How Can Litter Modify the Fluxes of CO2 and CH4 from Forest Soils? A Mini-Review
Abstract
1. Introduction
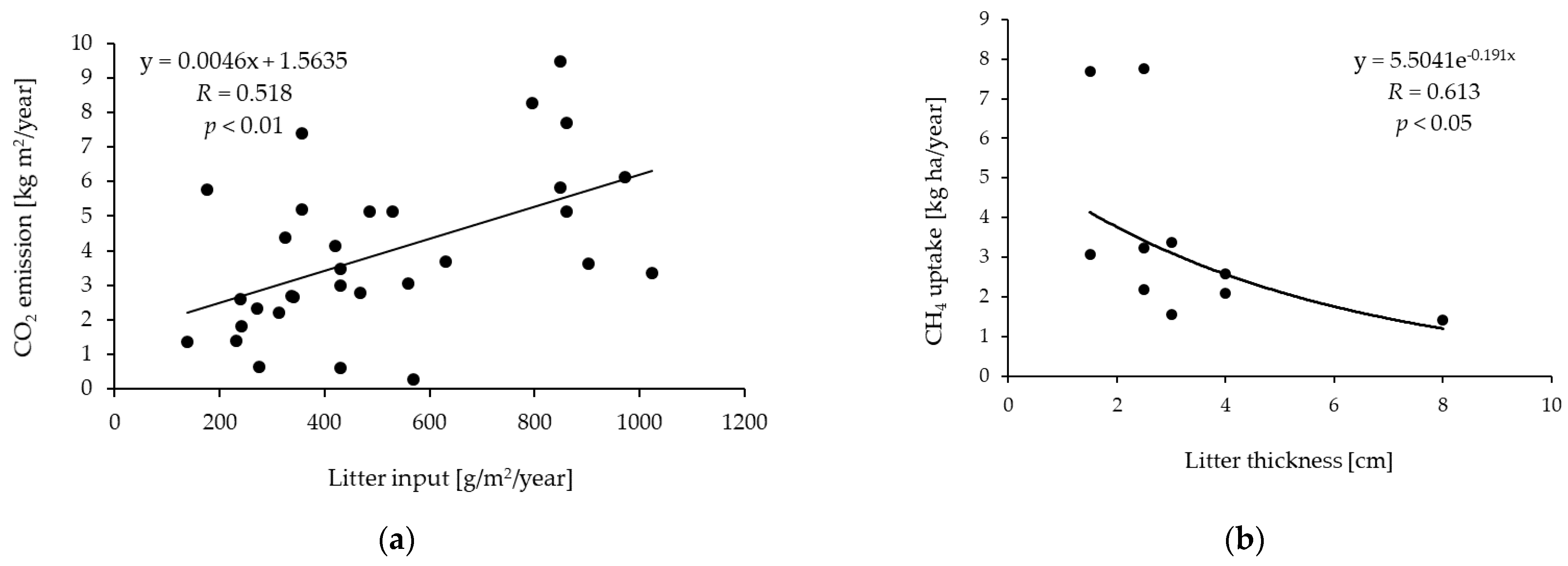
2. Litter as a Controller of GHG (CO2, CH4) Fluxes
2.1. Carbon Dioxide (CO2)
- (i)
- An alteration in the availability of substrates for soil microbes;
- (ii)
- modifications in soil microbial communities;
- (iii)
- a priming effect;
- (iv)
- the creation of a physical barrier that decreases gas exchange and/or water movement/water retention and acts as an insulating layer that modifies soil temperature.
2.2. Methane (CH4)
- (i)
- decrease the uptake by acting as a physical barrier to gas diffusion and reduced aeration due to faster litter decomposition in wet conditions;
- (ii)
- increase the uptake through the maintenance of soil gas diffusivity under wetter/high rainfall conditions;
- (iii)
- influence the capability of the soil for oxidizing CH4;
- (iv)
- provide a source of nutrients for methanotrophs;
- (v)
- improve the formation of macro-aggregates, which facilitates CH4 transport for methanotrophs.
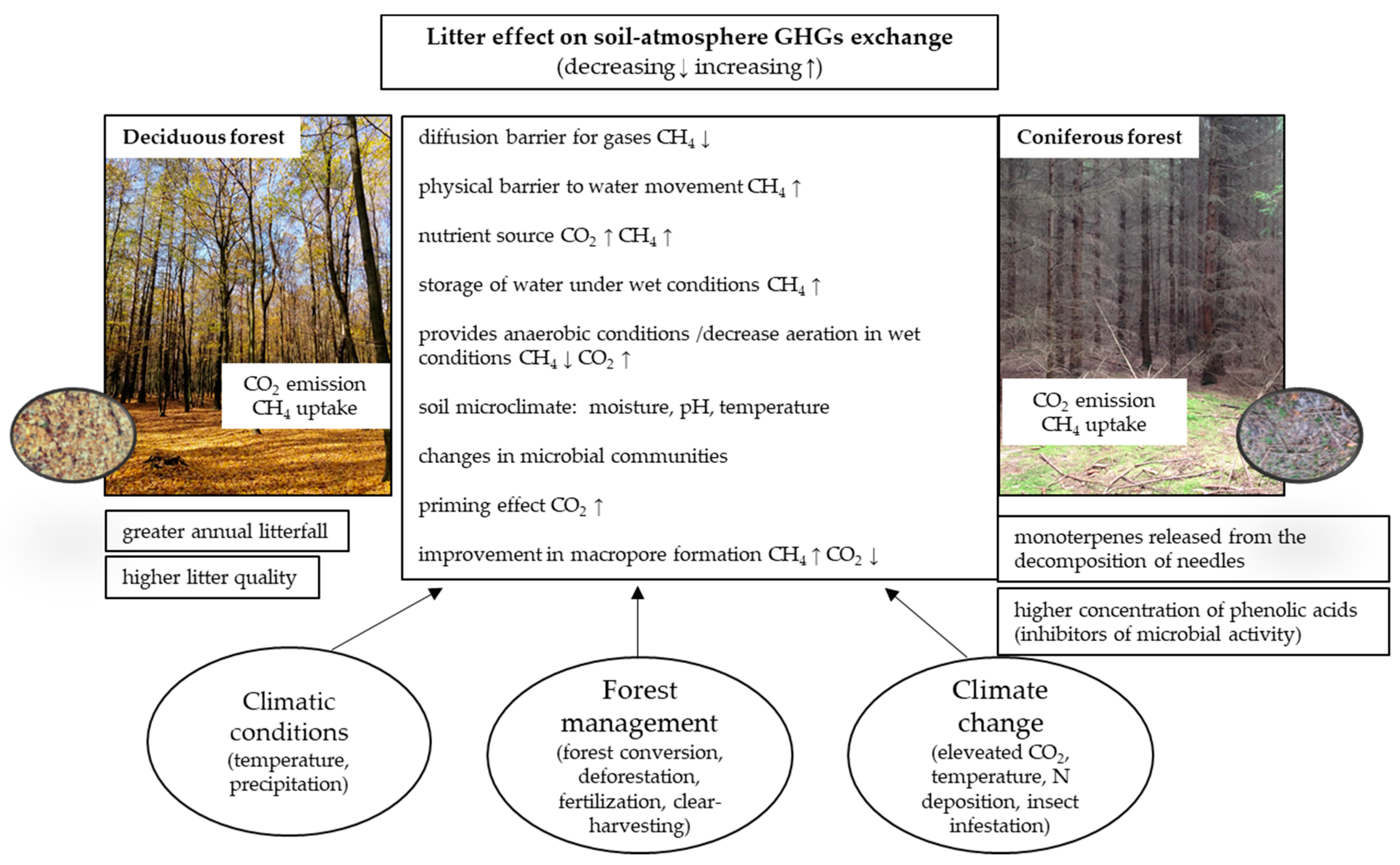
3. Tree Species-Specific Mechanisms of the Litter Effect on GHG Fluxes
3.1. Carbon Dioxide (CO2)
3.2. Methane (CH4)
4. Environmental Controllers of the Impact of Litter on GHG Fluxes
5. Forest Management
6. Effect of Climate Change
7. Future Research
- (i)
- Due to the sensitivity of soil CO2 efflux to climatic factors, it is important to focus on the multiple effects of climate change (e.g., increased CO2 and CH4 concentrations, increased temperature, drought, extreme precipitation events) on GHG exchange between the atmosphere and the litter-covered soil (or soil without litter) in various types of forests, globally. The litter may have direct or indirect effects on GHG emissions and decomposition processes [144], and even small changes can alter the global C balance, and atmospheric CO2 concentrations, or nutrient cycling, with the potential to exacerbate the effects of climate change [21,105].
- (ii)
- Equally noteworthy is the need to have a better understanding of global change-driven forest succession, where broad-leaved trees have begun to appear in needle-leaved ecosystems. Due to the shifts in tree composition, a number of ecosystem (e.g., litterfall rates, litter quality and soil-related processes, soil organic matter decomposition, and GHGs production) are changing [64].
- (iii)
- Climate change affects the primary productivity of forests, and elevated CO2 concentrations in the atmosphere may result in increased litterfall and increased organic matter inputs into the soil, resulting in increased C sequestration [52,144]. Large amounts of aboveground litter can also lead to a priming effect—a complex but not fully understood (especially in situ) soil–plant interaction [68]. As a result of the increased contribution of fresh organic matter to the soil, the decomposition processes are stimulated and the older stored C is released from the soil as CO2 [52]. A better understanding of this phenomenon is important in the context of future climatic scenarios, according to which litter inputs will be increased [52] and the occurrence of priming effects may intensify.
- (iv)
- The effect of monoterpenes on CH4 uptake is a largely uninvestigated topic and we propose several research areas that require attention: (a) the better recognition and identification of monoterpenes dominant in litter from different tree species, combined with the recognition of methanotroph responses; (b) understanding the longevity of any effects of monoterpenes in litter since recently fallen litter has a higher content of monoterpenes [129,130,164]; (c) finding out whether there are inhibitors that reduce the emissions of terpenes from litter, since monoterpenes can decrease the activity of methanotrophs; (d) the verification of whether methanotrophs produce monoterpenes [165] would be worthwhile with the objective of stimulating CH4 uptake in coniferous forests, as terpenes are generally widespread in both plant and bacterial metabolism [166]; (e) further work is also required on the effect of increasing the N deposition on monoterpene fluxes [98] in all climatic zones.
- (v)
- (vi)
- The identification of the underlying processes through which litter influences soil processes requires research into the species composition of microbial consortia occurring in different forest types in a range of climatic zones.
- (vii)
- Finally, the characteristics of the investigated litter needs to be specified in more detail, including the thickness of the litter, its morphology, temperature, number of layers and the degree of decomposition.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
References
- Lal, R. Forest soils and carbon sequestration. For. Ecol. Manag. 2005, 220, 242–258. [Google Scholar] [CrossRef]
- Forest Europe. State of Europe’s Forests 2020; Forest Europe: Bonn, Germany, 2020. [Google Scholar]
- Fan, J.; Luo, R.; McConkey, B.G.; Ziadi, N. Effects of nitrogen deposition and litter layer management on soil CO2, N2O, and CH4 emissions in a subtropical pine forestland. Nat. Sci. Rep. 2020, 10, 8959. [Google Scholar] [CrossRef]
- Kuzyakov, Y. Sources of CO2 efflux from soil and review of partitioning methods. Soil Biol. Biochem. 2006, 38, 425–448. [Google Scholar] [CrossRef]
- Baccini, A.; Goetz, S.J.; Walker, W.S.; Laporte, N.T.; Sun, M.; Sulla-Menashe, D.; Hackler, J.; Beck, P.S.A.; Dubayah, R.; Friedl, M.A.; et al. Estimated carbon dioxide emissions from tropical deforestation improved by carbon-density maps. Nat. Clim. 2012, 2, 182–185. [Google Scholar] [CrossRef]
- Carvalho, J.A., Jr.; Amaral, S.S.; Costa, M.A.M.; Soares Neto, T.G.; Veras, C.A.G.; Costa, F.S.; Van Leeuwen, T.T.; Krieger Filho, G.C.; Tourigny, E.; Forti, M.C.; et al. CO2 and CO emission rates from three forest fire controlled experiments in Western Amazonia. Atmos. Environ. 2016, 135, 73–83. [Google Scholar] [CrossRef]
- Waheed, R.; Chang, D.; Sarwar, S.; Chen, W. Forest, agriculture, renewable energy, and CO2 emission. J. Clean. Prod. 2018, 172, 4231–4238. [Google Scholar] [CrossRef]
- IPCC. Climate Change 2014: Synthesis Report. Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; IPCC: Geneva, Switzerland, 2014. [Google Scholar]
- Le Mer, J.; Roger, P. Production, oxidation, emission and consumption of methane by soils: A review. Eur. J. Soil Biol. 2001, 37, 25–50. [Google Scholar] [CrossRef]
- Wnuk, E.; Walkiewicz, A.; Bieganowski, A. Methanogenesis and aerobic methanotrophy in arable soils contaminated with cadmium. Catena 2020, 189, 104480. [Google Scholar] [CrossRef]
- Reay, D.S.; Nadwell, D.B. Methane oxidation in temperate soils: Effects of inorganic N. Soil Biol. Biochem. 2004, 36, 2059–2065. [Google Scholar] [CrossRef]
- Nazaries, L.; Murrell, J.C.; Millard, P.; Baggs, L.; Singh, B.K. Methane, microbes implication for landscape-integrated CH4 budget. Glob. Change Biol. 2013, 23, 966–976. [Google Scholar]
- Han, T.; Huang, W.; Liu, J.; Zhou, G.; Xiao, Y. Different soil respiration responses to litter manipulation in three subtropical successional forests. Sci. Rep. 2015, 5, 18166. [Google Scholar] [CrossRef]
- Sayer, E.J. Using experimental manipulation to assess the roles of leaf litter in the function of forest ecosystems. Biol. Rev. 2006, 81, 1–31. [Google Scholar] [CrossRef]
- Xu, S.; Liu, L.L.L.; Sayer, E.J. Variability of above-ground litter inputs alters soil physicochemical and biological processes: A meta-analysis of litterfall-manipulation. Biogeosciences 2013, 10, 7423–7433. [Google Scholar] [CrossRef]
- Krishna, M.P.; Mohan, M. Litter decomposition in forest ecosystems: A review. Energ. Ecol. Environ. 2017, 2, 236–249. [Google Scholar] [CrossRef]
- Thoms, C.; Gleixner, G. Seasonal differences in tree species’ influence on soil microbial communities. Soil Biol. Biochem. 2013, 66, 239–248. [Google Scholar] [CrossRef]
- Cheng, J.; Lee, X.; Zhou, Z.; Wang, B.; Xing, Y.; Cheng, H.; Tang, Y. The effects of litter layer and soil properties on the soil-atmosphere fluxes of greenhouse gases in karst forest, southwest China. Pol. J. Ecol. 2013, 61, 79–92. [Google Scholar]
- Wang, Y.; Wang, H.; Ma, Z.; Dai, X.; Wen, X.; Liu, Y.; Wang, Z.L. The litter layer acts a moisture-induced bidirectional buffers for atmospheric methane uptake by soil of a subtropical pine plantation. Soil Biol. Biochem. 2013, 66, 45–50. [Google Scholar] [CrossRef]
- Prévost-Bouré, N.C.; Soudani, K.; Damesin, C.; Berveiller, D.; Lata, J.C.; Dufrêne, E. Increase in aboveground fresh litter quantity over-stimulates soil respiration in a temperate deciduous forest. Appl. Soil Ecol. 2010, 46, 26–34. [Google Scholar] [CrossRef]
- Kotroczó, Z.; Veres, Z.; Biró, B.; Tóth, J.A.; Fekete, I. Influence of temperature and organic matter content on soil respiration in a deciduous oak forest. Eurasian, J. Soil Sci. 2014, 3, 303–310. [Google Scholar] [CrossRef]
- Holmes, K.W.; Chadwick, O.A.; Kyriakidis, P.C.; Silva de Filho, E.P.; Soares, J.V.; Roberts, D.A. Large-area spatially explicit estimates of tropical soil carbon stocks and response to land-cover change. Glob. Biogeochem. Cycles 2006, 20, GB3004. [Google Scholar] [CrossRef]
- Crow, S.E.; Lajtha, K.; Bowden, R.D.; Yano, Y.; Brant, J.B.; Caldwell, B.A.; Sulzman, E.W. Increased coniferous needle inputs accelerate decomposition of soil carbon in an old-growth forest. For. Ecol. Manag. 2009, 258, 2224–2232. [Google Scholar] [CrossRef]
- Huang, W.; Spohn, M. Effects of long-term litter manipulation on soil carbon, nitrogen, and phosphorus in a temperate deciduous forest. Soil Biol. Biochem. 2015, 83, 12–18. [Google Scholar] [CrossRef]
- Barrena, I.; Menéndez, S.; Duñabeitia, M.; Merino, P.; Stange, C.F.; Spott, O.; González-Muria, C.; Estavillo, J.M. Greenhouse gas fluxes (CO2, N2O and CH4) from forest soils in the Basque Country: Comparison of different tree species and growth stages. For. Ecol. Manag. 2013, 310, 600–611. [Google Scholar] [CrossRef]
- Mjöfors, K.; Strömgren, M.; Nohrstedt, H.Ö.; Gärdenäs, A.I. Impact of site-preparation on soil-surface CO2 fluxes and litter decomposition in a clear-cut in Sweden. Silva Fenn. 2015, 49, 1–20. [Google Scholar] [CrossRef]
- Wu, J.; Lu, M.; Feng, J.; Zhang, D.; Chen, Q.; Li, Q.; Long, C.; Zhang, Q.; Cheng, X. Soil net methane uptake rates in response to short-term litter input change in a coniferous forest ecosystem of central China. Agric. For. Meteorol. 2019, 271, 307–315. [Google Scholar] [CrossRef]
- Borken, W.; Beese, F. Methane and nitrous oxide fluxes of soils in pure and mixed stands of European beech and Norway spruce. Eur. J. Soil Sci. 2006, 57, 617–625. [Google Scholar] [CrossRef]
- Eickenscheidt, N.; Brumme, R. Regulation of N2O and NOx emission patterns in six acid temperate beech forest soils by soil gas diffusivity, N turnover, and atmospheric NOx concentrations. Plant Soil 2013, 369, 515–529. [Google Scholar] [CrossRef]
- Epron, D.; Nouvellon, Y.; Roupsard, O.; Mouvondy, W.; Mabiala, A.; Saint-André, L.; Joffre, R.; Jourdan, C.; Bonnefond, J.; Berbigier, P.; et al. Spatial and temporal variations of soil respiration in a Eucalyptus plantation in Congo. For. Ecol. Manag. 2004, 202, 149–160. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, H.; Zhu, Q.; Peng, C.; Wu, N.; Yang, G.; Zhu, D.; Tian, J.; Tian, L.; Kang, X.; et al. Soil methane uptake by grasslands and forests in China. Soil Biol. Biochem. 2014, 74, 70–81. [Google Scholar] [CrossRef]
- Leitner, S.; Sae-Tun, O.; Kranzinger, L.; Zechmeister-Boltenstern, S.; Zimmermann, M. Contribution of litter layer to soil greenhouse gas emissions in a temperate beech forest. Plant Soil 2016, 403, 455–469. [Google Scholar] [CrossRef]
- Liu, H.; Zhao, P.; Lu, P.; Wang, Y.S.; Lin, Y.B.; Rao, X.Q. Greenhouse gas fluxes from soils of different land-use types in a hilly area of South China. Agric. Ecosyst. Environ. 2008, 124, 125–135. [Google Scholar] [CrossRef]
- Andersson, M.; Kjøller, A.; Struwe, S. Microbial enzyme activities in leaf litter, humus and mineral soil layers of European forests. Soil Biol. Biochem. 2004, 36, 1527–1537. [Google Scholar] [CrossRef]
- Bond-Lamberty, B.; Wang, C.; Gower, S.T. A global relationship between the heterotrophic and autotrophic components of soil respiration? Glob. Change Biol. 2004, 10, 1756–1766. [Google Scholar] [CrossRef]
- Hanson, P.J.; Edwards, N.T.; Garten, C.T.; Andrews, J.A. Separating root and soil microbial contributions to soil respiration: A review of methods and observations. Biogeochemistry 2000, 48, 115–146. [Google Scholar] [CrossRef]
- Fekete, I.; Kotroczó, Z.; Varga, C.; Nagy, P.T.; Várbíró, G.; Bowden, R.D.; Tóth, J.A.; Lajtha, K. Alterations in forest detritus inputs influence soil carbon concentration and soil respiration in a Central-European deciduous forest. Soil Biol. Biochem. 2014, 74, 106–114. [Google Scholar] [CrossRef]
- Ivanov, A.V.; Braun, M.; Zamolodchikov, D.G.; Lynov, D.V.; Panfilova, E.V. Forest litters as a link in the carbon cycle in coniferous-broadleaved forests of southern far east of Russia. Eurasian Soil Sci. 2018, 51, 1164–1171. [Google Scholar] [CrossRef]
- Li, Y.; Xu, M.; Sun, O.J.; Cui, W. Effects of root and litter exclusion on soil CO2 efflux and microbial biomass in wet tropical forests. Soil Biol. Biochem. 2004, 36, 2111–2114. [Google Scholar] [CrossRef]
- Mukhopadhyay, S.; Maiti, S.K. Soil CO2 flux in grasslands, afforested land and reclaimed coalmine overburden dumps: A case study. Land Degrad. Develop. 2014, 25, 216–227. [Google Scholar] [CrossRef]
- Dou, X.; Zhou, W.; Zhang, Q.; Cheng, X. Greenhouse gas (CO2, CH4, N2O) emissions from soils following afforestation in central China. Atmos. Environ. 2016, 126, 98–106. [Google Scholar] [CrossRef]
- Wroński, K.T. Spatial variability of CO2 fluxes from meadow and forest soils in western part of Wzniesienia Łódzkie (Łódź Hills). For. Res. Pap. 2018, 79, 45–58. [Google Scholar] [CrossRef]
- Vasconcelos, S.S.; Zarin, D.J.; Capanu, M.; Littell, R.; Davidson, E.A.; Ishida, F.Y.; Santos, E.B.; Araújo, M.M.; Aragão, D.V.; Rangel-Vasconcelos, L.G.T.; et al. Moisture and substrate availability constrain soil trace gas fluxes in an eastern Amazonian regrowth forest. Glob. Biogeochem. Cycle 2004, 18, GB2009. [Google Scholar] [CrossRef]
- Konda, R.; Ohta, S.; Ishizuka, S.; Heriyanto, J.; Wicaksono, A. Seasonal changes in the spatial structures of N2O, CO2, and CH4 fluxes from Acacia mangium plantation soils in Indonesia. Soil Biol. Biochem. 2010, 42, 1512–1522. [Google Scholar] [CrossRef]
- Fuentes, J.P.; Bown, H.E.; Perez-Quezada, J.F.; Franck, N. Litter removal in a sclerophyll forest: Short- and medium-term consequences for soil properties. Soil Sci. Soc. Am. J. 2014, 78, 634–644. [Google Scholar] [CrossRef]
- Sun, W.; Maseyk, K.; Lett, C.; Seibt, U. Litter dominates surface fluxes of carbonyl sulfide in a Californian oak woodland. J. Geophys. Res. Biogeo. 2016, 121, 438–450. [Google Scholar] [CrossRef]
- Zimmermann, M.; Meir, P.; Bird, M.; Malhi, Y.; Ccahuana, A. Litter contribution to diurnal and annual soil respiration in a tropical montane cloud forest. Soil Biol. Biochem. 2009, 41, 1338–1340. [Google Scholar] [CrossRef]
- Borken, W.; Davidson, E.A.; Savage, K.; Gaudinski, J.; Trumbore, S.E. Drying and wetting effects on carbon dioxide release from organic horizons. Soil Sci. Soc. Am. J. 2003, 67, 1888–1896. [Google Scholar] [CrossRef]
- Goulden, M.L.; Miller, S.C.; Da Rocha, H.R.; Menton, M.C.; De Freitas, H.C.; Silva Figueira, M.; Dias de Sousa, C.A. Diel and seasonal patterns of tropical forest CO2 exchange. Ecol. Appl. 2004, 14, S42–S54. [Google Scholar] [CrossRef]
- Sayer, E.J.; Powers, J.S.; Tanner, E.V.J. Increased litterfall in tropical forests Boosts the transfer of soil CO2 to the atmosphere. PLoS ONE 2007, 2, e1299. [Google Scholar] [CrossRef]
- Sulzman, E.W.; Brant, J.B.; Bowden, R.D.; Lajtha, K. Contribution of aboveground litter, belowground litter, and rhizosphere respiration to total soil CO2 efflux in an old growth coniferous forest. Biogeochemistry 2005, 73, 231–256. [Google Scholar] [CrossRef]
- Bréchet, L.M.; Lopez-Sangil, L.; George, C.; Birkett, A.J.; Baxendale, C.; Castro Trujillo, B.; Sayer, E.J. Distinc responses of soil respiration to experimental litter manipulation in temperate woodland and tropical forest. Ecol. Evol. 2018, 8, 3787–3796. [Google Scholar] [CrossRef]
- Zhao, Q.; Classen, A.T.; Wang, W.W.; Zhao, X.R.; Mao, B.; Zeng, D.H. Asymmetric effects of litter removal and litter addition on the structure and function of soil microbial communities in a managed pine forest. Plant Soil 2017, 414, 81–93. [Google Scholar] [CrossRef]
- Keiblinger, K.M.; Hall, E.K.; Wanek, W.; Szukics, U.; Hämmerle, I.; Ellersdorfer, G.; Böck, S.; Strauss, J.; Sterflinger, K.; Richter, A.; et al. The effect of resource quantity and the resource stoichiometry on microbial carbon-use-efficiency. FEMS Microbiol. Ecol. 2010, 73, 430–440. [Google Scholar] [CrossRef]
- Singh, B.K.; Bardgett, R.D.; Smith, P.; Reay, D.S. Microorganisms and climate change: Terrestrial feedbacks and mitigation options. Nat. Rev. Microbiol. 2010, 8, 779–790. [Google Scholar] [CrossRef] [PubMed]
- Schneider, T.; Keiblinger, K.M.; Schmid, E.; Sterflinger-Gleixner, K.; Ellersdorfer, G.; Roschitzki, B.; Richter, A.; Eberl, L.; Zechmeister-Boltenstern, S.; Riedel, K. Who is who in litter decomposition? Metaproteomics reveals major microbial players and their biogeochemical functions. ISME J. 2012, 6, 1749–1762. [Google Scholar] [CrossRef]
- Bonanomi, G.; De Filippis, F.; Cesarano, G.; La Storia, A.; Zotti, M.; Mazzoleni, S.; Incerti, G. Linking bacterial and eukaryotic microbiota to litter chemistry: Combining next generation sequencing with 13C CPMAS NMR spectroscopy. Soil Biol. Biochem. 2019, 129, 110–121. [Google Scholar] [CrossRef]
- Janssens, I.A.; Luyssaert, S. Nitrogen’s carbon bonus. Nat. Geosci. 2009, 2, 318–319. [Google Scholar] [CrossRef]
- Reay, D.; Sabine, C.; Smith, P.; Hymus, G. Spring-time for sinks. Nature 2007, 446, 727–728. [Google Scholar] [CrossRef]
- Mo, J.; Zhang, W.; Zhu, W.; Fang, Y.; Li, D.; Zhao, P. Response of soil respiration to stimulated N deposition in a disturbed and rehabilitated tropical forest in southern China. Plant Soil 2007, 296, 125–135. [Google Scholar] [CrossRef]
- Manzoni, S.; Jackson, R.B.; Trofymow, J.A.; Porporatio, A. The global stoichiometry of litter nitrogen mineralization. Science 2008, 321, 684–686. [Google Scholar] [CrossRef]
- Smolander, A.; Loponen, J.; Suominen, K.; Kitunen, V. Organic matter characteristics and C and N transformations in the humus layer under two tree species, Betula pendula and Picea abies. Soil Biol. Biochem. 2005, 37, 1309–1318. [Google Scholar] [CrossRef]
- Ushio, M.; Balser, T.C.; Kitayama, K. Effects of condensed tannins in conifer leaves on the composition and activity of the soil microbial community in a tropical montane forest. Plant Soil 2013, 365, 157–170. [Google Scholar] [CrossRef]
- Fernández-Alonso, M.; Curiel Yuste, J.; Kitzler, B.; Oritz, C.; Rubio, A. Changes in litter chemistry associated with global change-driven forests succession resulted in time-decoupled responses of soil carbon and nitrogen cycles. Soil Biol. Biochem. 2018, 120, 200–211. [Google Scholar] [CrossRef]
- Tang, X.; Liu, S.; Zhou, G.; Zhang, D.; Zhou, C. Soil-atmospheric exchange of CO2, CH4, and N2O in three subtropical forest ecosystems in southern China. Glob. Chang. Biol. 2006, 12, 546–560. [Google Scholar] [CrossRef]
- Kuzyakov, Y.; Friedel, J.K.; Stahr, K. Review of mechanisms and quantification of priming effects. Soil Biol. Biochem. 2000, 32, 1485–1498. [Google Scholar] [CrossRef]
- Nottingham, A.T.; Griffiths, H.; Chamberlain, P.M.; Stott, A.W.; Tanner, E.V.J. Soil priming by sugar and leaf-litter substrates: A link to microbial groups. Appl. Soil Ecol. 2009, 42, 183–190. [Google Scholar] [CrossRef]
- Kuzyakov, Y. Priming effects: Interactions between living and dead organic matter. Soil Biol. Biochem. 2010, 42, 1363–1371. [Google Scholar] [CrossRef]
- Lyu, M.; Nie, Y.; Giardina, C.P.; Vadeboncoeur, M.A.; Ren, Y.; Fu, Z.; Wang, M.; Jin, C.; Liu, X.; Xie, J. Litter quality and site characteristics interact to affect the response of priming effect to temperature in subtropical forests. Funct. Ecol. 2019, 33, 2226–2238. [Google Scholar] [CrossRef]
- Naldini, M.B.; Harguindeguy, N.P.; Kowaljow, E. Soil carbon release enhanced by increased litter input in a degraded semi-arid forest soil. J. Arid Environ. 2021, 186, 104400. [Google Scholar] [CrossRef]
- Chao, L.; Liu, Y.; Freschet, G.T.; Zhang, W.; Yu, X.; Zheng, W.; Guan, X.; Yang, Q.; Chen, L.; Dijkstra, F.A. Litter carbon and nutrient chemistry control the magnitude of soil priming effect. Funct. Ecol. 2019, 33, 876–888. [Google Scholar] [CrossRef]
- Nottingham, A.T.; Turner, B.L.; Chamberlain, P.M.; Stott, A.W.; Tanner, E.V.J. Priming and microbial nutrient limitation in lowland tropical forest soils of contrasting fertility. Biogeochemistry 2012, 111, 219–237. [Google Scholar] [CrossRef]
- Luo, Y.; Zhou, X. Soil Respiration and the Environment; Academic Press: London, UK, 2006; p. 328. [Google Scholar]
- Khalid, M.S.; Shaaban, M.; Hu, R. N2O, CH4, and CO2 emissions from continuous flooded, wet, and flooded converted to wet soils. J. Plant Nutr. Soil Sci. 2019, 19, 342–351. [Google Scholar] [CrossRef]
- Price, S.J.; Sherlock, R.R.; Kelliher, F.M.; Mcseveny, T.M.; Tate, K.R.; Condron, L.M. Pristine New Zealand forest soil is a strong methane sink. Glob. Chang. Biol. 2004, 10, 16–26. [Google Scholar] [CrossRef]
- Marrero, T.R.; Mason, E.A. Gaseous diffusion coefficients. J. Phys. Chem. Ref. 1972, 1, 3–118. [Google Scholar] [CrossRef]
- Maurer, D.; Kolb, S.; Haumaier, L.; Borken, W. Inhibition of atmospheric methane oxidation by monoterpenes in Norway spruce and European beech soils. Soil Biol. Biochem. 2008, 40, 3014–3020. [Google Scholar] [CrossRef]
- Peichl, M.; Arain, M.A.; Ullah, S.; Moore, T.R. Carbon dioxide, methane, and nitrous oxide exchanges in an age-sequence of temperate pine forests. Glob. Change Biol. 2010, 16, 2198–2212. [Google Scholar] [CrossRef]
- Saari, A.; Heiskanen, J.; Martikainen, P.J. Effect of the organic horizon on methane oxidation and uptake in soil of a boreal Scots pine forest. FEMS Microbiol. Ecol. 1998, 26, 245–255. [Google Scholar] [CrossRef]
- Steinkamp, R.; Butterbach-Bahl, K.; Papen, H. Methane oxidation by soils of an N limited and N fertilized spruce forest in the Black Forest, Germany. Soil Biol. Biochem. 2001, 33, 145–153. [Google Scholar] [CrossRef]
- Dong, Y.; Scharffe, D.; Lobert, J.M.; Crutzen, P.J.; Sanhueza, E. Fluxes of CO2, CH4 and N2O from temperate forest soil: The effects of leaves and humus layers. Tellus B Chem. Phys. Meteorol. 1998, 50, 243–252. [Google Scholar] [CrossRef]
- Yan, Y.; Sha, L.; Cao, M.; Zheng, Z.; Tang, J.; Wang, Y.; Zhang, Y.; Wang, R.; Liu, G.; Wang, Y.; et al. Fluxes of CH4 and N2O from soil under a tropical seasonal rain forest in Xishuangbanna, Southwest China. J. Environ. Sci. 2008, 20, 207–215. [Google Scholar] [CrossRef]
- Xiao, D.M.; Wang, M.; Wang, Y.S.; Ji, L.Z.; Han, S.J. Fluxes of soil carbon dioxide, nitrous oxide and firedamp in broadleaved/Korean pine forest. J. For. Res. 2004, 15, 107–112. [Google Scholar]
- Pedersen, E.P.; Elberling, B.; Michelsen, A. Seasonal variations in methane fluxes in response to summer warming and leaf litter addition in a subarctic heath ecosystem. J. Geophys. Res. Biogeosci. 2017, 122, 2137–2153. [Google Scholar] [CrossRef]
- Liu, Y.; Cui, Z.; Huang, Z.; Miao, H.T.; Wu, G.L. The influence of litter crusts on soil properties and hydrological processes in a sandy ecosystem. Hydrol. Earth Syst. Sci. 2019, 23, 2481–2490. [Google Scholar] [CrossRef]
- Zhao, S.W.; Zhao, Y.G.; Wu, J.S. Quantitative analysis of soil pores under natural vegetation successions on the Loess Plateau. Sci. China Earth Sci. 2010, 53, 617–625. [Google Scholar] [CrossRef]
- Wolf, K.; Flessa, H.; Veldkamp, E. Atmospheric methane uptake by tropical montane forest soils and the contribution of organic layers. Biogeochemistry 2012, 111, 469–483. [Google Scholar] [CrossRef]
- Gritsch, C.; Egger, F.; Zehetner, F.; Zechmeister-Boltenstern, S. The effect of temperature and moisture on trace gas emissions from deciduous and coniferous leaf litter. Biogeosciences 2016, 121, 1339–1351. [Google Scholar] [CrossRef]
- Zhang, Y.; Zou, J.; Meng, D.; Dang, S.; Zhou, J.; Osborne, B.; Ren, Y.; Liang, T.; Yu, K. Effect of soil microorgnisms and labile C availability on soil respiration in response to litter inputs in forest ecosystems: A meta-analysis. Ecol. Evol. 2020, 10, 13602–13612. [Google Scholar] [CrossRef]
- Sullivan, B.W.; Selmants, P.C.; Hart, S.C. What is the relationship between soil methane oxidation and other C compounds? Glob. Change Biol. 2014, 20, 2381–2382. [Google Scholar] [CrossRef]
- Hensgens, G.; Lechtenfeld, O.J.; Guillemette, F.; Laudon, H.; Berggren, M. Impacts of litter decay on organic leachate composition and reactivity. Biogeochemistry 2021, 154, 99–117. [Google Scholar] [CrossRef]
- Liu, X.P.; Zhang, W.J.; Hu, C.S.; Tang, X.G. Soil greenhouse gas fluxes from different tree species on Taihang Mountain, North China. Biogeosciences 2014, 11, 1649–1666. [Google Scholar] [CrossRef]
- Li, Q.; Lee, Y.E.; Im, S. Characterizing the interception capacity of floor litter with rainfall simulation experiments. Water 2020, 12, 3145. [Google Scholar] [CrossRef]
- Erickson, H.; Davidson, E.A.; Keller, M. Former land-use and tree species affect nitrogen oxide emissions from a tropical dry forest. Oecologia 2002, 130, 297–308. [Google Scholar] [CrossRef]
- Hansen, K.; Vesterdal, L.; Schmidt, I.K.; Gundersen, P.; Sevel, L.; Bastrup-Birk, A.; Pedersen, L.B.; Bille-Hansen, J. Litter fall and nutrient return in five tree species in a common garden experiment. For. Ecol. Manag. 2009, 257, 2133–2144. [Google Scholar] [CrossRef]
- Guckland, A.; Brauns, M.; Flessa, H.; Thomas, F.; Leuschner, C. Acidity, nutrient stocks and organic matter content in soils of a temperate deciduous forest with different abundance of European beech (Fagus sylvatica L.). J. Plant Nutr. Soil Sci. 2009, 172, 500–511. [Google Scholar] [CrossRef]
- Longdoz, B.; Yernaux, M.; Aubinet, M. Soil CO2 efflux measurements in a mixed forest: Impact of chamber disturbances, spatial variability and seasonal evolution. Glob. Change Biol. 2000, 6, 907–917. [Google Scholar] [CrossRef]
- Huang, X.; Zheng, L.; Guo, P.; Yi, Z. Nitrogen addition inhibits total monoterpene emissions in subtropical forest floor of South China. Soil Ecol. Lett. 2021, 3, 63–72. [Google Scholar] [CrossRef]
- Huang, J.; Hammerbacher, A.; Weinhold, A.; Reichelt, M.; Gleixner, G.; Behrendt, T.; van Dam, N.M.; Sala, A.; Gershenzon, J.; Trumbore, S.E.; et al. Eyes on the future—Evidence for trade-offs between growth, storage and defense in Norway spruce. N. Phytol. 2019, 222, 144–158. [Google Scholar] [CrossRef] [PubMed]
- Vose, J.M.; Bolstad, P.V. Biotic and abiotic factors regulating forest floor CO2 flux across a range of forest age classes in the southern Appalachians. Pedobiologia 2007, 50, 577–587. [Google Scholar] [CrossRef]
- Parfitt, R.L.; Salt, G.J.; Saggar, S. Post-harvest residue decomposition and nitrogen dynamics in Pinus radiata plantations of different N status. For. Ecol. Manag. 2001, 154, 55–67. [Google Scholar] [CrossRef]
- Zhou, G.; Guan, L.; Wei, X.; Zhang, D.; Zhang, Q.; Yan, J.; Wen, D.; Liu, J.; Liu, S.; Huang, Z.; et al. Litterfall production along successional and altitudinal gradients of subtropical monsoon evergreen broadleaved forests in Guangdong, China. Plant Ecol. 2007, 188, 77–89. [Google Scholar] [CrossRef]
- Chen, H.; Gurmesa, G.A.; Liu, L.; Zhang, T.; Fu, S.; Liu, Z.; Dong, S.; Ma, C.; Mo, J. Effects of litter manipulation on litter decomposition in a successional gradients of tropical forests in southern China. PLoS ONE 2014, 9, e99018. [Google Scholar] [CrossRef] [PubMed]
- Guckland, A.; Flessa, H.; Prenzel, J. Controls of temporal and spatial variability of methane uptake in soils of a temperate deciduous forest with different abundance of European beech (Fagus sylvatica L.). Soil Biol. Biochem. 2009, 41, 1659–1667. [Google Scholar] [CrossRef]
- Borges Pinto, O., Jr.; Vourlitis, G.L.; De Souza Carneiro, E.M.; De França Dias, M.; Hentz, C.; De Souza Nogueira, J. Interactions between vegetation, hydrology, and litter inputs on decomposition and soil CO2 efflux of tropical forests in the Brazilian Pantanal. Forests 2018, 9, 281. [Google Scholar] [CrossRef]
- Townsend, A.R.; Asner, G.P.; Cleveland, C.C. The biogeochemical heterogeneity of tropical forests. Trends Ecol. Evol. 2008, 23, 424–431. [Google Scholar] [CrossRef]
- Leff, J.W.; Wieder, W.R.; Taylor, P.G.; Townsend, A.R.; Nemergut, D.R.; Grandy, A.S.; Cleveland, C.C. Experimental litterfall manipulation drives large and rapid changes in soil carbon cycling in a wet tropical forest. Glob. Change Biol. 2012, 18, 2969–2979. [Google Scholar] [CrossRef] [PubMed]
- Binkley, D.; Giardina, C. Why do tree species affect soils? The Warp and Woof of tree-soil interactions. Biogeochemistry 1998, 42, 89–106. [Google Scholar] [CrossRef]
- Hasselquist, N.J.; Allen, M.F.; Santiago, L.S. Water relations of evergreen and drought-deciduous trees along a seasonally dry tropical forest chronosequence. Oecologia 2010, 164, 881–890. [Google Scholar] [CrossRef] [PubMed]
- Kolb, S.; Knief, C.; Dunfield, P.F.; Conrad, R. Abundance and activity of uncultured methanotrophic bacteria involved in the consumption of atmospheric methane in two forest soils. Environ. Microbiol. 2005, 7, 1150–1161. [Google Scholar] [CrossRef] [PubMed]
- Kolb, S. The quest for atmospheric methane oxidizers in forest soils. Environ. Microbiol. Rep. 2009, 1, 336–346. [Google Scholar] [CrossRef] [PubMed]
- Menyailo, O.V.; Hungate, B.A. Interactive effects of tree species and soil moisture on methane consumption. Soil Biol. Biochem. 2003, 35, 625–628. [Google Scholar] [CrossRef]
- Degelmann, D.M.; Kolb, S.; Borken, W. Methane oxidation kinetics differ in European beech and Norway spruce soils. Eur. J. Soil Sci. 2009, 60, 499–506. [Google Scholar] [CrossRef]
- Zeng, L.; Tian, J.; Chen, H.; Wu, N.; Yan, Z.; Du, L.; Shen, Y.; Wang, X. Changes in methane oxidation ability and methanotrophic community composition across different climatic zones. J. Soils Sediments 2019, 19, 533–543. [Google Scholar] [CrossRef]
- Burgess-Conforti, J.R.; Moore, P.A., Jr.; Owens, P.R.; Miller, D.M.; Ashworth, A.J.; Hays, P.D.; Evans-White, M.A.; Anderson, K.R. Are soils beneath coniferous tree stands more acidic than soils beneath deciduous tree stands? Environ. Sci. Pollut. Res. Int. 2019, 15, 14920–14929. [Google Scholar] [CrossRef]
- Lau, E.; Ahmad, A.; Steudler, P.A.; Cavanaugh, C.M. Molecular characterization of methanotrophic communities in forest soils that consume atmospheric methane. FEMS Microbiol. Ecol. 2007, 60, 490–500. [Google Scholar] [CrossRef]
- Megonigal, J.P.; Guenther, A.B. Methane emissions from upland forest soils and vegetation. Tree Physiol. 2008, 28, 491–498. [Google Scholar] [CrossRef]
- Machacova, K.; Bäck, J.; Vanhatalo, A.; Halmeenmäki, E.; Kolari, P.; Mammarella, I.; Pumpanen, J.; Acosta, M.; Urban, O.; Pihlatie, M. Pinus sylvestris as a missing source of nitrous oxide and methane in boreal forest. Sci. Rep. 2016, 6, 23410. [Google Scholar] [CrossRef] [PubMed]
- Hanson, R.S.; Hanson, T.E. Methanotrophic bacteria. Microbiol. Rev. 1996, 60, 439–471. [Google Scholar] [CrossRef]
- Nguyen, N.L.; Yu, W.J.; Gwak, J.H.; Kim, S.J.; Park, S.J.; Herbold, C.W.; Kim, J.G.; Jung, M.Y.; Rhee, S.K. Genomic insights into the acid adaptation of novel methanotrophs enriched from acidic forest soils. Front. Microbiol. 2018, 9, 1982. [Google Scholar] [CrossRef]
- Ambus, P.; Zechmeister-Boltenstern, S.; Butterbach-Bahl, K. Sources of nitrous oxide emitted from European forest soils. Biogeosciences 2006, 3, 135–145. [Google Scholar] [CrossRef]
- Bodelier, P.L.E.; Laanbroek, H.J. Nitrogen as regulatory factor of methane oxidation in soils and sediments. FEMS Microbiol. Ecol. 2004, 47, 265–277. [Google Scholar] [CrossRef]
- Bodelier, P.L.E.; Steenbergh, A.K. Interactions between methane and the nitrogen cycle in light of climate change. Curr. Opin. Env. Sustain. 2014, 9, 26–36. [Google Scholar] [CrossRef]
- Mochizuki, Y.; Koba, K.; Yoh, M. Strong inhibitory effect of nitrate on atmospheric methane oxidation in forest soils. Soil Biol. Biochem. 2012, 50, 164–166. [Google Scholar] [CrossRef]
- Steudler, P.A.; Bowden, R.D.; Melillo, J.M.; Aber, J.D. Influence of nitrogen fertilization on methane uptake in temperate forest soils. Nature 1989, 341, 314–316. [Google Scholar] [CrossRef]
- Saari, A.; Rinnan, R.; Martikainena, P.J. Methane oxidation in boreal forest soils: Kinetics and sensitivity to pH and ammonium. Soil Biol. Biochem. 2004, 36, 1037–1046. [Google Scholar] [CrossRef]
- Reay, D.S.; Nedwell, D.B.; McNamara, N.; Ineson, P. Effect of tree species on methane and ammonium oxidation capacity in forest soils. Soil Biol. Biochem. 2005, 37, 719–730. [Google Scholar] [CrossRef]
- Li, Q.; Peng, H.; Zhang, J.; Li, Y.; Song, X. Nitrogen addition decreases methane uptake caused by methanotroph and methanogen imbalances in a Moso bamboo forest. Sci. Rep. 2021, 11, 5578. [Google Scholar] [CrossRef]
- Jo, G.G.; Kim, J.H. Changes in terpenes of three kinds of pine needles during litter decomposition. J. Ecol. Field Biol. 2010, 33, 175–186. [Google Scholar] [CrossRef]
- Wilt, F.M.; Miller, G.C.; Everett, R.L.; Hackett, M. Monoterpene concentrations in fresh, senescent and decaying foliage of single-leaf pinyon (Pinus monophylla Torr. & Frem.: Pinaceae) from the western great Basin. J. Chem. Ecol. 1993, 19, 185–194. [Google Scholar]
- Amaral, J.A.; Knowles, R. Inhibition of methane consumption in forest soils by monoterpenes. J. Chem. Ecol. 1998, 24, 723–734. [Google Scholar] [CrossRef]
- Wu, B.; Mu, C. Effects on Greenhouse Gas (CH4, CO2, N2O) Emissions of Conversion from Over-Mature Forest to Secondary Forest and Korean Pine Plantation in Northeast China. Forests 2019, 10, 788. [Google Scholar] [CrossRef]
- Pacheco, A.; Lindner, A.S. Effects of Alpha-pinene and trichloroethylene on oxidation potentials of methanotrophic bacteria. Bull. Environ. Contamin. Toxicol. 2005, 74, 133–140. [Google Scholar] [CrossRef]
- Dannenmann, M.; Gasche, R.; Ledebuhr, A.; Holst, T.; Mayer, H.; Papen, H. The effect of forest management on trace gas exchange at the pedosphere-atmosphere interface in beech (Fagus sylvatica L.) forests stocking on calcareous soils. Eur. J. For. Res. 2007, 126, 331–346. [Google Scholar] [CrossRef]
- Hanson, P.J.; O’Neill, E.G.; Chambers, M.L.S.; Riggs, J.S.; Joslin, J.D.; Wolfe, M.H. Soil respiration and litter decomposition. In North American Temperate Deciduous Forest Response to Changing Precipitation Regimes. Ecological Studies (Analysis and Synthesis); Hanson, P.J., Wullschleger, S.D., Eds.; Springer: New York, NY, USA, 2003; Volume 166, pp. 163–189. [Google Scholar]
- Ataka, M.; Kominami, Y.; Jomura, M.; Yoshimura, K.; Miyama, T.; Kosugi, Y.; Tani, M. CO2 efflux from decomposing leaf litter stack is influenced by the vertical distribution of leaf litter water content and its temporal variation. J. Agric. Meterol. 2015, 71, 263–270. [Google Scholar] [CrossRef]
- Wilson, T.B.; Kochendorfer, J.; Meyers, T.P.; Heuer, M.; Sloop, K.; Miller, J. Leaf litter water content and soil surface CO2 fluxes in a deciduous forest. Agric. For. Meteor. 2014, 192, 42–50. [Google Scholar] [CrossRef]
- Ataka, M.; Kominami, Y.; Yoshimura, K.; Miyama, T.; Jomura, M.; Tani, M. In situ CO2 efflux from leaf litter layer showed large temporal variation induced by rapid wetting and drying cycle. PLoS ONE 2014, 9, e108404. [Google Scholar] [CrossRef] [PubMed]
- Sardans, J.; Peñuelas, J. Drought decreases soil enzyme activity in a Mediterranean Quercus ilex L. forest. Soil Biol. Biochem. 2005, 37, 455–461. [Google Scholar] [CrossRef]
- Fekete, I.; Kotroczó, Z.; Varga, C.; Hargitai, R.; Townsend, K.; Csányi, G.; Várbiró, G. Variability of organic matter inputs affects soil moisture and soil biological parameters in a European detritus manipulation experiment. Ecosystems 2012, 15, 792–803. [Google Scholar] [CrossRef]
- Veres, Z.; Ktroczó, Z.; Magyaros, K.; Tóth, J.A.; Tóthmérész, B. Dehydrogenase activity in a litter manipulation experiment in temperate forest soil. Acta Silv. Lignaria Hung. 2013, 9, 25–33. [Google Scholar] [CrossRef][Green Version]
- Beni, A.; Soki, E.; Lajtha, K.; Fekete, I. An optimized HPLC method for soil fungal biomass determination and its application to a detritus manipulation study. J. Microbiol. Methods 2014, 103, 124–130. [Google Scholar] [CrossRef]
- Buchmann, N. Biotic and abiotic factors controlling soil respiration rates in Picea abies stands. Soil Biol. Biochem. 2000, 32, 1625–1635. [Google Scholar] [CrossRef]
- Yan, W.; Peng, Y.; Zhan, C.; Chen, X. The manipulation of aboveground litter input affects soil CO2 efflux in a subtropical liquidambar forest in China. iForest 2019, 12, 181–186. [Google Scholar] [CrossRef]
- Tate, K.R.; Ross, D.J.; Scott, N.A.; Rodda, N.J.; Townsend, J.A.; Arnold, G.C. Post-harvest patterns of carbon dioxide production, methane uptake and nitrous oxide production in a Pinus radiata D. Don plantation. For. Ecol. Manag. 2006, 228, 40–50. [Google Scholar] [CrossRef]
- Huang, Z.; Clinton, P.W.; Davis, M.R.; Yang, Y. Impacts of plantation forest management on soil organic matter quality. J. Soils Sediments 2011, 11, 1309–1316. [Google Scholar] [CrossRef]
- Molchanov, A.G.; Kurbatova, Y.A.; Olchev, A.V. Effect of clear-cutting on soil CO2 emission. Biol. Bull. 2017, 44, 218–223. [Google Scholar] [CrossRef]
- Wang, H.L.; Liu, J.; Jiang, P.K.; Zhou, G.M.; Li, Y.F.; Wu, J. Effect of management practices on methane uptake in forest soils. Sci. Silvae Sin. 2017, 53, 156–163. [Google Scholar]
- Ström, L.; Falk, J.M.; Skov, K.; Jackowicz-Korcynski, M.; Mastepanov, M.; Christensen, T.R.; Lund, M.; Schmidt, N.M. Controls of spatial and temporal variability in CH4 flux in a high arctic fen over three years. Biogeochemistry 2015, 125, 21–35. [Google Scholar] [CrossRef]
- Tate, K.R. Soil methane oxidation and land-use change-From process to mitigation. Soil Biol. Biochem. 2015, 80, 260–272. [Google Scholar] [CrossRef]
- Fontaine, S.; Bardoux, G.; Abbadie, L.; Mariotti, A. Carbon input to soil may decrease soil carbon content. Ecol. Lett. 2004, 7, 314–320. [Google Scholar] [CrossRef]
- Schaefer, D.A.; Feng, W.; Zou, X. Plant carbon inputs and environmental factors strongly affect soil respiration in a subtropical forest of southwestern China. Soil Biol. Biochem. 2009, 41, 1000–1007. [Google Scholar] [CrossRef]
- Graglia, E.; Jonasson, S.; Michelsen, A.; Schmidt, I.K.; Havström, M.; Gustavsson, L. Effects of environmental perturbations on abundance of subarctic plants after three, seven and ten years of treatments. Ecography 2001, 24, 5–12. [Google Scholar] [CrossRef]
- Cornelissen, J.H.; Van Bodegom, P.M.; Aerts, R.; Callaghan, T.V.; Van Logtestijn, R.S.P.; Alatalo, J.; Chapin, F.S.; Gerdol, R.; Gudmundsson, J.; Gwynn-Jones, D.; et al. Team. Global negative vegetation feedback to climate warming responses of leaf litter decomposition in cold biomes, Ecol. Lett. 2007, 10, 619–627. [Google Scholar]
- Rinnan, R.; Michelsen, A.; Bååth, E.; Jonasson, S. Mineralization and carbon turnover in subarctic heath soil as affected by warming and additional litter. Soil Biol. Biochem. 2007, 39, 3014–3023. [Google Scholar] [CrossRef]
- Rinnan, R.; Michelsen, A.; Jonasson, S. Effects of litter addition and warming on soil carbon, nutrient pools, and microbial communities in a subarctic heath ecosystem. Appl. Soil Ecol. 2008, 39, 271–281. [Google Scholar] [CrossRef]
- Venterea, R.T.; Groffman, P.M.; Verchot, L.V.; Magill, A.H.; Aber, J.D. Gross nitrogen process rates in temperate forest soils exhibiting symptoms of nitrogen saturation. For. Ecol. Manag. 2004, 194, 129–142. [Google Scholar] [CrossRef]
- Tuchman, N.C.; Wetzel, R.G.; Rier, S.T.; Wahtera, K.A.; Teeri, J.A. Elevated atmospheric CO2 lowers leaf litter nutritional quality for stream ecosystem food webs. Glob. Chang. Biol. 2002, 8, 163–170. [Google Scholar]
- Pancotto, V.A.; van Bodegom, P.M.; van Hal, J.; van Logtestijn, R.S.P.; Blokker, P.; Toet, S.; Aerts, R. N deposition and elevated CO2 on methane emissions: Differential responses of indirect effects compared to direct effects through litter chemistry feedbacks. J. Geophys. Res. 2010, 115, G02001. [Google Scholar]
- Brumme, R.; Borken, W. Site variation in methane oxidation as affected by atmospheric deposition and type of temperate forest ecosystem. Glob. Biogeochem. Cycles 1999, 13, 493–501. [Google Scholar] [CrossRef]
- Seidl, R.; Klonner, G.; Rammer, W.; Essl, F.; Moreno, A.; Neumann, M.; Dullinger, S. Invasive alien pests threaten the carbon stored in Europe’s forests. Nat. Commun. 2018, 9, 1626. [Google Scholar] [CrossRef]
- Flower, C.E.; Gonzalez-Meler, M.A. Responses of temperate forest productivity to insect and pathogen disturbances. Annu. Rev. Plant Biol. 2015, 66, 547–569. [Google Scholar] [CrossRef] [PubMed]
- Grüning, M.M.; Germeshausen, F.; Thies, C. Increased forest soil CO2 and N2O emissions during insect infestation. Forests 2018, 9, 612. [Google Scholar] [CrossRef]
- Kainulainen, P.; Holopainen, J.K. Concentrations of secondary compounds in Scots pine needles at different stages of decomposition. Soil Biol. Biochem. 2002, 34, 37–42. [Google Scholar] [CrossRef]
- Veraart, A.J.; Garbeva, P.; van Beersum, F.; Ho, A.; Hordijk, C.A.; Meima-Franke, M.; Zweers, A.J.; Bodelier, P.L.E. Living apart together-bacterial volatiles influence methanotrophic growth and activity. ISME J. 2018, 12, 1163–1166. [Google Scholar] [CrossRef]
- Yamada, Y.; Kuzuyama, T.; Komatsu, M.; Shin-ya, K.; Omura, S.; Cane, D.E.; Ikeda, H. Terpene synthases are widely distributed in bacteria. Proc. Natl. Acad. Sci. USA 2015, 112, 857–862. [Google Scholar] [CrossRef] [PubMed]
- Butterbach-Bahl, K.; Kock, M.; Willibald, G.; Hewett, B.; Buhagiar, S.; Papen, H.; Kiese, R. Temporal variations of fluxes of NO, NO2, N2O, CO2, and CH4 in a tropical rain forest ecosystem. Glob. Biogeochem. Cycles 2004, 18, GB3012. [Google Scholar] [CrossRef]
- Menyailo, O.V.; Hungate, B.A.; Abraham, W.-R.; Conrad, R. Changing land use reduces soil CH4 uptake by altering biomass and activity but not composition of high-affinity methanotrophs. Glob. Change Biol. 2008, 14, 2405–2419. [Google Scholar] [CrossRef]
- Bowden, R.D.; Nadelhoffer, K.J.; Boone, R.D.; Melillo, J.M.; Garrison, J.B. Contributions of aboveground litter, belowground litter, and root respiration to total soil respiration in a temperate mixed hardwood forest. Can. J. For. Res. 1993, 23, 1402–1407. [Google Scholar] [CrossRef]
- Sun, L.; Teramoto, M.; Liang, N.; Yazaki, T.; Hirano, T. Comparison of litter-bag and chamber methods for measuring CO2 emissions from leaf litter decomposition in a temperate forest. J. Agric. Meteorol. 2017, 73, 68–76. [Google Scholar] [CrossRef]
- Xiao, W.; Ge, X.; Zeng, L.; Huang, Z.; Lei, J.; Zhou, B.; Li., M. Rates of litter decompostion and soil respiration in relation to soil temperature and water in dfferent-aged Pinus massoniana forests in the Three Gorges Reservoir area, China. PLoS ONE 2014, 9, e101890. [Google Scholar]
- Yan, W.D.; Chen, X.Y.; Tian, D.L.; Peng, Y.Y.; Wang, G.J.; Zheng, W. Impacts of changed litter inputs on soil CO2 efflux in three forest types in central south China. Chin. Sci. Bull. 2013, 58, 750–757. [Google Scholar] [CrossRef]
- Ming, A.; Yang, Y.; Liu, S.; Wang, H.; Li, Y.; Li, H.; Nong, Y.; Cai, D.; Jia, H.; Tao, Y.; et al. Effects of near natural forest management on soil greenhouse gas flux in Pinus massoniana (Lamb.) and Cunninghamia lanceolata (Lamb.) Hook plantations. Forests 2018, 9, 229. [Google Scholar] [CrossRef]
- Wang, H.; Liu, S.; Mo, J.; Zhang, T. Soil-atmosphere exchange of greenhouse gases in subtropical plantations of indigenous tree species. Plant Soil 2010, 335, 213–227. [Google Scholar] [CrossRef]
| The Main Driver | Forest Type | Dominant Tree Species | Tree Age [Years] | Tree Height [m] | DBH [cm] | Tree Density [Trees/ha] | Litter Input [g/m2/year] | MAT [°C] | MAP [mm] | Soil Type | Soil Texture (Sand/Silt/Clay [%]) | Soil Temperature [°C] | Soil Moisture [%] | Effect of Litter on CO2 Fluxes | Landscape Type | Location | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Litter as a source of nutrient for microbes | Plantation | T. grandis (92%) | ~10 | n/a | 10.72 ± 2.1 | 429 | n/a | n/a | 1598 | n/a | n/a | 28.78 ± 1.75 | 10.60 ± 2.42 | Increased by 14.4% ** | n/a | Jharkhand, Eastern India | [40] |
| Plantation | Eucalyptus sp. | 3 | 12 | n/a | 700 | n/a | 25 | 1200 | Arenosol (FAO) | Sandy | ~24–33 | n/a | Increased ** | coastal | Pointe Noire, Southwestern Congo | [30] | |
| regrowth forest | L. pubescens, M. sylvatica, V. guianensis, C. scrobiculata (all species represent 71% of all stems in the stand) | 12 | 4.9 ± 0.4 | n/a | 21,300 | n/a | 24–27 | 2539 ± 280 | Distrophic Yellow Latosol Stony Phase I (Brazilian Classification), Sombriustox (U.S. Soil Taxonomy) | Sandy clay loam (74/6/20) | n/a | n/a | Increased by 28% ** | n/a | Northern Brazil (1°19′ S, 47°57′ W) | [43] | |
| Plantation | Ac. mangium | 8 | 23.6 | 22.5 | n/a | 20–270 (fresh litter); 780–1130 (decayed litter); 1050–1160 (fresh + decayed litter) in wet and dry season | 27.3 | 2750 | Acrisols (WRB 1998) | n/a | n/a | 55.5–66.3% WFPS | Increased * | Undulating topography (upper and lower plateau, upper and foot slope) | South Sumatra (3°52′40″ S, 103°58′40″ E) Indonesia | [44] | |
| Pine forest | P. massoniana | 30 | 5 | n/a | 2600 | n/a | 17.8 | 1785 | Ferric Acrisols (USDA soil taxonomy) | Loamy clay (21/43/36) | 24.2 | 60.3% WFPS | Increased by 24–32% * | Hilly region | Yingtan, Jiangxi Province, Southeastern China (28°15′ N, 116°55′ E) | [3] | |
| Sclerophyll forest | Cr.alba, Q. saponaria, Pe. boldus, L. caustica | n/a | 5.06 ± 0.87 | 6.51 ± 1.39 | 2600 ± 978 | 314 ± 30 | n/a | 503 | Pachic Humixerepts | Sandy (62.4/26.4/11.2) | n/a | n/a | Increased by 21.2–33% ** | Top slope (<4% slope) | Central Chile (34°7″ S, 71°11′18″ W) | [45] | |
| Mixed pine-broadleaf forest | Cs. chinensis (50.9%), S. superba, P. massoniana | 100 | n/a | n/a | n/a | 861 | 22.3 | 1680 | Ultisol (USDA soil taxonomy) | Lateritic | 17.5–24.0 | 20.8–27.8 | Increased by 33–38% ** | n/a | Dinghushan Biosphere Reserver, Southern China (23°09′21″ N–23°11′30″ N, 112°30′39″ E–112°33′41″ E) | [13] | |
| Pine forest | P. massoniana Lamb. (90%) | 50 | n/a | n/a | n/a | 356 | 22.3 | 1680 | Ultisol (USDA soil taxonomy) | Lateritic | 18.2–24.8 | 17.1–20.9 | Increased by 37–42% ** | n/a | |||
| Monsoon evergreen broadleaf forest | Cs. chinensis; Cr. chinensis, S. superba, Cr. concinna, Ap. yunnanensis, Ac. acuminatissima, G. subaequalis (all these species represent >60% of the community biomass) | >400 | n/a | n/a | n/a | 849 | 22.3 | 1680 | Ultisol (USDA soil taxonomy) | Lateritic | 16.2–23.1 | 26.4–28.7 | Increased by 29–35% ** | n/a | |||
| Enhancement of anaerobic conditions by litter | Plantation | Pl. orientalis | n/a | n/a | n/a | n/a | n/a | 15.7 | 834 | Yellow brown soil | Silty clay (11/41/48) | 16.31 ± 1.05 | 12.34 ± 0.80 | Increased by 18.84% * | n/a | Danjiangkou Reservoir, Central China (32°45′ N, 111°13′ E) | [41] |
| Soil moisture retention by litter | Mediterranean oak woodland | Qr. agrifolia | n/a | n/a | n/a | n/a | n/a | 19 | 180 | n/a | Gravelly loam | n/a | n/a | Increased by 34.2–44.8% *** | n/a | Santa Monica Mountains, California (34°05′38″ N, 118°39′26″ W) USA | [46] |
| Montane cloud forest | Clusiaceae, Cunoniaceae, Myrsinaceae, Rosaceae, Clethraceae families | n/a | n/a | n/a | n/a | n/a | 12.5 | n/a | n/a | Acidic | n/a | n/a | No effect ** | n/a | Peruvian Andes (13°11′28″ S, 71°35′24″ E) | [47] | |
| Pine forest | P. massoniana | 50 | n/a | n/a | n/a | 356 | 22.3 | 1680 | Ultisol (USDA soil taxonomy) | Lateritic | 18.2–24.8 | 17.1–20.9 | Increased by 37–42% ** | n/a | Dinghushan Biosphere Reserver, Southern China (23°09′21″ N–23°11′30″ N, 112°30′39″ E–112°33′41″ E) | [13] | |
| Mixed deciduous forest | Ar. rubrum, Qr. rubra | n/a | n/a | n/a | n/a | n/a | 8.5 | 1050 | Typic Dystrochrept | Fine sandy loam | n/a | n/a | Increased ** | n/a | Harvard Forest, Petersham, Massachusetts (42°32′ N, 72°11′ W) USA | [48] | |
| Old-growth semidecidous tropical forest | n/a | n/a | n/a | >35 | n/a | n/a | n/a | >2000 | n/a | n/a | n/a | n/a | Increased ** | Flat plateau (the planalto) | Pará, Northern Brazil (3°0′37 S, 54°34′53″ W) | [49] | |
| Priming effect | Old-growth moist lowland tropical forest | n/a | n/a | n/a | n/a | n/a | n/a | 27 | 2600 | Oxisol | n/a | n/a | n/a | Increased by 20% ** | n/a | Gigante Peninsula, central Panama (9°06′ N, 79°54′ W) | [50] |
| Undisturbed old-growth forest | Ts. heterophylla, Ps. menziesii | n/a | n/a | n/a | n/a | n/a | 8.7 | 2370 | Typic Hapludands | Coarse loamy | 9.5 | 29 | Increased ** | n/a | H.J. Andrews Experimental Forest, Oregon (44°13′ N, 122°13′ W) USA | [23] | |
| Undisturbed old-growth forest | P. menziesii, T. heterophylla | n/a | n/a | n/a | n/a | n/a | 8.7 | 2370 | Typic Hapludands | Coarse loamy (13% clay) | 9.5 | 29 | Increased by 19% and 58% ** | n/a | H.J. Andrews Experimental Forest, Oregon (44°15′ N, 122°10′ W) USA | [51] | |
| Temperate deciduous forest | Q. petraea (70%), Cp. betulus (30%) | 100–150 | n/a | n/a | n/a | n/a | 10.7 | 680 | Gleyic Luvisol (WRB 2006) | Loam (41.9/38.8/19.3) | 2.7 ± 0.5 | 20.4 ± 0.6 | Increased ** | n/a | Barbeau National Forest, Northern Central France (48°29′ N, 02°47′ E) | [20] | |
| Mixed deciduous temperate woodland | Ar. pseudoplatanus, Fr. excelsior | n/a | n/a | n/a | n/a | n/a | 10 | 714 | Stagni-vertic Cambisol (FAO/WRB) | Clay loam | n/a | n/a | Increased by 30% ** | n/a | Wytham Woods, Oxfordshire (51°43′42″ N, 1°19′42″ W) UK | [52] | |
| Semi-deciduous lowland tropical forest | Arecaceae, Burseraceae, Olacaceae families | 200 | n/a | ≥10 | n/a | n/a | 27 | 2600 | Clay-rich Oxisol | n/a | n/a | n/a | Increased by 10% ** | n/a | Gigante Peninsula, central Panama (9°06′ N, 79°54′ W) | [52] | |
| Litter can act as an insulating layer that also buffers the effects of variations in light, temperature and irradiation | Temperate deciduous forest | Qt. petraeae-cerris community | n/a | n/a | n/a | n/a | 2930 | 10.7 | 615.6 | Brown forest soil, Cambisols (FAO) | n/a | 9.94 | 25.4 | Reduction **** | n/a | Bükk Mountains, Northeastern Hungary (47°55′ N, 20°26′ E) | [21] |
| Temperate deciduous forest | Qt. petraeae-cerris community | n/a | n/a | n/a | n/a | 2754 ± 206 kg C ha−1 yr−1 | 10.8 | 599 | Cambisol | n/a | 11.4 ± 0.93–16.1 ± 0.78 | 12.8 ± 0.78–28.4 ± 1.39% v/v (soil) | Reduction **** | n/a | Bükk Mountains, Northeastern Hungary (47°55′ N, 20°26′ E) | [37] |
| The Main Driver | Forest Type | Dominant Tree Species | Tree Age [Years] | Tree Height [m] | DBH [cm] | Tree Density [Trees/ha] | Litter Specification (Thickness/Input) | MAT [°C] | MAP [mm] | Soil Type | Soil Texture (Sand/Silt/Clay [%]) | Soil Temperature [°C] | Soil Moisture | Effect of Litter on CH4 Uptake | Landscape Type | Location | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Litter as a diffusion barrier | Coniferous forest | P. strobus (87–100%) | 4–67 | n/a | 19.3 | 1265.5 (421–1683) | 2.5 cm/ 267–2324 g m−2 | 7.8 | 1010 | Brunisolic Gray Brown Luvisol and Gleyed Brunisolic Gray Brown Luvisol (Canadian System of Classification) | Sand, loamy sand (80–90/8–18/<5) | n/a | n/a | Reduction (in June–September period) * | Lake shore | Southern Ontario, Canada | [78] |
| Boreal coniferous forest | P. sylvestris | 27 | n/a | n/a | n/a | 2–3 cm/n/a | n/a | n/a | Podzol | Coarse sand | n/a | 10.9% | Reduction by 50% * | n/a | Central Finland (62°39′ N, 27°03′ E) | [79] | |
| Temperate coniferous forest | Pc. abies, Ab. alba, P. sylvestris | 110 | n/a | n/a | n/a | n/a | 6 | 1200 | Acid brown | n/a | n/a | n/a * | Reduction by 17% | n/a | Black Forest, Southwestern Germany (48°03′ N, 8°22′ E) | [80] | |
| Deciduous forest | Fg. sp., Qr. sp. | n/a | n/a | n/a | ~600 | 1–2 cm/~570 g dm m−2 yr−1 | n/a | n/a | Cambisol | Sandy | n/a | n/a | Reduction by 17% | n/a | South Central Germany, (49°86′ N, 8°65′ E) | [81] | |
| Temperate deciduous forest | Fg. sylvatica | n/a | n/a | n/a | n/a | n/a | 6.5 | 796 | Pseudo-gleyic Cambisol | n/a | n/a | n/a | Reduction by 16% * | n/a | Rosalien Mountains, Eastern Austria (47°42′26″ N, 16°17′59″ E) | [32] | |
| Karst forest | P. massoniana | n/a | n/a | 15 | 2000 | n/a | 14.8 | 1118 | Limestone soil | Sand silt | n/a | 76.0 ± 7.2% WFPS | Reduction by 24% * | Karst area | Guizhou Province, Southern China (26°32′ N, 106°46′ E) | [18] | |
| Lower aeration and limited diffusion of atmospheric CH4 due to fast litter decomposition | Tropical seasonal rain forest | Pm. tomentosa, Br. macrostachya, G. subaequalis, Tr. myriocarpa | n/a | 18.6 | ≥10 | 386 | n/a | 21.7 | 1557 | Oxisol | n/a | n/a | n/a | Reduction by 29% * | Plot located between two hills | Xishuangbanna, Southern China (21°56′ N, 101°1′ E) | [82] |
| Monoterpenes released from needles decomposition | Subtropical pine forest | P. massoniana | 30 | 5 | n/a | 2600 | n/a | 17.8 | 1785 | Ferric Acrisols (USDA soil taxonomy) | Loamy clay (21/43/36) | 24.2 | 60.3% WFPS in wet season | Reduction by 55% * | Hilly region | Yingtan, Jiangxi Province, Southeastern China (28°15′ N, 116°55′ E) | [3] |
| Temperate deciduous and coniferous forests | F. sylvatica Pc. abies | n/a | n/a | n/a | n/a | n/a | n/a | n/a | Haplic Cambisol | n/a | n/a | n/a | Reduction * | n/a | Steigerwald, South Central Germany (49°51′ N, 10°27′ E; 49°52′ N, 10°27′ E) | [77] | |
| Mechanism not known | Broad-leaved pine forest | P. koraiensis, Ar. mono, Tl. amurensis, Ul. mongolica, Fr. mandshurica, Qr. mongolica | n/a | 25 | 28.9 | 560 | −7.3–4.9 | 600–900 | Dark brown forest soil | n/a | n/a | n/a | Reduction* | Slope | Changbai Mountain. Antu County, Northeastern China (42°24′ N, 128°28′ E) | [83] | |
| A soil moisture > 15.8% v/v—dependence on soil water content | Plantation | P. elliottii | 20 | 15 | 16.1 | n/a | n/a | 17.9 | 1469 | Typic Dystrudepts (USDA Soil Taxonomy) | Sandy loam (68/17/15) | n/a | n/a | Increased * | n/a | Qianyanzhou Ecological Research Station, Jiangxi Province, Southeastern China (26°44′39″ N, 115°03′33″ E) | [19] |
| Litter as a source of labile C compounds and the improve formation of macro-aggregates | Coniferous forest | Pl. orientalis | n/a | n/a | n/a | n/a | 1–2 cm/n/a | 15.7 | 749.3 | Yellow-brown soil (Chinese soil classification), Haplic Luvisols (USDA Soil Taxonomy) | Sand (Silt and clay: 9.6%) | 19.24 ± 2.69 | 59.02 ± 3.81% WFPS | Increased by 37.7% * | n/a | Wulongchi Experiment Station, Hubei Province, Central China (32°45′ N, 111°13′ E) | [27] |
| Improving gas diffusion in soil surface due to water retention by litter | Temperate coniferous forest | Pc. abies (100%) | 121 | n/a | n/a | 317 | >8 cm/n/a | 7.5 | 900 | Dystric Cambisol (FAO) | Loamy silt | 9.8 | 0.42 cm3 cm−3 | Increased by 11.5% * | n/a | Solling (51°46′ N, 9°35′ W) Germany | [28] |
| Temperate deciduous forest | Fg. sylvatica (100%) | 130 | n/a | n/a | 342 | <3 cm/n/a | 7.5 | 900 | Dystric Cambisol (FAO) | Loamy silt | 10.0 | 0.48 cm3 cm−3 | Increased by 39% * | n/a | |||
| Temperate mixed forest | Pc. abies (70%), Fg. sylvatica (30%) | 121 | n/a | n/a | 96 | n/a | 7.5 | 900 | Dystric Cambisol (FAO) | Loamy silt | 9.8 | 0.39 cm3 cm−3 | Increased by 24.3% * | n/a | |||
| Temperate mixed forest | Pc. abies (30%), Fg. sylvatica (70%) | 129 | n/a | n/a | 93 | n/a | 7.5 | 900 | Dystric Cambisol (FAO) | Loamy silt | 9.9 | 0.42 cm3 cm−3 | Increased by 19.4% * | n/a | |||
| Plantation | P. massoniana | 20 | n/a | n/a | 3–5 cm/7.30 t h m−2 yr−1 | 21.7 | 1600 | Oxisol | Sandy clay loam | 7.7–30.1 | 4.67–36.91 cm3 H2O cm−3 | No effect * | Hilly area | Hesjan, Guangdong Province (112°54′ E, 22°41′ N) China | [33] | ||
| Pine forest | P. massoniana | 73 | n/a | n/a | n/a | n/a/1.8 mg C ha−2 yr−1 | 21.4 | 1927 | Lateritic red earth, Oxisol | Loamy | 21.8 ± 1.0 | 12.3 ± 1.9 cm3 H2O cm−3 | No effect * | Hilly land | Guangdong Province, Southern China (112°30′39″–112°33′41″ E, 23°09′21″–23°11′30″ N) | [65] | |
| Conifer and broadleaf mixed forest | P. massoniana; S. superba, C. chinensis, Cb. kwangtungense | n/a | n/a | n/a | n/a | n/a/4.3 mg C ha−1 yr−1 | 21.4 | 1927 | Lateritic red earth, Oxisol | Loamy | 20.1 ± 0.9 | 23.3 ± 1.5 cm3 H2O cm−3 | No effect * | n/a | |||
| Evergreen broadleaf forest | C. chinensis, Cs. chinensis, C. concinna, Er. fordii, Cy.podophylla | n.a | n/a | n/a | n/a | n/a/4.2 mg C ha−1 yr−1 | 21.4 | 1927 | Lateritic red earth, Oxisol | Loamy | 19.9 ± 0.9 | 26.1 ± 1.6 cm3 H2O cm−3 | No effect * | n/a | |||
| Plantation | Pl. orientalis | n/a | n/a | n/a | n/a | n/a | 15.7 | 834 | Yellow brown soil | Silty clay (11/41/48) | 16.31 ± 1.05 (soil) | 12.34 ± 0.80% | No effect * | n/a | Danjiangkou Reservoir, Central China (32°45′ N, 111°13′ E) | [41] | |
| Water retention by litter | Subarctic wet heath ecosystem | B. pubescens | n/a | n/a | n/a | n/a | n/a | 0.2 | 337 | Organic soil | n/a | n/a | n/a | Increased | Slightly slopin terrain ** | Northern Sweden (68°20′47″ N, 18°49′34″ E) | [84] |
| Acting as a diffusion barrier for soil moisture < 15.8% v/v | Plantation | P. elliottii | 20 | 15 | 16.1 | n/a | n/a | 17.9 | 1469 | Typic Dystrudepts (USDA taxonomy) | Sandy loam | n/a | n/a | Min Increased +0.7% | n/a * | Qianyanzhou Ecological Research Station, Jiangxi Province, Southeastern China (26°44′39″ N, 115°03′33″ E) | [19] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Walkiewicz, A.; Rafalska, A.; Bulak, P.; Bieganowski, A.; Osborne, B. How Can Litter Modify the Fluxes of CO2 and CH4 from Forest Soils? A Mini-Review. Forests 2021, 12, 1276. https://doi.org/10.3390/f12091276
Walkiewicz A, Rafalska A, Bulak P, Bieganowski A, Osborne B. How Can Litter Modify the Fluxes of CO2 and CH4 from Forest Soils? A Mini-Review. Forests. 2021; 12(9):1276. https://doi.org/10.3390/f12091276
Chicago/Turabian StyleWalkiewicz, Anna, Adrianna Rafalska, Piotr Bulak, Andrzej Bieganowski, and Bruce Osborne. 2021. "How Can Litter Modify the Fluxes of CO2 and CH4 from Forest Soils? A Mini-Review" Forests 12, no. 9: 1276. https://doi.org/10.3390/f12091276
APA StyleWalkiewicz, A., Rafalska, A., Bulak, P., Bieganowski, A., & Osborne, B. (2021). How Can Litter Modify the Fluxes of CO2 and CH4 from Forest Soils? A Mini-Review. Forests, 12(9), 1276. https://doi.org/10.3390/f12091276