 7.59 ± 0.05 | 7.71 ± 0.07 | 7.61 ± 0.11 | 7.95 ± 0.11 | 7.58 ± 0.06 | 7.75 ± 0.05 |
| min | 7.46 | 7.60 | 7.41 | 7.20 | 6.88 | 7.43 | 7.33 | 7.53 | |
| max | 8.17 | 8.08 | 7.86 | 8.04 | 8.11 | 8.35 | 7.89 | 8.01 | |
| EC, µS cm−1 | mean ± S.E. | 255 ± 5 | 262 ± 10 | 276 ± 9 | 335 ± 8 | 304 ± 16 | 326 ± 10 | 308 ± 9 | 282 ± 8 |
| min | 237 | 237 | 216 | 288 | 237 | 283 | 272 | 224 | |
| max | 283 | 354 | 315 | 376 | 438 | 374 | 377 | 309 | |
| TN, mg L−1 | mean ± S.E. | 0.34 ± 0.03 | 0.35 ± 0.04 | 0.63 ± 0.10 | 2.63 ± 0.44 | 1.55 ± 0.22 | 0.87 ± 0.09 | 1.86 ± 0.25 | 0.65 ± 0.13 |
| min | 0.22 | 0.20 | 0.33 | 0.84 | 0.63 | 0.45 | 0.89 | 0.17 | |
| max | 0.49 | 0.59 | 1.18 | 5.04 | 3.28 | 1.40 | 3.46 | 1.58 | |
| NO3−-N, mg L−1 | mean ± S.E. | 0.07 ± 0.03 | 0.10 ± 0.03 | 0.17 ± 0.09 | 2.52 ± 0.51 | 0.52 ± 0.21 | 0.27 ± 0.08 | 0.68 ± 0.30 | 0.54 ± 0.15 |
| min | <0.01 | <0.01 | <0.01 | 0.26 | <0.01 | <0.01 | <0.01 | <0.01 | |
| max | 0.29 | 0.32 | 1.05 | 6.38 | 2.07 | 0.74 | 3.31 | 1.49 | |
| NH4+-N, mg L−1 | mean ± S.E. | 0.05 ± 0.01 | 0.05 ± 0.01 | 0.04 ± 0.01 | 0.05 ± 0.01 | 0.07 ± 0.01 | 0.04 ± 0.01 | 0.28 ± 0.21 | 0.04 ± 0.01 |
| min | 0.02 | 0.01 | 0.01 | 0.02 | 0.03 | 0.01 | 0.03 | 0.01 | |
| max | 0.08 | 0.07 | 0.07 | 0.13 | 0.16 | 0.09 | 2.33 | 0.07 | |
| TP, mg L−1 | mean ± S.E. | 0.019 ± 0.002 | 0.021 ± 0.002 | 0.025 ± 0.004 | 0.042 ± 0.004 | 0.036 ± 0.004 | 0.025 ± 0.006 | 0.057 ± 0.018 | 0.017 ± 0.003 |
| min | 0.009 | 0.008 | 0.007 | 0.020 | 0.020 | 0.007 | 0.019 | <LOD | |
| max | 0.030 | 0.033 | 0.050 | 0.068 | 0.055 | 0.074 | 0.205 | 0.036 | |
| PO43−-P, mg L−1 | mean ± S.E. | 0.005 ± 0.002 | 0.010 ± 0.003 | 0.010 ± 0.004 | 0.015 ± 0.005 | 0.014 ± 0.006 | 0.008 ± 0.003 | 0.042 ± 0.016 | 0.006 ± 0.002 |
| min | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | |
| max | 0.016 | 0.048 | 0.040 | 0.054 | 0.065 | 0.029 | 0.163 | 0.018 | |
| SO42−-S, mg L−1 | mean ± S.E. | 1.76 ± 0.25 | 1.82 ± 0.27 | 1.86 ± 0.26 | 3.91 ± 0.50 | 2.64 ± 0.37 | 4.07 ± 0.51 | 2.70 ± 0.43 | 2.83 ± 0.37 |
| min | 0.63 | 0.63 | 1.03 | 1.69 | 1.80 | 2.23 | 1.21 | 1.54 | |
| max | 2.62 | 2.77 | 3.27 | 6.31 | 4.56 | 5.57 | 4.13 | 3.65 | |
| Cl−, mg L−1 | mean ± S.E. | 1.62 ± 0.04 | 1.60 ± 0.04 | 1.97 ± 0.29 | 2.20 ± 0.09 | 1.69 ± 0.13 | 1.60 ± 0.13 | 1.54 ± 0.06 | 1.80 ± 0.08 |
| min | 1.46 | 1.50 | 1.20 | 1.76 | 1.13 | 1.19 | 1.30 | 1.60 | |
| max | 1.74 | 1.77 | 3.15 | 2.44 | 1.98 | 2.07 | 1.69 | 2.11 | |
| DOC *, mg L−1 | mean ± S.E. | 13.0 | 21.9 ± 8.3 | 16.5 ± 0.9 | 29.5 ± 4.2 | 34.6 ± 3.5 | 29.2 ± 0.9 | 40.9 ± 3.3 | 13.7 ± 1.7 |
| min | 13.0 | 13.5 | 15.5 | 25.3 | 27.8 | 27.7 | 37.1 | 11.4 | |
| max | 13.0 | 38.6 | 18.2 | 33.7 | 39.5 | 30.7 | 47.4 | 16.9 | |
| TSS, mg L−1 | mean ± S.E. | 2.2 ± 0.4 | 14.0 ± 5.2 | 4.5 ± 0.7 | 33.6 ± 19.4 | 27.3 ± 12.8 | 10.6 ± 6.6 | 20.0 ± 11.5 | 6.3 ± 3.2 |
| min | <0.1 | <0.1 | 1.6 | 1.2 | 0.6 | <0.1 | <0.1 | <0.1 | |
| max | 5.3 | 57.0 | 7.6 | 242.4 | 136.8 | 76.5 | 130.7 | 36.8 | |
| ODO, mg L−1 | mean ± S.E. | 8.9 ± 0.6 | 9.9 ± 0.6 | 8.1 ± 1.0 | 9.1 ± 0.6 | 9.0 ± 1.1 | 12.1 ± 0.5 | 6.7 ± 1.0 | 9.5 ± 0.8 |
| min | 3.5 | 7.5 | 4.5 | 6.5 | 0.7 | 9.2 | 1.1 | 6.2 | |
| max | 10.9 | 13.4 | 12.1 | 12.0 | 12.6 | 14.4 | 11.2 | 13.3 | |
| ORP, mV | mean ± S.E. | 287 ± 12 | 272 ± 11 | 267 ± 6 | 286 ± 9 | 195 ± 33 | 229 ± 8 | 258 ± 8 | 257 ± 12 |
| min | 242 | 240 | 243 | 238 | -84 | 198 | 226 | 211 | |
| max | 346 | 349 | 300 | 310 | 270 | 282 | 294 | 337 | |
References
- Marttila, H.; Lepistö, A.; Tolvanen, A.; Bechmann, M.; Kyllmar, K.; Juutinen, A.; Wenng, H.; Skarbøvik, E.; Futter, M.; Kortelainen, P.; et al. Potential impacts of a future Nordic bioeconomy on surface water quality. Ambio 2020, 49, 1722–1735. [Google Scholar] [CrossRef] [PubMed]
- Hetemäki, L. The outlook for Nordic-Baltic forest bioeconomy to 2030. In The Forest Industry around the Baltic Sea Region: Future Challenges and Opportunities; Liuhto, K., Ed.; Centrum Balticum: Turku, Finland, 2020; pp. 14–24. [Google Scholar]
- Akselsson, C.; Westling, O.; Sverdrup, H.; Gindersen, P. Nutrient and carbon budgets in forest soils as decision support in sustainable forest management. For. Ecol. Manag. 2007, 238, 167–174. [Google Scholar] [CrossRef]
- Schelker, J.; Eklöf, K.; Bishop, K.; Laudon, H. Effects of forestry operations on dissolved organic carbon concentrations and export in boreal first-order streams. J. Geophys. Res. 2012, 117, G01011. [Google Scholar] [CrossRef]
- Akselsson, C.; Westling, O.; Alveteg, M.; Thelin, G.; Fransson, A.-M.; Hellsten, S. The influence of N load and harvest intensity on the risk of P limitation in Swedish forest soils. Sci. Total Environ. 2008, 404, 284–289. [Google Scholar] [CrossRef]
- Gundersen, P.; Schmidt, I.K.; Raulund-Rasmussen, K. Leaching of nitrate from temperate forests–effects of air pollution and forest management. Environ. Rev. 2006, 14, 1–57. [Google Scholar] [CrossRef]
- Löfgren, S.; Ring, E.; von Brömssen, C.; Sørensen, R.; Högbom, L. Short-term effects of clear-cutting on the water chemistry of two boreal streams in northern Sweden: A paired catchment study. Ambio 2009, 38, 347–356. [Google Scholar] [CrossRef]
- Palvianinen, M.; Finér, L.; Laurén, A.; Mattsson, T.; Högbom, L. A method to estimate the impact of clear-cutting on nutrient concentrations in boreal headwater streams. Ambio 2015, 44, 521–531. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schelker, J.; Sponseller, R.; Ring, E.; Högbom, L.; Löfgren, S.; Laudon, H. Nitrogen export from a boreal stream network following forest harvesting: Seasonal nitrate removal and conservative export of organic forms. Biogeosciences 2016, 13, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Sponseller, R.A.; Gundale, M.J.; Futter, M.; Ring, E.; Nordin, A.; Näsholm, T.; Laudon, H. Nitrogen dynamics in managed boreal forests: Recent advances and future research directions. Ambio 2016, 45, 175–187. [Google Scholar] [CrossRef] [Green Version]
- Porvari, P.; Verta, M.; Munthe, J.; Haapanen, M. Forestry practices increase mercury and methyl mercury output from boreal forest catchments. Environ. Sci. Technol. 2003, 37, 2389–2393. [Google Scholar] [CrossRef]
- Bishop, K.; Allan, C.; Bringmark, L.; Garcia, E.; Hellsten, S.; Högbom, L.; Johansson, K.; Lomander, A.; Meili, M.; Munthe, J.; et al. The Effects of Forestry on Hg Bioaccumulation in Nemoral/Boreal Waters and Recommendations for Good Silvicultural Practice. Ambio 2009, 38, 373–380. [Google Scholar] [CrossRef]
- Bishop, K.; Allan, C.J.; Bringmark, L.; Garcia, E.; Hellsten, S.; Högbom, L.; Johansson, K.; Lomander, A.; Meili, M.; Munthe, J.; et al. Forestry’s contribution to Hg bioaccumulation in freshwaters: Assessment of the available evidence. J. R. Swed. Acad. For. Agric. 2009, 148, 9–23. [Google Scholar]
- Eklöf, K.; Lidskog, R.; Bishop, K. Managing Swedish forestry’s impact on mercury in fish: Defining the impact and mitigation measures. Ambio 2016, 45, 163–174. [Google Scholar] [CrossRef] [PubMed]
- Kronberg, R.M.; Drott, A.; Jiskra, M.; Wiederhold, J.G.; Björn, E.; Skyllberg, U. Forest harvest contribution to Boreal freshwater methyl mercury load. Glob. Biogeochem. Cycles 2016, 30, 825–843. [Google Scholar] [CrossRef] [Green Version]
- Bishop, K.; Seibert, J.; Köhler, S.; Laudon, H. Resolving the Double Paradox of rapidly mobilized old water with highly variable responses in runoff chemistry. Hydrol. Process. 2004, 18, 185–189. [Google Scholar] [CrossRef]
- Creed, I.F.; Bergström, A.-K.; Trick, C.G.; Grimm, N.B.; Hessen, D.O.; Karlsson, J.; Kidd, K.A.; Kritzberg, E.; McKnight, D.M.; Freeman, E.C.; et al. Global change-driven effects on dissolved organic matter composition: Implications for food webs of northern lakes. Glob. Chang. Biol. 2018, 24, 3692–3714. [Google Scholar] [CrossRef]
- Nova, C.C.; Bozelli, R.L.; Spitzy, A.; Müller-Navarra, D. Living in a browning environment: Effects on Daphnia’s growth and fatty acid pattern. Limnol. Oceanogr. 2019, 64, 18–31. [Google Scholar] [CrossRef]
- Bieroza, M.; Baker, A.; Bridgeman, J. Relating freshwater organic matter fluorescence to organic carbon removal efficiency in drinking water treatment. Sci. Total Environ. 2009, 407, 1765–1774. [Google Scholar] [CrossRef]
- Rock, J.J. Formation of haloforms during chlorination of natural waters. Water Treat Exam 1974, 23, 234–243. [Google Scholar]
- Bishop, K.; Shanley, J.B.; Riscassi, A.; de Wit, H.A.; Eklöf, K.; Meng, B.; Mitchell, C.; Osterwalder, S.; Schuster, P.F.; Webster, J.; et al. Recent advances in understanding and measurement of mercury in the environment: Terrestrial Hg cycling. Sci. Total Environ. 2020, 721, 137647. [Google Scholar] [CrossRef]
- Laudon, H.; Sponseller, R.A.; Bishop, K. From legacy effects of acid deposition in boreal streams to future environmental threats. Environ. Res. Lett. 2021, 16, 015007. [Google Scholar] [CrossRef]
- Kreutzweiser, D.P.; Hazlett, P.W.; Gunn, J.M. Logging impacts on the biogeochemistry of boreal forest soils and nutrient export to aquatic systems: A review. Environ. Rev. 2008, 16, 157–179. [Google Scholar] [CrossRef]
- Lamontagne, S.; Carignan, R.; D’Arcy, P.; Prairie, Y.T.; Paré, D. Element export in runoff from eastern Canadian Boreal Shield drainage basins following forest harvesting and wildfires. Can. J. Fish. Aquat. Sci. 2000, 57, 118–128. [Google Scholar] [CrossRef]
- Nieminen, M.; Sarkkola, S.; Sallantaus, T.; Hasselquist, E.M.; Laudon, H. Peatland drainage—A missing link behind increasing TOC concentrations in waters from high latitude forest catchments? Sci. Total Environ. 2021, 774, 145150. [Google Scholar] [CrossRef] [PubMed]
- Conley, D.J.; Paerl, H.W.; Howarth, R.W.; Boesch, D.F.; Seitzinger, S.P.; Havens, K.E.; Lancelot, C.; Likens, G.E. Controlling eutrophication: Nitrogen and phosphorus. Science 2009, 323, 1014–1015. [Google Scholar] [CrossRef] [PubMed]
- Le Moal, M.; Gascuel-Odoux, C.; Ménesguen, A.; Souchon, Y.; Étrillard, C.; Levain, A.; Moatar, F.; Pannard, A.; Souchu, P.; Lefebvre, A.; et al. Eutrophication: A new wine in an old bottle? Sci. Total Environ. 2019, 651, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Ahtiainen, M.; Huttunen, P. Long-term effects of forestry managements on water quality and loading in brooks. Boreal Environ. Res. 1999, 4, 101–114. [Google Scholar]
- Nieminen, M.; Ahti, E.; Koivusalo, H.; Mattsson, T.; Sarkkola, S.; Laurén, A. Export of suspended solids and dissolved elements from peatland areas after ditch network maintenance in south-central Finland. Silva Fenn. 2010, 44, 39–49. [Google Scholar] [CrossRef] [Green Version]
- Vitousek, P.M.; Gosz, J.R.; Grier, C.C.; Melillo, J.M.; Reiners, W.A.; Todd, R.L. Nitrate losses from disturbed ecosystems. Science 1979, 204, 469–474. [Google Scholar] [CrossRef] [Green Version]
- Stednick, J.D. Monitoring the effects of timber harvest on annual water yield. J. Hydrol. 1996, 176, 79–95. [Google Scholar] [CrossRef]
- Paavolainen, L.; Smolander, A. Nitrification and denitrification in soil from a clear-cut Norway spruce (Picea abies) stand. Soil Biol. Biochem. 1998, 30, 775–781. [Google Scholar] [CrossRef]
- Smolander, A.; Kitunen, V.; Mälkönen, E. Dissolved soil organic nitrogen and carbon in a Norway spruce stand and an adjacent clear-cut. Biol. Fertil. Soils 2001, 33, 190–196. [Google Scholar] [CrossRef]
- Palviainen, M.; Finér, L.; Kurka, A.-M.; Mannerkoski, H.; Piirainen, S.; Starr, M. Decomposition and nutrient release from logging residues after clear-cutting of mixed boreal forest. Plant Soil 2004, 263, 53–67. [Google Scholar] [CrossRef]
- Bonsdorff, E. Eutrophication: Early warning signals, ecosystem-level and societal responses, and ways forward. Ambio 2021, 50, 753–758. [Google Scholar] [CrossRef] [PubMed]
- Mergler, D.; Anderson, H.A.; Chan, L.H.M.; Mahaffey, K.R.; Murray, M.; Sakamoto, M.; Stern, A.H. Methylmercury exposure and health effects in humans: A worldwide concern. Ambio 2007, 36, 3–11. [Google Scholar] [CrossRef]
- Mercury and Health. Available online: https://www.who.int/news-room/fact-sheets/detail/mercury-and-health (accessed on 22 July 2021).
- Mergler, D. Ecosystem approaches to mercury and human health: A way toward the future. Ambio 2021, 50, 527–531. [Google Scholar] [CrossRef] [PubMed]
- HELCOM Core Indicator Report. Available online: https://helcom.fi/wp-content/uploads/2019/08/Metals-HELCOM-core-indicator-2018.pdf (accessed on 22 July 2021).
- Korpinen, S.; Laamanen, M.; Andersen, J.H.; Asplund, L.; Berger, U.; Bignert, A.; Boalt, E.; Broeg, K.; Brzozowska, A.; Cato, I.; et al. Hazardous Substances in the Baltic Sea: An Integrated Thematic Assessment of Hazardous Substances in the Baltic Sea; Helsinki Commission: Helsinki, Finland, 2010; p. 116. [Google Scholar]
- Legal Acts of the Republic of Latvia. Regulations Regarding the Quality of Surface Waters and Groundwaters. Available online: https://likumi.lv/ta/en/en/id/60829-regulations-regarding-the-quality-of-surface-waters-and-groundwaters (accessed on 22 July 2021).
- LEGMC Report on Surface Water and Groundwater Condition in 2019. 2020. Available online: https://videscentrs.lvgmc.lv/files/Udens/udens_kvalitate/VPUK_2019_FINAL_26102020.pdf (accessed on 22 July 2021). (In Latvian).
- Castoldi, A.F.; Coccini, T.; Manzo, L. Neurotoxic and Molecular Effects of Methylmercury in Humans. Rev. Environ. Health 2003, 18, 19–31. [Google Scholar] [CrossRef]
- Compeau, G.C.; Bartha, R. Sulfate-Reducing Bacteria: Principal Methylators of Mercury in Anoxic Estuarine Sediment. Appl. Environ. Microbiol. 1985, 50, 498–502. [Google Scholar] [CrossRef] [Green Version]
- Gilmour, C.C.; Henry, E.A.; Mitchell, R. Sulfate stimulation of mercury methylation in freshwater sediments. Environ. Sci. Technol. 1992, 26, 2281–2287. [Google Scholar] [CrossRef]
- Fleming, E.J.; Mack, E.E.; Green, P.G.; Nelson, D.C. Mercury methylation from unexpected sources: Molybdate-inhibited freshwater sediments and an ironreducing bacterium. Appl. Environ. Microbiol. 2005, 72, 457–464. [Google Scholar] [CrossRef] [Green Version]
- Wood, J.M.; Kennedy, F.S.; Rosen, C.G. Synthesis of Methyl-mercury Compounds by Extracts of a Methanogenic Bacterium. Nature 1968, 220, 173–174. [Google Scholar] [CrossRef]
- Hamelin, S.; Amyot, M.; Barkay, T.; Wang, Y.; Planas, D. Methanogens: Principal Methylators of Mercury in Lake Periphyton. Environ. Sci. Technol. 2011, 45, 7693–7700. [Google Scholar] [CrossRef] [PubMed]
- Gilmour, C.C.; Podar, M.; Bullock, A.L.; Graham, A.M.; Brown, S.D.; Somenahally, A.C.; Johs, A.; Hurt, R.A.; Bailey, K.L.; Elias, D.A. Mercury Methylation by Novel Microorganisms from New Environments. Environ. Sci. Technol. 2013, 47, 11810–11820. [Google Scholar] [CrossRef] [PubMed]
- Hsu-Kim, H.; Eckley, C.S.; Achá, D.; Feng, X.; Gilmour, C.C.; Jonsson, S.; Mitchell, C.P.J. Challenges and opportunities for managing aquatic mercury pollution in altered landscapes. Ambio 2018, 47, 141–169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allan, C.J.; Heyes, A.; Mackereth, R.J. 2009. Changes to groundwater and surface water Hg transport flollowing clearcut logging: A Canadian case study. J. R. Swed. Acad. For. Agric. 2009, 148, 50–54. [Google Scholar]
- De Wit, H.A.; Granhus, A.; Lindholm, M.; Kainz, M.J.; Lin, Y.; Braaten, H.F.V.; Blaszczak, J. Forest harvest effects on mercury in streams and biota in Norwegian boreal catchments. For. Ecol. Manag. 2014, 324, 52–63. [Google Scholar] [CrossRef] [Green Version]
- Koskinen, M.; Tahvanainen, T.; Sarkkola, S.; Menberu, M.W.; Laurén, A.; Sallantaus, T.; Marttila, H.; Ronkanen, A.-K.; Parviainen, M.; Tolvanen, A.; et al. Restoration of nutrient-rich forestry-drained peatlands poses a risk for high exports of dissolved organic carbon, nitrogen, and phosphorus. Sci. Total Environ. 2017, 586, 858–869. [Google Scholar] [CrossRef]
- Nieminen, M.; Palviainen, M.; Sarkkola, S.; Laurén, A.; Marttila, H.; Finér, L. A synthesis of the impacts of ditch network maintenance on the quantity and quality of runoff from drained boreal peatland forests. Ambio 2018, 47, 523–534. [Google Scholar] [CrossRef] [PubMed]
- Joensuu, S.; Ahti, E.; Vuollekoski, M. Effects of ditch network maintenance on the chemistry of run-off water from peatland forests. Scand. J. For. Res. 2002, 17, 238–247. [Google Scholar] [CrossRef]
- Hynninen, A.; Sarkkola, S.; Laure’n, A.; Koivusalo, H.; Nieminen, M. Capacity of riparian buffer areas to reduce ammonium export originating from ditch network maintenance areas in peatlands drained for forestry. Boreal Environ. Res. 2011, 16, 430–444. [Google Scholar]
- Joensuu, S.; Vuollekoski, M.; Karosto, K. Kunnostusojitusten pitkäaikaisvaikutuksia. Suom. Ympäristö-Finn. Environ.-Miljön I Finl. 2006, 816, 83–90. (In Finnish) [Google Scholar]
- Åström, M.; Aaltonen, E.K.; Koivusaari, J. Impact of forest ditching on nutrient loadings of a small stream—A paired catchment study in Kronoby, W. Finland. Sci. Total Environ. 2002, 297, 127–140. [Google Scholar] [CrossRef]
- Hansen, K.; Kronnäs, V.; Zetterberg, T.; Setterberg, M.; Moldan, F.; Pettersson, P.; Munthe, J. The Effect of Ditch Cleaning on Discharge, Water Chemistry and Benthos in Forest Ecosystems; Report B2072; IVL Swedish Environmental Research Institute: Stockholm, Sweden, 2013. (In Swedish) [Google Scholar]
- Zālītis, P. The impact of the hydrological regime of the peat soils upon the growth potential of young spruce stands. Mezzinatne 2008, 17, 3–8. (In Latvian) [Google Scholar]
- Eriksson, L.; Johansson, E.; Kettapeh-Wold, N.; Wold, S. Introduction to Multiand Megavariate Data Analysis using Projection Methods (PCA & PLS); Umetrics: Umea, Sweden, 1999. [Google Scholar]
- Wold, S.; Sjöström, M.; Eriksson, L. PLS-regression: A basic tool of chemometrics. Chemom. Intell. Lab. Syst. 2001, 58, 109–130. [Google Scholar] [CrossRef]
- Kucheryavskiy, S. mdatools–R package for chemometrics. Chemom. Intell. Lab. Syst. 2020, 198, 103937. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing. Available online: http://www.r-project.org/ (accessed on 16 September 2021).
- Eklöf, K.; Schelker, J.; Sørensen, R.; Meili, M.; Laudon, H.; Von Brömssen, C.; Bishop, K. Impact of forestry on total and methyl-mercury in surface waters: Distinguishing effects of logging and site preparation. Environ. Sci. Technol. 2014, 48, 4690–4698. [Google Scholar] [CrossRef]
- Skyllberg, U.; Westin, M.B.; Meili, M.; Björn, E. Elevated Concentrations of Methyl Mercury in Streams after Forest Clear-Cut: A Consequence of Mobilization from Soil or New Methylation? Environ. Sci. Technol. 2009, 43, 8535–8541. [Google Scholar] [CrossRef] [PubMed]
- Eklöf, K.; Meili, M.; Åkerblom, S.; von Brömssen, C.; Bishop, K. Impact of stump harvest on run-off concentrations of total mercury and methylmercury. For. Ecol. Manag. 2013, 290, 83–94. [Google Scholar] [CrossRef]
- Kronberg, R.M.; Tjerngren, I.; Drott, A.; Björn, E.; Skyllberg, U. Net degradation of methyl mercury in alder swamps. Environ. Sci. Technol. 2012, 46, 13144–13151. [Google Scholar] [CrossRef]
- Porvari, P.; Verta, M.; Munthe, J. The Effect of Forestry Practices on Mercury and Methyl Mercury Output from Forest Catchment: A Follow-up Stud. Mater. Geoenviron. 2004, 51, 1316–1319. [Google Scholar]
- Ukonmaanaho, L.; Starr, M.; Kantola, M.; Laurén, A.; Piispanen, J.; Pietilä, H.; Perämäki, P.; Merilä, P.; Fritze, H.; Tuomivirta, T.; et al. Impacts of forest harvesting on mobilization of Hg and MeHg in drained peatland forests on black schist or felsic bedrock. Environ. Monit. Assess. 2016, 188, 228. [Google Scholar] [CrossRef]
- Zālītis, P. Forest and Water; LSFRI Silava: Salaspils, Latvia, 2012; 356p. (In Latvian) [Google Scholar]
- Kalvīte, Z.; Lībiete, Z.; Kļaviņš, I.; Bārdule, A.; Bičkovskis, K. The impact of beaver dam removal on the chemical properties of water in drainage ditches in peatland forests. Scand. J. For. Res. 2021, 36, 1–14. [Google Scholar] [CrossRef]
- Joensuu, S.; Ahti, E.; Vuollekoski, M. Discharge water quality from old ditch networks in Finnish peatland forests. Suo 2001, 52, 1–15. [Google Scholar]
- Manninen, P. Effects of forestry ditch cleaning and supplementary ditching on water quality. Boreal Environ. Res. 1998, 3, 23–32. [Google Scholar]
- Åström, M.; Aaltonen, E.K.; Koivusaari, J. Impact of ditching in a small forested catchment on concentrations of suspended material, organic carbon, hydrogen ions and metals in stream water. Aquat. Geochem. 2001, 7, 57–73. [Google Scholar] [CrossRef]
- Joensuu, S.; Ahti, E.; Vuollekoski, M. Long-term effects of maintaining ditch networks on runoff quality. Suo 2001, 52, 17–28. [Google Scholar]
- Åström, M.; Aaltonen, E.K.; Koivusaari, J. Changes in leaching patterns of nitrogen and phosphorus after artificial drainage of a boreal forest—A paired catchment study in Lappajärvi, western Finland. Boreal Environ. Res. 2005, 10, 67–78. [Google Scholar]
- Joensuu, S. Hydrological and Hydrochemical Impacts of Ditch Network Maintenance; TASO-Hanke-Kunnostusojituksen Vesiensuojelupäivä Jyväskylässä: Jyväskylässä, Finland, 16 April 2013; p. 1. (In Finnish) [Google Scholar]
- Tuukkanen, T.; Stenberg, L.; Marttila, H.; Finér, L.; Piirainen, S.; Koivusalo, H.; Kløve, B. Erosion mechanisms and sediment sources in a peatland forest after ditch cleaning. Earth Surf. Process. Landf. 2016, 41, 1841–1853. [Google Scholar] [CrossRef]
- Koivusalo, H.; Ahti, E.; Laurén, A.; Kokkonen, T.; Karvonen, T.; Nevalainen, R.; Finér, L. Impacts of ditch cleaning on hydrological processes in a drained peatland forest. Hydrol. Earth Syst. Sci. 2008, 12, 1211–1227. [Google Scholar] [CrossRef]
- Laine, J.; Silvola, J.; Tolonen, K.; Alm, J.; Nykänen, H.; Vasander, H.; Sallantaus, T.; Savolainen, I.; Sinisalo, J.; Martikainen, P.J. Effect of water-level drawdown on global climatic warming: Northern peatlands. Ambio 1996, 25, 179–184. [Google Scholar]
- Graydon, J.A.; St. Louis, V.L.; Lindberg, S.E.; Sandilands, K.A.; Rudd, J.W.M.; Kelly, C.A.; Harris, R.; Tate, M.T.; Krabbenhoft, D.P.; Emmerton, C.A.; et al. The role of terrestrial vegetation in atmospheric Hg deposition: Pools and fluxes of spike and ambient Hg from the METAALICUS experiment. Glob. Biogeochem. Cycles 2012, 26. [Google Scholar] [CrossRef] [Green Version]
- Witt, E.L.; Kolka, R.K.; Nater, E.A.; Wickman, T.R. Influence of the Forest Canopy on Total and Methyl Mercury Deposition in the Boreal Forest. Water Air Soil Pollut. 2009, 199, 3–11. [Google Scholar] [CrossRef]
- Brigham, M.E.; Wentz, D.A.; Aiken, G.R.; Krabbenhoft, D.P. Mercury cycling in stream ecosystems. 1. Water column chemistry and transport. Environ. Sci. Technol. 2009, 43, 2720–2725. [Google Scholar] [CrossRef]
- Shanley, J.B.; Kamman, N.C.; Clair, T.A.; Chalmers, A. Physical controls on total and methylmercury concentrations in streams and lakes of the northeastern USA. Ecotoxicology 2005, 14, 125–134. [Google Scholar] [CrossRef]
- Nagorski, S.A.; Engstrom, D.R.; Hudson, J.P.; Krabbenhoft, D.P.; Hood, E.; Dewild, J.F.; Aiken, G.R. Spatial distribution of mercury in southeastern Alaskan streams influenced by glaciers, wetlands, and salmon. Environ. Pollut. 2014, 184, 67–72. [Google Scholar] [CrossRef]
- Bravo, A.G.; Kothawala, D.N.; Attermeyer, K.; Tessier, E.; Bodmer, P.; Ledesma, J.L.J.; Audet, J.; Casas-Ruiz, J.P.; Catalán, N.; Cauvy-Fraunié, S.; et al. The interplay between total mercury, methylmercury and dissolved organic matter in fluvial systems: A latitudinal study across Europe. Water Res. 2018, 144, 172–182. [Google Scholar] [CrossRef] [PubMed]
- Drott, A.; Lambertsson, L.; Björn, E.; Skyllberg, U. Do Potential Methylation Rates Reflect Accumulated Methyl Mercury in Contaminated Sediments? Environ. Sci. Technol. 2008, 42, 153–158. [Google Scholar] [CrossRef] [PubMed]
- Eklöf, K.; Bishop, K.; Bertilsson, S.; Björn, E.; Buck, M.; Skyllberg, U.; Osman, O.A.; Kronberg, R.M.; Bravo, A.G. Formation of mercury methylation hotspots as a consequence of forestry operations. Sci. Total Environ. 2018, 613–614, 1069–1078. [Google Scholar] [CrossRef] [PubMed]
- Braaten, H.F.V.; de Wit, H.A. Effects of disturbance and vegetation type on total and methylmercury in boreal peatland and forest soils. Environ. Pollut. 2016, 218, 140–149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Munthe, J.; Hultberg, H. Mercury and Methylmercury in Runoff from a Forested Catchment—Concentrations, Fluxes, and Their Response to Manipulations. Water Air Soil Pollut. Focus Vol. 2004, 4, 607–618. [Google Scholar] [CrossRef]
- Stamenkovic, J.; Gustin, M.S.; Dennett, K.E. Net methyl mercury production versus water quality improvement in constructed wetlands: Trade-offs in pollution control. Wetlands 2005, 25, 748–757. [Google Scholar] [CrossRef]
- Selvendiran, P.; Driscoll, C.T.; Bushey, J.T.; Montesdeoca, M.R. Wetland influence on mercury fate and transport in a temperate forested watershed. Environ. Pollut. 2008, 154, 46–55. [Google Scholar] [CrossRef] [PubMed]
- Paranjape, A.R.; Hall, B.D. Recent advances in the study of mercury methylation in aquatic systems. FACETS 2017, 2, 85–119. [Google Scholar] [CrossRef]
- Roy, V.; Amyot, M.; Carignan, R. Beaver ponds increase methylmercury concentrations in Canadian shield streams along vegetation and pond-age gradients. Environ. Sci. Technol. 2009, 43, 5605–5611. [Google Scholar] [CrossRef] [PubMed]
- Levanoni, O.; Bishop, K.; Mckie, B.G.; Hartman, G.; Eklöf, K.; Ecke, F. Impact of Beaver Pond Colonization History on Methylmercury Concentrations in Surface Water. Environ. Sci. Technol. 2015, 49, 12679–12687. [Google Scholar] [CrossRef]
- Ecke, F.; Levanoni, O.; Audet, J.; Carlson, P.; Eklöf, K.; Hartman, G.; McKie, B.; Ledesma, J.; Segersten, J.; Truchy, A.; et al. Meta-analysis of environmental effects of beaver in relation to artificial dams. Environ. Res. Lett. 2017, 12, 113002. [Google Scholar] [CrossRef]
- Bergman, I.; Bishop, K.; Tu, Q.; Frech, W.; Åkerblom, S.; Nilsson, M. The Influence of Sulphate Deposition on the Seasonal Variation of Peat Pore Water Methyl Hg in a Boreal Mire. PLoS ONE 2012, 7, e45547. [Google Scholar] [CrossRef]
- Braaten, H.F.V.; Lindholm, M.; de Wit, H.A. Five decades of declining methylmercury concentrations in boreal foodwebs suggest pivotal role for sulphate deposition. Sci. Total Environ. 2020, 714, 136774. [Google Scholar] [CrossRef]
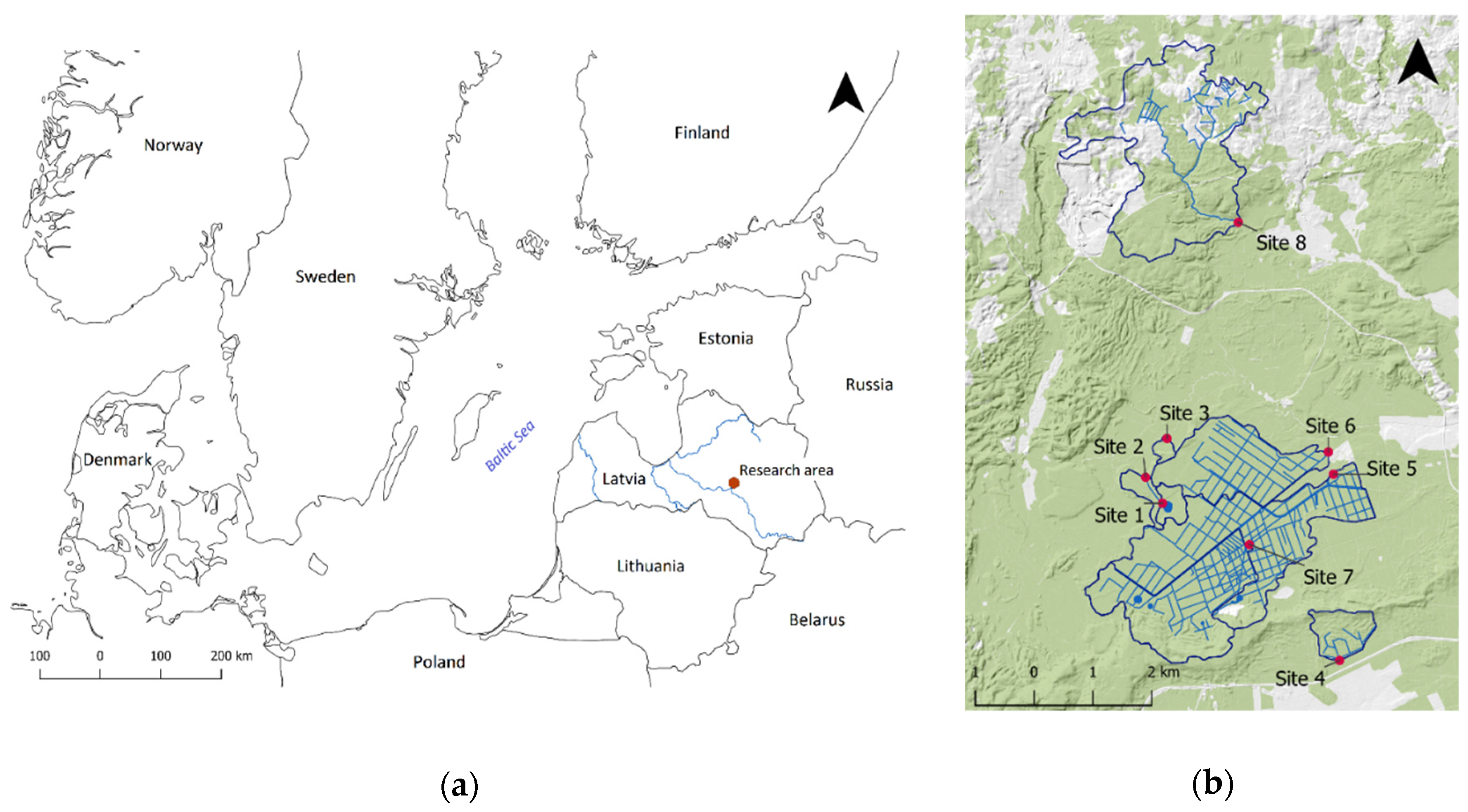
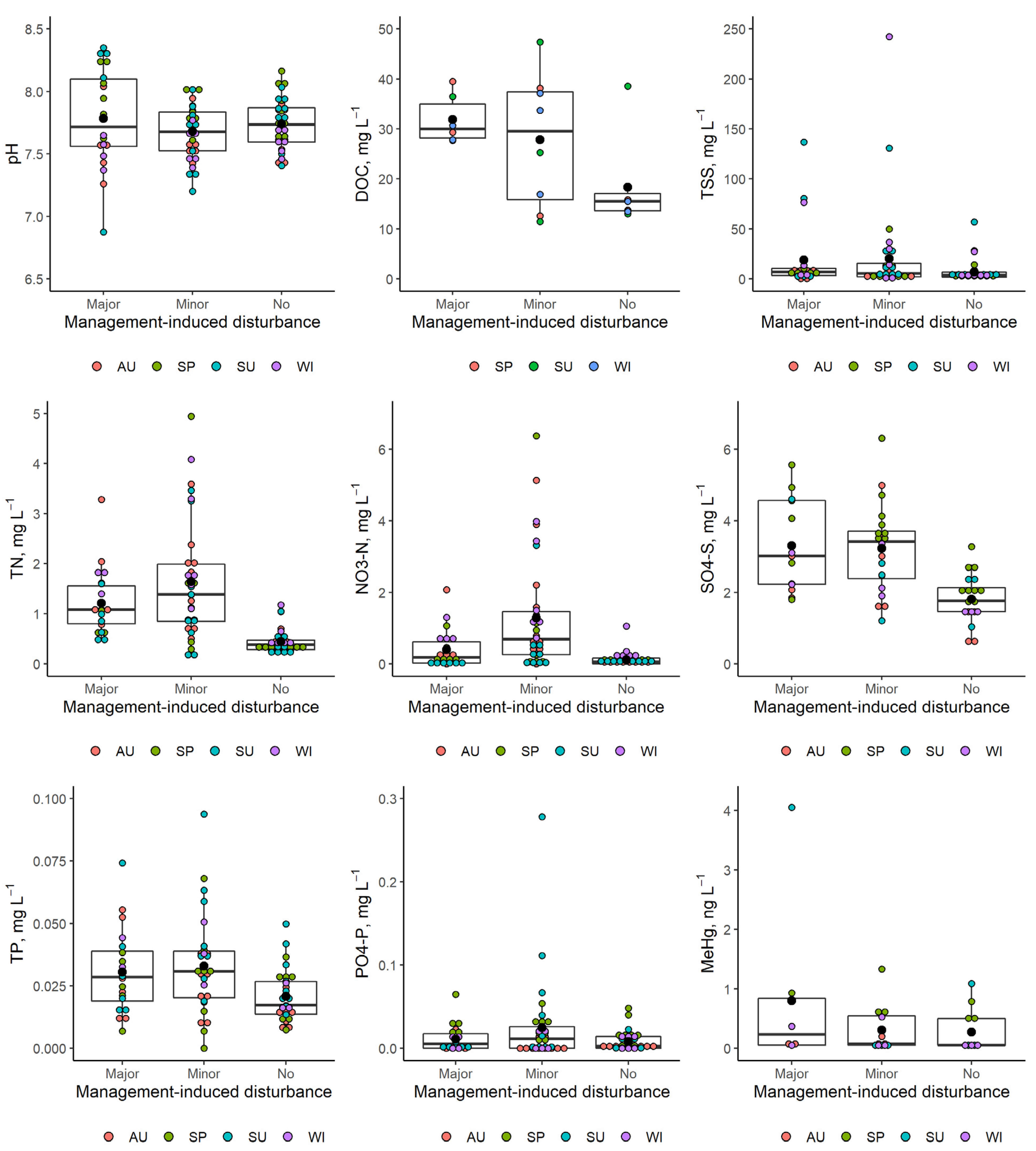
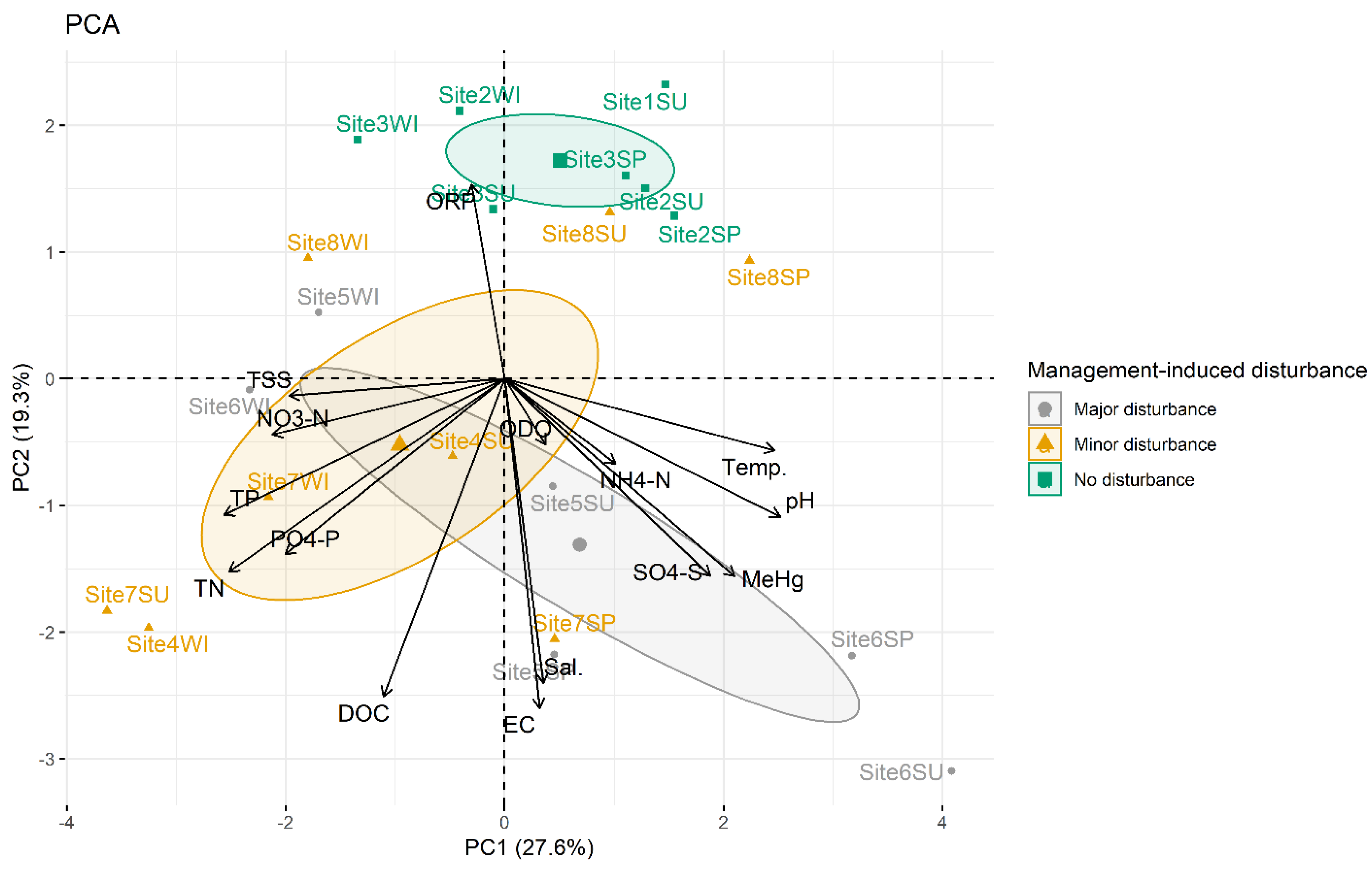
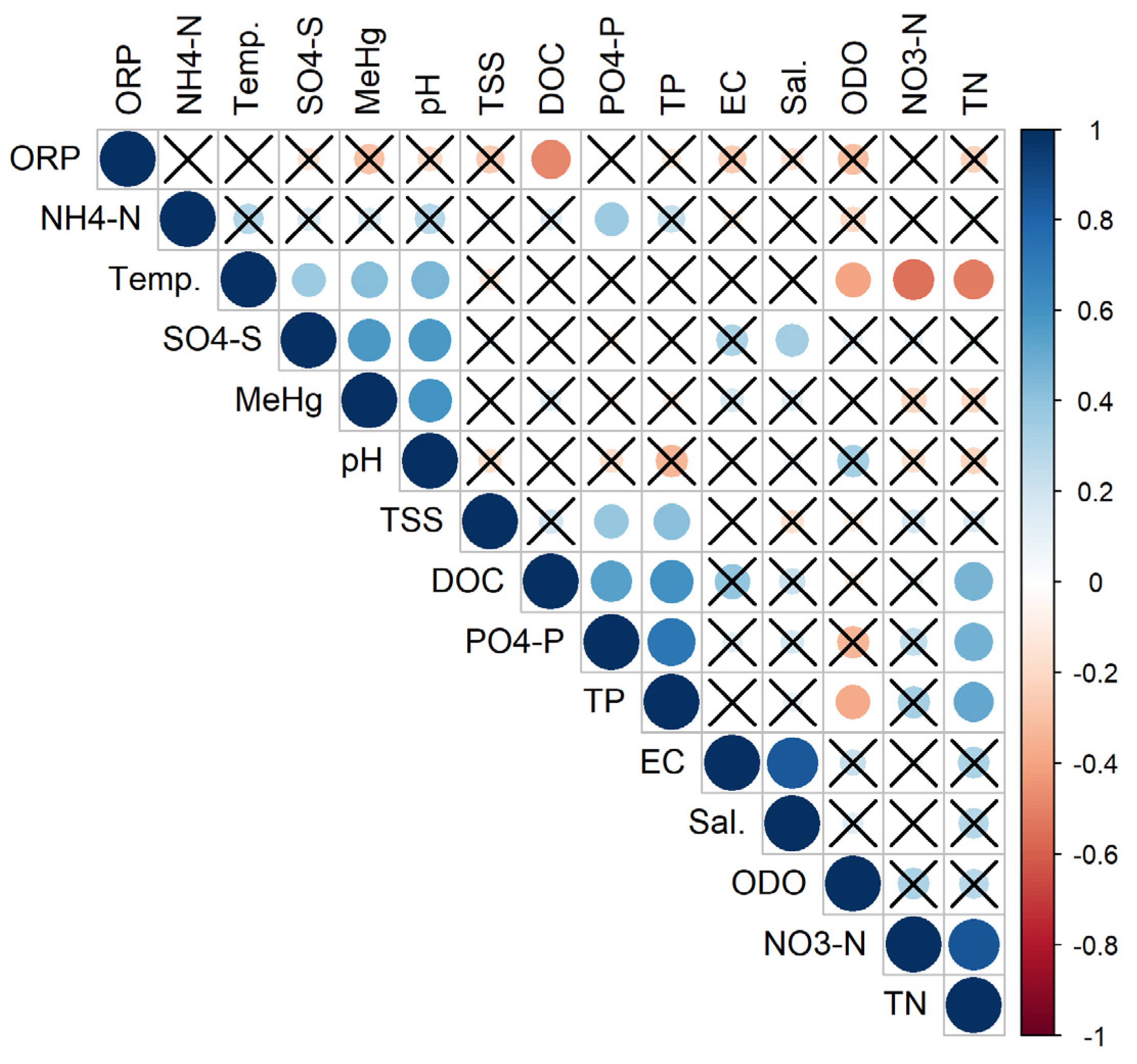
| Sampling Site | Sampling Point Coordinates (Latitude; Longitude) | Management-Induced Disturbance | Type of Disturbance | Organic Carbon Concentration (g kg−1) | Total Nitrogen Concentration (g kg−1) | Total Sulphur Concentration (g kg−1) | Total Phosphorus Concentration (g kg−1) |
|---|---|---|---|---|---|---|---|
| Site 1 | 56.689° N; 25.815° E | None | Natural stream, no disturbance | >400 | >15 | >5 | >0.5 |
| Site 2 | 56.693° N; 25.810° E | None | Natural stream, no disturbance | >400 | >15 | >5 | >0.5 |
| Site 3 | 56.699° N; 25.816° E | None | Natural stream, no disturbance | >400 | >15 | >5 | >0.5 |
| Site 4 | 56.664° N; 25.863° E | Minor | Drainage ditches >10 years since ditch cleaning | <100 | <10 | <2 | <0.5 |
| Site 5 | 56.693° N; 25.862° E | Major | Drainage ditch with beaver dam removal in 2017 | <100 | <10 | <2 | <0.5 |
| Site 6 | 56.696° N; 25.861° E | Major | Drainage ditch, ditch cleaning in 2016 | <100 | <10 | <2 | <0.5 |
| Site 7 | 56.682° N; 25.838° E | Minor | Drainage ditch, >10 years since ditch cleaning | <100 | <10 | <2 | <0.5 |
| Site 8 | 56.731° N; 25.839° E | Minor | Natural stream with careful beaver dam removal in recent years (2016) | <100 | <10 | <2 | <0.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kļaviņa, Z.; Bārdule, A.; Eklöf, K.; Bitenieks, K.; Kļaviņš, I.; Lībiete, Z. Carbon, Nutrients and Methylmercury in Water from Small Catchments Affected by Various Forest Management Operations. Forests 2021, 12, 1278. https://doi.org/10.3390/f12091278
Kļaviņa Z, Bārdule A, Eklöf K, Bitenieks K, Kļaviņš I, Lībiete Z. Carbon, Nutrients and Methylmercury in Water from Small Catchments Affected by Various Forest Management Operations. Forests. 2021; 12(9):1278. https://doi.org/10.3390/f12091278
Chicago/Turabian StyleKļaviņa, Zane, Arta Bārdule, Karin Eklöf, Krišs Bitenieks, Ivars Kļaviņš, and Zane Lībiete. 2021. "Carbon, Nutrients and Methylmercury in Water from Small Catchments Affected by Various Forest Management Operations" Forests 12, no. 9: 1278. https://doi.org/10.3390/f12091278
APA StyleKļaviņa, Z., Bārdule, A., Eklöf, K., Bitenieks, K., Kļaviņš, I., & Lībiete, Z. (2021). Carbon, Nutrients and Methylmercury in Water from Small Catchments Affected by Various Forest Management Operations. Forests, 12(9), 1278. https://doi.org/10.3390/f12091278






