Preference and Performance of the Pine-Tree Lappet Dendrolimus pini on Various Pine Species
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Species Characteristics
2.2. Study Design
2.2.1. Food Choice
2.2.2. Performance Test
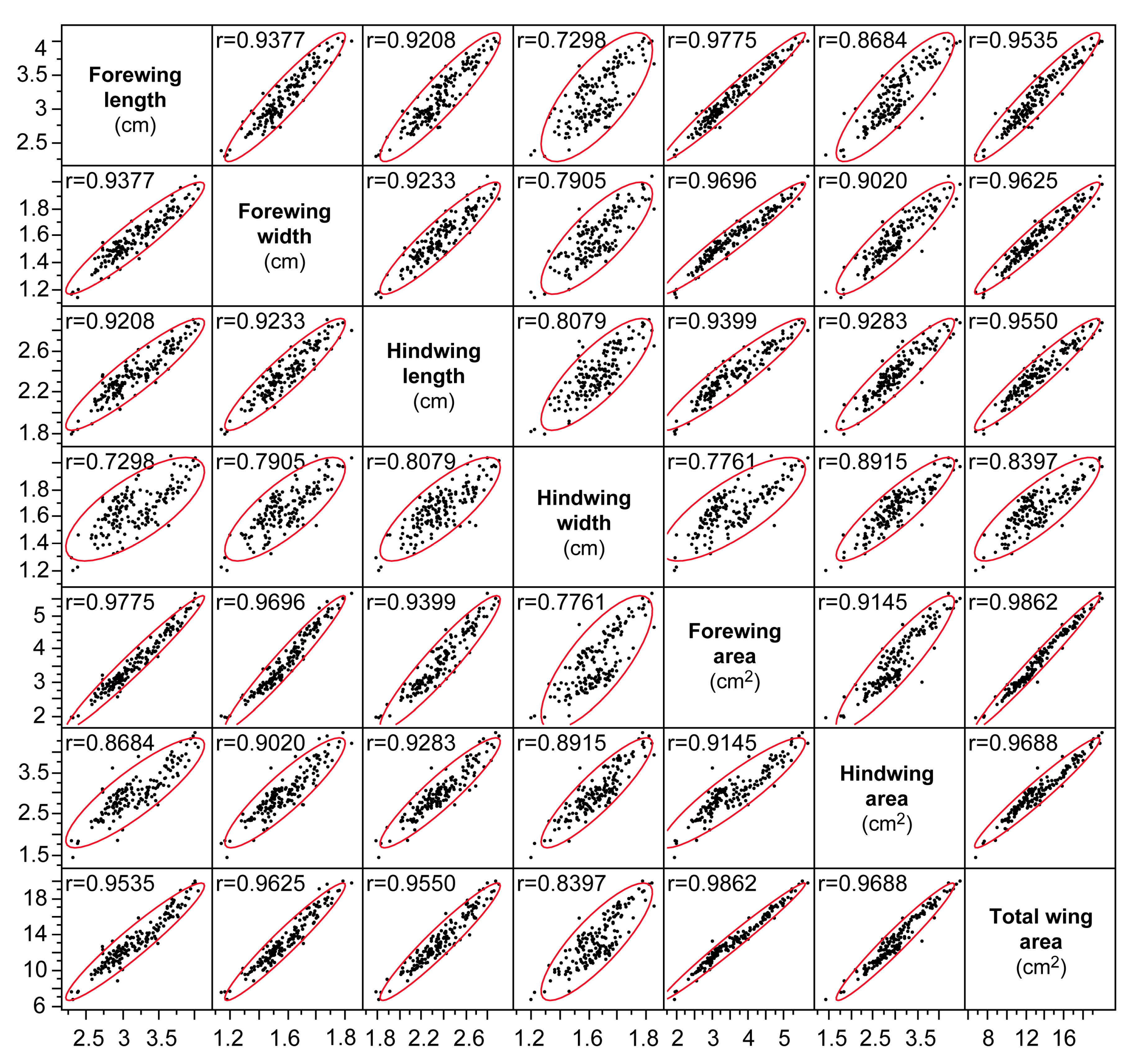
2.3. Chemical Analysis and Specific Leaf Area Determination
2.4. Statistical Analysis
3. Results
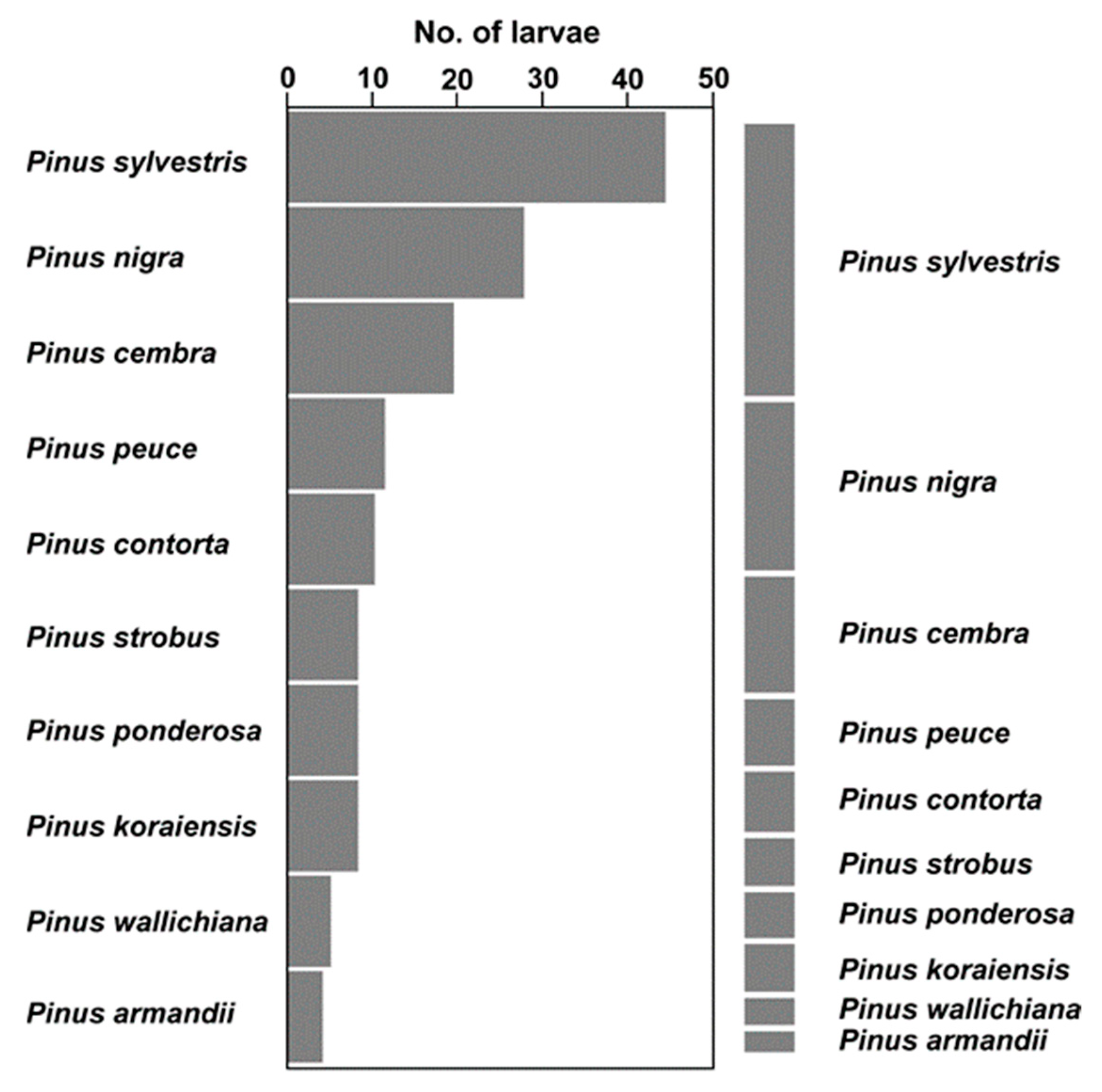
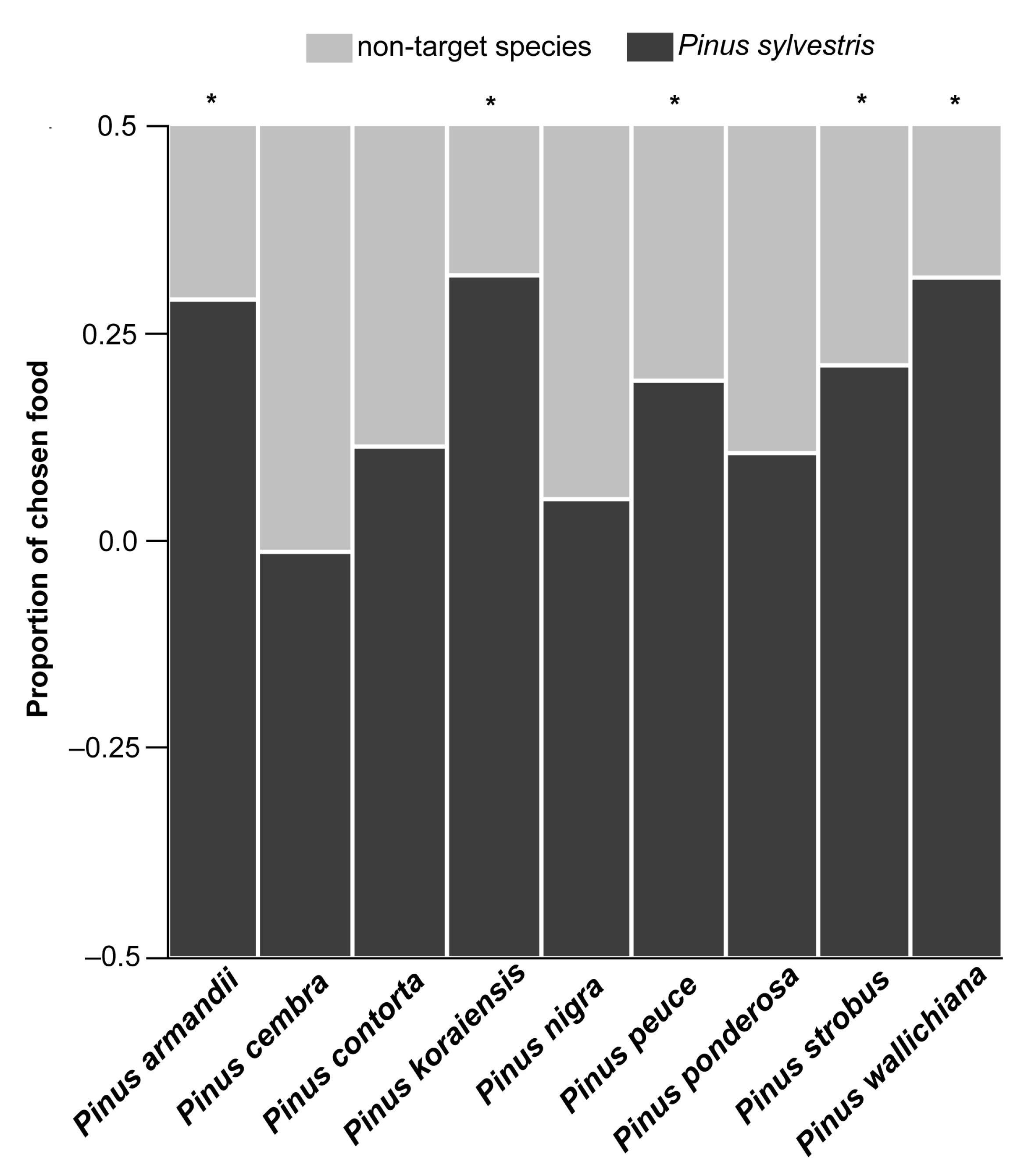
3.1. Food Choice
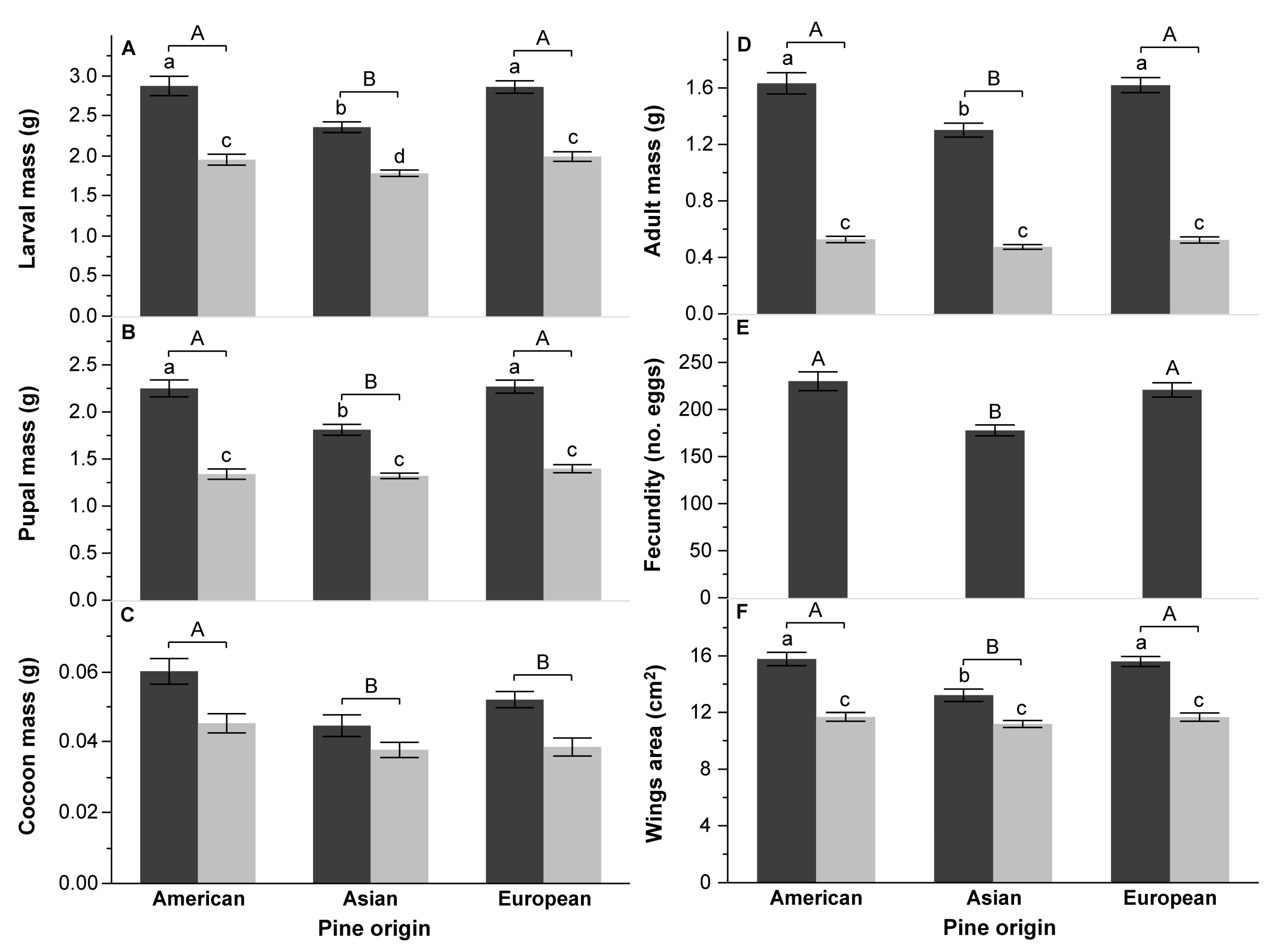
3.2. Survival and Performance Test
3.3. The Quality of Food
4. Discussion
4.1. Host Plant Preferences
4.2. Performance of DP on Various Pine Species
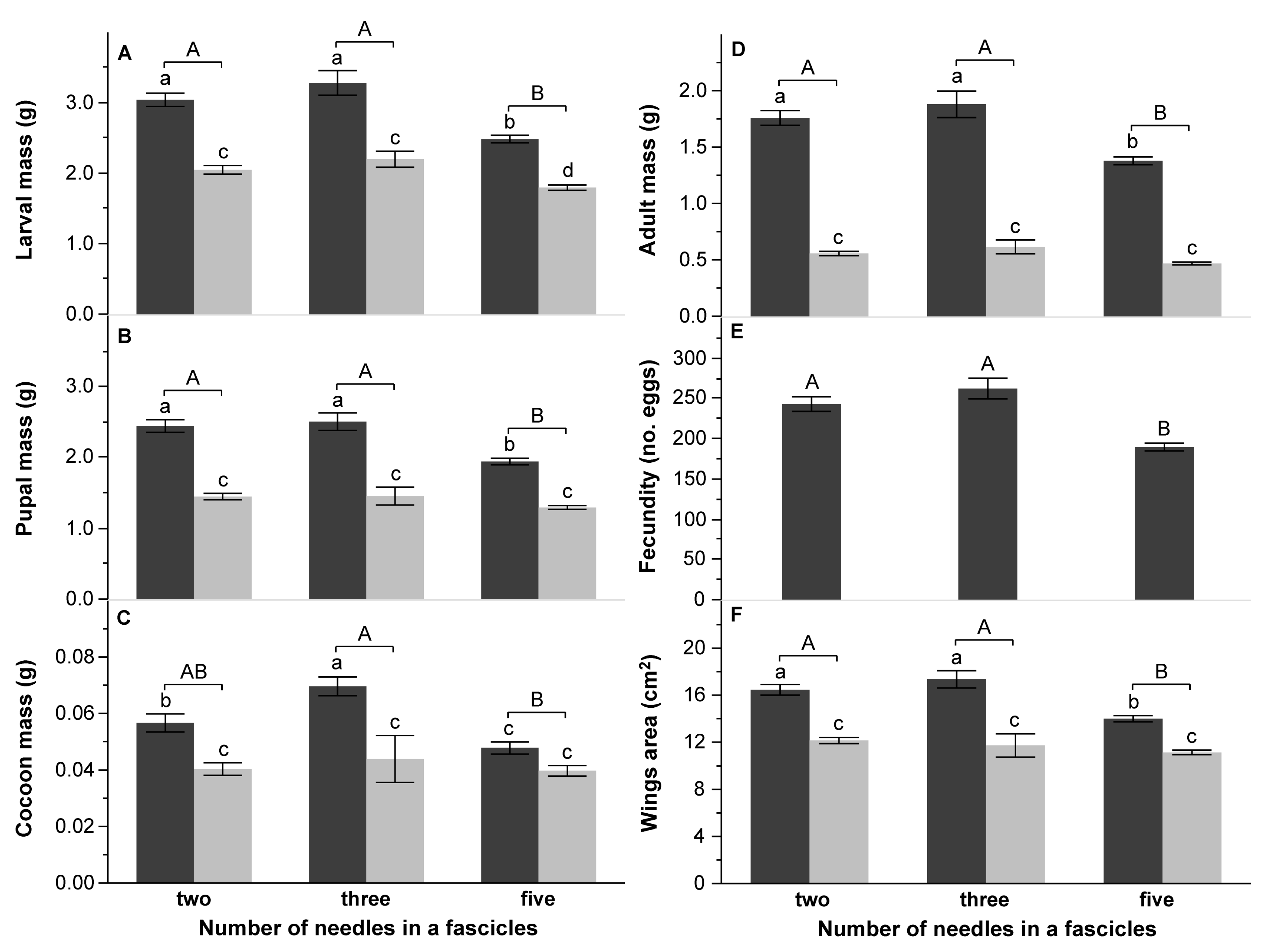
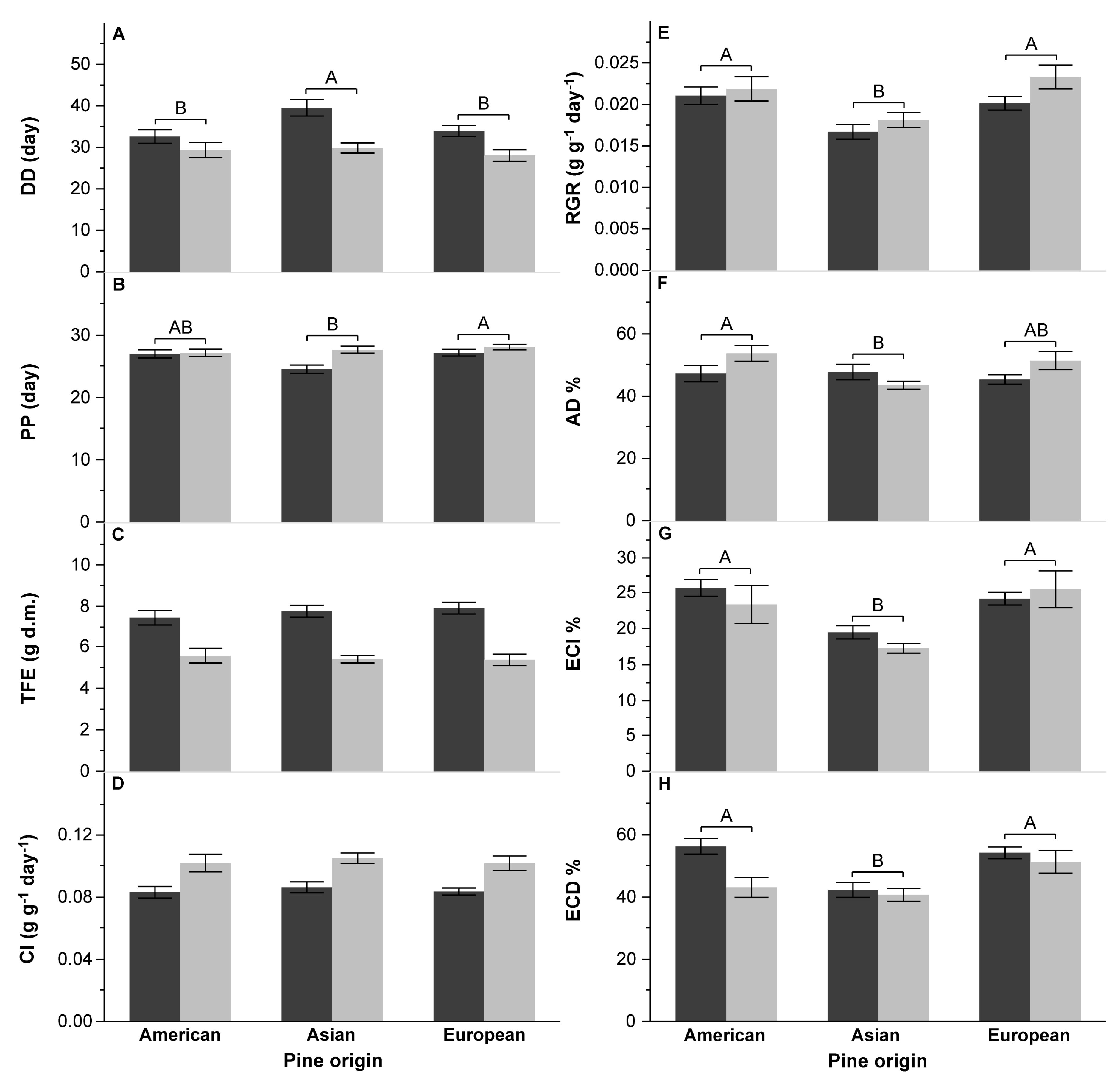
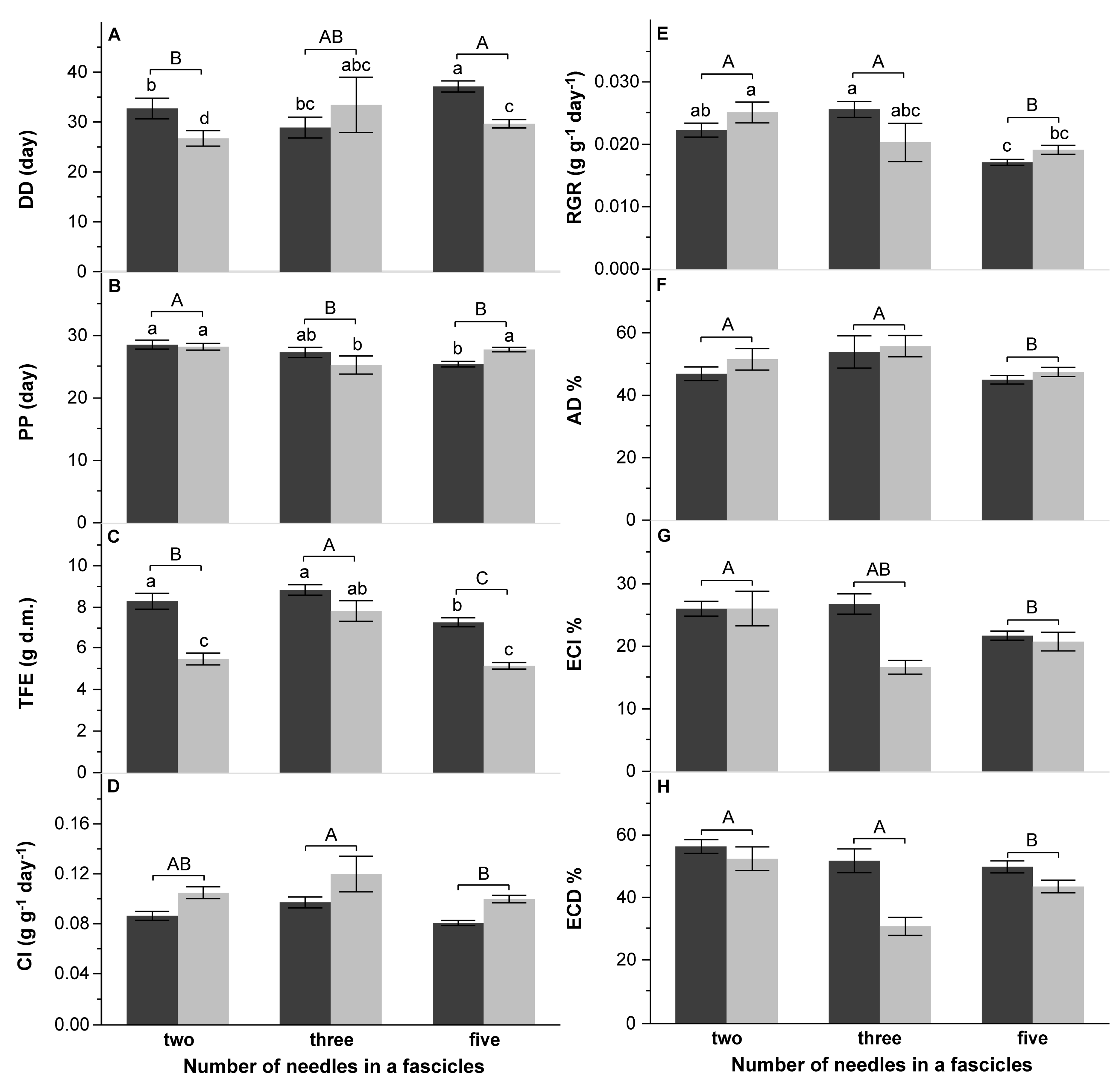
4.3. Effect of Pine Species Origin and the Number of Needles in a Fascicle on DP Performance
4.4. Influence of Chemical and Physical Characteristics of Pine Species on DP Performance
4.5. Application Aspects and Future Plans
4.6. Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Pine Species | N | Larval Mass (g) | Pupal Mass (g) | Cocoon Mass (mg) | Adult Mass (g) | Wings Area (cm2) | Fecundity (No. Eggs) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F | M | F | M | F | M | F | M | F | M | F | M | |||
| Pinus armandii | 5 | 11 | 2.33 ± 0.11 | 1.66 ± 0.07 | 1.77 ± 0.09 | 1.26 ± 0.06 | 43.3 ± 7.5 | 39.8 ± 3.1 | 1.24 ± 0.05 | 0.45 ± 0.03 | 11.92 ± 0.80 | 10.69 ± 0.60 | 164 ± 6 | |
| Pinus cembra | 10 | 8 | 2.79 ± 0.13 | 1.84 ± 0.10 | 2.19 ± 0.10 | 1.32 ± 0.08 | 49.8 ± 4.7 | 44.3 ± 8.0 | 1.55 ± 0.08 | 0.49 ± 0.04 | 15.07 ± 0.43 | 11.37 ± 0.54 | 209 ± 14 | |
| Pinus contorta | 5 | 11 | 2.94 ± 0.18 | 1.87 ± 0.08 | 2.30 ± 0.22 | 1.35 ± 0.08 | 57.1 ± 9.6 | 46.7 ± 3.6 | 1.58 ± 0.16 | 0.52 ± 0.02 | 15.26 ± 1.16 | 11.87 ± 0.34 | 227 ± 25 | |
| Pinus koraiensis | 5 | 12 | 2.14 ± 0.10 | 1.78 ± 0.05 | 1.70 ± 0.08 | 1.35 ± 0.05 | 36.2 ± 7.4 | 29.3 ± 2.8 | 1.24 ± 0.07 | 0.49 ± 0.03 | 13.07 ± 0.65 | 11.15 ± 0.26 | 183 ± 6 | |
| Pinus nigra | 7 | 12 | 3.30 ± 0.20 | 2.13 ± 0.08 | 2.59 ± 0.17 | 1.49 ± 0.06 | 51.3 ± 4.1 | 35.0 ± 2.9 | 1.86 ± 0.11 | 0.56 ± 0.04 | 17.01 ± 0.97 | 12.06 ± 0.38 | 237 ± 17 | |
| Pinus peuce | 9 | 7 | 2.52 ± 0.10 | 1.75 ± 0.11 | 1.94 ± 0.10 | 1.21 ± 0.10 | 46.4 ± 4.1 | 36.6 ± 5.0 | 1.33 ± 0.08 | 0.42 ± 0.04 | 14.01 ± 0.70 | 10.39 ± 0.52 | 184 ± 9 | |
| Pinus ponderosa | 10 | 7 | 3.28 ± 0.18 | 2.20 ± 0.11 | 2.50 ± 0.12 | 1.45 ± 0.13 | 69.6 ± 3.3 | 43.9 ± 8.3 | 1.88 ± 0.12 | 0.62 ± 0.06 | 17.35 ± 0.73 | 11.72 ± 0.98 | 262 ± 13 | |
| Pinus strobus | 10 | 9 | 2.42 ± 0.13 | 1.85 ± 0.14 | 1.97 ± 0.11 | 1.24 ± 0.08 | 53.2 ± 6.1 | 45.0 ± 3.4 | 1.41 ± 0.07 | 0.46 ± 0.02 | 14.45 ± 0.44 | 11.43 ± 0.35 | 200 ± 12 | |
| Pinus sylvestris | 10 | 7 | 2.91 ± 0.11 | 2.17 ± 0.16 | 2.42 ± 0.12 | 1.51 ± 0.09 | 60.1 ± 4.5 | 40.5 ± 4.8 | 1.78 ± 0.09 | 0.60 ± 0.03 | 16.73 ± 0.49 | 12.73 ± 0.86 | 255 ± 11 | |
| Pinus wallichiana | 11 | 8 | 2.46 ± 0.10 | 1.94 ± 0.06 | 1.88 ± 0.09 | 1.36 ± 0.04 | 49.7 ± 2.9 | 47.8 ± 2.5 | 1.36 ± 0.09 | 0.48 ± 0.02 | 13.93 ± 0.65 | 11.81 ± 0.21 | 181 ± 10 | |
| ANCOVA | df | F | p | F | p | F | p | F | p | F | p | F | p | |
| Pine species (Ps) | 9 | 13.4788 | <0.0001 | 9.7650 | <0.0001 | 3.9846 | <0.0001 | 11.1253 | <0.0001 | 8.2941 | <0.0001 | 9.3299 | <0.0001 | |
| Sex (S) | 1 | 174.7856 | <0.0001 | 240.4803 | <0.0001 | 11.5914 | 0.0009 | 942.35 | <0.0001 | 121.8399 | <0.0001 | |||
| Ps × S | 9 | 3.2063 | 0.0014 | 3.4518 | 0.0007 | 1.5574 | 0.1331 | 4.5734 | <0.0001 | 3.0862 | 0.0020 | |||
| Initial larval mass (cov) | 1 | 40.7382 | <0.0001 | 34.8416 | <0.0001 | 18.4386 | <0.0001 | 28.6974 | <0.0001 | 21.5775 | <0.0001 | 27.2014 | <0.0001 | |
| df error | 152 | 152 | 150 | 152 | 147 | 71 | ||||||||
Appendix B
| Pine Species | N | DD (day) | PP (day) | TFE (g d.m.) | CI (g g−1 day−1) | RGR (g g−1 day−1) | AD (%) | ECI (%) | ECD (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F | M | F | M | F | M | F | M | F | M | F | M | F | M | F | M | F | M | |
| Pinus armandii | 5 | 11 | 40.2 ± 4.7 | 32.9 ± 2.0 | 24.2 ± 1.7 | 28.6 ± 1.2 | 7.78 ± 0.72 | 5.49 ± 0.18 | 0.086 ± 0.010 | 0.103 ± 0.005 | 0.0140 ± 0.0015 | 0.0166 ± 0.0013 | 33.60 ± 1.93 | 39.35 ± 1.38 | 16.61 ± 1.59 | 15.93 ± 1.14 | 50.33 ± 5.86 | 41.27 ± 3.65 |
| Pinus cembra | 10 | 8 | 36.3 ± 2.1 | 29.8 ± 0.6 | 25.6 ± 0.8 | 27.5 ± 0.7 | 8.31 ± 0.41 | 5.93 ± 0.25 | 0.083 ± 0.002 | 0.110 ± 0.003 | 0.0177 ± 0.0010 | 0.0185 ± 0.0022 | 42.44 ± 1.10 | 49.89 ± 2.58 | 21.23 ± 0.82 | 17.22 ± 2.32 | 50.75 ± 3.18 | 36.38 ± 5.78 |
| Pinus contorta | 5 | 11 | 35.2 ± 4.6 | 27.4 ± 1.6 | 26.2 ± 2.0 | 28.0 ± 0.9 | 8.26 ± 0.40 | 5.20 ± 0.37 | 0.084 ± 0.008 | 0.103 ± 0.006 | 0.0191 ± 0.0020 | 0.0228 ± 0.0027 | 39.51 ± 1.08 | 52.13 ± 5.12 | 22.70 ± 1.24 | 24.20 ± 5.04 | 57.78 ± 4.20 | 45.12 ± 5.01 |
| Pinus koraiensis | 5 | 12 | 46.8 ± 3.4 | 30.9 ± 1.9 | 22.8 ± 1.7 | 27.9 ± 0.8 | 8.20 ± 0.84 | 5.26 ± 0.43 | 0.083 ± 0.007 | 0.096 ± 0.003 | 0.0135 ± 0.0014 | 0.0159 ± 0.0010 | 46.78 ± 2.89 | 44.54 ± 2.21 | 16.35 ± 0.91 | 16.85 ± 1.14 | 35.39 ± 2.58 | 38.95 ± 3.42 |
| Pinus nigra | 7 | 12 | 36.9 ± 5.1 | 25.3 ± 2.9 | 28.4 ± 1.2 | 28.3 ± 0.9 | 9.95 ± 0.65 | 5.77 ± 0.44 | 0.088 ± 0.008 | 0.115 ± 0.008 | 0.0218 ± 0.0024 | 0.0249 ± 0.0018 | 49.04 ± 5.20 | 40.53 ± 4.17 | 24.75 ± 1.61 | 22.10 ± 1.42 | 51.88 ± 3.51 | 59.63 ± 5.38 |
| Pinus peuce | 9 | 7 | 34.9 ± 1.6 | 30.6 ± 2.5 | 25.2 ± 0.9 | 28.3 ± 0.8 | 6.73 ± 0.37 | 4.17 ± 0.50 | 0.078 ± 0.004 | 0.081 ± 0.011 | 0.0170 ± 0.0008 | 0.0206 ± 0.0015 | 41.73 ± 2.74 | 53.73 ± 6.57 | 22.25 ± 1.31 | 31.11 ± 7.67 | 55.00 ± 4.38 | 54.98 ± 6.89 |
| Pinus ponderosa | 10 | 7 | 28.9 ± 2.1 | 33.4 ± 5.5 | 27.3 ± 0.8 | 25.3 ± 1.5 | 8.82 ± 0.25 | 7.80 ± 0.50 | 0.097 ± 0.004 | 0.120 ± 0.014 | 0.0256 ± 0.0013 | 0.0203 ± 0.0031 | 53.89 ± 5.15 | 55.75 ± 3.41 | 26.77 ± 1.62 | 16.65 ± 1.08 | 51.79 ± 3.82 | 30.76 ± 2.90 |
| Pinus strobus | 10 | 9 | 35.0 ± 2.5 | 28.3 ± 2.5 | 27.2 ± 1.1 | 27.8 ± 0.7 | 5.63 ± 0.32 | 4.31 ± 0.35 | 0.069 ± 0.004 | 0.087 ± 0.008 | 0.0175 ± 0.0009 | 0.0220 ± 0.0022 | 44.44 ± 3.29 | 54.07 ± 4.43 | 26.21 ± 2.33 | 27.79 ± 4.99 | 60.10 ± 4.42 | 50.54 ± 5.88 |
| Pinus sylvestris | 10 | 7 | 28.6 ± 1.2 | 28.3 ± 3.6 | 29.8 ± 0.8 | 28.4 ± 1.1 | 7.10 ± 0.38 | 5.38 ± 0.79 | 0.086 ± 0.004 | 0.091 ± 0.008 | 0.0241 ± 0.0013 | 0.0288 ± 0.0050 | 49.09 ± 2.76 | 69.54 ± 5.36 | 28.56 ± 2.06 | 35.42 ± 8.25 | 58.78 ± 3.69 | 50.51 ± 10.43 |
| Pinus wallichiana | 11 | 8 | 35.9 ± 2.4 | 24.0 ± 1.1 | 25.5 ± 0.6 | 26.1 ± 0.6 | 7.52 ± 0.32 | 5.56 ± 0.23 | 0.088 ± 0.005 | 0.122 ± 0.008 | 0.0193 ± 0.0010 | 0.0235 ± 0.0012 | 54.68 ± 2.72 | 47.63 ± 2.44 | 22.25 ± 1.03 | 19.69 ± 1.07 | 41.78 ± 3.03 | 42.58 ± 3.74 |
| ANCOVA | df | F | p | F | p | F | p | F | p | F | p | F | p | F | p | F | p | |
| Pine species (Ps) | 9 | 3.8039 | 0.0002 | 2.5756 | 0.0087 | 10.9865 | <0.0001 | 4.5752 | <0.0001 | 6.0806 | <0.0001 | 5.1063 | <0.0001 | 5.6397 | <0.0001 | 3.4757 | 0.0006 | |
| Sex (S) | 1 | 60.1113 | <0.0001 | 10.6704 | 0.0013 | 128.5158 | <0.0001 | 36.3141 | <0.0001 | 1.2958 | 0.2568 | 4.6539 | 0.0326 | 6.3559 | 0.0127 | 13.3744 | 0.0004 | |
| Ps × S | 9 | 1.8530 | 0.0632 | 2.5016 | 0.0107 | 2.2821 | 0.0199 | 0.8829 | 0.5421 | 1.3465 | 0.2176 | 2.3083 | 0.0185 | 1.3918 | 0.1964 | 1.9517 | 0.0487 | |
| Initial larval mass (cov) | 1 | 26.9481 | <0.0001 | 1.6807 | 0.1968 | 0.2121 | 0.6458 | 1.0073 | 0.3172 | 8.7103 | 0.0037 | 4.4652 | 0.0362 | 10.5231 | 0.0014 | 2.8394 | 0.0940 | |
| df error | 152 | 152 | 152 | 152 | 152 | 152 | 152 | 152 | ||||||||||
Appendix C
| Pine Species | N | Water (%) | Nitrogen (%) | Starch (%) | TNC (%) | TPh (µM g−1 d.m.) | Tannins (µM g−1 d.m.) | TT (mg−1 g d.m.) | SLA (cm-1 g d.m.) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| April | May | April | May | April | May | April | May | April | May | April | May | April | May | April | May | April | May | |
| Pinus armandii | 8 | 8 | 52.1 ± 0.6 | 51.2 ± 0.8 | 1.29 ± 0.07 | 1.33 ± 0.14 | 3.43 ± 0.43 | 6.77 ± 0.68 | 11.10 ± 0.50 | 13.92 ± 0.91 | 150.6 ± 12.3 | 103.7 ± 6.1 | 299.6 ± 54.0 | 235.9 ± 76.5 | 208.1 ± 1.9 | 158.1 ± 15.2 | 42.6 ± 1.2 | 43.3 ± 1.2 |
| Pinus cembra | 8 | 8 | 48.6 ± 0.7 | 51.2 ± 1.4 | 1.70 ± 0.15 | 1.49 ± 0.07 | 1.77 ± 1.01 | 2.15 ± 0.81 | 7.92 ± 1.62 | 7.33 ± 1.12 | 78.9 ± 2.4 | 101.2 ± 7.4 | 23.8 ± 5.7 | 18.5 ± 2.3 | 121.4 ± 4.5 | 112.7 ± 3.6 | 34.3 ± 0.8 | 38.5 ± 1.1 |
| Pinus contorta | 8 | 8 | 48.2 ± 1.1 | 51.2 ± 0.9 | 1.49 ± 0.13 | 1.26 ± 0.05 | 2.88 ± 0.38 | 2.98 ± 0.48 | 11.31 ± 0.51 | 9.84 ± 0.52 | 178.8 ± 11.2 | 173.1 ± 17.1 | 184.1 ± 22.9 | 224.6 ± 25.3 | 174.0 ± 7.7 | 170.6 ± 8.1 | 28.8 ± 0.6 | 27.6 ± 1.3 |
| Pinus koraiensis | 8 | 8 | 48.9 ± 0.6 | 47.7 ± 1.6 | 1.43 ± 0.07 | 1.30 ± 0.06 | 4.38 ± 1.18 | 5.18 ± 0.90 | 11.27 ± 1.24 | 11.48 ± 0.68 | 120.0 ± 8.9 | 114.6 ± 9.5 | 47.5 ± 10.4 | 50.2 ± 20.7 | 127.8 ± 6.0 | 128.5 ± 10.1 | 38.7 ± 1.0 | 37.6 ± 1.2 |
| Pinus nigra | 8 | 8 | 50.7 ± 0.8 | 47.9 ± 0.7 | 1.16 ± 0.05 | 1.07 ± 0.05 | 1.88 ± 0.43 | 5.67 ± 1.04 | 8.66 ± 0.53 | 12.21 ± 0.83 | 140.7 ± 8.1 | 123.4 ± 12.6 | 216.6 ± 24.6 | 147.1 ± 49.2 | 153.1 ± 1.8 | 128.4 ± 11.2 | 24.1 ± 1.0 | 22.1 ± 0.5 |
| Pinus peuce | 8 | 8 | 50.7 ± 0.6 | 48.3 ± 0.7 | 1.30 ± 0.05 | 1.39 ± 0.13 | 0.47 ± 0.04 | 3.02 ± 0.96 | 7.33 ± 0.48 | 9.51 ± 0.97 | 290.6 ± 12.4 | 202.8 ± 14.2 | 273.3 ± 61.8 | 118.2 ± 30.9 | 179.7 ± 4.2 | 177.8 ± 8.6 | 45.4 ± 1.6 | 43.7 ± 1.7 |
| Pinus ponderosa | 8 | 8 | 50.6 ± 0.4 | 55.9 ± 3.2 | 1.55 ± 0.05 | 1.63 ± 0.08 | 0.97 ± 0.43 | 2.01 ± 0.62 | 8.13 ± 0.56 | 9.01 ± 0.59 | 157.4 ± 16.2 | 143.8 ± 12.8 | 121.9 ± 33.3 | 135.1 ± 44.5 | 137.8 ± 3.7 | 145.2 ± 3.9 | 24.0 ± 0.6 | 29.4 ± 3.0 |
| Pinus strobus | 8 | 8 | 48.7 ± 0.4 | 46.9 ± 1.0 | 2.01 ± 0.06 | 1.97 ± 0.10 | 0.67 ± 0.10 | 4.05 ± 0.60 | 8.91 ± 0.36 | 13.58 ± 0.70 | 168.9 ± 6.0 | 130.0 ± 11.7 | 252.7 ± 45.8 | 178.5 ± 22.4 | 181.3 ± 4.5 | 174.8 ± 8.0 | 49.5 ± 3.1 | 45.0 ± 1.4 |
| Pinus sylvestris | 8 | 8 | 47.9 ± 0.6 | 48.6 ± 0.8 | 1.75 ± 0.09 | 1.51 ± 0.10 | 2.37 ± 0.49 | 5.51 ± 0.77 | 10.50 ± 0.65 | 13.35 ± 0.78 | 144.3 ± 6.6 | 155.4 ± 15.4 | 120.2 ± 23.4 | 125.5 ± 20.6 | 146.9 ± 4.5 | 144.3 ± 10.5 | 27.3 ± 1.0 | 27.9 ± 0.7 |
| Pinus wallichiana | 8 | 8 | 50.7 ± 0.9 | 47.6 ± 1.3 | 1.79 ± 0.15 | 1.50 ± 0.08 | 1.76 ± 0.35 | 4.70 ± 0.87 | 7.81 ± 0.67 | 10.59 ± 1.22 | 79.7 ± 11.3 | 79.7 ± 7.4 | 39.8 ± 10.0 | 44.2 ± 20.6 | 118.7 ± 6.3 | 121.9 ± 8.3 | 45.9 ± 1.2 | 42.8 ± 1.1 |
| ANCOVA | df | F | p | F | p | F | p | F | p | F | p | F | p | F | p | F | p | |
| Pine species (Ps) | 9 | 4.2619 | <0.0001 | 13.9862 | <0.0001 | 6.5146 | <0.0001 | 7.8032 | <0.0001 | 35.4660 | <0.0001 | 11.6377 | <0.0001 | 23.8333 | <0.0001 | 76.4862 | <0.0001 | |
| Month (M) | 1 | 0.0198 | 0.8883 | 6.4118 | 0.0124 | 47.2320 | <0.0001 | 23.0207 | <0.0001 | 13.4157 | 0.0004 | 3.7172 | 0.0559 | 7.6507 | 0.0064 | 0.1800 | 0.6720 | |
| Ps × M | 9 | 3.1534 | 0.0017 | 1.1726 | 0.3171 | 2.0219 | 0.0411 | 2.7440 | 0.0056 | 4.1476 | <0.0001 | 1.3375 | 0.2231 | 2.4973 | 0.0112 | 2.3633 | 0.0162 | |
| df error | 140 | 140 | 140 | 140 | 140 | 138 | 140 | 140 | ||||||||||
Appendix D
References
- Howe, G.A.; Jander, G. Plant Immunity to Insect Herbivores. Annu. Rev. Plant Biol. 2008, 59, 41–66. [Google Scholar] [CrossRef] [PubMed]
- Giertych, M.; Karolewski, P.; Grzebyta, J.; Oleksyn, J. Feeding behavior and performance of Neodiprion sertifer larvae reared on Pinus sylvestris needles. For. Ecol. Manag. 2007, 242, 700–707. [Google Scholar] [CrossRef]
- Coll, M.; Yuval, B. Larval Food Plants Affect Flight and Reproduction in an Oligophagous Insect Herbivore. Environ. Entomol. 2004, 33, 1471–1476. [Google Scholar] [CrossRef]
- Poelman, E.H. From induced resistance to defence in plant-insect interactions. Entomol. Exp. Appl. 2015, 157, 11–17. [Google Scholar] [CrossRef]
- Pagare, S.; Bhatia, M.; Tripathi, N.; Pagare, S.; Bansal, Y.K. Secondary metabolites of plants and their role: Overview. Curr. Trends Biotechnol. Pharm. 2015, 9, 293–304. [Google Scholar]
- Lucas, P.W.; Turner, I.M.; Dominy, N.; Yamashita, N. Mechanical Defences to Herbivory. Ann. Bot. 2000, 86, 913–920. [Google Scholar] [CrossRef]
- Kolosova, N.; Bohlmann, J. Conifer defense against insects and fungal pathogens. In Growth and Defence in Plants; Springer: Berlin/Heidelberg, Gaermany, 2012; pp. 85–109. ISBN 978-3-64230-645-7. [Google Scholar]
- Awmack, C.S.; Leather, S. Host Plant Quality and Fecundity in Herbivorous Insects. Annu. Rev. Entomol. 2002, 47, 817–844. [Google Scholar] [CrossRef]
- Koricheva, J.; Larsson, S.; Haukioja, E. Insect Performance on Experimentally Stressed Woody Plants: A Meta-Analysis. Annu. Rev. Entomol. 1998, 43, 195–216. [Google Scholar] [CrossRef]
- Tammaru, T.; Haukioja, E. Capital Breeders and Income Breeders among Lepidoptera: Consequences to Population Dynamics. Oikos 1996, 77, 561. [Google Scholar] [CrossRef]
- Gripenberg, S.; Mayhew, P.J.; Parnell, M.; Roslin, T. A meta-analysis of preference-performance relationships in phytophagous insects. Ecol. Lett. 2010, 13, 383–393. [Google Scholar] [CrossRef]
- Carvalho, M.R.M.; Vasconcellos-Neto, J. Host plant selection and larval performance in the Neotropical butterfly Mechanitis polymnia casabranca. Entomol. Exp. Appl. 2021, 169, 255–263. [Google Scholar] [CrossRef]
- Walczak, U.; Baraniak, E.; Zduniak, P. Survival, body mass and potential fecundity of the invasive moth Cameraria ohridella (Lepidoptera: Gracillariidae) on its original host plant Aesculus hippocastanum and Aesculus glabra. Eur. J. Entomol. 2017, 114, 295–300. [Google Scholar] [CrossRef][Green Version]
- Bertin, A.; Bortoli, L.C.; Botton, M.; Parra, J.R.P. Host Plant Effects on the Development, Survival, and Reproduction of Dysmicoccus brevipes (Hemiptera: Pseudococcidae) on Grapevines. Ann. Entomol. Soc. Am. 2013, 106, 604–609. [Google Scholar] [CrossRef]
- Colasurdo, N.; Gelinas, Y.; Despland, E. Larval nutrition affects life history traits in a capital breeding moth. J. Exp. Biol. 2009, 212, 1794–1800. [Google Scholar] [CrossRef]
- Kenis, M.; Auger-Rozenberg, M.-A.; Roques, A.; Timms, L.; Péré, C.; Cock, M.; Settele, J.; Augustin, S.; Lopez-Vaamonde, C. Ecological effects of invasive alien insects. Biol. Invasions 2008, 11, 21–45. [Google Scholar] [CrossRef]
- Perrings, C.; Williamson, M.; Barbier, E.B.; Delfino, D.; Dalmazzone, S.; Shogren, J.; Simmons, P.; Watkinson, A. Biological Invasion Risks and the Public Good: An Economic Perspective. Conserv. Ecol. 2002, 6, 1. [Google Scholar] [CrossRef]
- Roy, B.A.; Alexander, H.M.; Davidson, J.; Campbell, F.T.; Burdon, J.J.; A Sniezko, R.; Brasier, C. Increasing forest loss worldwide from invasive pests requires new trade regulations. Front. Ecol. Environ. 2014, 12, 457–465. [Google Scholar] [CrossRef]
- Schilthuizen, M.; Pimenta, L.P.S.; Lammers, Y.; Steenbergen, P.J.; Flohil, M.; Beveridge, N.G.; van Duijn, P.T.; Meulblok, M.M.; Sosef, N.; van de Ven, R.; et al. Incorporation of an invasive plant into a native insect herbivore food web. PeerJ 2016, 4, e1954. [Google Scholar] [CrossRef] [PubMed]
- Łukowski, A.; Janek, W.; Baraniak, E.; Walczak, U.; Karolewski, P. Changing Host Plants Causes Structural Differences in the Parasitoid Complex of the Monophagous Moth Yponomeuta evonymella, but Does Not Improve Survival Rate. Insects 2019, 10, 197. [Google Scholar] [CrossRef]
- D’Costa, L.; Koricheva, J.; Straw, N.; Simmonds, M.S. Oviposition patterns and larval damage by the invasive horse-chestnut leaf miner Cameraria ohridellaon different species of Aesculus. Ecol. Entomol. 2013, 38, 456–462. [Google Scholar] [CrossRef]
- Rogers, R.; Wallner, C.; Goodwin, B.; Heitland, W.; Weisser, W.; Brosius, H.-B. When do people take action? The importance of people’s observation that nature is changing for pro-environmental behavior within the field of impersonal, environmental risk. J. Integr. Environ. Sci. 2017, 14, 1–18. [Google Scholar] [CrossRef]
- Prestemon, J.P.; Zhu, S.; Turner, J.A.; Buongiorno, J.; Li, R. Forest Product Trade Impacts of an Invasive Species: Modeling Structure and Intervention Trade-Offs. Agric. Resour. Econ. Rev. 2006, 35, 128–143. [Google Scholar] [CrossRef][Green Version]
- Bradshaw, C.J.A.; Leroy, B.; Bellard, C.; Roiz, D.; Albert, C.; Fournier, A.; Barbet-Massin, M.; Salles, J.-M.; Simard, F.; Courchamp, F. Massive yet grossly underestimated global costs of invasive insects. Nat. Commun. 2016, 7, 12986. [Google Scholar] [CrossRef] [PubMed]
- Wiatrowska, B.; Łukowski, A.; Karolewski, P.; Danielewicz, W. Invasive Spiraea tomentosa: A new host for monophagous Earias clorana? Arthropod-Plant Interact. 2018, 12, 423–434. [Google Scholar] [CrossRef]
- Brockerhoff, E.G.; Liebhold, A.M. Ecology of forest insect invasions. Biol. Invasions 2017, 19, 3141–3159. [Google Scholar] [CrossRef]
- Ray, D.; Peace, A.; Moore, R.; Petr, M.; Grieve, Y.; Convery, C.; Ziesche, T. Improved prediction of the climate-driven outbreaks of Dendrolimus pini in Pinus sylvestris forests. Forestry 2016, 89, 230–244. [Google Scholar] [CrossRef]
- Kirichenko, N.; Flament, J.; Baranchikov, Y.; Grégoire, J.-C. Larval performances and life cycle completion of the Siberian moth, Dendrolimus sibiricus (Lepidoptera: Lasiocampidae), on potential host plants in Europe: A laboratory study on potted trees. Eur. J. For. Res. 2011, 130, 1067–1074. [Google Scholar] [CrossRef]
- Phillips, S.J.; Anderson, R.P.; Schapire, R.E. Maximum entropy modeling of species geographic distributions. Ecol. Model. 2006, 190, 231–259. [Google Scholar] [CrossRef]
- Möykkynen, T.; Pukkala, T. Modelling of the spread of a potential invasive pest, the Siberian moth (Dendrolimus sibiricus) in Europe. For. Ecosyst. 2014, 1, 10. [Google Scholar] [CrossRef]
- Kanturski, M.; Bugaj-Nawrocka, A.; Wieczorek, K. Pine pest aphids of the genus Eulachnus (Hemiptera: Aphididae: Lachninae): How far can their range extend? Agric. For. Entomol. 2016, 18, 398–408. [Google Scholar] [CrossRef]
- Keena, M.A. Survival and Development of Lymantria monacha (Lepidoptera: Lymantriidae) on North American and Introduced Eurasian Tree Species. J. Econ. Entomol. 2003, 96, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Skrzecz, I.; Ślusarski, S.; Tkaczyk, M. Integration of science and practice for Dendrolimus pini (L.) management—A review with special reference to Central Europe. For. Ecol. Manag. 2020, 455, 117697. [Google Scholar] [CrossRef]
- Le Mellec, A.; Michalzik, B. Impact of a pine lappet (Dendrolimus pini) mass outbreak on C and N fluxes to the forest floor and soil microbial properties in a Scots pine forest in Germany. Can. J. For. Res. 2008, 38, 1829–1841. [Google Scholar] [CrossRef]
- Björkman, C.; Lindelöw, Å.; Eklund, K.; Kyrk, S.; Klapwijk, J.; Fedderwitz, F.; Nordlander, G. A rare event—An isolated outbreak of the pine-tree lappet moth (Dendrolimus pini) in the Stockholm archipelago. Entomol. Tidskr. 2013, 134, 1–9. [Google Scholar]
- Sukovata, L.; Kolk, A.; Jaroszynska, J.; Krajewska, U.; Purzynska, A.; Isidorov, V. Host-tree preferences of the pine moth (Lepidoptera: Lasiocampidae) and pine beauty moth (Lepidopera: Noctuidae) larvae in relation to needle quality. Ecol. Surv. Manag. For. Insects Proc. 2003, 311, 98–106. [Google Scholar]
- Kolk, A.; Starzyk, J.R. Atlas of Forest Insect Pests; MULTICO Publishing House Ltd.: Warsaw, Poland, 1996. [Google Scholar]
- Śliwa, E. Barczatka Sosnówka [Pine-Tree Lappet Moth]; PWRiL: Warsaw, Poland, 1992. [Google Scholar]
- Kovač, M.; Lacković, N.; Pernek, M. Effect of Beauveria bassiana Fungal Infection on Survival and Feeding Behavior of Pine-Tree Lappet Moth (Dendrolimus pini L.). Forests 2020, 11, 974. [Google Scholar] [CrossRef]
- Matek, M.; Pernek, M. First Record of Dendrolimus pini Outbreak on Aleppo Pine in Croatia and Severe Case of Population Collapse Caused by Entomopathogen Beauveria bassiana. South-East Eur. For. 2018, 9, 91–96. [Google Scholar] [CrossRef]
- Keena, M.A.; Shi, J. Effects of Temperature on First Instar Lymantria (Lepidoptera: Erebidae) Survival and Development With and Without Food. Environ. Entomol. 2019, 48, 655–666. [Google Scholar] [CrossRef]
- Osier, T.; Jennings, S. Variability in host-plant quality for the larvae of a polyphagous insect folivore in midseason: The impact of light on three deciduous sapling species. Entomol. Exp. Appl. 2007, 123, 159–166. [Google Scholar] [CrossRef]
- Milanovic, S.; Lazarević, J.; Popović, Z.; Miletić, Z.; Kostić, M.; Radulović, Z.; Karadžić, D.; Vuleta, A. Preference and performance of the larvae of Lymantria dispar (Lepidoptera: Lymantriidae) on three species of European oaks. Eur. J. Entomol. 2014, 111, 371–378. [Google Scholar] [CrossRef]
- Łukowski, A.; Adamczyk, D.; Karolewski, P. Survival and Recovery of the Pine-Tree Lappet Dendrolimus pini When Subjected to Simulated Starvation. Insects 2020, 11, 67. [Google Scholar] [CrossRef] [PubMed]
- Elkinton, J.S.; Cardé, R.T. Distribution, Dispersal, and Apparent Survival of Male Gypsy Moths1 as Determined by Capture in Pheromone-Baited Traps2. Environ. Entomol. 1980, 9, 729–737. [Google Scholar] [CrossRef]
- Sierpińska, A. Towards an integrated management of Dendrolimus pini L. In Proceedings of the Population Dynamics, Impacts, and Integrated Management of Forest Defoliating Insects; Faculty Publications: Nacogdoches, TX, USA, 1998; Volume 226, pp. 129–142. [Google Scholar]
- Sukovata, L. A Comparison of Three Approaches for Larval Instar Separation in Insects—A Case Study of Dendrolimus pini. Insects 2019, 10, 384. [Google Scholar] [CrossRef] [PubMed]
- Grodner, J.; Zander, R. Sex propheromone of the pine tree lappet moth Dendrolimus pini and its use in attractant-based monitoring system. Pesticides 2010, 1–4, 43–49. [Google Scholar]
- Diaz, J.H. The evolving global epidemiology, syndromic classification, management, and prevention of caterpillar envenoming. Am. J. Trop. Med. Hyg. 2005, 72, 347–357. [Google Scholar] [CrossRef]
- Jagiełło, R.; Łakomy, P.; Łukowski, A.; Giertych, M.J. Spreading-the-risk hypothesis may explain Cameraria ohridella oviposition in relation to leaf blotch disease. Arthropod-Plant Interact. 2019, 13, 787–795. [Google Scholar] [CrossRef]
- Waldbauer, G. The consumption and utilization of food by insects. Adv. Insect Phys. 1968, 5, 229–288. [Google Scholar] [CrossRef]
- Mąderek, E.; Łukowski, A.; Giertych, M.J.; Karolewski, P. Influence of native and alien Prunus species and light conditions on performance of the leaf beetle Gonioctena quinquepunctata. Entomol. Exp. Appl. 2015, 155, 193–205. [Google Scholar] [CrossRef]
- Łukowski, A.; Giertych, M.; Zadworny, M.; Mucha, J.; Karolewski, P. Preferential Feeding and Occupation of Sunlit Leaves Favors Defense Response and Development in the Flea Beetle, Altica brevicollis coryletorum—A Pest of Corylus avellana. PLoS ONE 2015, 10, e0126072. [Google Scholar] [CrossRef]
- Hansen, J.; Møller, I. Percolation of starch and soluble carbohydrates from plant tissue for quantitative determination with anthrone. Anal. Biochem. 1975, 68, 87–94. [Google Scholar] [CrossRef]
- Haissig, B.E.; Dickson, R.E. Starch Measurement in plant tissue using enzymatic hydrolysis. Physiol. Plant. 1979, 47, 151–157. [Google Scholar] [CrossRef]
- Johnson, G.; Schaal, L.A. Accumulation of phenolic substances and ascorbic acid in potato tuber tissue upon injury and their possible role in disease resistance. Am. J. Potato Res. 1957, 34, 200–209. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Price, M.L.; Van Scoyoc, S.; Butler, L.G. A critical evaluation of the vanillin reaction as an assay for tannin in sorghum grain. J. Agric. Food Chem. 1978, 26, 1214–1218. [Google Scholar] [CrossRef]
- Ghorai, N.; Chakraborty, S.; Gucchait, S.; Saha, S.K.; Biswas, S. Estimation of total terpenoids concentration in plant tissues using a monoterpene, Linalool as standard reagent. Protoc. Exch. 2012, 1–7. [Google Scholar] [CrossRef]
- Cornelissen, J.H.C.; Lavorel, S.; Garnier, E.; Díaz, S.; Buchmann, N.; Gurvich, D.E.; Reich, P.; ter Steege, H.; Morgan, H.D.; Van Der Heijden, M.G.A.; et al. A handbook of protocols for standardised and easy measurement of plant functional traits worldwide. Aust. J. Bot. 2003, 51, 335–380. [Google Scholar] [CrossRef]
- Garnier, E.; Shipley, B.; Roumet, C.; Laurent, G. A standardized protocol for the determination of specific leaf area and leaf dry matter content. Funct. Ecol. 2001, 15, 688–695. [Google Scholar] [CrossRef]
- Bliss, C.I. The transformation of percentages for use in the analysis of variance. Ohio J. Sci. 1938, 38, 9–12. [Google Scholar]
- Luo, D.; Lai, M.; Xu, C.; Shi, H.; Liu, X. Life history traits in a capital breeding pine caterpillar: Effect of host species and needle age. BMC Ecol. 2018, 18, 24. [Google Scholar] [CrossRef] [PubMed]
- Kirichenko, N.; Baranchikov, Y.N.; Vidal, S. Performance of the potentially invasive Siberian moth Dendrolimus superans sibiricuson coniferous species in Europe. Agric. For. Entomol. 2009, 11, 247–254. [Google Scholar] [CrossRef]
- Füldner, K. Entwicklungserfolg von kiefernspinner (Dendrolimus pini Linnaeus, 1758: Lepidoptera, Lasiocampidae) an douglasie (Pseudotsuga menziesii), fichte (Picea abies) und kiefer (Pinus sylvestris) unter laborbedingungen. Allg. Forst. Jagdzeitung 2001, 172, 221–225. [Google Scholar]
- Knolhoff, L.M.; Heckel, D. Behavioral Assays for Studies of Host Plant Choice and Adaptation in Herbivorous Insects. Annu. Rev. Entomol. 2014, 59, 263–278. [Google Scholar] [CrossRef] [PubMed]
- Moreau, G.; Quiring, D.T.; Eveleigh, E.S.; Bauce, É. Advantages of a mixed diet: Feeding on several foliar age classes increases the performance of a specialist insect herbivore. Oecologia 2003, 135, 391–399. [Google Scholar] [CrossRef]
- Muller, K.; Thiéry, D.; Moret, Y.; Moreau, J. Male larval nutrition affects adult reproductive success in wild European grapevine moth (Lobesia botrana). Behav. Ecol. Sociobiol. 2014, 69, 39–47. [Google Scholar] [CrossRef]
- Singer, M.; Bernays, E.; Carrière, Y. The interplay between nutrient balancing and toxin dilution in foraging by a generalist insect herbivore. Anim. Behav. 2002, 64, 629–643. [Google Scholar] [CrossRef]
- Mumm, R.; Hilker, M. Direct and indirect chemical defence of pine against folivorous insects. Trends Plant Sci. 2006, 11, 351–358. [Google Scholar] [CrossRef]
- Ikeda, T.; Matsumura, F.; Benjamin, D.M. Mechanism of feeding discrimination between matured and juvenile foliage by two species of pine sawflies. J. Chem. Ecol. 1977, 3, 677–694. [Google Scholar] [CrossRef]
- Johns, R.C.; Tobita, H.; Hara, H.; Ozaki, K. Adaptive advantages of dietary mixing different-aged foliage within conifers for a generalist defoliator. Ecol. Res. 2015, 30, 793–802. [Google Scholar] [CrossRef]
- Beukeboom, L.W. Size matters in insects—An introduction. Entomol. Exp. Appl. 2018, 166, 2–3. [Google Scholar] [CrossRef]
- Łukowski, A.; Mąderek, E.; Giertych, M.J.; Karolewski, P. Sex Ratio and Body Mass of Adult Herbivorous Beetles Depend on Time of Occurrence and Light Conditions. PLoS ONE 2015, 10, e0144718. [Google Scholar] [CrossRef]
- Lee, K.P.; Roh, C. Temperature-by-nutrient interactions affecting growth rate in an insect ectotherm. Entomol. Exp. Appl. 2010, 136, 151–163. [Google Scholar] [CrossRef]
- Kaplan, I.; McArt, S.H.; Thaler, J.S. Plant Defenses and Predation Risk Differentially Shape Patterns of Consumption, Growth, and Digestive Efficiency in a Guild of Leaf-Chewing Insects. PLoS ONE 2014, 9, e93714. [Google Scholar] [CrossRef] [PubMed]
- Honěk, A. Intraspecific Variation in Body Size and Fecundity in Insects: A General Relationship. Oikos 1993, 66, 483. [Google Scholar] [CrossRef]
- Blanckenhorn, W.U. The evolution of body size: What keeps organisms small? Q. Rev. Biol. 2000, 75, 385–407. [Google Scholar] [CrossRef]
- Niinemets, U.; Ellsworth, D.S.; Lukjanova, A.; Tobias, M. Dependence of needle architecture and chemical composition on canopy light availability in three North American Pinus species with contrasting needle length. Tree Physiol. 2002, 22, 747–761. [Google Scholar] [CrossRef]
- An, P.-C.; Tang, D.-L.; Chen, H.; Yang, Q.; Ding, S.-T.; Wu, J.-Y. Pliocene white pine (Pinus subgenus Strobus) needles from western Yunnan, southwestern China. Hist. Biol. 2018, 31, 1–11. [Google Scholar] [CrossRef]
- Singh, L.; Dixit, P.; Srivastava, R.P.; Pandey, S.; Verma, P.C.; Saxena, G. Morpho-anatomical variation and their phylogenetic implications in native and exotic species of Pinus L. growing in the Indian Himalayas. An. Biol. 2020, 2020, 105–114. [Google Scholar] [CrossRef]
- Pelcastre, C.E.; Hernández-León, S.; Gernandt, D.S.; Arce-Cervantes, O.; Rodríguez-Laguna, R.; González-Ávalos, J. Taxonomic identification key with leaf anatomical characters for Pinus L. species in Hidalgo. Rev. Mex. Cienc. For. 2018, 9, 28–49. [Google Scholar] [CrossRef]
- Ghimire, B.; Lee, C.; Yang, J.; Heo, K. Comparative leaf anatomy of native and cultivated Pinus (Pinaceae) in Korea: Implication for the subgeneric classification. Plant Syst. Evol. 2015, 301, 531–540. [Google Scholar] [CrossRef]
- Karolewski, P.; Giertych, M.J.; Żmuda, M.; Jagodzinski, A.M.; Oleksyn, J. Season and light affect constitutive defenses of understory shrub species against folivorous insects. Acta Oecol. 2013, 53, 19–32. [Google Scholar] [CrossRef]
- Hatcher, P.E. Seasonal and age-related variation in the needle quality of five conifer species. Oecologia 1990, 85, 200–212. [Google Scholar] [CrossRef]
- Hanley, M.E.; Lamont, B.B.; Fairbanks, M.M.; Rafferty, C.M. Plant structural traits and their role in anti-herbivore defence. Perspect. Plant Ecol. Evol. Syst. 2007, 8, 157–178. [Google Scholar] [CrossRef]
- Karolewski, P.; Łukowski, A.; Walczak, U.; Baraniak, E.; Mucha, J.; Giertych, M.J. Larval food affects oviposition preference, female fecundity and offspring survival in Yponomeuta evonymellus. Ecol. Entomol. 2017, 42, 657–667. [Google Scholar] [CrossRef]
- Sukovata, L.; Asztemborska, M.; Rudziński, K.J.; Cieślak, M.; Staszek, D.; Janiszewski, W.; Szmigielski, R.; Kolk, A.; Raczko, J. Effect of dispenser type, trap design and placement on catches of pine-tree lappet moth, Dendrolimus pini. Phytoparasitica 2020, 48, 63–74. [Google Scholar] [CrossRef]
- Venette, R.C. Exotic Pine Pests: Survey Reference. USDA Forest Service, 2008. Available online: https://www.fs.usda.gov/Internet/FSE_DOCUMENTS/fsbdev2_026444.pdf (accessed on 30 June 2021).
- Hernández-Baz, F.; Romo, H.; González, J.M.; Hernández, M.D.J.M.; Pastrana, R.G. Maximum Entropy Niche-Based Modeling (Maxent) of Potential Geographical Distribution of Coreura albicosta (Lepidoptera: Erebidae: Ctenuchina) in Mexico. Fla. Entomol. 2016, 99, 376–380. [Google Scholar] [CrossRef]
- Scharf, I. The multifaceted effects of starvation on arthropod behaviour. Anim. Behav. 2016, 119, 37–48. [Google Scholar] [CrossRef]
- McCue, M.D. Starvation physiology: Reviewing the different strategies animals use to survive a common challenge. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2010, 156, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Gergs, A.; Jager, T. Body size-mediated starvation resistance in an insect predator. J. Anim. Ecol. 2014, 83, 758–768. [Google Scholar] [CrossRef]


| Scientific Name | Common Name | Place of Origin | Number of Needles in Fascicles | |
|---|---|---|---|---|
| 1. | Pinus armandii Franch. | Chinese white pine Eastern | Eastern Asia | Five |
| 2. | Pinus cembra L. | Stone pine | Europe | Five |
| 3. | Pinus contorta Dougl. ex Loud. | Lodgepole pine | North America | Two |
| 4. | Pinus koraiensis Siebold and Zucc. | Korean pine | Eastern Asia | Five |
| 5. | Pinus nigra Arn. | Black pine | Europe | Two |
| 6. | Pinus peuce Griseb. | Macedonian pine | Europe | Five |
| 7. | Pinus ponderosa Douglas ex C. Lawson | Ponderosa pine | North America | Three |
| 8. | Pinus strobus L. | White pine | North America | Five |
| 9. | Pinus sylvestris L. | Scots pine | Europe | Two |
| 10. | Pinus wallichiana A.B. Jacks | Himalayan pine | Eastern Asia | Five |
| A: Pine Origin | Larval Mass (g) | Pupal Mass (g) | Cocoon Mass (mg) | Adult Mass (g) | Wings Area (cm2) | Fecundity (no. Eggs) | DD (Day) | PP (Day) | TFE (g d.m.) | CI (g g−1 Day−1) | RGR (g g−1 Day−1) | AD (%) | ECI (%) | ECD (%) | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ANCOVA | df | F | p | F | p | F | p | F | p | F | p | F | p | F | p | F | p | F | p | F | p | F | p | F | p | F | p | F | p |
| Pine origin (Po) | 2 | 21.7357 | <0.0001 | 15.6451 | <0.0001 | 9.8975 | <0.0001 | 16.0361 | <0.0001 | 15.0088 | <0.0001 | 11.9165 | <0.0001 | 6.0039 | 0.0031 | 3.5827 | 0.0301 | 0.0017 | 0.9983 | 0.2910 | 0.7479 | 12.3612 | <0.0001 | 3.4169 | 0.0352 | 13.9226 | <0.0001 | 6.9901 | 0.0012 |
| Pine species [Po] | 7 | 9.9749 | <0.0001 | 7.3411 | <0.0001 | 3.4956 | 0.0016 | 8.5158 | <0.0001 | 6.2753 | <0.0001 | 8.1890 | <0.0001 | 3.9936 | 0.0005 | 1.8012 | 0.0905 | 12.8156 | <0.0001 | 5.7314 | <0.0001 | 5.6737 | <0.0001 | 5.1784 | <0.0001 | 3.3027 | 0.0026 | 2.6268 | 0.0136 |
| Sex (S) | 1 | 163.2014 | <0.0001 | 234.0973 | <0.0001 | 12.4169 | 0.0006 | 861.8713 | <0.0001 | 119.4269 | <0.0001 | 57.9123 | <0.0001 | 8.0228 | 0.0052 | 116.3617 | <0.0001 | 41.3346 | <0.0001 | 1.4211 | 0.2350 | 2.3150 | 0.1301 | 7.4947 | 0.0069 | 12.0833 | 0.0007 | ||
| Po × S | 2 | 3.1348 | 0.0462 | 7.0945 | 0.0011 | 1.0654 | 0.3471 | 6.2480 | 0.0024 | 5.2590 | 0.0062 | 1.6033 | 0.2045 | 3.0142 | 0.0519 | 2.3347 | 0.1001 | 0.2399 | 0.7870 | 1.5459 | 0.2163 | 1.5808 | 0.2090 | 0.9614 | 0.3846 | 2.7967 | 0.0640 | ||
| Initial larval mass (cov) | 1 | 30.6088 | <0.0001 | 25.8380 | <0.0001 | 13.8404 | 0.0003 | 16.7247 | <0.0001 | 13.1889 | 0.0004 | 27.2014 | <0.0001 | 26.9124 | <0.0001 | 0.4294 | 0.5132 | 0.4661 | 0.4958 | 1.9916 | 0.1601 | 10.0633 | 0.0018 | 9.7943 | 0.0021 | 13.4641 | 0.0003 | 1.7707 | 0.1852 |
| df error | 159 | 159 | 157 | 159 | 154 | 71 | 159 | 159 | 159 | 159 | 1590 | 159 | 159 | 159 | |||||||||||||||
| B: Number of needles in fascicles | Larval mass (g) | Pupal mass (g) | Cocoon mass (mg) | Adult mass (g) | Wings area (cm2) | Fecundity (no. eggs) | DD (day) | PP (day) | TFE (g d.m.) | CI (g g−1 day−1) | RGR (g g−1 day−1) | AD (%) | ECI (%) | ECD (%) | |||||||||||||||
| ANCOVA | df | F | p | F | p | F | p | F | p | F | p | F | p | F | p | F | p | F | p | F | p | F | p | F | p | F | p | F | p |
| Number of needles (Nn) | 2 | 45.0919 | <0.0001 | 33.1481 | <0.0001 | 8.1693 | 0.0004 | 38.9873 | <0.0001 | 26.0645 | <0.0001 | 32.8685 | <0.0001 | 8.3982 | 0.0003 | 6.6218 | 0.0017 | 20.9735 | <0.0001 | 6.6418 | 0.0017 | 20.4456 | <0.0001 | 5.5261 | 0.0048 | 6.7040 | 0.0016 | 5.7426 | 0.0039 |
| Pine species [Nn] | 7 | 3.0357 | 0.0050 | 1.7341 | 0.1046 | 4.1680 | 0.0003 | 1.9163 | 0.0702 | 2.4977 | 0.0186 | 2.1141 | 0.0529 | 3.2732 | 0.0028 | 0.9980 | 0.4348 | 7.4770 | <0.0001 | 3.9222 | 0.0006 | 3.4258 | 0.0019 | 4.7192 | <0.0001 | 5.3125 | <0.0001 | 2.7553 | 0.0100 |
| Sex (S) | 1 | 162.7075 | <0.0001 | 225.0700 | <0.0001 | 23.8280 | <0.0001 | 761.0352 | <0.0001 | 134.4132 | <0.0001 | 18.1564 | <0.0001 | 0.1903 | 0.6633 | 65.7105 | <0.0001 | 27.0208 | <0.0001 | 0.0089 | 0.9248 | 1.4248 | 0.2344 | 12.3074 | 0.0006 | 15.2884 | 0.0001 | ||
| Nn × S | 2 | 7.6766 | 0.0007 | 10.3064 | <0.0001 | 5.7117 | 0.0040 | 14.0336 | <0.0001 | 8.8260 | 0.0002 | 6.7481 | 0.0015 | 5.6230 | 0.0044 | 4.2452 | 0.0160 | 0.0966 | 0.9080 | 4.0842 | 0.0186 | 0.1044 | 0.9010 | 2.9468 | 0.0554 | 2.3127 | 0.1023 | ||
| Initial larval mass (cov) | 1 | 39.9266 | <0.0001 | 36.1313 | <0.0001 | 18.0245 | <0.0001 | 26.0921 | <0.0001 | 20.7870 | <0.0001 | 27.2014 | <0.0001 | 35.4491 | <0.0001 | 1.4658 | 0.2278 | 0.8288 | 0.3640 | 2.1871 | 0.1411 | 7.7213 | 0.0061 | 11.4024 | 0.0009 | 11.6252 | 0.0008 | 0.7647 | 0.3832 |
| df error | 159 | 159 | 157 | 159 | 154 | 71 | 159 | 159 | 159 | 159 | 1590 | 159 | 159 | 159 | |||||||||||||||
| A: Pine Origin | Water (%) | Nitrogen (%) | Starch (%) | TNC (%) | TPh (µM g−1 d.m.) | Tannins (µM g−1 d.m.) | TT (mg g−1 d.m.) | SLA (cm−2 g−1 d.m.) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ANCOVA | df | F | p | F | p | F | p | F | p | F | p | F | p | F | p | F | p |
| Pine origin (Po) | 2 | 0.6347 | 0.5316 | 14.1773 | <0.0001 | 16.8918 | <0.0001 | 4.5196 | 0.0124 | 38.4868 | <0.0001 | 23.0966 | <0.0001 | 14.2578 | <0.0001 | 90.8466 | <0.0001 |
| Pine species [Po] | 7 | 4.6250 | 0.0001 | 16.8374 | <0.0001 | 8.0918 | <0.0001 | 7.6947 | <0.0001 | 25.4490 | <0.0001 | 24.5070 | <0.0001 | 21.4331 | <0.0001 | 84.1642 | <0.0001 |
| Month (M) | 1 | 0.7262 | 0.3955 | 7.2648 | 0.0079 | 44.3518 | <0.0001 | 19.7187 | <0.0001 | 7.6109 | 0.0065 | 5.9364 | 0.0160 | 6.8408 | 0.0098 | 0.0340 | 0.8540 |
| Po × M | 2 | 3.2981 | 0.0397 | 0.2117 | 0.8095 | 0.8666 | 0.4225 | 0.2567 | 0.7739 | 0.4161 | 0.6604 | 0.8556 | 0.4272 | 1.4703 | 0.2332 | 0.4595 | 0.6325 |
| df error | 146 | 147 | 147 | 147 | 147 | 145 | 147 | 147 | |||||||||
| B: Number of needles in fascicles | Water (%) | Nitrogen (%) | Starch (%) | TNC (%) | TPh (µM g−1 d.m.) | Tannins (µM g−1 d.m.) | TT (mg g−1 d.m.) | SLA (cm−2 g−1 d.m.) | |||||||||
| ANCOVA | df | F | p | F | p | F | p | F | p | F | p | F | p | F | p | F | p |
| Number of needles (Nn) | 2 | 5.8304 | 0.0037 | 10.5012 | <0.0001 | 12.5336 | <0.0001 | 5.8964 | 0.0034 | 11.5970 | <0.0001 | 17.9745 | <0.0001 | 1.1192 | 0.3293 | 366.3140 | <0.0001 |
| Pine species [Nn] | 7 | 3.1526 | 0.0039 | 18.3808 | <0.0001 | 9.2315 | <0.0001 | 7.3169 | <0.0001 | 33.1239 | <0.0001 | 25.6868 | <0.0001 | 25.1421 | <0.0001 | 14.1982 | <0.0001 |
| Month (M) | 1 | 0.6602 | 0.4178 | 2.2768 | 0.1335 | 26.2360 | <0.0001 | 8.8520 | 0.0034 | 3.1828 | 0.0765 | 2.1777 | 0.1422 | 1.3638 | 0.2448 | 3.5949 | 0.0599 |
| Nn × M | 2 | 3.0163 | 0.0520 | 1.9519 | 0.1457 | 0.2591 | 0.7721 | 0.3847 | 0.6814 | 0.4002 | 0.6709 | 0.5676 | 0.5682 | 1.3383 | 0.2655 | 6.3288 | 0.0023 |
| df error | 146 | 147 | 147 | 147 | 147 | 145 | 147 | 147 | |||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Łukowski, A.; Giertych, M.J.; Adamczyk, D.; Mąderek, E.; Karolewski, P. Preference and Performance of the Pine-Tree Lappet Dendrolimus pini on Various Pine Species. Forests 2021, 12, 1261. https://doi.org/10.3390/f12091261
Łukowski A, Giertych MJ, Adamczyk D, Mąderek E, Karolewski P. Preference and Performance of the Pine-Tree Lappet Dendrolimus pini on Various Pine Species. Forests. 2021; 12(9):1261. https://doi.org/10.3390/f12091261
Chicago/Turabian StyleŁukowski, Adrian, Marian J. Giertych, Dawid Adamczyk, Ewa Mąderek, and Piotr Karolewski. 2021. "Preference and Performance of the Pine-Tree Lappet Dendrolimus pini on Various Pine Species" Forests 12, no. 9: 1261. https://doi.org/10.3390/f12091261
APA StyleŁukowski, A., Giertych, M. J., Adamczyk, D., Mąderek, E., & Karolewski, P. (2021). Preference and Performance of the Pine-Tree Lappet Dendrolimus pini on Various Pine Species. Forests, 12(9), 1261. https://doi.org/10.3390/f12091261







